Abstract
We showed that nitric oxide (NO) signaling is decreased in the pulmonary vasculature before the development of endothelial dysfunction in a lamb model of congenital heart disease and increased pulmonary blood flow (Shunt). The elucidation of the molecular mechanism by which this occurs was the purpose of this study. Here, we demonstrate that concentrations of the endogenous NO synthase (NOS) inhibitor, asymmetric dimethylarginine (ADMA), are elevated, whereas the NOS cofactor tetrahydrobiopterin (BH4) is decreased in Shunt lambs. Our previous studies demonstrated that ADMA decreases heat shock protein–90 (Hsp90) chaperone activity, whereas other studies suggest that guanosine-5′-triphosphate cyclohydrolase 1 (GCH1), the rate-limiting enzyme in the generation of BH4, may be a client protein for Hsp90. Thus, we determined whether increases in ADMA could alter GCH1 protein and activity. Our data demonstrate that ADMA decreased GCH1 protein, but not mRNA concentrations, in pulmonary arterial endothelial cells (PAECs) because of the ubiquitination and proteasome-dependent degradation of GCH1. We also found that Hsp90–GCH1 interactions were reduced, whereas the association of GCH1 with Hsp70 and the C-terminus of Hsp70-interacting protein (CHIP) increased in ADMA-exposed PAECs. The overexpression of CHIP potentiated, whereas a CHIP U-box domain mutant attenuated, ADMA-induced GCH1 degradation and reductions in cellular BH4 concentrations. We also found in vivo that Hsp90/GCH1 interactions are decreased, whereas GCH1–Hsp70 and GCH1–CHIP interactions and GCH1 ubiquitination are increased. Finally, we found that supplementation with l-arginine restored Hsp90–GCH1 interactions and increased both BH4 and NOx concentrations in Shunt lambs. In conclusion, increased concentrations of ADMA can indirectly alter NO signaling through decreased cellular BH4 concentrations, secondary to the disruption of Hsp90–GCH1 interactions and the CHIP-dependent proteasomal degradation of GCH1.
Keywords: proteasome, ubiquitination, Hsp90, Hsp70, mitochondrial dysfunction
Nitric oxide (NO), produced by endothelial NO synthase (eNOS), plays a role in controlling vascular tone. Accumulating evidence suggests that a decrease in NO signaling causes endothelial dysfunction in a number of cardiovascular diseases. We recently found, using a lamb model of congenital heart disease (CHD) with increased pulmonary blood flow (Shunt), that NO signaling is compromised before the development of endothelial dysfunction, and this correlates with an increase in eNOS uncoupling. NOS uncoupling is a complex process that can be induced by decreases in the availability of the substrate l-arginine or the cofactor tetrahydrobiopterin (BH4), or as we recently showed, by increases in the cellular concentrations of asymmetric dimethyarginine (ADMA) (1). A large body of evidence supports the conclusion that increased concentrations of ADMA contribute to the development of endothelial dysfunction (2). ADMA is constantly formed during physiologic protein turnover, and it is released from the hydrolysis of methylated proteins (3, 4). Increased ADMA concentrations were reported in a variety of pathologic conditions affecting the cardiovascular system (3, 4), including pulmonary hypertension (5). However, the mechanisms by which ADMA exerts its effects on endothelial function have not been adequately elucidated.
A large number of studies showed that suboptimal concentrations of BH4 induce endothelial dysfunction through eNOS uncoupling (6). In cells, BH4 can be produced by two different metabolic pathways: the de novo and the salvage pathways. GTP cyclohydrolase I (GCH1) is the first, rate-limiting enzyme in the de novo pathway of BH4 biosynthesis (7). Recent studies suggested that GCH1 may be a client protein for Hsp90 (8), and the conditions that reduce concentrations of BH4 are also the conditions that reduce Hsp90 chaperone activity (1, 9), suggesting that the activity of GCH1 may be influenced by its interactions with Hsp90. We previously showed that increasing the cellular concentrations of ADMA l decreases Hsp90 chaperone activity in pulmonary arterial endothelial cells (PAECs) (1). Thus, the purpose of this study was to begin elucidating the molecular mechanisms that underlie the increase in eNOS uncoupling that we previously showed in our Shunt model, and to determine if a link existed between increases in ADMA concentrations and alterations in BH4 biosynthesis.
Here we report, both in vitro and in vivo, that increased concentrations of ADMA result in decreased BH4 concentrations through the disruption of the Hsp90–GCH1 complex, leading to the C-terminus of Hsp70-interacting protein (CHIP)–dependent ubiquitin-mediated proteasomal degradation of GCH1. Together, our data indicate that ADMA can both directly, through competition with l-arginine for the active site of eNOS, and indirectly, through its modulation of Hsp90 chaperone activity and decreased BH4, produce eNOS uncoupling.
MATERIALS AND METHODS
Surgical Preparation and Care
Eighteen mixed-breed Western pregnant ewes (at 137–141 days of gestation; term, 145 days) were operated upon, as previously described (10) (see also the online supplement). All protocols and procedures were approved by the Committees on Animal Research at the University of California at San Francisco, the Medical College of Georgia, and the German Heart Center.
Hemodynamic Measurements
Pulmonary arterial and right and left atrial pressures were measured as described in the online supplement.
Measurement of ADMA Concentrations
Concentrations of ADMA were analyzed using high-performance liquid chromatography (HPLC), as we described elsewhere (1).
Measurement of Dimethylarginine Dimethylaminohydrolase Activity
The activity of dimethylarginine dimethylaminohydrolase (DDAH) in tissues was assessed directly by measuring the amount of ADMA metabolized by this enzyme, as previously described (11).
Cell Culture and Treatment
Primary cultures of ovine PAECs were isolated, as described previously (12), and were treated as described in the online supplement.
Quantification of Biopterin Concentrations According to HPLC
Concentrations of BH4 were determined using the differential iodine oxidation method, as we previously described (13, 14). Levels were normalized for protein concentration, using the Bradford assay.
Purification of Recombinant Human eNOS
Recombinant human eNOS was purified from Escherichia coli strain BL21 (DE3) pLysS (EMD4Biosciences, San Diego, CA) containing the poly-His-pCWeNOS vector, as previously described (15).
