Abstract
Several studies provide empirical evidence for the association between impulsivity and time perception. However, little is known about the neural substrates underlying this function. This investigation examined the influence of impulsivity on neural activation patterns during the encoding and reproduction of intervals with durations of 3, 9 and 18 seconds using event-related functional magnetic resonance imaging (fMRI). Twenty-seven subjects participated in this study, including 15 high impulsive subjects that were classified based on their self-rating. FMRI activation during the duration reproduction task was correlated with measures of two self-report questionnaires related to the concept of impulsivity (Barratt Impulsiveness Scale, BIS; Zimbardo Time Perspective Inventory, ZTPI). Behaviorally, those individuals who under-reproduced temporal intervals also showed lower scores on the ZTPI future perspective subscale and higher scores on the BIS. FMRI activation revealed an accumulating pattern of neural activity peaking at the end of the 9- and 18-s interval within right posterior insula. Activations of brain regions during the reproduction phase of the timing task, such as those related to motor execution as well as to the ‘core control network’ – encompassing the inferior frontal and medial frontal cortex, the anterior insula as well as the inferior parietal cortex – were significantly correlated with reproduced duration, as well as with BIS and ZTPI subscales. In particular, the greater activation in these regions the shorter were the reproduced intervals, the more impulsive was an individual and the less pronounced the future perspective. Activation in the core control network, thus, may form a biological marker for cognitive time management and for impulsiveness.
Keywords: time perception, duration reproduction, impulsivity, time perspective, fMRI
1. Introduction
Empirical evidence suggests associations between impulsiveness, impulsive decision making, and an altered sense of time (Berlin and Rolls, 2004; Rubia et al., 2009; Wittmann et al., 2007). Although impulsivity in general can be characterized by an individual’s rapid response without appropriate forethought (Sweitzer et al., 2008), different methods of assessment – ranging from questionnaires to various behavioral tasks – do not necessarily correlate (Carillo-de-la-Peña et al., 1993; Gerbing and Patton, 1987). These dissociations imply that the concept of impulsivity consists of multiple components (Arce and Santisteban, 2006). Nevertheless, impulsivity can be defined as a pattern of unplanned actions without regard for the negative consequences that might follow, i.e. to prefer immediate gains over long-term consequences; this construct has been successfully linked to many psychiatric syndromes including substance use and dependence (Lane et al., 2003b; Moeller et al., 2001). We have proposed that impulsive choices such as opting for smaller and sooner rewards over larger but delayed rewards are due to the subjective overestimation of the duration of the delay (Wittmann and Paulus, 2008; 2009). In particular, highly impulsive person process time differently, i.e. they overestimate duration. Delays are experienced as too high of a cost, which becomes apparent in premature responses, a decreased tolerance to delays, poor foresight and the selection of relatively smaller rewards that can be consumed earlier (Rubia et al., 2009). Impulsive patients from psychiatric populations devalue (discount) temporally delayed rewards more strongly than comparison subjects (Crean et al., 2000; Kirby et al., 1999). Moreover, a stronger present time perspective and a less pronounced future time perspective predicts impulsive behavior and drug use (Keough et al., 1999). Time perspective is a fundamental dimension in the construction of subjective time partitioning human experience into past, present, and future. Drug-dependent persons, who show stronger impulsive behavior in decision making, score significantly lower on a future orientation scale and their future perspective is less extended (Petry et al., 1998; Smart, 1968).
Regarding the estimation of duration in the seconds-to-minutes range, several studies have shown that impulsive individuals overestimate and under-produce time intervals. An under-production of an interval is indicative of an overestimation of time; if more time has passed subjectively for an individual she will indicate earlier that a given duration has passed (Melges and Fougerousse, 1966). Patients with borderline personality disorder as well as patients with orbitofrontal cortex lesions, individuals who are highly impulsive, under-produced and overestimated time intervals in the multiple seconds range (Berlin and Rolls, 2004; Berlin et al., 2004). Cocaine and methamphetamine dependent patients participating in an inpatient alcohol and drug treatment program overestimated the duration of a 53 s interval, estimates that were mediated by higher self-reported impulsivity (Wittmann et al., 2007). Sleep-deprived subjects, compared to when they were well rested, discounted delayed rewards more strongly and under-produced as well as under-reproduced time intervals of multiple seconds duration (Reynolds and Schiffbauer, 2004). Children with attention deficit hyperactivity disorder (ADHD) show an altered timing performance in several domains of time perception and at the same time show stronger discounting of delayed rewards (Barkley et al., 2001a; Smith et al., 2002), findings that have led some investigators to propose that impulsiveness can essentially be described as a deficit in temporal processing (Rubia et al., 2009).
There is considerable uncertainty on how and where in the brain time is processed (Rubia and Smith, 2004; Wittmann, 2009a; b). The lack of agreement as to which mechanisms account for the perception of time is evident by the number of different psychological and neural models (Wittmann and van Wassenhove, 2009). While there is evidence suggesting that the processing of duration relies on the integrity of the whole brain (Coslett et al., 2009), specific neural models have been proposed for the perception of time in the milliseconds-to-seconds range. Among these models are the coincidence detection model using oscillatory signals in cortico-striatal circuits (Matell and Meck, 2004), generalized magnitude processing for time, space and number in the right posterior parietal cortex (Bueti and Walsh, 2009), event timing and temporal prediction in the cerebellum (Ivry et al., 2002), working memory related integration in the right prefrontal cortex (Lewis and Miall, 2006), as well as the integration of self- and body processes in the anterior insula (Craig, 2008; 2009a). Other investigators assume memory-loss components as intrinsic features in theoretical models of time perception (Staddon, 2005; Wackermann and Ehm, 2006), or propose that the amount of energy spent during cognitive processing defines the subjective experience of duration (Eagleman and Pariyadath, 2009). In a recent event-related functional magnetic resonance imaging (fMRI) study we reported that activation in the dorsal posterior insular cortex was linked to the perception of time in a duration reproduction task using intervals of 9 and 18 s (Wittmann et al., 2010b). Neural time-activity curves showed that activation in the posterior insula increased linearly during the encoding interval of the task (i.e., during presentation of the tone that had to be temporally reproduced). A similar linear increase in activation was seen during the reproduction interval of the task in the anterior insula, inferior frontal and medial frontal cortex bilaterally. We suggested that this accumulator-like activity in the posterior insula during the encoding interval might signify an integration of body signals over time that could be used to represent duration.
Since temporal processing deficits are assumed to be associated with impulsivity, neuroimaging studies of duration processing in impulsive individuals could provide insight into the neural basis of time perception and of impulsiveness. To this end we selected a subset of student subjects with large variability on self-rated impulsivity for a functional magnetic resonance imaging (fMRI) study while they completed a duration reproduction task that had been used in a preceding functional imaging study with healthy controls (Wittmann et al., 2010b). We related performance in the duration reproduction task and related brain activation with self-report measures of impulsiveness (Barratt Impulsiveness Scale; BIS) (Barratt et al., 1999), of the temporal perspective (Zimbardo Time Perspective Inventory; ZTPI) (Zimbardo and Boyd, 1999), and performance in a delay discounting task – all of which have been shown to be related to trait impulsiveness (Barkley et al., 2001b; Rubia et al., 2009). Subjects were selected on the basis that they were healthy young students with a wide range of trait impulsivity. We, therefore, conducted careful psychiatric and medical assessments to make sure that the students did not fulfill any DSM-IV criteria for psychiatric diagnoses.
Specifically, we examined whether trait impulsivity would relate to neural activation of accumulator-like activity in the posterior insula during the encoding of intervals in the timing task or whether impulsivity would relate to more frontal brain regions during the reproduction phase of the task. We used a task with three durations that had to be reproduced: 3 s, 9 s, and 18 s. The longest time interval is of a magnitude that has so far not been employed in an fMRI study with impulsive individuals. Most behavioral studies showing an overestimation of duration in impulsive individuals used intervals in the multiple-seconds-to-minutes range. With the choice of 9 s and 18 s intervals we compromised in having comparably long intervals and at the same time having a feasible duration for an fMRI setting. Moreover, in having both shorter and longer time intervals, we were able to specifically probe whether brain activation for the two longer durations correlates more with impulsivity measures than the shorter interval. Specifically, evidence suggests that temporal intervals up to around 3 seconds are governed by different mechanisms than intervals exceeding this approximate time range (Pöppel, 2009, Ulbrich et al., 2006).
