Summary
Background
Lowering LDL cholesterol with statin regimens reduces the risk of myocardial infarction, ischaemic stroke, and the need for coronary revascularisation in people without kidney disease, but its effects in people with moderate-to-severe kidney disease are uncertain. The SHARP trial aimed to assess the efficacy and safety of the combination of simvastatin plus ezetimibe in such patients.
Methods
This randomised double-blind trial included 9270 patients with chronic kidney disease (3023 on dialysis and 6247 not) with no known history of myocardial infarction or coronary revascularisation. Patients were randomly assigned to simvastatin 20 mg plus ezetimibe 10 mg daily versus matching placebo. The key prespecified outcome was first major atherosclerotic event (non-fatal myocardial infarction or coronary death, non-haemorrhagic stroke, or any arterial revascularisation procedure). All analyses were by intention to treat. This trial is registered at ClinicalTrials.gov, NCT00125593, and ISRCTN54137607.
Findings
4650 patients were assigned to receive simvastatin plus ezetimibe and 4620 to placebo. Allocation to simvastatin plus ezetimibe yielded an average LDL cholesterol difference of 0·85 mmol/L (SE 0·02; with about two-thirds compliance) during a median follow-up of 4·9 years and produced a 17% proportional reduction in major atherosclerotic events (526 [11·3%] simvastatin plus ezetimibe vs 619 [13·4%] placebo; rate ratio [RR] 0·83, 95% CI 0·74–0·94; log-rank p=0·0021). Non-significantly fewer patients allocated to simvastatin plus ezetimibe had a non-fatal myocardial infarction or died from coronary heart disease (213 [4·6%] vs 230 [5·0%]; RR 0·92, 95% CI 0·76–1·11; p=0·37) and there were significant reductions in non-haemorrhagic stroke (131 [2·8%] vs 174 [3·8%]; RR 0·75, 95% CI 0·60–0·94; p=0·01) and arterial revascularisation procedures (284 [6·1%] vs 352 [7·6%]; RR 0·79, 95% CI 0·68–0·93; p=0·0036). After weighting for subgroup-specific reductions in LDL cholesterol, there was no good evidence that the proportional effects on major atherosclerotic events differed from the summary rate ratio in any subgroup examined, and, in particular, they were similar in patients on dialysis and those who were not. The excess risk of myopathy was only two per 10 000 patients per year of treatment with this combination (9 [0·2%] vs 5 [0·1%]). There was no evidence of excess risks of hepatitis (21 [0·5%] vs 18 [0·4%]), gallstones (106 [2·3%] vs 106 [2·3%]), or cancer (438 [9·4%] vs 439 [9·5%], p=0·89) and there was no significant excess of death from any non-vascular cause (668 [14·4%] vs 612 [13·2%], p=0·13).
Interpretation
Reduction of LDL cholesterol with simvastatin 20 mg plus ezetimibe 10 mg daily safely reduced the incidence of major atherosclerotic events in a wide range of patients with advanced chronic kidney disease.
Funding
Merck/Schering-Plough Pharmaceuticals; Australian National Health and Medical Research Council; British Heart Foundation; UK Medical Research Council.
Introduction
Chronic kidney disease is associated with an increased risk of cardiovascular disease,1,2 but little is known about the prevention of cardiovascular disease in patients with chronic kidney disease.3 Meta-analyses of randomised trials undertaken mainly in patients without chronic kidney disease have shown that statin therapy reduces the risks of major coronary events (myocardial infarction or death from coronary heart disease), ischaemic strokes, and coronary revascularisations by about one fifth for each 1 mmol/L reduction in LDL cholesterol, while producing little effect on haemorrhagic strokes or vascular causes of death other than coronary heart disease.4,5 In people with an estimated glomerular filtration rate (eGFR) greater than 60 mL/min per 1·73 m2, in whom the cause of cardiovascular disease is typically atherosclerotic, the proportional effects of statin therapy on vascular events seem to be independent of renal function.5,6 But, when eGFR falls below about 30 mL/min per 1·73 m2, a different cardiovascular pathology emerges, with vascular stiffness and calcification, structural heart disease, and sympathetic overactivity contributing to an increasing risk of cardiac arrhythmia and heart failure.2 A key question, therefore, is whether LDL-cholesterol-lowering therapy remains effective as renal impairment progresses.