Plasmids and Transient Transfection of PAECs
The pcDNA3 expression constructs encoding the Myc epitope-tagged human wild-type and point mutant (K260Q) CHIP were described previously (16, 17). PAECs were seeded onto 6-well plates at a density of approximately 16,000 cells/cm2. Cells were transiently transfected with wild-type CHIP, a U-box domain mutant CHP, or the parental vector (pcDNA3), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Immunoprecipitation and Western Blot Analysis
Immunoprecipitation and Western blot analysis were performed as described in the online supplement.
Determination of mRNA Concentrations
For cell-culture studies to determine concentrations of GCH1 mRNA, semiquantitative RT-PCR was used as described in the online supplement. For in vivo studies using peripheral lung tissue, quantitative RT-PCR, involving SYBR green I dye for the specific detection of double-stranded DNA, was used as described in the online supplement.
Shear Stress
Laminar shear stress (20 dyn/cm2) was applied using a cone-plate viscometer, as described previously (18, 19).
Determination of NOx Concentrations
A chemiluminescence method was used, as described in the online supplement.
Detection of Ubiquitinated GCH1
Ubiquitinated GCH1 was enriched using a ubiquitinated protein enrichment kit (Calbiochem, Gibbstown, NJ), according to the manufacturer's guidelines. Samples were separated by SDS-PAGE and analyzed by Western blotting, using a specific antiserum raised against GCH1 (13).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 4.01 for Windows (GraphPad Software, La Jolla, CA). Means ± SEMs were calculated for all samples, and significance was determined either by unpaired t test (for two groups) or by ANOVA (for ≥ 3 groups), with Newman-Keuls post hoc testing. When data were non-normally distributed, nonparametric testing was used (with the Wilcoxon signed-rank test for two groups, and the Kruskal-Wallis test for ≥ 3 groups). P < 0.05 was considered significant.
RESULTS
Hemodynamics
The hemodynamic data for the 2-week-old control and Shunt lambs are shown in Table 1. Pulmonary arterial pressure, left atrial pressure, and left pulmonary blood flow were significantly greater in Shunt lambs than in age-matched control lambs (Table 1). In addition, a significant difference in pulmonary-to-systemic blood flow ratio occurred in Shunt lambs (Table 1). No significant differences were evident in mean blood pressure, heart rate, and right atrial pressure (Table 1).
TABLE 1.
HEMODYNAMIC MEASUREMENTS
| 2-Week Shunt Lambs | 2-Week Control Lambs | |
|---|---|---|
| PAP (mean, mm Hg) | 21.6 ± 4.3* | 15.4 ± 3.2 |
| LAP (mean, mm Hg) | 6.5 ± 4.1* | 3.1 ± 1.9 |
| RAP (mean, mm Hg) | 4.9 ± 3.2 | 2.5 ± 1.4 |
| Mean BP (mm Hg) | 75.8 ± 31.5 | 65.0 ± 7.2 |
| Left pulmonary flow (ml per minute/kg) | 166.0 ± 15.7* | 55.5 ± 15.9 |
| Heart rate (beats/minute) | 162.7 ± 30.4 | 177.4 ± 26.7 |
| Qp/Qs | 3.4 ± 1.2* | 1.1 ± 0.1 |
Definition of abbreviations: PAP, pulmonary arterial pressure; LAP, left atrial pressure; RAP, right atrial pressure; BP, systemic blood pressure; Qp/Qs, pulmonary-to-systemic blood flow ratio (n = 6 for each group). Data are mean ± SD
Indicates P ≤ 0.05 vs. 2-week control lambs.
Peripheral Lung Concentrations of ADMA and Tetrahydrobiopterin
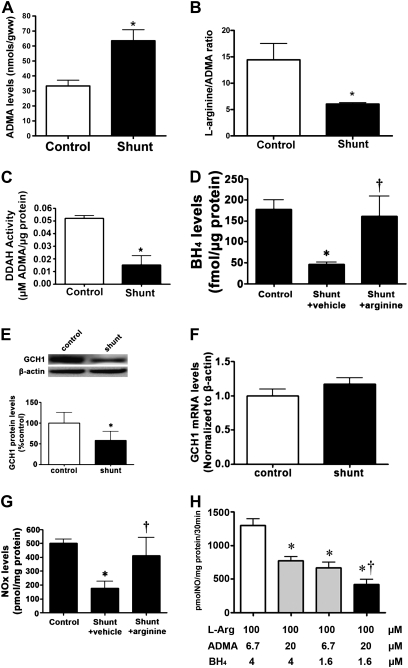
Our previous studies showed an early increase in eNOS uncoupling in Shunt lambs, although the mechanisms remain unclear (1, 20). To begin elucidating the mechanism underlying this uncoupling process, we initially determined whether alterations occurred in either ADMA or BH4 in the Shunt lambs. Our data indicate that Shunt lambs have significantly higher concentrations of peripheral lung-tissue ADMA (Figure 1A), resulting in a significant decrease in the peripheral lung l-arginine/ADMA ratio (Figure 1B). This increase in ADMA concentrations correlates with a decrease in DDAH activity (Figure 1C). In addition, Shunt lambs exhibit a significant decrease in BH4 concentrations (Figure 1D), correlating with a significant decrease in GCH1 protein concentrations (Figure 1E) but not GCH1 mRNA (Figure 1F). Together, these changes significantly decrease tissue NOx concentrations in Shunt lambs (Figure 1G). To begin determining the relative effects of these changes in ADMA and BH4 on NO signaling, we used human recombinant protein and matched the ADMA/l-arginine ratio and BH4 concentrations in control and Shunt lambs, both individually and in tandem. Our data demonstrate that increasing the ratio of ADMA to l-arginine to match that of Shunt lambs significantly decreased the generation of NO to a similar extent as when matching the decrease in BH4 concentrations (Figure 1H). When we matched the increase in ADMA and the decrease in BH4 observed in Shunt lambs, a synergistic decrease in the generation of NO was evident (Figure 1H).
Figure 1.