2. Results
2.1. Behavioral results
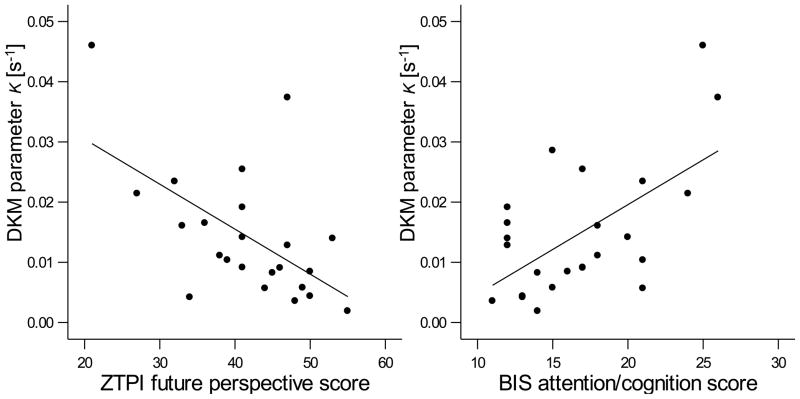
Across all 27 subjects, the mean of the reproduced intervals for the 3-s condition was 2.881 s (S.D.: 0.50 s) and with increasing interval lengths was progressively under-reproduced relative to physical time: 7.956 s (S.D. = 1.39 s) for the 9-s interval and 13.245 s (S.D. = 2.99 s) for the 18-s interval (see Supporting Figure 1), this behavioral signature being in accordance with former studies employing the temporal reproduction method (Noulhiane et al., 2009; Sawyer et al., 1994). The coefficients of variation for duration reproduction performance across groups were in a similar range and they did not decrease with increasing interval length (see Table 2), an indication that subjects did not systematically use counting strategies (Clément and Droit-Volet, 2006). The DKM parameter κ, measuring the loss rate of internal duration representation (Wackermann and Ehm, 2006), and which is responsible for the progressive under-reproduction of durations, had an average value of 0.0142 s−1 (S.D. = 0.0108 s−1, range = 0.00189 s−1 – 0.0460 s−1), which is in a typical range found in young individuals (Wackermann et al., 2008). Parameter κ correlated negatively with the future perspective subscale of the ZTPI (Pearson’s r = −0.578, p < 0.004; partial correlation with subjects’ drug use factored out: r = −0.669, p < 0.011), this association indicating that the stronger the under-reproduction of temporal intervals, the weaker was the future perspective (Fig. 1). Positive correlations between κ and the BIS (Fig. 1) showed how higher impulsivity related to a stronger under-reproduction of duration (BIS attention/cognition: r = 0.645, p < 0.001; partial r = 0.593, p < 0.007).
Table 2.
Mean DKM parameter κ (s-1), mean reproduced length (s) and coefficient of variation of the duration reproduction task for three durations, as well as discounting parameter k compared between stimulant-using, impulsive subjects as well as stimulant-naïve controls.
| Ever used Popular types |
Stimulant users (n = 10) | Impulsive (n = 5) | Controls (n = 12) | |||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| BIS non-planning | 25.2 | 6.6 | 26 | 0.7 | 21 | 4 |
| BIS motor | 24.8 | 6.2 | 32 | 5.6 | 21 | 4.1 |
| BIS attention/cognition | 16.6 | 3.8 | 21.4 | 4.4 | 15 | 3.2 |
| ZTPI present-hedonistic | 54.3 | 4.5 | 59.2 | 5.8 | 54.8 | 6.4 |
| ZTPI present fatalistic | 22 | 5.2 | 27.2 | 3.7 | 21 | 5.1 |
| ZTPI future | 40.9 | 7.7 | 35.6 | 9.8 | 44.6 | 6.3 |
| DKM parameter κ | 0.0128 | 0.0052 | 0.0302 | 0.0141 | 0.0092 | 0.0007 |
| 3-s mean reproduction | 2.821 | 0.312 | 2.706 | 0.762 | 3.004 | 0.523 |
| 3-s CV reproduction | 30.6 | 11.1 | 24.3 | 9.1 | 19.6 | 4.6 |
| 9-s mean reproduction | 8.042 | 1.238 | 7.178 | 1.982 | 8.209 | 1.249 |
| 9-s CV reproduction | 26.9 | 10.1 | 30.1 | 10.2 | 22.7 | 9.0 |
| 18-s mean reproduction | 13.760 | 1.940 | 11.939 | 5.503 | 13.360 | 2.455 |
| 18-s CV reproduction | 29.9 | 13.6 | 23.2 | 5.0 | 30.5 | 12.2 |
| Discounting parameter k | 0.488 | 0.473 | 0.053 | 0.036 | 0.188 | 0.119 |
Figure 1.
Significant Pearson correlations between the behavioral measure of duration reproduction (DKM parameter κ) and the scores of the questionnaire subscales ZTPI future perspective and BIS attention/cognition (r = 0.578, p < 0.004). The more subjects under-reproduce the temporal intervals (indicated by a larger κ) the less pronounced is the future time perspective and the more impulsiveness is reported (r = 0.645, p < 0.001).
Individual subject data of the delay discounting task were fitted to the hyperbolic equation and explained on average 91.1% of the variance; the inter-individual average k value representing the discounting rate was 0.277 (S.D. = 0.343, range = 0.011 – 1.15). The supporting Fig. S2 in the supplementary content shows the hyperbolic function derived by fitting the mean indifference points of all 27 subjects across the delay values. The larger the delay of the reward when choosing between the immediate and the delayed reward, the less likely subjects chose the delayed reward. Discounting rate k did not significantly correlate with any of the questionnaire scores. A larger AUC in the delay discounting task (typically associated with more self-control) was marginally related to stronger under-reproduction, that is, to a larger value of DKM parameter κ (r = 0.435, p < 0.038; partial r = 0.412, p < 0.079).
In addition, the BIS subscales correlated with two ZTPI subscales. The more impulsive subjects were according to their self reported impulsivity, the less pronounced was their future perspective: negative correlations of the future perspective existed with non-planning impulsivity (r = −0.685, p < 0.0001; partial r = −0.758, p < 0.0001) and with motor impulsivity (r = −0.52, p < 0.008; partial r = −0.56, p < 0.01). In addition, the more impulsive individuals were, the more they were present fatalistic: positive correlations of the present fatalistic subscale existed with non-planning impulsivity (r = 0.660, p < 0.0001; partial r = 0.674, p < 0.001) and with attention/cognition impulsivity (r = 0.557, p < 0.004; partial r = 0.608, p < 0.003).
2.2. fMRI results
2.2.1 Areas of significant activation
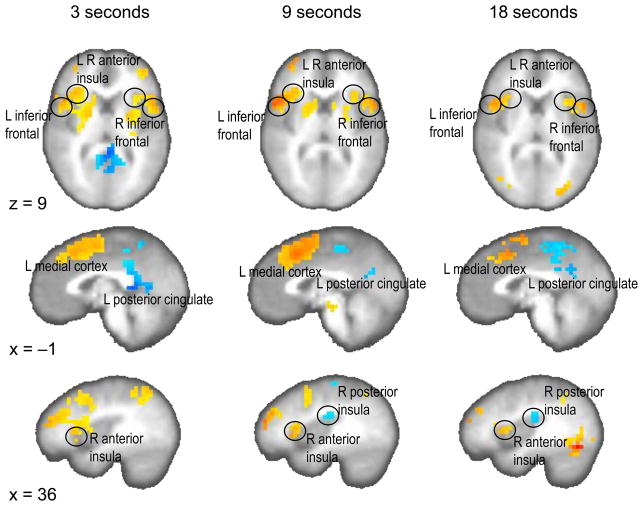
Clustered results for the encoding interval versus control interval contrast and the reproduction interval versus control interval contrast are provided in the supplementary content (Supporting Tables S1, S2; supporting Figs. S3, S4). Many of the regions found in these two contrasts showed notable overlap with the reproduction versus encoding contrast results (Fig. 2, Supporting Table S3). These results provide a strong replication of prior work (Wittmann et al., 2010b). Across all three durations, the left and right medial frontal region and the left and right anterior frontal cortices were activated more during the reproduction interval than during the encoding condition. Moreover, the left and right anterior insula was significantly activated in the reproduction versus encoding phases across all three interval lengths. More positive activation in the encoding as compared to the reproduction phase was consistently found across all three durations in the posterior cingulate cortex and precuneus (both left- and right-sided). In the 9 s and 18 s duration condition of the encoding phase, the right posterior insula was activated. In summary, more posterior regions of the brain (posterior insula, posterior cingulate cortex) were activated in the encoding phase, whereas more frontal regions were activated (inferior frontal cortex, anterior insula) in the reproduction phase of the timing task (Fig. 2).
Figure 2.
Significant brain activation for the contrast reproduction phase versus encoding phase (p < 0.01, corrected) on one axial (z = 9) and two sagittal (x = −1, x = 36) planes separately for the three temporal intervals. Stronger activation in the reproduction phase is colored in yellow to red, stronger activation in the encoding phase is coded in blue.
The comparison of the reproduction versus encoding contrast with the corresponding encoding versus control and reproduction versus control contrasts (Supporting Tables S1, S2) corroborated that mainly areas of the inferior frontal cortex, the anterior insula and medial frontal areas were activated in the reproduction phase and that (among others) the posterior insula and the posterior cingulate cortex were activated in the encoding phase (Supporting Table S3). Moreover, in order to investigate brain activation independent of duration, we tested the duration-independent contrast ‘reproduction phase versus encoding phase’ by collapsing all three time intervals. Complementing the duration-dependent contrasts, stronger activation in the reproduction as compared to the encoding phase can be found in left and right medial frontal, left and right inferior frontal, left and right anterior insula. Stronger activation in the encoding phase as compared to the reproduction phase is seen in left and right posterior cingulate cortex and precuneus (Supporting Table S4).
