Abstract
Sox2, a transcription factor critical for the maintenance of embryonic stem cells and induction of pluripotent stem cells, is expressed exclusively in the conducting airway epithelium of the lung, where it is required for differentiation of nonciliated, goblet, and ciliated cells. To determine the role of Sox2 in respiratory epithelial cells, Sox2 was selectively and conditionally expressed in nonciliated airway epithelial cells and in alveolar type II cells in the adult mouse. Sox2 induced epithelial cell proliferation within 3 days of expression. Epithelial cell proliferation was associated with increased Ki-67 and cyclin D1 staining. Expression of cell cycle genes, including FoxM1, Ccna2 (Cyclin A2), Ccnb2 (Cyclin B2), and Ccnd1 (Cyclin D1), was increased. Consistent with a role in cell proliferation, Sox2 activated the transcription of FoxM1 in vitro. In alveoli, Sox2 caused hyperplasia and ectopic differentiation of epithelial cells to those with morphologic and molecular characteristics of conducting airway epithelium. Sox2 induced the expression of conducting airway epithelial specific genes, including Scgb1a1, Foxj1, Tubb3, and Cyp2f2. Although prolonged expression of Sox2 caused cell proliferation and epithelial hyperplasia, Sox2 did not induce pulmonary tumors. Sox2 induces proliferation of respiratory epithelial cells and, subsequently, partially reprograms alveolar epithelial cells into cells with characteristics of the conducting airways.
Keywords: lung, transcription, progenitor cell, differentiation, tumorigenesis
CLINICAL RELEVANCE.
These studies demonstrate a novel role for the transcription factor Sox2 in the regulation of airway epithelial cell fate decisions. Sox2 is required for normal airway epithelial cell proliferation and the differentiation of distinct subsets of cells lining bronchioles, including ciliated, goblet, and nonciliated secretory cells. Sox2 is dynamically regulated after injury of the airways, activating cell proliferation and enhancing differentiation of airway progenitor cells, processes critical for homeostasis and repair of the respiratory epithelium.
The respiratory tract consists of distinct structural compartments, including the conducting airways, trachea, bronchi, and bronchioles, that terminate in the alveoli, where gas exchange occurs. The numbers and characteristics of epithelial cells lining the airways vary among species and along the proximal-distal and dorso-ventral axes of the lung. For example, cartilaginous airways are lined by a pseudostratified epithelium composed of multiple cell types, including Clara, ciliated, basal, and goblet cells, whose abundance varies among species and with age. Noncartilaginous airways are lined by a simple columnar cell layer that, in the mouse, consists primarily of nonciliated bronchiolar cells (Clara cells) and ciliated cells that play important roles in innate host defense and mucociliary clearance of the lung, respectively. Alveoli are lined by squamous type I cells and cuboidal type II cells, the latter producing pulmonary surfactant that prevents alveolar collapse during the respiratory cycle. Lineage tracing studies demonstrate that bronchial, bronchiolar, and alveolar epithelial cells are derived from common progenitors during mouse lung morphogenesis (1). Although the genes and processes regulating the specification and differentiation of various proximal and peripheral respiratory epithelial cell types are incompletely understood, there is strong evidence that basal and Clara cells play critical roles as progenitor cells mediating repair of the conducting airways after injury (2–6). Alveolar type II cells serve as progenitors for alveolar type I and type II cells after alveolar cell injury (7, 8). Although generally nonproliferative, the respiratory epithelium in the adult lung is capable of remarkable proliferation and repopulation after lung injury. In mice, basal, Clara, and alveolar type II cells rapidly proliferate and differentiate into various airway epithelial cell types (5–11).
Sox2 is a member of a large family of transcription factors that play diverse roles in development and disease (reviewed in References 12 and 13). Sox2 is required for maintenance of embryonic stem cells and the induction of pluripotent stem cells (iPS cells) from various differentiated cell types (14). In mice, Sox2 is required for normal morphogenesis and homeostasis of diverse tissues, including neural stem cells; retinal stem cells taste buds; hair sensory follicles in the ear; and epithelia of trachea, lung, and esophagus (15–18). In humans, Sox2 mutations are associated with eye and tracheal/esophageal malformations (18–20). Recent studies demonstrated that Sox2 is amplified in human lung and esophageal squamous cell carcinomas (21–23). In the lung, Sox2 is selectively expressed in the conducting airway epithelium and is not detected in the alveolus (24). Recent loss-of-function experiments demonstrated that Sox2 is a positive regulator of mouse tracheal, bronchial, and bronchiolar epithelial proliferation and differentiation (11, 18). Expression of transgenic Sox2 in respiratory epithelial cells of the embryonic mouse lung disrupted normal branching morphogenesis and epithelial cell differentiation (24).
In normal mouse lung, Sox2 is exclusively expressed in conducting airway epithelial cells. To assess the roles of Sox2 in cell proliferation, pulmonary oncogenesis, and differentiation, we used the Scgb1a1 promoter to conditionally drive expression of Sox2 in the lungs of adult mice. Although conditional expression of Sox2 increased epithelial cell proliferation and produced hyperplastic lesions throughout the alveoli, it was not sufficient to induce pulmonary tumors. Instead, Sox2 induced partial reprogramming of alveolar epithelial cells into cells with morphological and molecular features of the conducting airway epithelium.