Concentrations of asymmetric dimethyarginine (ADMA) and tetrahydrobiopterin (BH4) are altered in lambs with increased pulmonary blood flow. ADMA (A), the l-arginine/ADMA ratio (B), and dimethylarginine dimethylaminohydrolase (DDAH) activity (C) were determined in peripheral lungs of Shunt and control lambs. Concentrations of ADMA are significantly increased in Shunt lambs, whereas the l-arginine/ADMA ratio and DDAH activity are significantly decreased. In addition, concentrations of BH4 were also significantly decreased in Shunt lambs, whereas concentrations of BH4 were restored by supplementation with l-arginine (D). Protein extracts or total RNA were also prepared from the peripheral lungs of Shunt and control lambs. GTP cyclohydrolase I (GCH1) protein (E) and mRNA (F) were then determined by Western blot analysis or quantitative RT-PCR, respectively. The Western blot analysis was performed using an antibody raised against GCH1. A representative image is shown. Loading was normalized by reprobing the membranes with an antibody specific to β-actin. GCH1 protein concentrations were significantly decreased in 2-week-old Shunt lambs (E). However, concentrations of GCH1 mRNA were unchanged (F). These changes correlated with a decrease in tissue NOx concentrations in Shunt lambs. NOx concentrations were restored to control levels by supplementation with l-arginine (G). Values represent mean ± SEM (n = 6). *P < 0.05 versus control lambs. To define the relative roles of increased ADMA and decreased BH4 in the inhibition of NO synthesis, purified human endothelial NO synthase (eNOS) (80 ng) was incubated with 4 μM (control concentrations) or 1.6 μM (Shunt concentrations) of BH4 and 0.1 mM l-arginine in the presence of ADMA levels that matched the l-arginine/ADMA ratios we observed in either control or Shunt lambs. The generation of NOx was then determined. Shunt concentrations of ADMA or BH4 alone significantly decreased the generation of NOx (H), whereas a synergistic decrease occurred in NOx concentrations when Shunt concentrations of BH4 and ADMA were added in combination (H). Values represent means ± SEM (n = 6). *P < 0.05 versus control concentrations of BH4 and ADMA. †P < 0.05 versus Shunt concentrations of ADMA or BH4 alone.
Effect of ADMA on BH4 and GCH1 Concentrations in PAECs
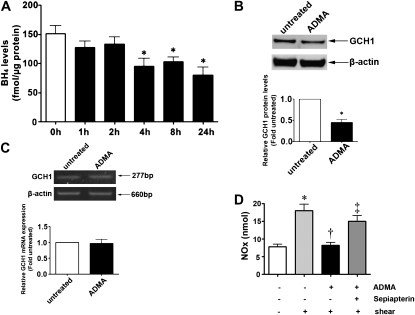
Recent studies suggested that GCH1 may be a client protein for Hsp90 (8), and we previously showed that increasing the cellular concentrations of ADMA decreased Hsp90 chaperone activity in PAECs (1). Thus, we initiated a series of cell-culture studies to determine if increased concentrations of ADMA were modulating BH4 concentrations through alterations in Hsp90 chaperone activity. A time-course analysis of PAECs exposed to increased concentrations of ADMA demonstrated that BH4 concentrations were significantly decreased after 4 hours of exposure, and this decrease was sustained through 24 hours (Figure 2A). We also analyzed the effects of ADMA on both GCH1 mRNA and protein concentrations, to determine if any changes were detectable before the decrease in BH4 concentrations. Our data indicate that exposure of PAECs to ADMA for 2 hours significantly lowered the protein concentrations of GCH1 (Figure 2B), but as in the Shunt lambs, GCH1 mRNA concentrations were unchanged (Figure 2C). These changes resulted in a decrease in the generation of NO in response to shear stress that could be reversed by the addition of the BH4 precursor, sepiapterin (Figure 2D).
Figure 2.
Effects of increased ADMA concentrations on expression of GCH1 and BH4 concentrations in pulmonary arterial endothelial cells (PAECs). PAECs were exposed to an l-arginine/ADMA ratio of 5:1 to mimic the concentrations seen in Shunt lambs for up to 24 hours. Concentrations of BH4 in cell extracts were then measured by high-performance liquid chromatography (HPLC) (A). Concentrations of BH4 significantly decreased after 4 hours, and this decrease persisted through 24 hours of exposure (A). In addition, protein extracts and total RNA were prepared from cells exposed to ADMA for 2 hours (a time that preceded the decrease in BH4 concentrations). Western blot (B) and semiquantitative RT-PCR (C) analyses were also performed. A representative image is shown, along with a densitometric analysis indicating that ADMA decreases GCH1 protein concentrations (B). Loading was normalized by reprobing the membranes with an antibody specific to β-actin. GCH1 mRNA concentrations were unchanged (C). Values represent mean ± SEM (n = 4). *P < 0.05 versus untreated cells. The decrease in concentrations of BH4 induced by ADMA resulted in a significant decrease in the generation of NO in response to shear stress (15 minutes, 20 dyn/cm2), and this was reversed by pretreatment with the BH4 precursor, sepiapterin (10 μM) (D). Values represent mean ± SEM (n = 6). *P < 0.05 versus no shear. †P < 0.05 versus shear alone. ‡P < 0.05 versus shear + ADMA.
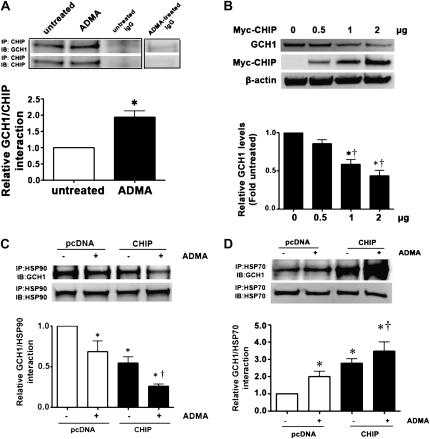
ADMA Disrupts the Hsp90–GCH1 Complex and Increases Hsp70–GCH1 Interactions in PAECs
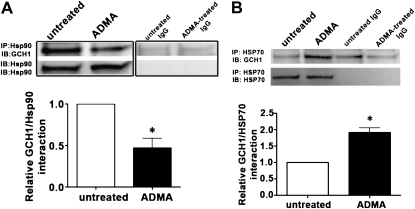
Using an immunoprecipitation–Western blot technique, we revealed that GCH1 forms a complex with Hsp90 in PAECs (Figure 3A), whereas the exposure of cells to ADMA led to a significant decrease in this association (Figure 3A). Hsp90 client proteins are usually brought into the complex with Hsp90 through multiprotein Hsp90/Hsp70–based chaperone machinery (21). Further, Hsp70 appears to be involved in both the folding and degradation of Hsp90-client proteins (22). Again, using an immunoprecipitation–Western blot technique, we found that exposure to ADMA significantly increased the interaction of GCH1 with Hsp70 (Figure 3B).
Figure 3.