2.2.2 Time activity curves
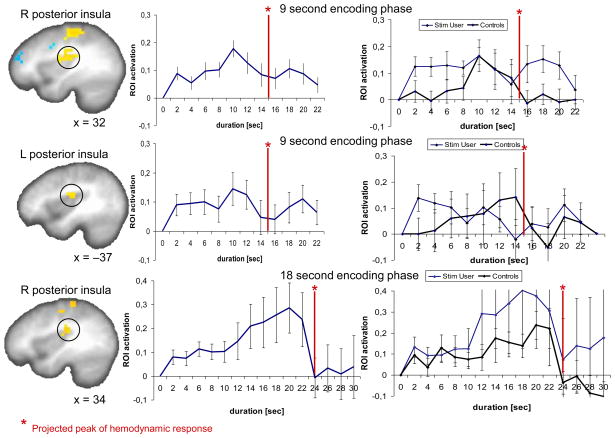
Time activity curves across the encoding phase were plotted for each ROI defined by the significant contrast in the encoding phase versus control condition for the 9-s and the 18-s condition. Two temporal signatures of brain activity were seen in both the 9-s and the 18-s encoding conditions (Supporting Figs. S5, S6): (1) in the majority of identified ROI a rise of activity was detected that peaks at 10 s (4 s into the interval plus 6 s delay of the hemodynamic function) and then gradually decreased towards the end of the interval. However, in the posterior insula a different type of activity was seen. Especially, in the left posterior insula in the 9-s condition and the right posterior insula in the 18-s condition there was a steady increase in activity that peaked close to the end of the interval (Fig. 3). This climbing activity could be seen for the average of all 27 subjects in the 18-s condition for the right posterior insula as well as when inspecting only the time activity curve of the left posterior insula for the control subjects. In analyzing the slopes for the sites of climbing brain activity up to the time point where activity breaks down (Fig. 3), one-sample t tests on the individual linear regression slopes showed that the slopes rose significantly in the 9-s condition in the right posterior insula (t27 = 3.397, p < 0.001, 22/27 subjects had a positive slope between T0 to T12) and in the left posterior insula (t27 = 1.979, p < 0.029, 21/27 subjects had a positive slope between T0 to T12). 24 out 27 subjects had a positive slope between T0 to T22 in the right posterior insula in the 18-s condition (one-sample t test: t27 = 1.757, p < 0.045). Across all 27 subjects, the slopes as well as the peak activations did not correlate with any of the behavioral or questionnaire variables, that is, self-reported impulsivity was not related to the increase of activation in the encoding phase. In the reproduction phase, time activity in most ROI exhibited a similar temporal profile, i.e. showing a monotonic rise followed by a drop at around the button press (Supporting Figs. S7, S8 online).
Figure 3.
Time activity curves during the encoding phases of the 9-s (right posterior insula, x = 32) and 18-s duration reproduction task (left and right posterior insula, x = −37, x = 34, respectively). The red line represents the end of the stimulus (= interval length plus 6 s delay of the hemodynamic function). On the left side activation plots across all 27 subjects are presented, on the right side time activity curves are plotted separately for the control subjects and the stimulant using individuals (the two subject groups with comparable group size).
2.2.3. Correlations between behavioral and fMRI results – duration dependent
Correlations between outward timing performance and activation in contrast-dependent ROI were more pronounced in the reproduction versus encoding contrasts (as presented below, see also Supporting Table S3) than for the respective reproduction versus control and encoding versus control contrasts (Supporting Tables S1, S2 online), separately assessed for the three interval conditions. All correlations were also tested for significance after false discovery rate (FDR) correction. Those correlations that are significant after this correction method are accordingly labeled as “significant after FDR correction”.
fMRI and duration reproduction
DKM parameter κ correlated positively with activations in the reproduction phase (as compared with the encoding phase) of the 9-s condition in the left inferior parietal cortex (r = 0.551, p < 0.005; partial r = 0.578, p < 0.01) and the right cerebellum (r = 0.632, p < 0.001, significant after FDR correction; partial r = 0.644, p < 0.003) (Fig. 4); in the 18-s condition DKM parameter κ correlated positively with activation in the reproduction phase in the left anterior insula (r = 0.562, p < 0.004; partial r = 0.609, p < 0.006), the left pre-post central cortex (r = 0.618, p < 0.001; partial r = 0.614, p < 0.005), and the left and right medial frontal cortex (r = 0.468, p < 0.021; partial r = 0.593, p < 0.007) (Fig. 5). All these correlations indicated that more pronounced activation in the respective ROI was associated with a stronger under-reproduction of intervals.
Figure 4.
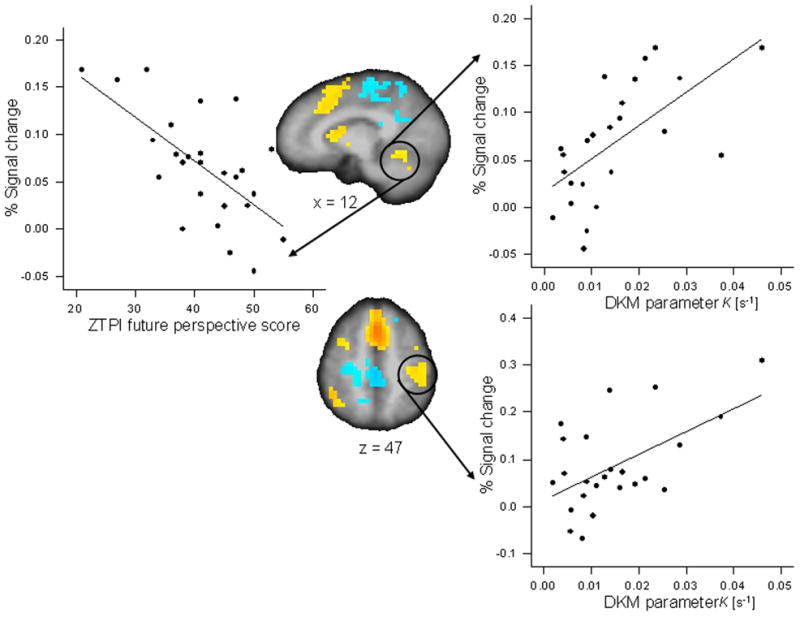
Significant Pearson correlations between activation in regions of interest for the reproduction versus encoding contrast for the 9-s duration condition and behavioral variables. Activation in the right cerebellum (x = 12) correlated with DKM parameter κ of duration reproduction (r = 0.632, p < 0.001) and with the ZTPI future perspective score (r = −0.647, p < 0.0001). Activation in the left inferior parietal and post-central cortex (z = 47) correlated with DKM parameter κ (r = 0.551, p < 0.005).
Figure 5.
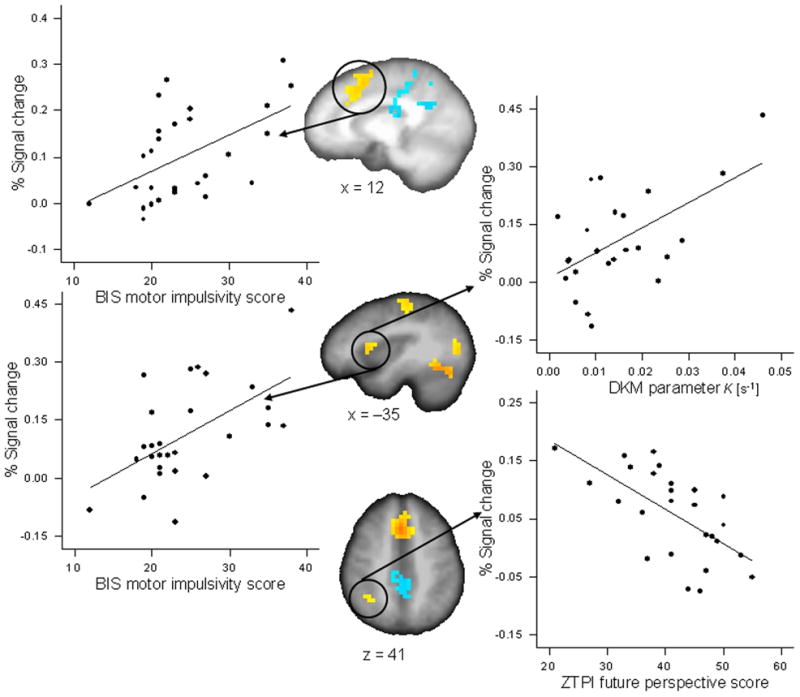
Significant Pearson correlations between activation in regions of interest for the reproduction versus encoding contrast for the 18-s duration condition and behavioral variables. The BIS subscale motor impulsivity correlated with activation in the left and right medial frontal cortex (x = 12) (r = 0.521, p < 0.005) and with activation in the left anterior insula and inferior frontal cortex (x = −35) (r = 0.564, p < 0.002). DKM parameter κ correlated with activation in the left anterior insula and inferior frontal cortex (x = −35) (r = 0.562, p < 0.004). The ZTPI future perspective score correlated with activation in right inferior parietal cortex (z = 41) (r = −0.636, p < 0.0001).