MATERIALS AND METHODS
Transgenic Animals
Transgenic mice bearing the (tetO)7CMVSox2 construct were produced by injecting one-cell zygotes with a plasmid construct consisting of cDNAs encoding full-length mouse Sox2 (NM_011443) isolated from the pCIG-Sox2 plasmid (kind gift of A.P. McMahon, Harvard University, Cambridge, MA). The linearized fragment was microinjected into the pronucleus of fertilized eggs from FVB/N mice. The Sox2 transgene was identified by PCR using the primers 5′-CAT CCA CGC TGT TTT GAC CT-3′ and 5′-CGG GCT GTT CTT CTG GTTG-3′. To target the respiratory epithelium, the 2.3-kb rat Scgb1a1 promoter was used to drive the reverse tetracycline transactivator (rtTA). rCCSP-rtTA mice (line 2) (25) were mated to (tetO)7CMVSox2 mice to generate double transgenic mice rCCSP-rtTAtg/wt, (tetO)7CMVSox2tg/wt (hereafter referred to as rSox2 mice). To induce Sox2 transgene expression in respiratory epithelial cells of double transgenic mice, doxycycline was administered in the food at a concentration of 625 mg/kg dry weight (Harlan Teklad, Madison, WI) starting at 4 weeks of age for 3 days, 1 week, and 6 months. Mice were maintained in a pathogen-free environment in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Cincinnati Children's Hospital Research Foundation. All animals were housed in humidity- and temperature-controlled rooms on a 14 hour–10 hour light/dark cycle. Mice were allowed food and water ad libitum. There was no serological evidence of pulmonary pathogens or bacterial infections in sentinel mice maintained within the colony. Mice survived normally in the vivarium for up to 6 months despite extensive induction of ectopic, conducting-airway–like cells in the alveoli.
Tissue Preparation and Immunostaining
Lung tissue preparation, immunohistochemistry, and immunofluorescence were performed essentially as described previously (11). Immunohistochemical antibodies used were rabbit antiSox2 (WRAB-Sox2, 1:6,000; Seven Hills Bioreagents, Cincinnati, OH), Scgb1a1 (WRAB-Ccsp, 1:4,000; Seven Hills Bioreagents), FoxJ1 (generated internally, 1:6,000), acetylated α-tubulin (Clone6–11B-1, 1:15,000; Sigma, St. Louis, MO), p63 (sc-8344, 1:200; Santa Cruz Biotechnology Inc., Santa Cruz, CA), Ki-67 (m7249, 1:500; Dako, Carpintera, CA), CGRP (catalog #8198, 1:3,000; Sigma), Nkx2.1 (WRAB-Ttf1, 1:2,000; Seven Hills Bioreagents), FoxM1 (sc-500, 1:1,000; Santa Cruz), cyclin D1 (1:350; Abcam, Cambridge, MA), CD3-ɛ (sc-1127, 1:1,000; Santa Cruz), and proSP-C (1:4,000; Seven Hills Bioreagents). Primary and secondary antibodies used in immunofluorescence were guinea pig Sox2 (generated internally), rabbit proSP-C (1:1,000; Seven Hills Bioreagents), rabbit Scgb1a1 (1:1,000; Seven Hills Bioreagents), acetylated α-tubulin (Clone6–11B-1, 1:2,000; Sigma), hamster T1-α (8.1.1, 1:1,000; Developmental Studies Hybridoma Bank, Iowa City, IA), Alexa Fluor anti-guinea pig 594, anti-rabbit 350, anti-rabbit 488, anti-hamster 488, and anti-mouse IgG2B 488 (1:200, A11076, A10039, A11008, A21110, A21141; Invitrogen). Z-stack black and white images were obtained and pseudo-colored on a Zeiss Axioplan2 microscope equipped with AxioVision Software (Thornwood, NY) and deconvoluted using Autoquant X software (MediaCybernetics, Bethesda, MD).
Additional materials and methods are provided in the online supplement.
RESULTS
Expression of Sox2 In Vivo
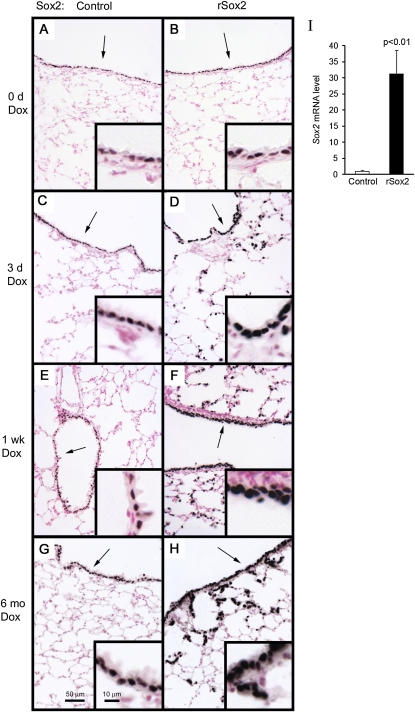
To conditionally express Sox2 in the respiratory epithelium, mice containing a (tetO)7CMVSox2 transgene were bred to transgenic mice expressing the tetracycline transactivator (rtTA) under control of the rat Scgb1a1 promoter (line 2). Previous studies demonstrated that rtTA expression was highly selective and efficient in targeting most nonciliated epithelial cells in the conducting airways and in a subset of alveolar type II cells (25). To induce Sox2 expression, 4-week-old double transgenic mice and single transgenic littermate control mice were treated with doxycycline for specified lengths of time and then killed. Consistent with previous findings in normal mice, immunohistochemical staining of Sox2 was observed exclusively in all cells of the conducting airways of single transgenic and untreated double transgenic mice (Figures 1A and 1B). Compared with control mice, robust transgenic Sox2 expression was observed in bronchiolar and alveolar epithelial cells of double transgenic (rSox2) mice after 3 days, 1 week, and 6 months of doxycycline treatment (Figures 1C–3H). Quantitative PCR demonstrated an increase in Sox2 mRNA (Figure 1I).
Figure 1.
Conditional expression of Sox2 in the respiratory epithelium. Four-week-old mice were treated with doxycyline for the times shown and then killed. Immunohistochemical staining for Sox2 was performed on adult control (A, C, E, G) and double transgenic mice rCCSP-rtTAtg/wt, (tetO)7CMVSox2tg/wt (rSox2) (B, D, F, H). Endogenous and exogenous Sox2 expression was distinguished by choosing an anti-Sox2 titer (1:6,000) that selectively stained transgenic Sox2 (A–H). Arrows indicate inset regions. Sox2 mRNA was assessed by quantitative RT-PCR in total lung from adult control or double transgenic mice after 3 days of doxycycline (I). Results were normalized to 18S rRNA and expressed as the means ± SE of three or four animals per group. Sox2 mRNA was significantly increased after 3 days of doxycycline.