The SHARP (Study of Heart and Renal Protection) trial aimed to assess the safety and efficacy of reducing LDL cholesterol in more than 9000 patients with chronic kidney disease. To achieve an average reduction in LDL cholesterol of about 1 mmol/L without the use of high statin doses (which are associated with an increased risk of myopathy,7 especially in patients with impaired renal function8), a low dose of a statin (simvastatin 20 mg daily) was combined with a cholesterol-absorption inhibitor9 (ezetimibe 10 mg daily). The biochemical efficacy and tolerability of this regimen was first confirmed in the UK-HARP pilot studies.10,11
Methods
Trial design and participants
Details of the SHARP trial objectives, design, and methods have been reported previously.12 Patients aged 40 years and older were eligible to participate if they had chronic kidney disease with more than one previous measurement of serum or plasma creatinine of at least 150 μmol/L (1·7 mg/dL) in men or 130 μmol/L (1·5 mg/dL) in women, whether receiving dialysis or not. Potentially eligible patients attended a screening visit at which medical history and other eligibility criteria were checked, written informed consent obtained, and non-fasting blood samples taken for local laboratory assays. Single-blind study placebo tablets were provided for a 6-week run-in period to identify potential non-compliers who could be excluded before randomisation, with a consequent improvement in statistical sensitivity.13 Ethical approval was obtained from all study sites prior to enrolment.
Randomisation and masking
At the end of the run-in period, patients who agreed to continue were allocated the study treatment by the local study laptop computer with minimised randomisation14 (which balanced for age, sex, ethnic origin, dialysis vs non-dialysis, prior vascular disease, previous diabetes, systolic blood pressure, creatinine, and total cholesterol). Patients entering SHARP were initially randomised three ways between simvastatin 20 mg plus ezetimibe 10 mg daily, simvastatin 20 mg daily, and placebo to assess the safety of adding ezetimibe to simvastatin during the first year (with no safety concerns identified12) and those initially allocated simvastatin alone were then rerandomised to simvastatin 20 mg plus ezetimibe 10 mg daily versus placebo after 1 year (figure 1). A double-dummy method ensured that patients and study staff were unaware of the treatment allocation, with all patients taking two tablets during the first year (an active simvastatin plus ezetimibe tablet with a placebo simvastatin tablet; a placebo simvastatin plus ezetimibe tablet with an active simvastatin tablet; or a placebo simvastatin plus ezetimibe tablet with a placebo simvastatin tablet) and, after the first year, one tablet (active or placebo simvastatin plus ezetimibe).
Figure 1.