fMRI and BIS Questionnaire
The BIS subscore motor impulsivity correlated positively with activation in the left and right medial frontal cortex (r = 0.521, p < 0.005; partial r = 0.706, p < 0.0001; significant after FDR correction) and within the left anterior insula and pre-central cortex (r = 0.564, p < 0.002; partial r = 0.442, p < 0.045) in the 18-s reproduction phase; the BIS subscore attention/cognition impulsivity correlated positively with activation in the left pre-post central cortex in the 18-s reproduction phase (r = 0.502, p < 0.008; partial r = 0.526, p < 0.014) (Fig. 5); that is, more activation in this region during the timing task was associated with participants’ degree of impulsivity.
fMRI and ZTPI Questionnaire
The ZTPI future perspective correlated negatively with activation in the right inferior frontal cortex in the 3-s reproduction condition (r = −0.678, p < 0.0001, significant after FDR correction; partial r = −0.685, p < 0.001), it correlated negatively with activation in the right cerebellum in the 9-s reproduction condition (r = −0.647, p < 0.0001, significant after FDR correction; partial r = −0.669, p < 0.001), and it correlated negatively with activation in the left inferior parietal cortex (r = −0.636, p < 0.0001, significant after FDR correction; partial r = −0.632, p < 0.003) in the 18-s reproduction condition (Figs. 4 and 5, Supporting Table S3). That is, activity in these brain regions was associated with a weaker subjects’ future perspective.
Correlations between behavioral and fMRI results – duration independent
fMRI and duration reproduction
DKM parameter κ correlated positively with activation in the reproduction phase (as compared to the encoding phase) in the left inferior parietal cortex (r = 0.558, p < 0.005; partial r = 0.508, p < 0.011) and a region encompassing the left inferior parietal cortex and the pre-central cortex (r = 0.569, p < 0.004; partial r = 0.527, p < 0.009).
fMRI and BIS Questionnaire
The BIS subscore attention/cognition impulsivity correlated positively with activation in the right posterior cingulate in the encoding phase (r = 0.542, p < 0.004; partial r = 0.413, p < 0.019)
fMRI and ZTPI Questionnaire
The ZTPI future perspective correlated negatively with activation in right inferior parietal cortex in the reproduction phase (r = −0.596, p < 0.001; partial r = 0.578, p < 0.001). The ZTPI present fatalistic correlated negatively with activation in the right posterior cingulate in the encoding phase (r = −0.541, p < 0.004; partial r = −0.446, p < 0.010).
3. Discussion
This study examining the relationship between impulsivity and duration processing yielded three main results: First, more impulsive individuals under-reproduced temporal intervals more strongly and had a less pronounced future time perspective. Second, accumulating brain activation in the posterior insula during the encoding phase was observed, which confirms our previous findings and implies a potential generating process for the representation of time (Wittmann et al., 2010b). Third, the degree of brain activation in motor execution areas and the ‘core control network’ (see below) during the duration reproduction task correlated with both self-rated impulsivity and with behavioral performance in the duration reproduction task.
In our “enriched” student group of subjects, timing behavior in the duration reproduction task correlated with self-rated impulsivity and the future perspective, that is, the more impulsive individuals were and the lower their future perspective score the more they under-reproduced the durations (a larger DKM parameter κ) in the multiple-seconds range. Thus, one could argue that parameter κ – representing the progressive under-reproduction of duration –, impulsivity as personality trait and the future time perspective seem to be inter-related measures of an individual. The delay discounting task did not relate to the duration reproduction task or to the other measures of impulsivity. It is a common finding that correlations across different methods of assessing impulsivity, especially between self-rating questionnaires and behavioral tasks, if at all existent, are at most modest (Carillo-de-la-Peña et al., 1993; Lane et al., 2003b). It is also possible that since the delays of the higher rewards in the seconds range were relatively short, impulsive individuals did not exhibit delay aversion (Scheres et al., 2006). We had selected our delay discounting task because the delays are comparable to the intervals used in the duration reproduction task. It seems that for a delay discounting task to be sensitive to trait impulsivity in young healthy individuals longer delays are required.
Regarding the correlations of the three inter-related measures with brain activation during the reproduction phase of the task, these measures associated with similar regions of interest. That is, the shorter the reproduced duration the more activity was found in left and right inferior frontal cortex as well as in the left parietal cortex, regions that are typically involved in executive functions such as working memory, attention and inhibitory control (Aron et al., 2003; Roberts and Garavan, 2010), but also have been implicated in time perception of multiple seconds (Koch et al., 2009). In addition, the left anterior insula and pre-post central cortex were more activated when people produced relatively shorter intervals. Moreover, regions such as the cerebellum (which have also been discussed as locus of control for temporal processes in perception and action; Ivry et al., 2002) and the pre-post central cortex, related to motor execution, were found to be more active when subjects produced relatively shorter intervals. Activations in the left and right medial frontal cortex, anterior insula and the left pre-post central cortex were associated with increased self-reported impulsivity in individuals. A less pronounced future perspective was associated with more activation in left inferior parietal and inferior frontal cortex as well as the right cerebellum.
To sum up these diverse findings, two functional clusters of brain activation were activated during the reproduction phase of the timing task and positively related to timing behavior and impulsivity scores. These brain areas are related to motor execution (left pre-post central cortex, right cerebellum) as well to the ‘core control network’ (inferior frontal, parietal, medial frontal cortex, anterior insula). These latter brain regions have recently been identified as coactive regions which form a functionally connected cognitive core control network, a system for task-dependent control of sensory information and goal-directed behavior (Cole and Schneider, 2007; Craig, 2009b). Less activation in these areas has shown to be related to dysfunctions in timing behavior in children with ADHD (Rubia et al., 2009). Increased (anterior insula) and decreased (fronto-parietal) activation related to executive control has been reported in adults with ADHD (Schneider et al., 2010). Moreover, a recent study has shown that the cognitive core control network is more activated in impulsive drug users relative to control subjects performing a task of response inhibition (Roberts and Garavan, 2010). In our study, the cognitive control network was engaged during the processing of time, and activation was increased in more impulsive individuals. In the context of dysfunction, a decrease in fMRI-related activation is typically interpreted as a sign of co-occurring deficient processing, an increase in activation is considered as indicative of an increased recruitment of neural networks in order to compensate for a dysfunction.
The question remains why in our study impulsive individuals had stronger performance-related activation in the core control network, whereas in a lot of other neuroimaging studies on time perception impulsive subjects exhibit deactivations in the involved neural structures (for an overview, see Rubia et al., 2009). First, we have to point to differences in the subject population; our subjects, as opposed to other subject populations diagnosed with impulsivity, for example, in individuals with attention-deficit hyperactivity disorder or with drug dependence, were psychiatrically and neurologically healthy. Secondly, in former neuroimaging studies with impulsive participants the temporal intervals were in the range of milliseconds to a few seconds. Regarding the tone intervals with 9 and 18 s, individuals in our study were confronted with much longer durations that so far have not been employed in studies probing for duration related neural activation in impulsive individuals. Only with longer intervals, the experience of boredom and emotional distress, i.e. the feeling of impatience can emerge. The reports of impulsive individuals overestimating duration and of producing or reproducing shorter intervals, can be explained by increased arousal levels (Droit-Volet and Meck, 2007; Wittmann and Paulus, 2008); and increased arousal levels could have lead to stronger activation in the cognitive control network. That is, in the context of cognitive models of duration processing, besides an assumed internal clock component, many additional processing components are involved in timing tasks such as attention, working memory, decision making as well as motor preparation and execution, functions which are related to the core control network (Church, 1984; Zakay and Block, 1997). To be able to accurately reproduce temporal intervals the whole cognitive machinery has to function. Arousal dependent greater activation of the cognitive control network, therefore, could have been positively associated with smaller reproduced intervals and greater impulsivity.
Regarding the components of the cognitive control network in relation to the experience of time, the anterior insula and the medial frontal cortex have repeatedly been identified as underlying duration processing in the sub- and supra-seconds range (Craig, 2008; 2009a; Macar et al., 2004; Morillon et al., 2009; Wiener et al., 2010; Wittmann et al., 2010b;c). It has been suggested that the anterior insula is a locus of unified meta-representation of homeostatic feelings which constitutes the experienced self at one moment, providing a continuity of subjective awareness across time through a series of elementary emotional moments (Craig, 2009a;b). In this model, the anterior insula and the medial frontal cortex are conjointly engaged during task performance as complementary limbic sensory and limbic motor regions that work together. Both regions were also found to be activated in the reproduction phase of our timing study.