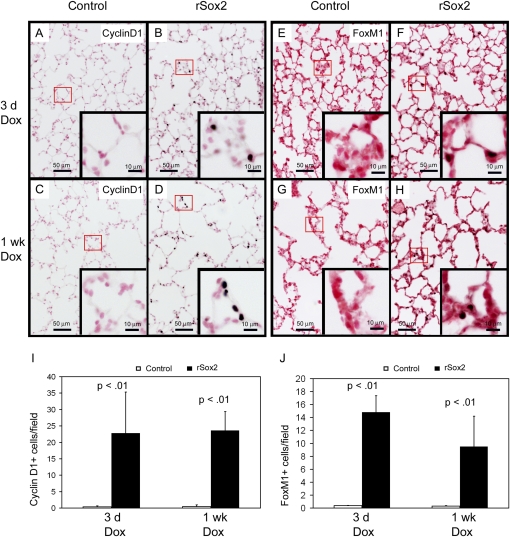
Figure 3.
Exogenous Sox2 induced cell cycle proteins. Immunohistochemical staining and quantification of lung sections from mice treated with doxycycline for the time periods shown demonstrated increased protein expression for cyclin D1 (A–D, I) and FoxM1 (E–H, J). Figures are representative of n = 3 mice per group at each time.
Sox2 Induced Cell Proliferation and Caused Alveolar Cell Hyperplasia
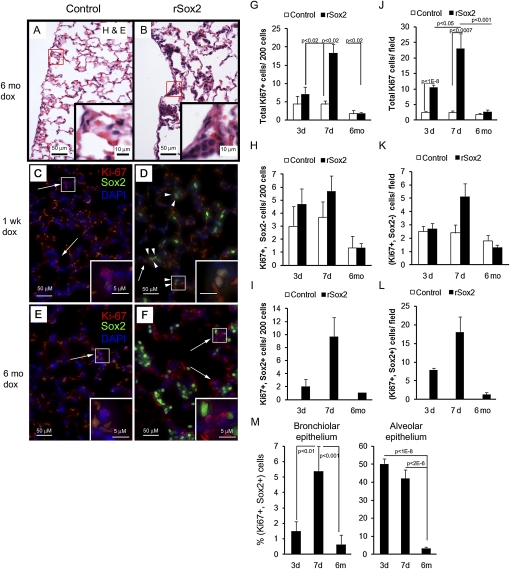
Hematoxylin-eosin staining of lungs from mice treated with doxycycline for 6 months demonstrated normal bronchial and alveolar morphology in single transgenic mice. Abnormal clusters of epithelial cells were observed in the alveoli of double transgenic littermates treated with doxycyline. The lesions consisted of clusters of cuboidal cells that were present throughout the alveoli (Figures 2A and 2B). Alveolar clusters containing 10 or more cells were rare in control animals (mean, 0.3 clusters per 200× microscopic field; SD, 0.6) and common in rSox2 animals (mean, 6.7 clusters per 200× microscopic field; SD, 1.4).
Figure 2.
Exogenous Sox2 caused cell proliferation and epithelial hyperplasia in the respiratory epithelium. Four-week-old mice were treated with doxycyline for the times shown and then killed. (A, B) Hematoxylin-eosin staining of lung sections from mice treated with doxycycline for 6 months demonstrated hyperplastic clusters in the alveolar region of rSox2 mice. (C, D) Dual immunofluorescence for Sox2 and the proliferation marker Ki-67 was used to analyze the effect of transgenic Sox2 expression on proliferation; the intensity of Sox2 fluorescence in rSox2 mice was adjusted to visualize only transgenic Sox2 (Sox2tg). Proliferative cells (Ki-67+) with Sox2tg (arrowheads) or without Sox2tg (arrows) are shown (C–F); pale orange autofluorescence is derived from red blood cells. Three days of doxycyline treatment induced alveolar proliferation (J), and 7 days of treatment induced bronchiolar and alveolar proliferation (G and J). No increase in proliferation was observed in either compartment after 6 months of doxycycline (G, J). The number of proliferating epithelial cells without Sox2tg expression (Ki-67+, Sox2−) was not statistically different in control and rSox2 mice (H, K). Therefore, the increased proliferation was associated with proliferating Sox2tg + cells (Ki-67+, Sox2+) (I, L). Not all Soxtg+ cells were proliferative (M). G, H, I = bronchiolar epithelium; J, K, L = alveolar epithelium.
Compared with control mice, increased numbers of Ki-67 reactive cells were observed in the conducting airways and alveoli of double transgenic mice after 3 days or 1 week of doxycyline treatment but not after 6 months of treatment (Figures 2C–2F, 2G, and 2J). At 3 and 7 days, dual immunofluorescence staining for Sox2 and Ki-67 demonstrated that most of the increased proliferation was due to cells coexpressing Ki-67 and high levels of Sox2 (Figures 2G–2M). Staining for the cell cycle initiator (cyclin D1) and the late cell cycle regulator (FoxM1) was increased in conducting airway (data not shown) and alveolar epithelial cells of the rSox2 mice (Figures 3A–3J), consistent with the sites and extent of proliferation induced by the transgene.