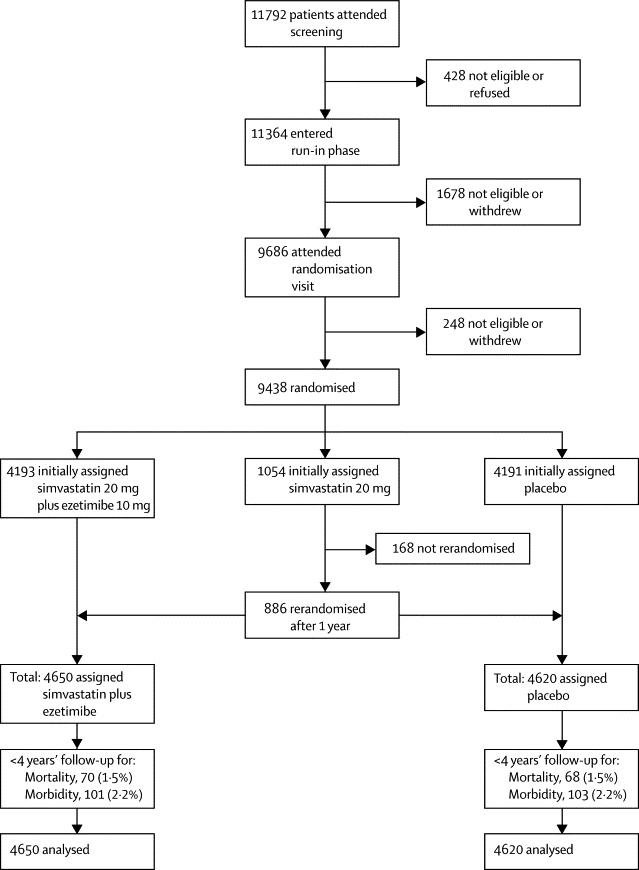
Trial profile
Procedures
After randomisation between August, 2003, and August, 2006, participants were to be seen in the study clinics for routine follow-up checks and blood safety monitoring at 2, 6, and 12 months, and then every 6 months for at least 4 years in total, until the final follow-up visits between March and August, 2010. An early recall visit could also be arranged for any participant requiring additional review. At each follow-up, compliance with study treatment was estimated, any unexplained muscle pain and non-study treatment recorded, and weight and blood pressure measured. Samples of non-fasting blood were taken for local laboratory assay of creatine kinase, liver transaminase, and creatinine. Central laboratory assays of lipid profile were conducted at randomisation in samples obtained from all participants, in about 10% of participants attending study visits at 1 and 4 years after the initial randomisation, and from all participants attending the 2·5-year visit. Differences between the treatment groups in average blood lipid concentrations were based on comparisons between all patients allocated simvastatin plus ezetimibe and all allocated placebo, irrespective of whether they were still compliant (with any missing data imputed from the initial randomisation values, on the basis of the assumption of non-compliance).
Information was recorded at each follow-up about any suspected myocardial infarction, stroke, vascular procedure, cancer, other reasons for hospital admissions, or other serious adverse events. If a participant became unwilling or unable to attend the follow-up visits, information about serious adverse events was obtained from them (or their relative or carer) by telephone or from their own doctors until the scheduled end of the study. Local study staff then sought extra information from hospital records and other appropriate sources about all reports of serious adverse events that might relate to study outcomes (ie, death, myocardial infarction, cardiac arrest, angina, heart failure, stroke, transient ischaemic attack, revascularisation procedures, angiography, amputation, initiation of dialysis, kidney transplant, renal failure, cancer, myopathy, rhabdomyolysis, hepatobiliary conditions). This information was sent to the international coordinating centre for central adjudication, in accordance with prespecified definitions, by trained clinicians who were masked to study treatment allocation.
Statistical analysis
The annual incidence of major vascular events in SHARP (defined as non-fatal myocardial infarction or any cardiac death, any stroke, or any arterial revascularisation excluding dialysis access procedures) was projected to be about 3·7%. A study with at least 1100 such events and all patients followed up for at least 4 years was estimated to have 90% power to detect a 20% proportional reduction at p<0·01. For the reasons given in the statistical analysis plan published before unmasking,12 the steering committee decided that the key study outcome should be changed to major atherosclerotic events (defined as non-fatal myocardial infarction or coronary death, non-haemorrhagic stroke, or arterial revascularisation excluding dialysis access procedures) and the key comparison should be between all patients ever allocated simvastatin plus ezetimibe versus placebo (including those initially allocated simvastatin; figure 1). Assessments of efficacy and safety were to be intention-to-treat comparisons.15,16 Time-to-event analyses used log-rank methods15,16 to calculate two-sided p values, event rate ratios, and 95% CIs. Exploratory analyses that made allowance for variation in the size of the LDL cholesterol reductions achieved within subgroups of patients involved rate ratios per mmol/L reduction derived through weighting by subgroup-specific LDL cholesterol differences at the study midpoint (2·5 years).4,5 Analyses were done with in-house C++ programs and verified with SAS version 9.1 and R version 2.2.1.