More specifically, and similar to a previous study employing the same duration reproduction task (Wittmann et al., 2010b), activation curves over time showed an accumulating pattern of neural activity, which peaked at the end of the interval within the posterior insula cortex during the presentation of 9- and 18-s tone intervals. These time activity curves are similar to those reported in neurophysiological animal studies where climbing neuronal activity, interpreted as representing a temporal integrator function, encoded duration (Leon and Shadlen, 2003; Reutimann et al., 2004). We also interpreted this accumulator-type signature as indicative of activation important for the encoding of duration. Given the close connection between the dorsal posterior insula and ascending internal body signals (see below), we suggested that this activation pattern may represent the accumulation of physiological changes in body states constituting our experience of time (Wittmann et al., 2010b). The conjecture that interoception might be at the base of time perception is supported by a recent study by Meissner and Wittmann (2011), recording physiological signals (heart periods, skin conductance levels, respiratory periods) during a similar duration reproduction task, and showing a positive relationship between duration reproduction accuracy and the slope of cardiac slowing during the encoding of temporal intervals. In addition, in this study a correlation between duration reproduction accuracy and subjects’ ability to perceive their own heart beats was detected.
The posterior-to-anterior progression of insula activation corresponding to the encoding and reproduction phases of the timing task – together with the dominant association of assessed psychological variables with activation in the reproduction phase – finds correspondence in the general functional assignment of the insular cortex. The insula is considered the primary interoceptive cortex, that is, the receptive area for physiological states of the body (Craig, 2002; Critchley et al., 2004). Conscious awareness of complex feeling states and the self is realized by a posterior-to-anterior progression of bodily representations in the insula, this progressive integration encompassing cognitive and motivational information culminating in the anterior insula (Craig, 2009b; Singer et al., 2009). Potentially related, the encoding phase of the task had no explicit verbal (or other motor) response related to time. In contrast, in the reproduction phase a comparison between the first and the second tone was required, a decision had to be made when to press the button and a motor response was given. All these additional cognitive processes required a stronger functional integration of activation with other brain systems, which therefore may have been associated with the anterior insula with its integrative function.
Moreover, neuroimaging data on the neural basis of impulsivity in healthy individuals as well as in patient groups shows the involvement of a network of fronto-limbic structures, among other regions the insular cortex and the anterior cingulate cortex (Boes et al., 2009; Paulus, 2007; Takahashi et al., 2009). Therefore, the proposed link between time perception, cognitive processes and impulsivity as being partly based on these two structures (among others) is consistent with the literature. Functionally, the insular and the medial frontal regions are critical components of the decision-making neural network, integrating visceral sensations and emotional states to modulate decisions, in the current study potentially involved to sense the right moment for pressing the button in the duration reproduction task. The specific activation of these and other brain structures in the representation of temporal intervals is discussed in various models as relying on the integrity of involved cognitive functions such as attention and working-memory demands (Koch et al., 2009; Rubia et al., 2009). Similar to this interpretation we argue that neural systems related to the core control network are involved in “impulsive” or “self controlled” cognitive time management. The way in which we conceptualize the experience of time could play a decisive role in future research attempting to form a common framework for the understanding of impulsivity.
Several methodological caveats have to be mentioned. First of all, since we did not study impulsivity and time perception in a clinical context, i.e. in patients with psychiatric or neurologic diagnoses, presented associations are comparably moderate. Further studies with patient populations might reveal stronger associations between time perception measures and related neural activation as well as disclose further associations between delay discounting, self-reported impulsivity and time perception. A further limiting factor can be seen in our choice of including stimulant-using students. Although these individuals did not fulfill any DSM-IV criteria, neither for drug dependence or any other psychiatry diagnoses, and their comparably stronger usage of cannabis did not lead to dependency according to the criteria assessed with the SSAGA (Bucholz et al., 1994), it is known that occasional stimulant users, next to having higher impulsivity scores, exhibit subtle executive dysfunctions (Reske et al., 2011). Neuroimaging studies with stimulant users reveal attenuated neural activation related to cognitive processes but also increased activation that is discussed as related to the compensation of inefficient processing in other brain regions (Paulus et al., 2008; Tomasi et al., 2007). Moreover, regular cannabis users exhibit altered patterns of brain activation during cognitive tasks, a decrease in brain activation in task-related regions or an increase in activation in compensatory regions (Quickfall and Crockford, 2006; Gonzales, 2007). Although we statistically controlled for drug use it is possible that some found associations could be mediating effects of drug use. Third, we employed a secondary working memory task in order to prevent subjects from counting. Through the presentation of an identical secondary task in the control task we aimed at subtracting out this factor (the classical fMRI approach). Nevertheless, it is possible that brain activation in the reproduction phase was still attributable to an interaction between time perception and working memory processes of the secondary task, since the reproduction phase took place with a larger delay after the presentation of the numbers as compared to the encoding phase or to the control task (see Fig. 6). A candidate area for such working-memory related activation would be the pre-frontal and parietal cortex. In the reproduction versus encoding and reproduction versus control contrasts, inferior frontal cortex activity could potentially relate to working-memory function (Cappell et al., 2010). Last but not least, we used an auditory duration reproduction task. So far only one fMRI task has been published employing a temporal reproduction task with comparable durations in the visual domain (Jech et al., 2005). Using a different analysis method by correlating the length of the reproduced intervals with brain activation, several regions of interest were disclosed which correlated positively with performance, namely the dorsolateral prefrontal cortex, the primary motor cortex, the supplementary motor area and the striatum. Only a study employing stimulus durations with both modalities and using the same analysis technique will address the question of which areas of the brain are involved in time perception across both modalities and which areas are modality-specific.
Figure 6.
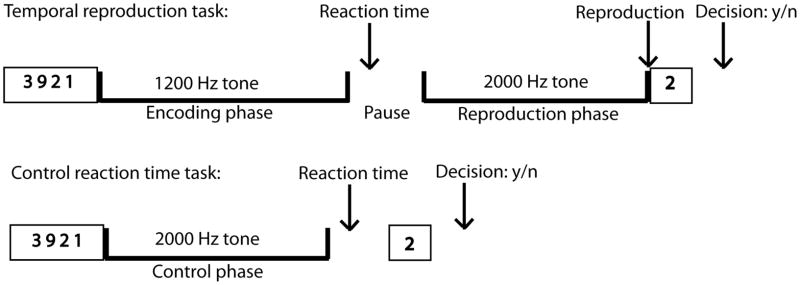
Experimental Design. Trial events in the duration reproduction and the control reaction time task: First, subjects saw for 3 s four numbers on the screen. Then a continuous 1.2 Hz tone was presented for one of three durations (3-, 9-, 18-s). After the tone had stopped subjects had to press a button as fast as possible. In the duration reproduction task, after a short pause a continuous 2 Hz tone was presented that had to be stopped by pressing a button when the subjects thought that it has lasted as long as the first stimulus. After both the duration reproduction and the control task one single number appeared at the end of each trial on the screen and subjects had to decide by pressing one of two buttons whether it was one of the four numbers seen at the beginning of the trial.
In summary, the posterior insula seemed be involved in the integration, and thus the encoding, of duration, and did not map to measures of impulsivity. Other more frontal brain structures such as the medial frontal cortex, the inferior frontal cortex and the anterior insula showed correlations with impulsivity measures. Potentially, activity in these frontal areas of the brain can be used as a biological marker for cognitive time management as well as for impulsiveness.
4. Materials and methods
4.1 Participants
Twenty-seven students from local colleges (12 female; age: 21.1 ± 2.2 years) and participating in an ongoing study examining the neural substrates underlying risk for stimulant abuse took part in this study. Twelve were stimulant-naïve control subjects (6 male, 6 female; age: 21.7 ± 2.3 years; education: 14.9 ± 1.5 years), ten were stimulant-using individuals (8 male, 2 female; age: 21.7 ± 1.8 years; education: 15 ± 1 years), and five subjects (1 male, 4 female; age: 19 ± 1.4 years; education: 12.6 ± 0.9 years) who were not using stimulants, were selected through a screening procedure using the Barratt impulsivity scale (BIS; Barratt et al., 1999). They were included as having a BIS sum score of at least 74 corresponding to the 75th percentile of a representative San Diego student sample. Thus, we have an “enriched” sample of students with varying degrees of impulsivity.
The 12 control subjects had never used drugs (other than cannabis, nicotine or alcohol) and thus formed a typical student sample of control subjects. The ten stimulant-using subjects were selected on the following criteria: (i) having used recreational stimulants (such as powder cocaine) or prescription stimulants for recreational use (amphetamines or methylphenidate) at least 3 times over the past 6 months, (ii) no lifetime stimulant (or any other drug) dependence (see Table 1 for group details on drug use). Previous research has shown that these individuals are on average more impulsive than comparison subjects (Leland and Paulus, 2005; see Table 2 for BIS scores across groups). Participants had no lifetime history of Axis I disorder, no history of antisocial personality disorder (ASPD) or any current severe medical disorder. Mental health problems, physical difficulties, and substance-related problems were determined by using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Buchholz et al., 1994). Moreover, subjects were instructed to abstain from illicit substance use for 48 h prior to the experimental session. Urine toxicology was obtained for all subjects before testing and revealed no evidence of recent drug use. The study was approved by the University of California San Diego Human Research Protection Program and subjects gave informed and written consent and were paid for participation.