GCH1 is present in a complex with heat shock protein–90 (Hsp90) in PAECs, and ADMA disrupts this interaction. PAECs were exposed or not exposed to ADMA for 2 hours, and then whole-cell lysates (1 mg) were subjected to immunoprecipitation (IP), using an antibody specific to Hsp90 (A) or Hsp70 (B) (2 μg), followed by Western blot (IB) analysis using a specific antiserum raised against GCH1. A representative image is shown for each Western blot, including the control with IgG alone. Blots were also stripped and reprobed for Hsp90 or Hsp70, to normalize for the efficiency of immunoprecipitation. Densitometric values were then obtained for each. GCH1 is present in PAECs in a complex with both Hsp90 and Hsp70, and ADMA reduces the interaction of GCH1 with Hsp90 and enhances its interaction with Hsp70. Values represent mean ± SEM (n = 4). *P < 0.05 versus control.
ADMA Mediates GCH1 Degradation via a Ubiquitination–Proteasome Pathway in PAECs
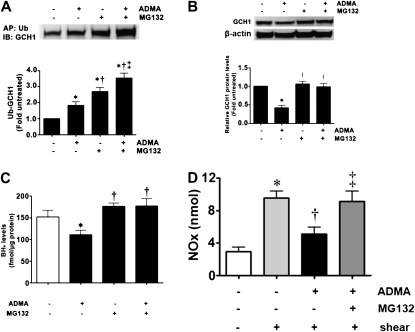
Increasing evidence suggests that the selective degradation of many proteins in eukaryotic cells is mediated by protein ubiquitination (23). To investigate the role of ubiquitination in the decrease in GCH1 protein concentrations associated with increased concentrations of ADMA, PAECs were pretreated with the proteasome inhibitor MG132 in the presence or absence of increased concentrations of ADMA. Exposure of PAECs to ADMA, or MG132 alone, significantly increased the ubiquitination of GCH1 (Figure 4A), suggesting that the ADMA-mediated degradation of GCH1 occurs via a ubiquitin-dependent mechanism. To confirm this, we measured the effects of proteasome inhibition on the ADMA-induced degradation of GCH1 and on cellular BH4 concentrations. Our data indicate that the inhibition of proteasome attenuates the ADMA-induced decrease in GCH1 protein concentrations (Figure 4B), and attenuates the reduction in BH4 concentrations (Figure 4C). Similarly, the decrease in NO generation in response to ADMA was reversed in the presence of MG132 (Figure 4D).
Figure 4.
ADMA induces GCH1 ubiquitination and proteasomal degradation in PAECs. PAECs were exposed or not to ADMA in the presence or absence of the proteasomal inhibitor MG132 (10 μM) for 2 hours, and then whole-cell lysates were subjected to ubiquitinated protein enrichment (AP), followed by Western blotting with an anti-GCH1 antibody (IB). A representative image is shown. In addition, the effect of MG132 on the ADMA-mediated decrease in GCH1 protein concentrations was determined by Western blot analysis, using an antibody raised against GCH1. Loading was normalized by reprobing the membranes with an antibody specific to β-actin. A representative image is shown. Densitometric values represent mean ± SEM (n = 6). *P < 0.05 versus untreated cells. †P < 0.05 versus ADMA alone. Finally, PAECs were exposed or not exposed to ADMA in the presence or absence of MG132 for 4 hours, and the effect on cellular BH4 concentrations was determined. The presence of MG132 attenuated the ADMA-mediated decrease in BH4 concentrations. Values represent mean ± SEM (n = 6). *P < 0.05 versus untreated cells. †P < 0.05 versus ADMA alone. ‡P < 0.05 versus MG132 alone. The decreases in concentrations of NO induced by ADMA in response to shear stress (15 minutes, 20 dyn/cm2) were reversed by MG132 (D). Values represent mean ± SEM (n = 6). *P < 0.05 versus no shear. †P < 0.05 versus shear alone. ‡P < 0.05 versus shear + ADMA.
CHIP Facilitates Both Basal and ADMA-Mediated GCH1 Degradation in PAECs
Previous studies identified CHIP as a ubiquitin ligase that directs client protein degradation via the ubiquitin–proteasome pathway (17). To assess whether CHIP is involved in the ADMA-induced degradation of GCH1, we determined whether GCH1 interacts with CHIP and the effects of ADMA on this interaction. Our data indicate that ADMA increased the interaction of GCH1 with CHIP in PAECs (Figure 5A). Next, we examined the effects of CHIP overexpression on GCH1 protein concentrations in PAECs. Cells were transiently transfected with an expression construct for wild-type CHIP (pcDNA-myc-CHIP) or the parental vector (pCDNA3), and the effects on GCH1 protein concentrations were determined by Western blot analysis. The overexpression of CHIP decreased GCH1 protein concentrations in a dose-dependent manner (Figure 5B). Further, we determined possible synergistic effects between CHIP and ADMA on the interactions of GCH1 with Hsp90 and Hsp70 in PAECs. Again, cells were transiently transfected with wild-type CHIP and exposed to ADMA. We found that the overexpression of CHIP in combination with the addition of ADMA further reduced the Hsp90–GCH1 interaction, compared with the overexpression of CHIP or ADMA treatment alone (Figure 5C). The overexpression of CHIP, in combination with the addition of ADMA, also significantly increased the Hsp70–GCH1 interaction, compared with the overexpression of CHIP or ADMA treatment alone (Figure 5D). Taken together, our results suggest that CHIP or ADMA can individually modulate Hsp90–GCH1 and Hsp70–GCH1 interactions, and that in combination, a synergistic effect on the interactions of GCH1 with Hsp90 and Hsp70 occurs.
Figure 5.