Sox2 Induced Expression of mRNAs Associated with Cell Proliferation
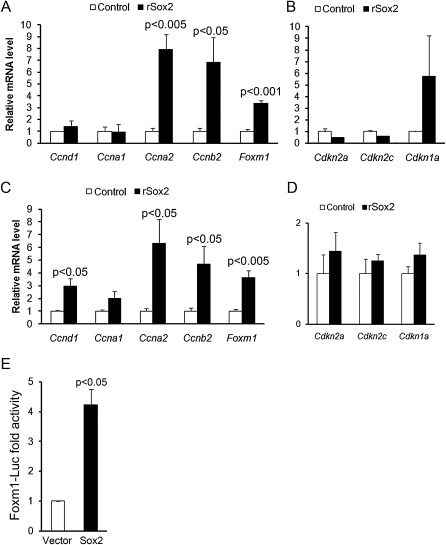
The levels of mRNAs encoding selected cell cycle genes were assessed by qRT-PCR of whole lung homogenates. Messenger RNA for the transcription factor FoxM1 and the cyclins Ccna2 and Ccnb2 were significantly increased after expression of Sox2 (Figures 4A–4D). Similarly, Ccnd1 mRNA was significantly increased after 7 days of doxycycline. Messenger RNA levels of the cell cycle inhibitors Cdkn1a, Cdkn2a, and Cdkn2c (cyclin-dependent kinase inhibitors) were not altered.
Figure 4.
Sox2 induced mRNAs associated with cell proliferation and activated Foxm1 gene expression. Messenger RNAs encoding selected genes associated with cell proliferation were assessed by quantitative RT-PCR in total lung from adult control or rSox2 mice after doxycycline treatment for 3 (A, B) and 7 (C, D) days. Results were normalized to 18S rRNA and expressed as the means ± SE of three or four animals per group. Ccna2, Ccnb2, and Foxm1 mRNAs were induced by expression of Sox2 after 3 or 7 days (A, C). Ccnd1 mRNA was induced by expression of Sox2 after 7 days (C). (B, D) Messenger RNA encoding cell cycle inhibitors was not significantly changed. (E) Effects of Sox2 on Foxm1 promoter activity were assessed by transfection of human bronchial epithelial cells with a 0.78-kb mFoxm1-Luc reporter construct containing an intron 1/2 regulatory sequence, and a Sox2 expression vector or control vector. Results are expressed as the means ± SE of three separate experiments performed in triplicate. Sox2 expression induced transcriptional activity of the 0.78 kb mFoxm1 reporter construct.
Sox2 Activated the Foxm1 Promoter
To identify possible mechanisms by which Sox2 influenced cell proliferation in the lung, we sought to determine if FoxM1, a transcription factor that promotes proliferation, is a downstream target of Sox2. A Foxm1-luciferase reporter construct containing a regulatory sequence from the first intron, 780 bp upstream of the start codon, was cotransfected with a vector expressing Sox2 in human bronchial epithelial cells. Transcriptional activity of the −0.78 kb Foxm1 was significantly induced by cotransfection with Sox2 (Figure 4E). These data support the concept that Sox2 induces FoxM1 expression, providing insight into a potential mechanism by which increased levels of Sox2 promote cell proliferation.
Sox2 Inhibited Scgb1a1 Expression in Conducting Airway Epithelial Cells
Sox2 is required for the expression of Scgb1a1 and for the differentiation of Clara and ciliated cells in conducting airways (11, 18). Staining for Scgb1a1 in conducting airways was inhibited by the increased expression of Sox2 (Figures 5A–5F). In contrast, FoxJ1 and α-tubulin staining was maintained in ciliated cells in the conducting airways (see Figures E1A–E1F in the online supplement). Staining for the neuroendocrine marker, CGRP, was distributed normally in rSox2 mice (data not shown). Staining for proSP-C was readily detected in the alveolar epithelium in control and rSox2 mice (Figures E1G and E1H), whereas staining in the bronchioles for proSP-C was negative (not shown).
Figure 5.
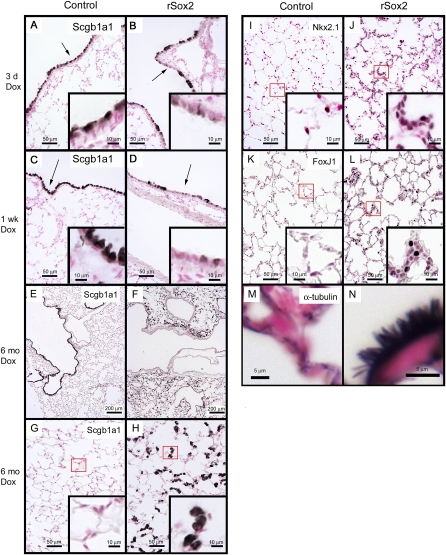
Sox2 inhibited expression of the Clara cell marker Scgb1a1 in conducting airways and induced abnormal alveolar clusters expressing markers of conducting airway epithelium. (A–H) Immunohistochemical staining for the Clara cell marker protein Scgb1a1 was performed on lung tissue from mice treated with doxycycline for 3 days, 1 week, or 6 months. Sox2 caused loss of Scgb1a1 from conducting airway epithelium. In contrast, Scgb1a1 was readily detected in subsets of alveolar epithelial cells after 6 months of doxycycline treatment (H) but was not detected at 3 and 7 days (data not shown). (I, J) After 6 months of doxycycline treatment, alveolar epithelial cells were positive for Nkx2.1, consistent with their respiratory epithelial cell phenotype. Staining for ciliated cell markers FoxJ1 (K, L), α-tubulin (M, N), and the Clara cell marker Scgb1a1 (E–H) are shown. (N) Arrows indicate ectopic cilia in the alveoli of rSox2 mice. Arrows and boxes denote areas in insets.
Sox2 Induced Conducting Airway Cell Differentiation in the Alveoli
Increased Sox2 expression caused widespread focal alveolar hyperplasia, inducing formation of abnormal clusters of highly differentiated cells with characteristics of airway epithelia in the peripheral lung. The hyperplastic alveolar epithelial lesions expressed TTF-1, consistent with maintenance of a respiratory epithelial cell phenotype (Figures 5I and 5J). Immunohistochemical staining of the lesions for the lymphocytic marker CD3 was negative (data not shown), indicating that clusters are not composed of inflammatory infiltrates. Hyperplastic lesions consisted of distinct subsets of cuboidal cells that expressed FoxJ1 and α-tubulin (Figures 5K–5N) or the Clara cell marker Scgb1a1 (Figures 5E–5H). Ciliated cells were present throughout the alveoli and within the hyperplastic alveolar lesions (Figures 5K–5N). Some alveolar cells expressed proSP-C, consistent with maintenance of a subset of alveolar type II cells (Figures E1E and E1F). Cells staining for proSP-C (an alveolar type II cell marker) or T1-α (an alveolar type I cell marker) were located primarily outside the lesions (Figures E2A–E2D) and did not colocalize with Sox2.