Table 1.
Average amount of drug use, average scores in the three BIS and the three ZTPI subscales compared between stimulant-using, impulsive subjects as well as stimulant-naïve controls.
| Ever used Popular types |
Stimulant users (n = 10) | Impulsive (n = 5) | Controls (n = 12) | |||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | |
|
Amphetamine-like stimulants Adderall, Ritalin |
16.8 | 16.2 | 0 | 0 | 0 | 0 |
| Cocaine | 19.1 | 21.0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Marijuana | 952.2 | 1233.3 | 68.2 | 151.9 | 15.5 | 30.6 |
|
| ||||||
| Ecstasy | 9.6 | 22.8 | 0 | 0 | 0 | 0 |
|
| ||||||
|
Opiates Vicodin, Codeine |
1.2 | 2.0 | 0 | 0 | 4.2 | 14.4 |
|
| ||||||
|
Sedatives Xanax, Valium |
0.2 | 0.4 | 0 | 0 | 1.6 | 5.8 |
|
| ||||||
|
Hallucinogens LSD, Mushrooms |
1.8 | 3.2 | 0 | 0 | 0 | 0 |
|
| ||||||
| PCP | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
|
Inhalants Nitrous oxide, glue |
2.0 | 6.3 | 0 | 0 | 0 | 0 |
4.2. Tasks
4.2.1. fMRI task: duration reproduction
Subjects performed a duration reproduction task that was previously utilized in an fMRI study using normal controls (Wittmann et al., 2010b). They were instructed to reproduce tone intervals with 3, 9, and 18 s duration during fMRI (see Fig. 6). Each trial consisted of two consecutive phases: the encoding and the reproduction interval. In the encoding phase, participants listened to a 1.2 kHz tone. After a short variable pause with an average of 6 s (durations generated randomly from an exponential distribution with a mean of 6 s), the reproduction phase was started and consisted of the presentation of a 2 kHz tone. In this phase, participants had to stop the presentation of the second tone when they estimated that it had reached the length of the first tone. We used two different frequencies during the task in order to provide an additional cue indicating which phase of the task subjects were in. Subjects were requested not to count but to rely on their subjective feeling of elapsed time. Chronometric counting leads to a substantially smaller variation in performance than pure time estimation and is also guided by different brain structures (Clément and Droit-Volet, 2006; Hinton et al., 2004). To further reduce the possibility of counting, subjects were given a secondary working-memory task. Just before the initial tone, a group of four numbers was presented for subjects to memorize. After the second (reproduction) tone had been switched off by the subject one number appeared (for 3 s) and the subjects responded whether the presented number was one of the four presented at the beginning of the trial by pressing one of two buttons. The inter-trial interval was adjusted to the subjects’ response times in order to have identical run durations for each subject (11 min. 55 s). The maximum allowable duration of the reproduction tone was set to 150% of the encoding tone duration. An extra inter-trial duration with variable time randomly generated from an exponential distribution with a mean of 1.5 s was added to avoid having the inter-trial interval dependent on subjects’ timing.
In the ‘dual klepsydra’ model’ (DKM) by Wackermann and Ehm (2006), two inflow–outflow units, which act as ‘leaky accumulators’ of their respective inflows, are allocated for the representation of duration during the encoding phase and the reproduction phase, respectively. In this model, a subject’s response function across a range of stimulus durations is reduced to the parameter kappa (κ), which represents the ‘loss rate’ of the leaky accumulator. The DKM fits well with the ‘progressive shortening’ of reproduced durations, i.e. an increasing under-reproduction with longer stimulus duration. The greater parameter κ the more pronounced is the under-reproduction of intervals expressed by a greater negative curvature of the response function. We calculated κ according to Wackermann and Ehm (2006) and thus obtained a value representing the degree of under-reproduction.
4.2.2. fMRI control task: reaction time
In the reaction time control task subjects listened to 2 kHz tones with durations of around 3 s, 9 s, and 18 s and to press the button immediately after the tone stopped (Fig. 6). The tone durations were directly taken from the reproduced durations in a behavioral reproduction task acquired from a previous behavioral session. Each participant was instructed to respond as quickly as possible to the end of the tone in the control task to better match the attention and motor preparation demands of the temporal reproduction task. The same working-memory task (digit recognition), as described above, was used in the control task. In order to have identical run times across all subjects (8 min. 44 s) the inter-trial interval was adjusted by subtracting the individually performed reaction time to the tone and the decision time in the memory task from a constant (24 s) and adding a jittered duration of around 1.5 s (duration retrieved from an exponential function; as done in the temporal reproduction task above).
4.2.3. Behavioral task: delay discounting
Subjects completed a delay discounting task during fMRI, based on a paradigm previously utilized by Lane et al. (2003a) and Wittmann et al. (2010a). Only the behavioral results are reported here. Subjects were repeatedly presented with two options of (1) a nearly immediate reward and (2) a delayed reward. They had to choose one of the two by pressing a corresponding button on a response box. The immediate option consisted of a reward value of 1¢, 6¢, 12¢, 18¢, or 24¢ always available after 2 s. The delayed option had a constant reward value of 24¢, but the waiting period within one block was either 4 s, 10 s, 30 s, or 60 s. After the selection of an option, subjects waited through the chosen delay period and then had to press the same button on the response box. That way, the running total of the subject’s earnings increased and was shown at all times in the upper center portion of the display screen. Subjects were informed that they would receive the final sum at the end of the scanning session.
The delay periods were presented to subjects with each of the five varying immediate reward options (1¢, 6¢, 12¢, 18¢, or 24¢) presented in either ascending or descending order within one block (defined by the delay period). All subjects received the same sequence of blocks in the following order (indicated by the delay period): 4 s, 30 s, 10 s, 60 s. This sequence of blocks was repeated four times with ascending (A) and descending (D) immediate reward options ordered as ADDA. The standard variable of delay discounting tasks is the discounting parameter k representing the rate of discounting of delayed rewards. To obtain parameter k a point of subjective indifference for each delay is defined which is calculated as the mid-point (in monetary amount) when the individual switches from choosing the larger amount/longer delay to the smaller amount/shorter delay. The indifference points are then plotted across each delay value. These data for each individual can be fitted to the following hyperbolic equation (Myerson and Green, 1995):
| (1) |
V represents the subjective value of the reward in ¢, or “indifference” point at which the subject prefers the immediate smaller and delayed larger rewards equally. A represents the value in ¢ of the larger reward, D represents the delay in s of the larger reward, and k represents the rate at which the function declines as the delay increases. This hyperbolic equation was fitted to individual subject data and the parameter k is taken per individual as measure of steepness of discounting; the larger k the stronger a subject discounts delayed rewards. An alternative measure of discounting is obtained by calculating the area under the discounting curve (AUC) by summing up the value of indifference points across delays, thus producing a scoring range constrained between 0 and 1. The AUC procedure avoids several issues related to the goodness-of-fit with individual (potentially inconsistent) responses (Lane et al., 2003a; Myerson et al., 2001).
4.2.4. Self-report scale of impulsivity (BIS)
The Barratt Impulsiveness Scale (BIS-11) (Barratt et al., 1999) consists of 30 items with 4-point scales ranging from 1 (rarely) to 4 (almost always) which subjects rate on how often they think or act as described in the item. The items are grouped into three subscales: non-planning impulsivity (“I plan tasks carefully”, “I change jobs”), motor impulsivity (“I do things without thinking”, “I buy things on impulse”), and attention/cognition impulsivity (“I concentrate easily”, “I get easily bored when solving thought problems”) (Patton et al., 1995).
4.2.5. Zimbardo Time Perspective Inventory (ZTPI)
The Zimbardo Time Perspective Inventory (ZTPI) has 56 5-point scale items ranging from 1 (very untrue) to 5 (very true) that are grouped into five subscales: past-negative, present-hedonistic, future, past-positive, and present-fatalistic (Zimbardo and Boyd, 1999). The three subscales relating to the future and the present are used here.
4.3. Imaging procedure
Stimulus presentation and response registration in the duration reproduction and the reaction time control task were controlled using the WinVis toolbox (Neurometrics Institute) for MATLAB (Version 5.3, MathWorks Inc.). The duration reproduction (R) and the control task (C) were presented twice in alternating order, either as RCRC or CRCR design. A run (= scan) of each task (reproduction, control) contained six presentations of each of the three durations, which adds up to 36 duration presentation in each task (2 runs × 6 presentations × 3 intervals). The different durations in the tasks were presented in randomized order across the recording session as well as across subjects. Following these four runs, a fifth run containing the delay discounting task was conducted.