This study is registered at ClinicalTrials.gov, NCT00125593, and ISRCTN54137607.
Role of the funding source
The main funding source (Merck/Schering-Plough Pharmaceuticals) participated in initial discussions about trial design, contributed two non-voting observers to the steering committee, and had a right to comment on (but not require changes to) study reports. It had no involvement in data collection, analysis, and interpretation, report writing, or the decision to submit for publication, and will not receive an unmasked copy of the trial database. Voting members of the steering committee, all of whom are authors, accept full responsibility for the content of this paper.
Results
Overall, 9270 patients were randomly assigned to simvastatin plus ezetimibe (4650 patients, 4193 initially plus 457 after 1 year) versus placebo (4620 patients, 4191 initially plus 429 after 1 year; figure 1). Among these patients, all variables were well balanced between randomised groups (table 1). Mean age was 62 years (SD 12), 5800 (63%) were male, mean blood pressure was 139/79 mm Hg, mean body-mass index was 27 (SD 6) kg/m2, 2094 (23%) had diabetes, and 1393 (15%) had a history of vascular disease (angina, stroke, or peripheral vascular disease). Self-reported ethnic origin was 6646 (72%) white, 264 (3%) black, 2086 (23%) Asian, and 274 (3%) other or unspecified. Mean baseline non-fasting plasma concentrations were 4·9 (SD 1·2) mmol/L for total cholesterol, 2·8 (SD 0·9) mmol/L for directly measured LDL cholesterol, 1·1 (SD 0·3) mmol/L for HDL cholesterol, and 2·3 (SD 1·7, IQR 1·3–2·8) mmol/L for triglycerides (table 1).
Table 1.
Baseline demographic features and laboratory measurements by treatment allocation
| Simvastatin plus ezetimibe (n=4650) | Placebo (n=4620) | |||
|---|---|---|---|---|
| Previous vascular disease* | 711 (15%) | 682 (15%) | ||
| Diabetes* | 1054 (23%) | 1040 (23%) | ||
| Men | 2915 (63%) | 2885 (62%) | ||
| Age at randomisation (years)* | 62 (12) | 62 (12) | ||
| Current smoker | 626 (13%) | 608 (13%) | ||
| Diastolic blood pressure (mm Hg)* | 79 (13) | 79 (13) | ||
| Systolic blood pressure (mm Hg)* | 139 (22) | 139 (22) | ||
| Total cholesterol (mmol/L) | 4·88 (1·20) | 4·90 (1·17) | ||
| LDL cholesterol (mmol/L) | 2·77 (0·88) | 2·78 (0·87) | ||
| HDL cholesterol (mmol/L) | 1·12 (0·35) | 1·11 (0·34) | ||
| Triglycerides (mmol/L) | 2·31 (1·76) | 2·34 (1·68) | ||
| Body-mass index (kg/m2)* | 27·1 (5·7) | 27·1 (5·6) | ||
| Renal status | ||||
| On dialysis | 1533 (33%) | 1490 (32%) | ||
| Haemodialysis | 1275 (27%) | 1252 (27%) | ||
| Peritoneal dialysis | 258 (6%) | 238 (5%) | ||
| Not on dialysis† | 3117 (67%) | 3130 (68%) | ||
| MDRD-estimated GFR (mL/min per 1·73 m2)*‡§ | ||||
| Mean (SD) | 26·6 (12·9) | 26·6 (13·1) | ||
| ≥60 | 44 (1%) | 44 (1%) | ||
| ≥30 to <60 | 1100 (37%) | 1055 (35%) | ||
| ≥15 to <30 | 1246 (41%) | 1319 (44%) | ||
| <15 | 614 (20%) | 607 (20%) | ||
| Not available | 113 | 105 | ||
| Urinary albumin:creatinine ratio (mg/g)ठ| ||||
| Median (IQR) | 217 (44–788) | 196 (43–748) | ||
| <30 | 545 (20%) | 562 (20%) | ||
| ≥30 to ≤300 | 1032 (37%) | 1076 (39%) | ||
| >300 | 1203 (43%) | 1156 (41%) | ||
| Not available | 337 | 336 | ||
Data are n (%), mean (SD), or median (IQR). MDRD=Modified Diet in Renal Disease.17 GFR=glomerular filtration rate.