The ADMA-induced degradation of GCH1 is the dependent on the C-terminus of Hsp70-interacting protein (CHIP) in PAECs. PAECs were exposed or not to ADMA for 2 hours, and then whole-cell lysates were subjected to immunoprecipitation (IP), using an antibody specific to CHIP, and then analyzed by Western blot analysis, using the specific antiserum raised against GCH1 protein (A). A representative image is shown, including the control with IgG alone. Blots were then stripped and reprobed for CHIP, to normalize for the efficiency of immunoprecipitation. Densitometric analysis indicates that ADMA enhances the association of GCH1 with CHIP (A). PAECs were also transiently transfected with increasing amounts (0, 0.5, 1, and 2 μg) of pcDNA-Myc-carboxy terminus of Hsc70 interacting protein (pcDNA-Myc-CHIP). Forty hours after tranfection, whole-cell lysates (20 μg) were prepared, and protein concentrations of GCH1 and Myc-CHIP were determined by immunoblotting with anti-GCH1 and anti-Myc. A representative image is shown, along with the densitometric analysis indicating that the overexpression of CHIP dose-dependently decreases GCH1 protein concentrations (B). Loading was normalized by reprobing the membranes with an antibody specific to β-actin. In addition, whole-cell lysates prepared form PAECs transfected with pcDNA-Myc-CHIP or the parental vector (PCDNA3, 2 μg) were subjected to immunoprecipitation (IP), using an antibody specific to Hsp90 (C) or Hsp70 (D), and then analyzed by Western blotting (IB), using a specific antiserum raised against GCH1. A representative image is shown for each Western blot. Blots were also stripped and reprobed for Hsp90 or Hsp70, to normalize for the efficiency of immunoprecipitation. Densitometric values were then obtained for each band. The overexpression of CHIP significantly reduces the interaction of GCH1 with Hsp90, and enhances its interaction with Hsp70. Values represent mean ± SEM (n = 4–6). *P < 0.05 versus empty vector transfection without ADMA treatment. †P < 0.05 versus overexpression of CHIP or empty vector transfection with ADMA treatment.
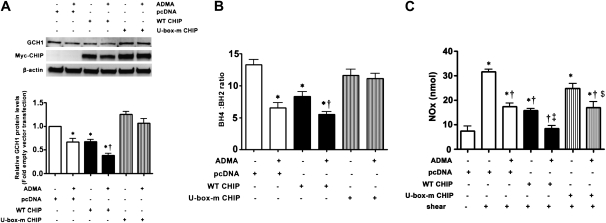
The U-Box Domain of CHIP Is Essential for ADMA-Mediated GCH1 Degradation in PAECs
CHIP has a C-terminal U-box domain that is responsible for its ubiquitin ligase activity (17). To test the effects of this domain on the CHIP-facilitated, ADMA-mediated degradation of GCH1, we used a U-box domain mutant CHIP (U-box-mCHIP) that lacks the ability to ubiquitinate protein (17). PAECs were trasnsiently transfected with wild-type CHIP, U-box-mCHIP, or the parental vector (pCDNA3), and were then exposed or not to ADMA. GCH1 protein concentrations and BH4/BH2 ratio were subsequently determined by Western blot analysis or HPLC. Western blot analysis demonstrated that U-box-mCHIP exerted a dominant negative effect on the ADMA-mediated decrease in GCH1 protein concentrations (Figure 6A). Our results also indicated that the overexpression of wild-type CHIP but not U-box-mCHIP significantly lowered the BH4/BH2 ratio in PAECs (Figure 6B). In the presence of ADMA, the BH4/BH2 ratio was further reduced in cells overexpressing wild-type CHIP (Figure 6B). However, when cells were transfected with U-box-mCHIP and exposed to ADMA, the BH4/BH2 ratio was not altered (Figure 6B). We also found that the overexpression of wild-type CHIP significantly decreased the shear-mediated generation of NOx (Figure 6C). Further, the overexpression of CHIP in combination with ADMA further reduced the production of NOx, compared with the overexpression of CHIP or ADMA treatment alone (Figure 6C). Conversely, the overexpression of the U-box-mCHIP significantly attenuated the ADMA-mediated decrease in NOx generation (Figure 6C).
Figure 6.
The U-box domain of CHIP is required for the ADMA-mediated degradation of GCH1 in PAECs. PAECs were transiently transfected with pcDNA-Myc-CHIP, a U-box domain mutant CHIP (U-box-mCHIP), or the parental vector (pcDNA3). Forty hours after transfection, cells were exposed or not exposed to ADMA for 2 hours, and then whole-cell lysates (20 μg) were prepared and protein concentrations of GCH1 and Myc-CHIP were determined by immunoblotting with anti-GCH1 or anti-Myc, respectively. β-actin was used as an SDS-PAGE loading control. A representative image is shown (A). The U-box mutant CHIP attenuates the ADMA-mediated degradation of GCH1 (A). In addition, concentrations of BH4 and BH2 were determined by HPLC. Although no synergistic effect of CHIP and ADMA occurred in decreasing cellular BH4 concentrations, a significant synergistic decrease in the BH4/BH2 ratio did occur. Values represent mean ± SEM (n = 6). *P < 0.05 versus pcDNA3. †P < 0.05 versus overexpression of CHIP. The overexpression of CHIP alone decreased concentrations of NO in response to shear stress (15 minutes, 20 dyn/cm2), and this was potentiated in the presence of ADMA (D). The overexpression of the U-box mutant CHIP attenuated the negative effects of ADMA on the generation of NO in response to shear stress (D). Values represent mean ± SEM (n = 6). *P < 0.05 versus no shear. †P < 0.05 versus shear alone. ‡P < 0.05 versus wild-type (WT) CHIP + shear. $P < 0.05 versus wild-type CHIP + shear + ADMA.
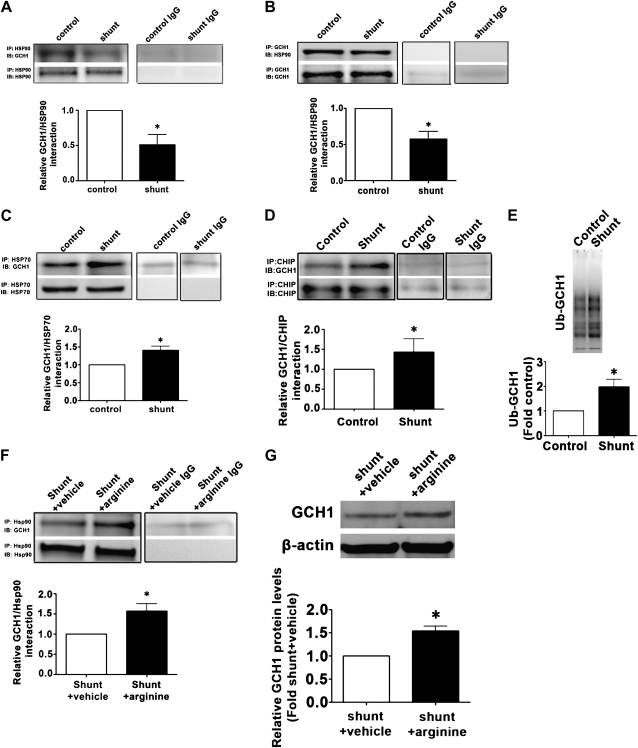
The CHIP–Ubiquitin Pathway Modifies GCH1 in a Lamb Model of Pulmonary Hypertension Secondary to Increased Pulmonary Blood Flow
Finally, we reexamined our lamb model to determine if the changes in GCH1 protein concentrations (Figure 1) were attributable to the same Hsp70–CHIP–ubiquitin pathway we elucidated in culture. Our data indicate that the interaction of GCH1 with Hsp90 is significantly decreased in the Shunt lamb (Figures 7A and 7B), whereas Hsp70–GCH1 (Figure 7C) and CHIP–GCH1 (Figure 7D) interactions are significantly increased in Shunt lambs. Consistent with these findings, the ubiquitination of GCH1 is significantly increased in Shunt lambs (Figure 7E).