4.3.1. fMRI-Data Acquisition
Participants were scanned in a 3T GE Signa scanner using an 8-channel head array coil. Each scanning session consisted of a three-plane scout scan, a sagittally acquired spoiled gradient recalled (SPGR) sequence for acquiring T1-weighted images (field of view: 25 cm; matrix: 192 × 256; 172 slices; thickness: 1 mm; TR: 8 ms; TE: 3 ms; flip angle: 12°) and a T2*-weighted axially acquired echo-planar imaging (EPI) scan to measure Blood Oxygen Level Dependency (BOLD) functional activity. The parameters for the five EPI scans were 3.43 × 3.43 × 2.6 mm with a 1.4 mm gap, TR = 2 s, TE = 32 ms, flip angle of 90°, and 30 slices (whole brain). Cushions were arranged around the head and neck to maximize comfort and minimize motion.
4.3.2. fMRI protocol analysis pathway
All image processing and analysis of this event-related fMRI study was done with the Analysis of Functional Neuroimages Software (AFNI) package (Cox, 1996). For preprocessing, EPI images were interpolated to correct for three-dimensional motion, time-corrected for non-simultaneous slice acquisition, and normalized to Talairach coordinates (Talairach and Tournoux, 1988). The event-related time series data for each individual was then analyzed using a multiple regression model.
For the duration reproduction and control reaction time task, nine response regressors were generated for each of the three durations (3-, 9-, 18-s) for (1) the encoding phase and (2) the reproduction phase of the duration reproduction task, as well as for (3) the duration in the control task. Additionally, for all tasks three movement-related nuisance regressors were used to account for residual motion (in the roll, pitch, and yaw directions). A further regressor was included for filtering out activation attributable to noise. This white matter regressor was generated in the following manner: (1) a grey-white matter mask was generated based on the associated high level anatomical scan, (2) this mask was down-sampled to the resolution of the echoplanar image, (3) the mask was used to obtain an overall average across all white matter voxels for each time point, (4) the white matter regressor was normalized. The goal of this approach was to eliminate signal fluctuations that are not due to BOLD-signal changes but are due to undulating echoplanar signal variation or physiological variance. The regressors of interest were convolved with a modified gamma variate function to account for the delay and dispersion relating presumed neural activation to hemodynamic changes measured by the BOLD response (Boynton et al., 1996). The AFNI program 3dDeconvolve was used to calculate the estimated voxel-wise response amplitude and a Gaussian filter of 6 mm full width at half maximum (FWHM) was applied to voxel-wise percent signal change data to account for individual anatomical variations.
4.3.3. Task-related analyses of fMRI
With respect to the duration reproduction and control reaction time task, a voxel-based two-way (fixed factor: duration, random factor: subject) analyses of variance (ANOVA) were conducted to test the differences of activation for the three contrasts: (1) encoding phase versus control condition, (2) reproduction phase versus control condition as well as (3) reproduction phase versus encoding phase. In addition, voxel-wise two-sample paired t tests were used to identify brain areas in which the signal data differed significantly between the contrasted conditions for each of the three intervals (3-, 9-, 18-s). A threshold adjustment method based on Monte-Carlo simulations was used in a whole-brain cluster analysis (Foreman et al., 1995). Based on these simulations, a voxel-wise a-priori probability of 0.01 (0.001) results in a corrected cluster-wise activation probability of 0.01 (0.001) and a voxel-wise a-posteriori probability of 4.96 × 10−6 if a minimum volume of 704 μl (512 μl) and a connectivity radius of 4.0 mm is considered.
Areas of significant activation as found for the contrasts in the duration reproduction and control task (encoding phase versus control condition, reproduction phase versus control condition) were defined as region of interest (ROI) for plotting time activity curves across the time intervals of interest (9 s and 18s in the encoding and the reproduction phase). Time activity curves are plotted in intervals of 2 s which corresponds to the acquisition time of brain activation in the scanner (repetition time; TR). With a TR of 2 s, time activity curves can not be shown for the 3-s encoding interval, i.e. there are not sufficient data points to furnish interpretable curves. In order to compare individual BOLD signals across subjects at each time point during the encoding phase, values represent the difference of activation between time points T1, T2, T3 etc. and T0 (the onset of activation), respectively. Regarding time activity curves for the reproduction phase individual time activity curves for the 9- and 18-s reproduction phases were aligned to the actual individual reproduction times of the participants (stopping the tone in the reproduction). Significance levels for one-sample t tests probing whether linear regression slopes have positive values, thus indicating an increase in activity, are set to p < 0.05.
Significance levels for Pearson’s correlations between behavior and self-report questionnaires were set to p < 0.05. Concerning Pearson’s correlations between performance and self-report scales on the one hand and the multiple ROI related to brain activation on the other hand significance levels were initially also set to p < 0.05. To resolve the risk of Type I errors (incorrect rejection of null hypothesis) within multiple comparisons, one can correct the alpha level according to Bonferroni. This, however, increases the possibility of Type II error (false acceptance of null hypothesis). We, therefore, chose a conservative alpha level of 0.01. In addition, however, we applied the false discovery rate (FDR) method, a multiple comparisons correction procedure developed by Benjamini and Hochberg (1995, see p. 294f for a step-by-step example of this procedure) which constitutes a more rigorous method. The seven behavioral variables, namely DKM parameter κ of the duration reproduction task, the three subscales of the BIS self-report impulsivity scale plus the three time perspective subscale of the ZTPI (the scores all of which are interrelated) were correlated with the ROI for the reproduction versus encoding contrast separately for the three duration conditions. For application of the FDR method we thus had seven times the number of ROI for each duration condition as total number of correlations (3s = 14 ROI, 9s = 19 ROI, 18s = 14 ROI). Finally, we also present partial correlations where the use of drugs, namely the number of life time (1) stimulants (sum of amphetamine-like stimulants and cocaine) and (2) marijuana have been factored out (see Table 1 for participants’ drug use).
Supplementary Material
Research Highlights.
We present evidence of an association and the underlying neural substrates of time perception and impulsivity.
More impulsive subjects under-reproduce duration more strongly and they have a less pronounced future time perspective.
Accumulating brain activation in the posterior insula during the encoding phase of the duration reproduction task implies a potential generating process for the representation of time.
More frontal brain structures (core control network: anterior insula, medial and inferior frontal cortex) are related to maintaining the representation of temporal duration across time.
Core control network + motor areas that are related to brain activation during the duration reproduction phase are associated with measures of impulsiveness.
→ Activation in the core control network forms a biological marker for cognitive time management and for impulsivity.
Acknowledgments
We are grateful to Carolyn Eidt, Elena Kosheleva, Heather Donovan and Bettina Friedrich for help in logistical matters of this study. This study was supported by grants from the National Institute on Drug Abuse (R01DA016663, R01DA018307, R01DA015392, 1R03DA020687-01A1), the National Institute on Alcohol Abuse and Alcoholism (grant R01AA016965), and the Kavli Institute for Brain and Mind (07-33).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arce E, Santisteban C. Impulsivity: a review. Psicothema. 2006;18:213–220. [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore T, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Bush T. Time perception and reproduction in young adults with attention deficit hyperactivity disorder. Neuropsychology. 2001a;15:351–360. doi: 10.1037//0894-4105.15.3.351. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J Abnorm Child Psychol. 2001b;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Stanford MS, Dowdy L, Liebman MJ, Kent TA. Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Res. 1999;86:163–173. doi: 10.1016/s0165-1781(99)00024-4. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- Berlin HA, Rolls ET. Time perception, impulsivity, emotionality, and personality in self-harming borderline personality disorder patients. J Pers Disord. 2004;18:358–378. doi: 10.1521/pedi.18.4.358.40349. [DOI] [PubMed] [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Soc Cogn Affect Neurosci. 2009;4:1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Bueti D, Walsh V. The parietal cortex and the respresentation of time, space, number and other magnitudes. Philos Trans R Soc Lond B Biol Sci. 2009;364:1831–1840. doi: 10.1098/rstb.2009.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefrontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-de-la-Peña MT, Otero JM, Romero E. Comparison among various methods of assessment of impulsiveness. Percept Mot Skills. 1993;77:567–575. doi: 10.2466/pms.1993.77.2.567. [DOI] [PubMed] [Google Scholar]
- Church RM. Properties of the internal clock. In: Gibbon J, Allan L, editors. Timing and Time Perception. Vol. 423. New York: New York Academy of Sciences; 1984. pp. 566–582. [DOI] [PubMed] [Google Scholar]
- Clément A, Droit-Volet S. Counting in a time discrimination task in children and adults. Behav Process. 2006;71:164–171. doi: 10.1016/j.beproc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Shenton J, Dyer T, Wiener M. Cognitive timing: Neuropsychology and anatomic basis. Brain Res. 2009;1254:38–48. doi: 10.1016/j.brainres.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception and emotion: a neuroanatomical perspective. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of Emotion. Guilford; New York: 2008. pp. 272–288. [Google Scholar]
- Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci. 2009a;364:1933–1942. doi: 10.1098/rstb.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel – now? The anterior insula and human awareness. Nature Rev Neurosci. 2009b;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crean JP, de Wit H, Richards JB. Reward discounting as a measure of impulsive behavior in a psychiatric outpatient population. Exp Clin Psychopharmacol. 2000;8:155–162. doi: 10.1037//1064-1297.8.2.155. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Meck WH. How emotions colour our perception of time. Trends Cogn Sci. 2007;11:504–513. doi: 10.1016/j.tics.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Eagleman D, Pariyadath V. Is subjective duration a signature for coding efficiency? Philos Trans R Soc Lond B Biol Sci. 2009;364:1841–1852. doi: 10.1098/rstb.2009.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gerbing DW, Patton JH. Toward a conceptualization of impulsivity: components across the behavioral and self-report. Multivar Behav Res. 1987;22:357–379. doi: 10.1207/s15327906mbr2203_6. [DOI] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev. 2007;17:347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Harrington DL, Binder JR, Durgerian S, Rao SM. Neural systems supporting timing and chronometric counting: an fMRI study. Brain Res Cogn Brain Res. 2004;21:183–192. doi: 10.1016/j.cogbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. [DOI] [PubMed] [Google Scholar]
- Jech R, Dusek P, Wackermann J, Vymazal J. Cumulative blood oxygenation-level-dependent signal changes support the ‘time accumulator’ hypothesis. Neuroreport. 2005;16:1467–1471. doi: 10.1097/01.wnr.0000175616.00936.1c. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. Timing in the absence of clocks: encoding time in neural network states. Neuron. 2007;53:427–438. doi: 10.1016/j.neuron.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough KA, Zimbardo PG, Boyd JN. Who’s smoking, drinking, and using drugs? Time perspective as a predictor of substance use. Basic Appl Soc Psychol. 1999;21:149–164. [Google Scholar]
- Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philos Trans R Soc Lond B Biol Sci. 2009;364:1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, Tcheremissine OV. Measurement of delay discounting using trial-by-trial consequences. Behav Processes. 2003a;64:287–303. doi: 10.1016/s0376-6357(03)00143-8. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Pietras CJ, Rhoades HR, Tcheremissine OV. Relationships among laboratory and psychometric measures of impulsivity: Implications in substance abuse and dependence. Addict Disord Their Treat. 2003b;2:33–40. [Google Scholar]
- Leland DS, Paulus MP. Increased risk-taking decision-making but not altered response to punishment in stimulant-using young adults. Drug Alcohol Depend. 2005;78:83–90. doi: 10.1016/j.drugalcdep.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. A right hemispheric prefrontal system for cognitive time measurement. Behav Processes. 2006;71:226–234. doi: 10.1016/j.beproc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Macar F, Anton JL, Bonnet M, Vidal F. Timing functions of the supplementary motor area: an event-related fMRI study. Brain Res Cogn Brain Res. 2004;21:206–215. doi: 10.1016/j.cogbrainres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Brain Res Cogn Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Meissner K, Wittmann M. Body signals, cardiac awareness, and the perception of time. Biol Psychol. 86:289–297. doi: 10.1016/j.biopsycho.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Melges FT, Fougerousse CE. Time sense, emotions, and acute mental illness. J Psychiatric Res. 1966;4:127–139. doi: 10.1016/0022-3956(66)90025-2. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Morillon B, Kell CA, Giraud AL. Three stages and four neural systems in time estimation. J Neurosci. 2009;29:14803–14811. doi: 10.1523/JNEUROSCI.3222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L. Discounting of delayed rewards models of individual choice. J Exp Anal Behav. 1995;64:263–276. doi: 10.1901/jeab.1995.64-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noulhiane M, Pouthas V, Samson S. Is time reproduction sensitive to sensory modalities? Eur J Cogn Psychol. 2009;21:18–34. [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry Altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Lovero K, Wittmann M, Leland D. Reduced behavioral and neural activation in stimulant users to different error rates during decision-making. Biol Psych. 2008;63:1054–1060. doi: 10.1016/j.biopsych.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Pöppel E. Pre-semantically defined window for cognitive processing. Philos Trans R Soc Lond B Biol Sci. 2009;364:1887–1896. doi: 10.1098/rstb.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: A review. J Neuropsych Clin Neurosci. 2006;18:318–332. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Reske M, Delis DC, Paulus MP. Evidence for subtle verbal fluency deficits in occasional stimulant users: Quick to play loose with verbal rules. J Psychiatr Res. 2011;45:361–368. doi: 10.1016/j.jpsychires.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutimann J, Yakovlev V, Fusi S, Senn W. Climbing neuronal activity as an event-based cortical representation of time. J Neurosci. 2004;24:3295–3303. doi: 10.1523/JNEUROSCI.4098-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Roberts GMP, Garavan H. Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. NeuroImage. 2010;52:429–435. doi: 10.1016/j.neuroimage.2010.04.192. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp. 2004;64:329–340. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sci. 2009;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer TF, Meyers PJ, Huser SJ. Contrasting task demands alter the perceived duration of brief time intervals. Percept Psychophys. 1994;56:649–657. doi: 10.3758/bf03208358. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Castellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Schneider MF, Krick CM, Retz W, Hengesch G, Retz-Junginger P, Reith W, Rösler M. Impairment of fronto-striatal and parietal cerebral networks correlates with attention deficit hyperactivity disorder (ADHD) psychopathology in adults – A functional magnetic resonance imaging (fMRI) study. Psychiatry Res Neuroimag. 2010;183:75–84. doi: 10.1016/j.pscychresns.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Smart RG. Future time perspectives in alcoholics and social drinkers. J Abnorm Psychol. 1968;73:81–83. doi: 10.1037/h0025449. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Rogers JW, Newman S, Rubia K. Evidence for a pure time perception deficit in children with ADHD. J Child Psychol Psychiatry. 2002;43:529–542. doi: 10.1111/1469-7610.00043. [DOI] [PubMed] [Google Scholar]
- Staddon JER. Interval timing: memory, not a clock. Trends Cogn Sci. 2005;9:312–314. doi: 10.1016/j.tics.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Allen PA, Kaut KP. Relation of individual differences in impulsivity to nonclinical emotional decision making. J Int Neuropsychol Soc. 2008;14:878–882. doi: 10.1017/S1355617708080934. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Chanen AM, Wood SJ, Yücel M, Tanino R, Suzuki M, Velakoulis D, Pantelis C, McGorry PD. Insular cortex volume and impulsivity in teenagers with first-presentation borderline personality disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1395–1400. doi: 10.1016/j.pnpbp.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme, Stuttgart; New York: 1988. [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: Implications in attention and perception. Psych Res Neuroimag. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich P, Churan J, Fink M, Wittmann M. Temporal reproduction: further evidence for two processes. Acta Psychologica. 2006;125:51–65. doi: 10.1016/j.actpsy.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Ehm W. The dual klepsydra model of internal time representation and time reproduction. J Theor Biol. 2006;239:482–493. doi: 10.1016/j.jtbi.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Wittmann M, Hasler F, Vollenweider FX. Effects of varied doses of psilocybin on time interval reproduction in human subjects. Neurosci Lett. 2008;435:51–55. doi: 10.1016/j.neulet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Wiener M, Turkeltaub P, Coslett HB. The image of time: a voxel-wise meta-analysis. Neuroimage. 2010;49:1728–1740. doi: 10.1016/j.neuroimage.2009.09.064. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland D, Churan J, Paulus MP. Impaired time perception and motor timing in stimulant-dependent subjects. Drug Alcohol Depend. 2007;90:183–192. doi: 10.1016/j.drugalcdep.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Decision making, impulsivity, and time perception. Trends Cogn Sci. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Temporal horizons in decision making. J Neurosci Psychol Econ. 2009;2:1–11. [Google Scholar]
- Wittmann M. The inner sense of time. Philos Trans R Soc Lond B Biol Sci. 2009a;364:1955–1967. doi: 10.1098/rstb.2009.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M. Die Neuropsychologie der Zeit - Kognitive und emotionale Modulatoren der zeitlichen Erfahrung. Z Med Psychol. 2009b;18:28–39. [Google Scholar]
- Wittmann M, van Wassenhove V. The experience of time: neural mechanisms and the interplay of emotion, cognition and embodiment. Philos Trans R Soc Lond B Biol Sci. 2009;364:1809–1813. doi: 10.1098/rstb.2009.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Lovero KL, Lane SD, Paulus MP. Now or later? Striatum and insula activation to immediate versus delayed rewards. J Neurosci Psychol Econ. 2010a;3:15–26. doi: 10.1037/a0017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Simmons AN, Aron J, Paulus MP. Accumulation of neural activity in the posterior insula encodes the passage of time. Neuropsychologia. 2010b;48:3110–3120. doi: 10.1016/j.neuropsychologia.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, van Wassenhove V, Craig AD, Paulus MP. The neural substrates of subjective time dilation. Front Hum Neurosci. 2010c;4:2. doi: 10.3389/neuro.09.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner K, Wittmann M. Body signals, cardiac awareness, and the perception of time. Biological Psychology. 2011;86:289–297. doi: 10.1016/j.biopsycho.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Zakay D, Block RA. Temporal cognition. Curr Dir Psychol Sci. 1997;6:12–16. [Google Scholar]
- Zimbardo PG, Boyd JN. Putting time in perspective: a valid, reliable individual-difference metric. J Pers Soc Psychol. 1999;77:1271–1288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.