Variables updated at 1 year for patients originally allocated simvastatin only who were rerandomised to simvastatin plus ezetimibe or placebo.
Five versus five patients received a transplant before rerandomisation.
Percentages exclude participants for whom data were not available for that category.
For patients not on dialysis.
3023 (33%) patients were receiving maintenance dialysis at randomisation (2527 [27%] haemodialysis and 496 [5%] peritoneal dialysis; table 1). Mean LDL cholesterol concentration was lower in patients on dialysis than in those who were not (2·6 [SD 0·9] vs 2·9 [SD 0·9] mmol/L; p<0·0001). Among the 6029 (97%) of 6247 patients not on dialysis with centrally measured creatinine, the average eGFR estimated with the Modification of Diet in Renal Disease equation17 was 26·6 (SD 13·0) mL/min per 1·73 m2 (table 1): 2155 (36%) had Kidney Disease Outcomes Quality Initiative18 stage 3 disease (eGFR 30–59 mL/min per 1·73 m2), 2565 (43%) stage 4 disease (eGFR 15–29 mL/min per 1·73 m2), and 1221 (20%) stage 5 disease (eGFR <15 mL/min per 1·73 m2). Among the 5574 (89%) of 6247 patients not on dialysis with a centrally measured urinary albumin-to-creatinine ratio (ACR), 1107 (20%) had ACR lower than 30 mg/g, 2108 (38%) had ACR 30–300 mg/g, and 2359 (42%) had ACR higher than 300 mg/g (table 1).
The median duration of follow-up was 4·9 years for surviving patients. During the scheduled treatment period, slightly fewer patients allocated simvastatin plus ezetimibe discontinued study treatment (1533 [33·0%] simvastatin plus ezetimibe vs 1669 [36·1%] placebo); this finding was chiefly attributable to slightly more placebo-allocated patients commencing non-study statin therapy (337 [9·6%] vs 513 [14·6%]; p<0·0001). Among patients allocated simvastatin plus ezetimibe, there were no significant excesses of discontinuations due to suspected serious adverse reactions (17 [0·4%] vs 12 [0·3%]), other serious adverse events (297 [6·4%] vs 307 [6·6%]), non-serious adverse events (165 [3·5%] vs 131 [2·8%]), or other reasons (1054 [22·7%] vs 1219 [26·4%]).
Compliance was defined as at least 80% of the scheduled simvastatin plus ezetimibe or placebo tablets having been taken since the previous follow-up. Among the patients allocated simvastatin plus ezetimibe, 3403 (77%) of 4435 at the end of the first year of follow-up and 2397 (68%) of 3512 at the end of the fourth year remained compliant or were taking a non-study statin and, at the study midpoint (2·5 years), 2864 (71%) of 4058 were taking simvastatin plus ezetimibe or a non-study statin (table 2). By contrast, in patients allocated placebo, 124 (3%) of 4162 patients at the end of the first year and 447 (14%) of 3278 at the end of the fourth year were taking a non-study statin and, at the study midpoint, the average use was 9% (341 of 3735 patients). Hence, the average difference in the proportion taking simvastatin plus ezetimibe or non-study statin was 61% (table 2). As a result, the intention-to-treat comparisons assess the effects of around two-thirds of participants actually taking LDL-cholesterol-lowering treatment daily, which yielded an average LDL cholesterol difference of 0·85 mmol/L (SE 0·02; table 2). The average use of simvastatin plus ezetimibe or non-study statin did not vary much among different types of patient (webappendix p 1), except that the average use was lower in patients who were on dialysis than in those who were not (54% vs 65%), which, taken together with the lower baseline LDL cholesterol concentration in patients on dialysis (2·6 vs 2·9 mmol/L), yielded a smaller average LDL cholesterol reduction (0·60 vs 0·96 mmol/L; webappendix p 1).