Cellular Heterogeneity of Alveolar Lesions Induced by Sox2
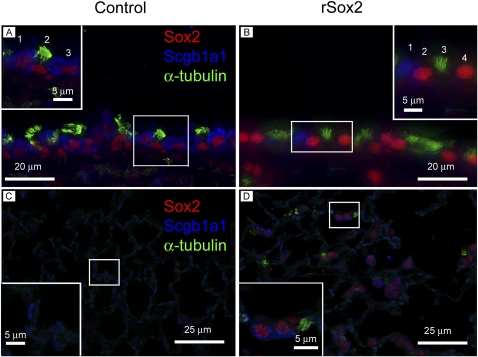
To test the differentiation status of diverse epithelial cells located within the hyperplastic lesions induced by Sox2, triple immunofluorescence staining was performed on mice treated for 6 months with doxycycline. In conducting airways, epithelial cells of control mice coexpressed Sox2 and Scgb1a1 or α-tubulin (Figure 6A). In the bronchioles of double transgenic mice, epithelial cells expressing high levels of Sox2 lacked Scgb1a1 (the Clara cell marker) and α-tubulin (Figure 6B). High levels of exogenous Sox2 observed in conducting airway epithelial cells of double transgenic mice precluded observation of lower endogenous Sox2 levels in Scgb1a1-positive and α-tubulin–positive epithelial cells (Figure 6B). In alveolar epithelium, control mice were negative for Sox2, Scgb1a1, and α-tubulin (Figure 6C). Ectopic alveolar clusters in double transgenic mice contained heterogeneous cell types, including many cells expressing Sox2 and Scgb1a1 (Figure 6D) and less frequently Sox2+, α-tubulin+, Scgb1a1− cells. The weaker Scgb1a1 intensity (Figure 6D) was due to the weaker expression of Scgb1a1 in the alveolar compartment compared with the bronchiolar compartment of the double transgenic mice.
Figure 6.
Sox2-expressing cells in conducting airway lack differentiation markers. Expression of airway differentiation markers was determined in bronchiolar (A, B) and alveolar epithelium (C, D) from control (A, C) and rSox2 mice (B, D) after 6 months of doxycycline treatment. Triple immunofluorescence for Sox2, Scgb1a1, and α-tubulin demonstrated that high levels of Sox2 in bronchiolar epithelium was associated with the absence of expression of Clara cell (Scgb1a1) or ciliated (α-tubulin) markers (B, inset). High levels of Sox2 seen in (B) precluded observation of lower Sox2 levels in Scgb1a1-positive and α-tubulin–positive cells in the bronchioles of rSox2 mice. In contrast, (Sox2+, Scgb1a1+) and (Sox2+, α-tubulin+) cells were observed in control mice (A, inset). In the alveolar epithelium of rSox2 mice, most Sox2+ cells were Scgb1a1+ and α-tubulin−, whereas some Sox2+ cells were Scgb1a1− and α-tubulin+ (D). Scgb1a1 expression in the alveolar epithelium of rSox2 mice (D) was weaker than in bronchioles (A, B).
Ultrastructural Changes Induced by Sox2
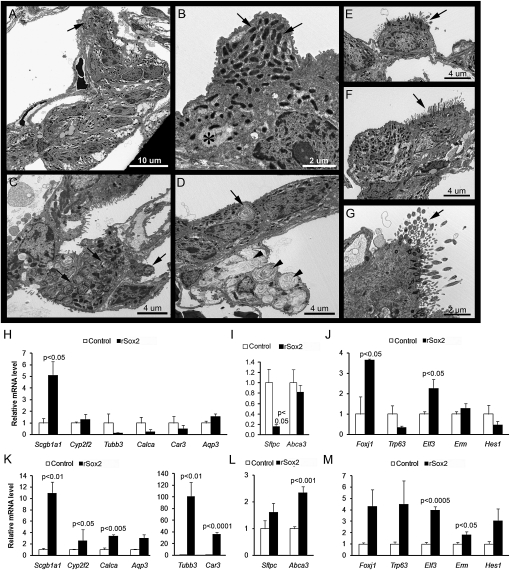
The effects of Sox2 on the ultrastructure of the alveolar epithelium were assessed in control and rSox2 mice after 5 months of doxycycline exposure (Figure 7). The abnormal epithelial cell clusters induced in the alveoli consisted primarily of nonciliated cells with one or two visible ciliated cells and, more rarely, one or two mature type II cells with multiple lamellar bodies (LBs) (Figures 7A–7C), as well as transitional cells containing one or two residual LBs and type II cells with fusing and/or abnormally enlarged LBs (Figure 7D). The nonciliated cells contained numerous mitochondria, smooth endoplasmic reticulum, and glycogen but no rough endoplasmic reticulum or secretory granules. Ciliated cells, however, were well differentiated and readily detected along the alveolar walls as single cells (Figure 7E) or in pairs containing one ciliated and one nonciliated cell (Figure 7F). Cilia consisted of the usual nine pairs of microtubules, arranged concentrically around the periphery, with two central microtubules (Figure 7G); basal bodies exhibited the normal arrangement of nine sets of three microtubules. These abnormal epithelial cell clusters had features of alveolar and bronchiolar epithelial cells, consistent with partial reprogramming of alveolar cells in response to increased Sox2 expression.
Figure 7.