Figure 7.
The decrease in Hsp90–GCH1 interactions, and the increase in CHIP–GCH1 interactions and protein ubiquitination, are recapitulated in lambs with increased pulmonary blood flow. Protein extracts were prepared from peripheral lungs of Shunt and control lambs, and subjected to immunoprecipitation (IP), using antibodies specific to Hsp90, Hsp70, or CHIP, and then analyzed by Western blotting (IB), using a specific antiserum raised against GCH1. In addition, a reverse IP was performed using CGH1, and then analyzed by IB for Hsp90 (B). Tissue lysates were also subjected to ubiquitinated protein enrichment (AP), followed by IB with an anti-GCH1 antibody. The concentrations of GCH1 associated with Hsp90 (A and B) are significantly reduced in Shunt lambs, whereas the interactions of GCH1 with Hsp70 (C) and CHIP (D) are significantly increased. A significant increase in ubquitinated (Ub) GCH1 also occurred in Shunt lambs (E). Supplementation with l-arginine significantly increased both GCH1–Hsp90 interactions (F) and GCH1 protein concentrations (G) in Shunt lambs. Densitometric values represent mean ± SEM (n = 6). *P < 0.05 compared with age-matched control lambs (A–E) or vehicle-treated Shunt lambs (F and G).
Supplementation with l-Arginine Preserves the Hsp90–GCH1 Interaction and BH4 Concentrations, and Restores Endothelial Function in a Lamb Model of Pulmonary Hypertension Secondary to Increased Pulmonary Blood Flow
Finally, we determined whether restoring the l-arginine/ADMA ratio would restore BH4 concentrations and NO signaling in our lamb model. Our data indicate that supplementation with l-arginine enhanced the interaction of GCH1 with Hsp90 (Figure 7F), significantly increased GCH1 protein concentrations (Figure 7G), and restored concentrations of both BH4 (Figure 1D) and NOx (Figure 1G).
DISCUSSION
This study produced several important findings: (1) ADMA mediates the degradation of GCH1, secondary to the disruption of the Hsp90–GCH1 complex; (2) the ubiquitin–proteasome pathway is involved in the ADMA-mediated degradation of GCH1; (3) the ADMA-mediated degradation of GCH1 is facilitated by the E3 ligase CHIP; (4) decreases in the formation of the Hsp90–GCH1 complex lead to ubiquitination and the degradation of GCH1, causing BH4 concentrations to decrease; and (5) supplementation with l-arginine restores endothelial function in a lamb model of pulmonary hypertension secondary to increased pulmonary blood flow. Further, we validated these events both in vitro using cultured PAECs, and in vivo using a lamb model of CHD with increased pulmonary blood flow, and we showed that these alterations lead to a decrease in NO signaling. Therefore, our study provides insights into a novel mechanism by which ADMA can indirectly reduce NO signaling, at least in part, by stimulating the CHIP-dependent degradation of GCH1 and reductions in BH4 concentrations. Recent evidence suggests that pulmonary vascular tone is regulated by a complex interaction of vasoactive substances that are locally produced by vascular endothelium. Endothelial injury, secondary to increased pulmonary blood flow or pressure, may disrupt these regulatory mechanisms, and is a potential factor in the development of pulmonary hypertension. Our previous studies showed that uncoupled eNOS occurs before overt endothelial dysfunction (24). The data in this study shed light on the complex signaling pathways that regulate the generation of NO from eNOS, by demonstrating that ADMA-induced eNOS uncoupling in shunt lambs is, at least in part, attributable to a BH4 deficiency secondary to the disruption of Hsp90–GCH1 interactions and the induction of a CHIP-dependent proteasomal degradation of GCH1.
ADMA is a newly identified cardiovascular risk factor, and increasing evidence suggests that impaired vasodilation in a variety of cardiovascular diseases is linked to the inhibition of NO generation by ADMA (3, 4). Our recent studies showed that ADMA can exert a direct inhibitory effect on NO signaling by competing with l-arginine for binding to the active site of eNOS (1). However, we also found that ADMA can exert an indirect effect on eNOS coupling through its ability to cause mitochondrial dysfunction and a decrease in Hsp90 chaperone activity (1). Interestingly, recent studies suggest that GCH1 may also be a client protein of Hsp90 (8). GCH1 is the first, rate-limiting enzyme of BH4 biosynthesis (7), and accumulating evidence indicates that optimal concentrations of BH4 are fundamental for the normal function of eNOS in endothelial cells. A number of previous studies demonstrated that the regulation of GCH1 can occur at a variety of levels, including transcriptional, posttranscriptional, and posttranslational (6). The data we present here, both in vitro and in vivo, demonstrate that GCH1 can be posttranslationally regulated through a ubiquitin-dependent degradation of the GCH1 protein that is independent of changes in GCH1 mRNA concentrations, and that in vivo, restoring the ADMA/l-arginine ratio can prevent these alterations in GCH1 and BH4 and preserve NO signaling. Our findings are supported by previous studies where exposure of human umbilical vascular endothelial cells to high amounts of glucose was found to increase 26S proteasome activity, resulting in ubiquitinated GCH1 degradation (25). Moreover, 4-hydroxy-2-nonenal increases superoxide anion radical concentrations in bovine aortic endothelial cells via GCH1 proteasomal degradation (9). Our data add to this knowledge, both by identifying CHIP as the key ubiquitin ligase regulating GCH1 degradation, and by demonstrating the key role of Hsp90 in regulating normal GCH1 function. Interestingly, our data also suggest that increasing BH4 concentrations through supplementation with sepiapterin overcomes the negative regulation of ADMA on NO signaling, suggesting that therapies based on stimulating BH4 biosynthesis or preventing its degradation through oxidant stress mechanisms could have clinical utility. However, further studies will be required to test this possibility.