Table 2.
Average use of study simvastatin plus ezetimibe or non-study statin and average change in plasma LDL cholesterol from baseline, by period of follow-up
|
LDL-cholesterol-lowering drug use |
LDL cholesterol difference (mmol/L)* |
|||||
|---|---|---|---|---|---|---|
| Simvastatin plus ezetimibe | Placebo | Absolute difference | Simvastatin plus ezetimibe | Placebo | Absolute difference (SE) | |
| 8–13 months | 77% | 3% | 74% | −1·08 | 0·02 | −1·09 (0·06) |
| 26–31 months | 71% | 9% | 61% | −1·00 | −0·15 | −0·85 (0·02) |
| 44–49 months | 68% | 14% | 55% | −0·84 | −0·08 | −0·77 (0·06) |
In patients initially allocated to simvastatin, no 1-year sample was collected, while samples scheduled for collection at 2·5 and 4 years were collected at 1·5 and 3 years after rerandomisation.
During the scheduled treatment period, there were 526 (11·3%) first major atherosclerotic events (non-fatal myocardial infarction or coronary death, non-haemorrhagic stroke, or arterial revascularisation) among the 4650 participants allocated simvastatin plus ezetimibe compared with 619 (13·4%) among the 4620 allocated placebo, corresponding to a significant 17% proportional reduction (RR 0·83, 95% CI 0·74–0·94; log-rank p=0·0021; figure 2 and figure 3). There was also a significant one-sixth reduction in major vascular events (ie, major atherosclerotic events plus non-coronary cardiac deaths and haemorrhagic strokes: 701 [15·1%] vs 814 [17·6%]; RR 0·85, 95% CI 0·77–0·94; p=0·0012; webappendix p 2), even when the patients initially allocated simvastatin alone were excluded (639 [15·2%] vs 749 [17·9%]; RR 0·84, 0·75–0·93; p=0·001).
Figure 2.

Life-table plot of effects of allocation to simvastatin plus ezetimibe versus placebo on major atherosclerotic events
Numbers remaining at risk of a first major atherosclerotic event at the beginning of each year are shown for both treatment groups.
Figure 3.
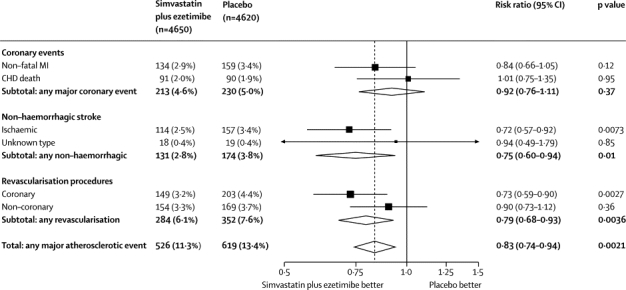
Major atherosclerotic events subdivided by type
MI=myocardial infarction. CHD=coronary heart disease.
Allocation to simvastatin plus ezetimibe was associated with non-significantly fewer first major coronary events (213 [4·6%] vs 230 [5·0%]; RR 0·92, 95% CI 0·76–1·11; p=0·37), which reflected non-significantly fewer non-fatal myocardial infarctions (134 [2·9%] vs 159 [3·4%]; RR 0·84, 0·66–1·05; p=0·12; figure 3), but no significant difference in coronary mortality (91 [2·0%] vs 90 [1·9%]; RR 1·01, 0·75–1·35; p=0·95).