Sox2 induced structural features and expression of markers of the conducting airway epithelium in the alveolar epithelium. Ultrastructural features of alveolar epithelial cells were assessed by transmission electron microscopy in rSox2 mice treated with doxycycline for 5 months (A–G). (A) Abnormal epithelial cell clusters, containing 2 to 15 cells per cluster, were easily detected in the alveolar epithelium. (B) These cell clusters consisted primarily of nonciliated cells with features similar to Clara cells and contained numerous mitochondria (arrows), smooth endoplasmic reticulum, and glycogen (asterisk) but no secretory granules. (C) Scattered type II cells with normal lamellar bodies (LBs) (arrows) were found throughout the alveolar epithelium but rarely in association with cell clusters (A, arrow). (D) Transitional cells with residual lamellar LBs (arrow) were found infrequently. Type II cells with fusing and enlarged LBs (arrowheads) were also found throughout the alveolar epithelium (D). Ciliated cells were detected as single cells (E, arrow) and, more often, in small clusters of two or three cells (F, arrow). (G) Cilia contained the normal arrangement of 9+2 pairs of microtubules (arrow). Messenger RNAs associated with respiratory epithelial cell differentiation were assessed by quantitative RT-PCR in total lung from adult control or rSox2 mice after doxycycline treatment for 3 (H–J) and 7 (K–M) days. Results were normalized to 18S rRNA and expressed as the means ± SE of three or four animals per group. After 3 days of doxycycline, Clara cells (Scgb1a1, Elf3) and ciliated cells (Foxj1) associated mRNAs were induced by Sox2, whereas Sftpc mRNA, an alveolar type cell marker, was decreased. After 7 days of doxycycline, Clara cells (Scgb1a1, Cyp2f2, Car3, Elf3) and ciliated cells (Foxj1, Tubb3) associated mRNAs were induced by Sox2. Abca3 and Erm mRNAs were slightly increased in rSox2 mice compared with control mice.
Sox2 Induced the Expression of Genes Expressed in Conducting Airways
The abundance of mRNAs of genes related to bronchiolar (Scgb1a1, Cyp2f2, Tubb3, Calca, Car3, Aqp3, Foxj1, Trp63, Elf3, Hes1) and alveolar epithelia (Sftpc, Abca3, Erm) was assessed by qRT-PCR in whole lung homogenates (Figure 7). Sox2 increased expression of mRNAs for Clara (Scgb1a1, Elf3) and ciliated (Foxj1) cell markers after 3 days of exposure to doxycycline (Figures 7H and 7J). Expression of the alveolar cell marker Sftpc was decreased 3 days after induction with doxycycline (Figure 7I). After 7 days of doxycycline, Clara cell (Scgb1a1, Cyp2f2, Car3) and ciliated cell (Foxj1, Tubb3) markers were significantly increased, likely indicating the differentiation of proliferative cells induced earlier by Sox2 (Figures 7K and 7M). Trp63 mRNA, a marker for basal cells, and Calca mRNA, a marker for neuroendocrine cells, were modestly increased after 7 days of doxycycline (Figures 7K and 7M).
Lack of Pulmonary Tumors after Expression of Sox2
Pulmonary tumors expressing Sox2 were not detected after 6 months of Sox2 expression (n = 6 control and n = 6 rSox2 mice). Single pulmonary adenomatous tumors were seen in one control and in one rSox2 mouse. Each tumor expressed proSP-C, lacked Sox2, and had morphologic features typical of the spontaneous tumors seen with relatively high frequency in the FVB/N strain (26).
DISCUSSION
The numbers and characteristics of respiratory epithelial cells vary remarkably from the proximal to peripheral regions of the lung, where they play critical roles in barrier formation, mucociliary clearance, innate defense, gas exchange, and repair. Recent studies demonstrated that Sox2 is selectively expressed in the conducting airway, as compared with alveolar epithelial cells, and is required for the normal proliferation and differentiation of basal and nonciliated respiratory epithelial cells (11, 18). A potential role for Sox2 in pulmonary oncogenesis was supported by recent findings that Sox2 was commonly amplified in squamous cell carcinomas of the esophagus and lung (21–23). Consistent with this proposed role of Sox2 in tumorigenesis, the present study demonstrates that Sox2 rapidly induced the proliferation of mouse bronchiolar and alveolar epithelial cells in vivo. Sox2 induced FoxM1 and associated cell cycle regulators in association with increased cell proliferation. In adult mice, Sox2 caused the partial reprogramming of alveolar epithelial cells to those with ciliated and nonciliated conducting airway epithelial cell characteristics. The temporal changes caused by increased expression of Sox2 support the concept that Sox2 initiates proliferation in mouse respiratory epithelial cells followed by partial differentiation into cells with features of airway cells. Sox2 was not sufficient to induce tumor formation at the expression levels produced by our transgenic system.
The Role of Sox2 in Respiratory Epithelial Cell Proliferation
Although Sox2 induced Ki-67 staining in the bronchiolar epithelium in the present model, bronchiolar cell hyperplasia or adenomas were not observed after expression of Sox2. The present findings demonstrated that Sox2 induced cell proliferation that was associated with the increased expression of S-phase–associated cyclin A2 and mitotic-associated cyclin B2. Sox2 also enhanced the expression of FoxM1 in vivo and increased transcriptional activity of a FoxM1 intronic regulatory element in vitro. FoxM1 is known to be expressed before the S-phase and promotes the expression of a number of genes critical for entry into S-phase and, ultimately, mitosis. Thus, in the present study, Sox2 induced FoxM1 and a number of cell cycle proteins, including cyclins D1, A2, and B2. The timing of this induction is consistent with an important role of FoxM1 in the proliferative process. In previous studies, overexpression of FoxM1 in transgenic mice induced DNA replication and cell mitosis and increased cyclin A2 and B1 expression (27, 28). The early induction of cyclins A2 and B2, observed at 3 days after induction of Sox2, likely plays a critical role in the proliferative response seen after increased expression of Sox2. Cyclin D1, also a key regulator of cell proliferation, is known to be activated by FoxM1 (29). Cyclin D1 accumulates until the G1/S phase boundary, when levels rapidly decline. The finding that Sox2 activated the FoxM1 promoter supports the likelihood that Sox2 activates FoxM1, in turn activating the cyclins A2, B2, and D1 to induce cell proliferation. The findings are consistent with previous in vitro studies that Sox2 positively regulates cell proliferation in breast cancer cell lines (30). Likewise, Sox17 induced cell proliferation, activated FoxM1, and induced ectopic differentiation of airway cells in the alveoli of transgenic mice (31). In contrast to the effects of Sox17, Sox2 did not alter the expression of cell cycle inhibitors in vivo (31). The finding that cell proliferation in rSox2 mice was similar to that of control mice after 6 months of doxycycline supports the concept that the cells induced by Sox2 had differentiated into ciliated- and Clara-like cells and were no longer proliferative.