Hsp90 was shown to interact with a number of proteins that are required for the efficient biosynthesis of NO, including eNOS (26), soluble guanylate cyclase (27), and (as we showed here) GCH1 (8). In cells, GCH1 is a pentamer that forms a further complex structure with a pentamer of the GCH1 feedback regulatory protein (7). An examination of this multimeric structure implies regions that are similar in charge and hydrophobicity to those found in eNOS, and that interact with Hsp90 according to Fontana and colleagues (28). This suggests that Hsp90 may be necessary to maintain this complex in a conformation required for the biosynthesis of BH4. Consistent with previous studies, our present data demonstrate that Hsp90 appears to be a newly described client protein for GCH1, and that the levels of this interaction are reduced in ADMA-treated PAECs and in a lamb model of CHD with increased pulmonary blood flow and elevated concentrations of ADMA. This may be the case because ADMA decreases the generation of ATP (1), and the interaction of Hsp90 with its client proteins is ATP-dependent (29). Many Hsp90 client proteins are brought into complex with Hsp90 by a multiprotein Hsp90/Hsp70–based chaperone machinery (21), and Hsp70 appears to be involved in both the folding and degradation of Hsp90-client proteins (22). Our data show that an increased interaction of Hsp70 with GCH1 correlates with increases in GCH1 ubiquitination and degradation, both in vitro and in vivo. Most proteins are targeted for degradation by the 26S proteasome after ubiquitin has been covalently attached in the form of a polyubiquitin chain, with linkages involving lysine 48 functioning as a degradation signal (30). Our data, along with previous studies, indicate that the ubiquitin–proteasomal pathway is involved in the degradation of GCH1 (9, 25). Ubiquitin-dependent proteasomal degradation is a highly regulated process. The selectivity of protein degradation is determined primarily at the stage of ligation to ubiquitin (23). Ubiquitin–protein ligation requires a cascade of three enzymes, E1 ubiquitin–activiting enzyme, E2 ubiquitin–conjugating enzyme, and E3 ubiquitin ligases (23). CHIP is an E3 ubiquitin ligase, and was recently identified as an Hsp90 cofactor (26). Although CHIP is a ubiquitous protein, its high level of expression in heart and endothelial cells suggests that CHIP may interact with proteins playing important roles in cardiovascular function (26). Our results indicate that in PAECs, CHIP is associated with GCH1 in a complex with Hsp90 and Hsp70, and the association of GCH1 and CHIP is increased when concentrations of ADMA are elevated. We also found that the overexpression of CHIP decreased endogenous GCH1 protein concentrations in PAECs. Furthermore, the overexpression of CHIP acted synergistically with ADMA to enhance the degradation of GCH1. A similar role for CHIP was reported in the degradation of soluble guanylyl cyclase and neuronal NO synthase (27, 31). CHIP has an important and unique U-box domain in the C-terminal region that is responsible for ubiquitin ligase activity (17). To evaluate the effects of the U-box domain of CHIP on the ADMA-mediated degradation of GCH1, a U-box domain point mutant CHIP was produced that lacks the ability to ubiquitinate ligase. Because the U-box domain mutant CHIP is a dominant-negative protein (17), the binding and sequestration of GCH1 from endogenous CHIP by the overexpression of the U-box domain mutant CHIP resulted in abolished CHIP-facilitated GCH1 degradation in both the presence and absence of ADMA and the maintenance of NO signaling. However, in contrast to the similar effects of CHIP and ADMA on decreased GCH1 protein concentrations, CHIP overexpression alone did not significantly decrease concentrations of BH4, although the BH4/BH2 ratio was decreased. One possible explanation could be that, unlike ADMA, CHIP over-expression does not change concentrations of reactive oxygen species (ROS), and BH4 is a molecular target of ROS (32). Furthermore, the regulation of net BH4 bioavailability in endothelial cells is likely the result of a complex equilibrium between the de novo synthesis pathway and the salvage pathway. In the salvage pathway, sepiapterin can be reduced by sepiapterin reductase to BH2, and further reduced by dihydrofolate reductase (DHFR) to form BH4 (6). Recent studies revealed that DHFR plays a critical role in maintaining the BH4/BH2 ratio (33), which in turn plays an important role in regulating eNOS coupling.
In conclusion, our data elucidate the effects of ADMA on BH4 deficiency both in vitro and in vivo, and suggest an important role for the CHIP-mediated ubiquitin–proteasomal degradation of GCH1 in the regulation of eNOS uncoupling. Our study suggests that preventing or attenuating the ADMA-induced degradation of GCH1 using l-arginine supplementation constitutes a potential therapeutic approach for the treatment of endothelial dysfunction associated with pulmonary hypertension.
Supplementary Material
Acknowledgments
The authors thank Michael Johengen and Cynthia Harmon for excellent technical support.