Sox2 and Respiratory Epithelial Cell Differentiation
Previous studies demonstrated the loss of Clara, ciliated, and goblet cell markers in conducting airway cells after Sox2 deletion (11, 18). Thus, the finding that increased expression of Sox2 decreased Scgb1a1 in conducting airways was surprising and contrasted sharply with the induction of Scgb1a1 by Sox2 in hyperplastic cell clusters found in the alveolar regions of the transgenic mice. Taken together, the data support the concept that normal differentiation depends on the precise control of Sox2 levels in airway epithelial cells.
Sox2 induced ectopic clusters of cuboidal cells in the alveolus, which expressed markers normally confined to the conducting airway epithelium. Cells with distinct ciliated and nonciliated bronchiolar cell features as well as “transitional cells” containing rare lamellar bodies were observed at the ultrastructural level. These observations support the concept that Sox2 can partially reprogram alveolar type II cells into various conducting airway cell types.
Sox2 is dynamically regulated during repair of the bronchiolar epithelium (32). After naphthalene-induced bronchiolar cell injury, Sox2 is ubiquitously expressed in the remaining bronchiolar epithelium, its expression being restricted after several days of repair, during which progenitor cells have proliferated and differentiated. The dynamic regulation of Sox2 is likely critical for proliferation and reestablishment of ciliated and Clara cell lineages during the bronchiolar repair process. The finding that high levels of Sox2 inhibited Scgb1a1 in the bronchioles suggests that Sox2 could influence Scgb1a1 expression because proliferation is induced in airway progenitor cells from which ciliated and Clara cells are derived.
Sox2 caused partial reprogramming of alveolar epithelial cells to those with ciliated and nonciliated conducting airway epithelial cell characteristics. Scgb1a1 and Foxj1 mRNA levels were markedly induced after 3 and 7 days of doxycycline treatment in rSox2 mice. These results are supported by previous data demonstrating that Sox2 is required for Clara, goblet, and ciliated cell differentiation from a common precursor (11). The reduction of Sftpc mRNA seen after 3 days of Sox2 expression is consistent with widespread proliferation and loss of alveolar type II phenotype in cells that express the Sox2 transgene. The concept that Sox2 partially reprogrammed alveolar type II cells into bronchiolar-like epithelium is consistent with absence of proSP-C and lamellar bodies in the abnormal alveolar cluster cells and the expression of the lung epithelial lineage marker Nkx2.1.
The temporal changes in gene expression of conducting airway epithelial cell markers in the rSox2 transgenic mice are consistent with early proliferation and subsequent differentiation of alveolar epithelium in response to transgenic Sox2 expression. Expression of cell selected markers increased after 7 days of Sox2 expression and included Cyp2f2 (cytochrome P450, which is responsible for the specific toxicity of naphthalene in Clara cells), Aqp3 (aquaporin 3, a water channel enriched in Clara cells), Car3 (carbonic anhydrase 3, playing a role in CO2 detoxification), and Tubb3 (tubulin3, β-tubulin isoform, participating in the formation of cilia) mRNAs. In addition, Hes1 mRNA, a transcription factor important for Clara cell maintenance, was modestly increased (33). Calca (CGRP, neuroendocrine cell) and Trp63 (p63, basal cell) mRNAs were modestly increased by Sox2 (although not significantly for Trp63) (Figure 7). These findings are consistent with a recent study in which Sox2 was expressed in the embryonic lung using the SPC-rtTA driver (24). In the present study, mRNA of selected alveolar epithelial genes (Abca3, Erm) were slightly increased at 7 days doxycycline, perhaps indicating compensation of remaining alveolar epithelial cells to maintain surfactant homeostasis and respiratory function. This concept is supported by the observation of type II cells containing enlarged and/or fusing, phospholipid-laden LBs. Taken together, these findings indicate that Sox2 partially reprogrammed alveolar type II cells into cells with features of ciliated and nonciliated conducting airway epithelial cells in a process dependent on the induction of Sox2 expression.
Sox2 and Pulmonary Tumorogenesis
Recent studies demonstrated that amplification of the Sox2 gene and increased staining for Sox2 are associated with human squamous cell carcinomas of lung and esophagus, whereas Sox2 expression is absent in most pulmonary adenocarcinomas (22, 23, 34, 35). In the present study, widespread epithelial cell proliferation was induced by Sox2, but no tumors or squamous cell differentiation were observed. Mouse strain, as well as the timing, cell specificity, or levels of Sox2 expression, may have influenced the current findings. In our model, Sox2 expression levels were increased overall but were markedly increased in peripheral lung at sites where Sox2 is not normally expressed. The marked differences between mouse and human airways, the former having little pseudostratified columnar epithelia with p63+ basal cells, may account in part for the lack of squamous differentiation or tumorigenesis.
During the review of the present study, a report featuring a Cre-mediated system for expressing Sox2 was published (36). In that report, dose-dependent expression of Sox2 induced hyperplasia or ectopic epithelial cell differentiation similar to the present findings. In contrast to our data, peripheral lung tumors were observed after expression of Sox2.
Summary
Sox2 induced proliferation in the alveolar epithelium, followed by dramatic induction of ectopic clusters of cuboidal cells with markers of the conducting airway epithelium. Rapid induction of the cell cycle regulators FoxM1, Cyclin A2, and Cyclin B2 was observed in vivo, and Sox2 activated a FoxM1-Luciferase construct in vitro. Conditional Sox2 transgene expression driven by rScgb1a1-rtTA was insufficient to induce pulmonary tumors. The present study supports the concept that Sox2 is a positive regulator of proliferation and differentiation in respiratory epithelium.