This study was supported by National Institutes of Health grants HL60190 (S.M.B.), HL67841 (S.M.B.), HL72123 (S.M.B.), HL70061 (S.M.B.), R21HD057406 (S.M.B.), HL61284 (J.R.F.), and 5T32-HL-06699 (J.D.C. and R.R.), by the Fondation Leducq (S.M.B., J.R.F., S.F., and J.H.), by the American Heart Association Southeast Affiliates Beginning Grant-in-Aid Award 09BGIA2310050 (S.S.), and by Seed Awards (S.S. and S.K.) from the Cardiovascular Discovery Institute of the Medical College of Georgia.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0467OC on October 1, 2010
Author Disclosure: S.M.B. received a sponsored grant from the National Institutes of Health for more than $100,001. S.F. received lecture fees from Actelion for $1,001–$5,000. S.K. received a sponsored grant from the Medical College of Georgia for $10,001–$50,000. S.S. received a sponsored grant from the Medical College of Georgia for $10,001–$50,000, and from the American Heart Association for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits Hsp90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol 2008;294:C1407–C1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangoni AA. The emerging role of symmetric dimethylarginine in vascular disease. Adv Clin Chem 2009;48:73–94. [DOI] [PubMed] [Google Scholar]
- 3.Boger RH. Association of asymmetric dimethylarginine and endothelial dysfunction. Clin Chem Lab Med 2003;41:1467–1472. [DOI] [PubMed] [Google Scholar]
- 4.Boger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “l-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 2004;134(Suppl 10):2842S–2847S, 2853S. [DOI] [PubMed] [Google Scholar]
- 5.Arrigoni FI, Vallance P, Haworth SG, Leiper JM. Metabolism of asymmetric dimethylarginines is regulated in the lung developmentally and with pulmonary hypertension induced by hypobaric hypoxia. Circulation 2003;107:1195–1201. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. [DOI] [PubMed] [Google Scholar]
- 7.Auerbach G, Nar H. The pathway from GTP to tetrahydrobiopterin: three-dimensional structures of GTP cyclohydrolase I and 6-pyruvoyl tetrahydropterin synthase. Biol Chem 1997;378:185–192. [PubMed] [Google Scholar]
- 8.Swick L, Kapatos G. A yeast 2-hybrid analysis of human GTP cyclohydrolase I protein interactions. J Neurochem 2006;97:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitsett J, Picklo MJ Sr, Vasquez-Vivar J. 4-Hydroxy-2-nonenal increases superoxide anion radical in endothelial cells via stimulated GTP cyclohydrolase proteasomal degradation. Arterioscler Thromb Vasc Biol 2007;27:2340–2347. [DOI] [PubMed] [Google Scholar]
- 10.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, Fineman JR. In utero placement of aortopulmonary shunts: a model of postnatal pulmonary hypertension with increased pulmonary blood flow in lambs. Circulation 1995;92:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 2002;106:987–992. [DOI] [PubMed] [Google Scholar]
- 12.Kelly LK, Wedgwood S, Steinhorn RH, Black SM. Nitric oxide decreases endothelin-1 secretion through the activation of soluble guanylate cyclase. Am J Physiol Lung Cell Mol Physiol 2004;286:L984–L991. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Sun X, Sharma S, Aggarwal S, Ravi K, Fineman JR, Black SM. GTP cyclohydrolase I expression is regulated by nitric oxide: role of cyclic AMP. Am J Physiol Lung Cell Mol Physiol 2009;297:L309–L317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainwright MS, Arteaga E, Fink R, Ravi K, Chace DH, Black SM. Tetrahydrobiopterin and nitric oxide synthase dimer levels are not changed following hypoxia–ischemia in the newborn rat. Brain Res Dev Brain Res 2005;156:183–192. [DOI] [PubMed] [Google Scholar]
- 15.Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by Hsp90. Am J Physiol Lung Cell Mol Physiol 2007;293:L1444–L1453. [DOI] [PubMed] [Google Scholar]
- 16.Daicho T, Yagi T, Abe Y, Ohara M, Marunouchi T, Takeo S, Tanonaka K. Possible involvement of mitochondrial energy–producing ability in the development of right ventricular failure in monocrotaline-induced pulmonary hypertensive rats. J Pharmacol Sci 2009;111:33–43. [DOI] [PubMed] [Google Scholar]
- 17.Xu W, Marcu M, Yuan X, Mimnaugh E, Patterson C, Neckers L. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for C-Erbb2/Neu. Proc Natl Acad Sci USA 2002;99:12847–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewey CF Jr, Bussolari SR, Gimbrone MA Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 1981;103:177–185. [DOI] [PubMed] [Google Scholar]
- 19.Wedgwood S, Bekker JM, Black SM. Shear stress regulation of endothelial NOS in fetal pulmonary arterial endothelial cells involves PKC. Am J Physiol Lung Cell Mol Physiol 2001;281:L490–L498. [DOI] [PubMed] [Google Scholar]
- 20.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, Rau T, Wilham J, Harmon C, Oishi P, et al. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2008;294:L46–L56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the Hsp90/Hsp70–based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–133. [DOI] [PubMed] [Google Scholar]
- 22.Bercovich B, Stancovski I, Mayer A, Blumenfeld N, Laszlo A, Schwartz AL, Ciechanover A. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J Biol Chem 1997;272:9002–9010. [DOI] [PubMed] [Google Scholar]
- 23.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998;67:425–479. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Grobe AC, Wiseman DA, Kumar S, Englaish M, Najwer I, Benavidez E, Oishi P, Azakie A, Fineman JR, et al. Lung antioxidant enzymes are regulated by development and increased pulmonary blood flow. Am J Physiol Lung Cell Mol Physiol 2007;293:L960–L971. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Wu Y, Song P, Zhang M, Wang S, Zou MH. Proteasome-dependent degradation of guanosine 5′-triphosphate cyclohydrolase I causes tetrahydrobiopterin deficiency in diabetes mellitus. Circulation 2007;116:944–953. [DOI] [PubMed] [Google Scholar]
- 26.Jiang J, Cyr D, Babbitt RW, Sessa WC, Patterson C. Chaperone-dependent regulation of endothelial nitric–oxide synthase intracellular trafficking by the co-chaperone/ubiquitin ligase CHIP. J Biol Chem 2003;278:49332–49341. [DOI] [PubMed] [Google Scholar]
- 27.Xia T, Dimitropoulou C, Zeng J, Antonova GN, Snead C, Venema RC, Fulton D, Qian S, Patterson C, Papapetropoulos A, et al. Chaperone-dependent E3 ligase chip ubiquitinates and mediates proteasomal degradation of soluble guanylyl cyclase. Am J Physiol Heart Circ Physiol 2007;293:H3080–H3087. [DOI] [PubMed] [Google Scholar]
- 28.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of Hsp90 serves as a molecular scaffold to regulate AKT-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res 2002;90:866–873. [DOI] [PubMed] [Google Scholar]
- 29.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones 1998;3:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glickman MH, Ciechanover A. The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 2002;82:373–428. [DOI] [PubMed] [Google Scholar]
- 31.Peng HM, Morishima Y, Jenkins GJ, Dunbar AY, Lau M, Patterson C, Pratt WB, Osawa Y. Ubiquitylation of neuronal nitric-oxide synthase by CHIP, a chaperone-dependent E3 ligase. J Biol Chem 2004;279:52970–52977. [DOI] [PubMed] [Google Scholar]
- 32.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in APOE-knockout mice. Arterioscler Thromb Vasc Biol 2004;24:445–450. [DOI] [PubMed] [Google Scholar]
- 33.Crabtree MJ, Tatham AL, Hale AB, Alp NJ, Channon KM. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric oxide synthase coupling: relative importance of the de novo biopterin synthesis vs. salvage pathways. J Biol Chem 2009;284:28128–28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.