Acknowledgments
The authors thank Gail Macke for technical assistance and Dr. K.A. Wikenheiser-Brokamp (Cincinnati Children's Hospital Medical Center and University of Cincinnati College of Medicine) for suggestions and advice.
This work was supported by National Institutes of Health grants HL090156 and HL099580 (J.A.W. and S.E.W.) and by partial fulfillment of the graduate thesis in the Cancer Cell Biology program of the University of Cincinnati College of Medicine (D.H.T.).
This article contains an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0149OC on September 20, 2010
Author Disclosure: J.W. has a patent from the Cincinnati Children's Hospital Medical Center for the use of SP-D for treatment of lung disease and has received sponsored grants from March of Dimes for $10,001 to $50,000 and NIH for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol 2005;33:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development 2006;133:2455–2465. [DOI] [PubMed] [Google Scholar]
- 3.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 2004;164:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of scgb1a1+ clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009;106:12771–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal basal cells: a facultative progenitor cell pool. Am J Pathol 2010;177:362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans MJ, Cabral LC, Stephens RJ, Freeman G. Acute kinetic response and renewal of the alveolar epithelium following injury by nitrogen dioxide. Chest 1974;65:62S–65S. [DOI] [PubMed] [Google Scholar]
- 8.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to no2. Exp Mol Pathol 1975;22:142–150. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. Spdef is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 2009;119:2914–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 2002;161:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar clara, ciliated, and goblet cells. PLoS ONE 2009;4:e8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chew LJ, Gallo V. The yin and yang of sox proteins: activation and repression in development and disease. J Neurosci Res 2009;87:3277–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell 2002;3:167–170. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 15.Graham V, Khudyakov J, Ellis P, Pevny L. Sox2 functions to maintain neural progenitor identity. Neuron 2003;39:749–765. [DOI] [PubMed] [Google Scholar]
- 16.Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature 2005;434:1031–1035. [DOI] [PubMed] [Google Scholar]
- 17.Okubo T, Pevny LH, Hogan BL. Sox2 is required for development of taste bud sensory cells. Genes Dev 2006;20:2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for sox2 in the developing and adult mouse trachea. Development 2009;136:1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ioannides AS, Massa V, Ferraro E, Cecconi F, Spitz L, Henderson DJ, Copp AJ. Foregut separation and tracheo-oesophageal malformations: The role of tracheal outgrowth, dorso-ventral patterning and programmed cell death. Dev Biol 2009;337:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider A, Bardakjian T, Reis LM, Tyler RC, Semina EV. Novel sox2 mutations and genotype-phenotype correlation in anophthalmia and microphthalmia. Am J Med Genet A 2009;149A:2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et al. Sox2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 2009;41:1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembele D, Martinet N, Thibault C, Huelsken J, Brambilla E, et al. Sox2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS ONE 2010;5:e8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCaughan F, Pole JC, Bankier AT, Konfortov BA, Carroll B, Falzon M, Rabbitts TH, George PJ, Dear PH, Rabbitts PH. Progressive 3q amplification consistently targets sox2 in preinvasive squamous lung cancer. Am J Respir Crit Care Med 2010;182:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: branching morphogenesis and epithelial cell differentiation. Dev Biol 2008;317:296–309. [DOI] [PubMed] [Google Scholar]
- 25.Whitsett JA, Perl AK. Conditional control of gene expression in the respiratory epithelium: a cautionary note. Am J Respir Cell Mol Biol 2006;34:519–520. [DOI] [PubMed] [Google Scholar]
- 26.Mahler JF, Stokes W, Mann PC, Takaoka M, Maronpot RR. Spontaneous lesions in aging fvb/n mice. Toxicol Pathol 1996;24:710–716. [DOI] [PubMed] [Google Scholar]
- 27.Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, Yoder HM, Costa RH. Ubiquitous expression of the forkhead box m1b transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem 2003;278:37888–37894. [DOI] [PubMed] [Google Scholar]
- 28.Leung TW, Lin SS, Tsang AC, Tong CS, Ching JC, Leung WY, Gimlich R, Wong GG, Yao KM. Over-expression of foxm1 stimulates cyclin b1 expression. FEBS Lett 2001;507:59–66. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box m1b transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci USA 2001;98:11468–11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Shi L, Zhang L, Li R, Liang J, Yu W, Sun L, Yang X, Wang Y, Zhang Y, et al. The molecular mechanism governing the oncogenic potential of sox2 in breast cancer. J Biol Chem 2008;283:17969–17978. [DOI] [PubMed] [Google Scholar]
- 31.Lange AW, Keiser AR, Wells JM, Zorn AM, Whitsett JA. Sox17 promotes cell cycle progression and inhibits tgf-beta/smad3 signaling to initiate progenitor cell behavior in the respiratory epithelium. PLoS ONE 2009;4:e5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 2006;34:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito T, Udaka N, Yazawa T, Okudela K, Hayashi H, Sudo T, Guillemot F, Kageyama R, Kitamura H. Basic helix-loop-helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development 2000;127:3913–3921. [DOI] [PubMed] [Google Scholar]
- 34.Sholl LM, Long KB, Hornick JL. Sox2 expression in pulmonary non-small cell and neuroendocrine carcinomas. Appl Immunohistochem Mol Morphol 2010;18:55–61. [DOI] [PubMed] [Google Scholar]
- 35.Yuan P, Kadara H, Behrens C, Tang X, Woods D, Solis LM, Huang J, Spinola M, Dong W, Yin G, et al. Sex determining region y-box 2 (sox2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLoS ONE 2010;5:e9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL, Onaitis MW. Evidence that sox2 overexpression is oncogenic in the lung. PLoS ONE 2010;5:e11022. [DOI] [PMC free article] [PubMed] [Google Scholar]