Allocation to simvastatin plus ezetimibe produced a significant reduction in non-haemorrhagic stroke (131 [2·8%] vs 174 [3·8%]; RR 0·75, 95% CI 0·60–0·94; p=0·01; figure 3), due chiefly to a significant reduction in strokes that were definitely ischaemic (114 [2·5%] vs 157 [3·4%]; RR 0·72, 0·57–0·92; p=0·0073). There were non-significantly more patients with haemorrhagic stroke (45 [1·0%] vs 37 [0·8%]; RR 1·21, 95% CI 0·78–1·86; p=0·4; webappendix p 2). There was a significant reduction in the risk of any type of stroke (171 [3·7%] vs 210 [4·5%]; RR 0·81, 95% CI 0·66–0·99; p=0·04).
Allocation to simvastatin plus ezetimibe significantly reduced the incidence of any arterial revascularisation (284 [6·1%] vs 352 [7·6%]; RR 0·79, 95% CI 0·68–0·93; p=0·0036; figure 3), with no evidence of statistical heterogeneity (χ12=2·0, p=0·2) between the reductions in coronary revascularisations (149 [3·2%] vs 203 [4·4%]; RR 0·73, 0·59–0·90), which was statistically significant (p=0·0027), and non-coronary revascularisations (ie, carotid, aortic or leg, but not haemodialysis access procedures), which was not (154 [3·3%] vs 169 [3·7%]; RR 0·90, 0·73–1·12; p=0·36). Among coronary revascularisation procedures, there was no evidence of statistical heterogeneity (χ12=0·1, p=0·8) between the reductions in percutaneous coronary intervention procedures (106 [2·3%] vs 148 [3·2%]; RR 0·71, 95% CI 0·56–0·91; p=0·0063) and coronary artery bypass grafts (50 [1·1%] vs 66 [1·4%]; RR 0·75, 0·52–1·09; p=0·13).
SHARP was not expected to have sufficient statistical power to allow reliable estimation of effects on major atherosclerotic events in particular clinical circumstances, so subgroup analyses were planned only as tertiary assessments.12 There was not good evidence that the proportional effects on major atherosclerotic events differed between patients on dialysis and not (χ12=1·3, p=0·25; figure 4), and nor were there trends towards smaller proportional reductions in patients not on dialysis with lower eGFR (trend χ12=0·12, p=0·73) or higher urinary albumin excretion (trend χ12=0·38, p=0·54; figure 4). Conventionally significant trends in the proportional effect of allocation to simvastatin plus ezetimibe were observed for subgroups defined by total cholesterol (trend χ12=9·01, p=0·0027) and body-mass index (trend χ12=4·04, p=0·04). After adjustment for the subgroup-specific LDL cholesterol reductions, the χ12 statistics were reduced but remained conventionally significant for total cholesterol (p=0·02; webappendix p 3).
Figure 4.

Major atherosclerotic events by baseline characteristics
χ2 tests on 1 degree of freedom are shown for heterogeneity between rate ratios within dichotomous categories and for trend within other categories. BP=blood pressure. MDRD=Modified Diet in Renal Disease formula.17 GFR=glomerular filtration rate.
Allocation to simvastatin plus ezetimibe was associated with non-significantly fewer cardiac deaths (253 [5·4%] vs 272 [5·9%]; RR 0·93, 95% CI 0·78–1·10; p=0·38; figure 5) and stroke deaths (68 [1·5%] vs 78 [1·7%]; RR 0·87, 0·63–1·20, p=0·39), but with similar numbers of deaths due to other vascular causes (40 [0·9%] vs 38 [0·8%]; RR 1·05, 0·67–1·64, p=0·83); overall, there were non-significantly fewer deaths due to any vascular cause (361 [7·8%] vs 388 [8·4%]; RR 0·93, 0·80–1·07; p=0·30; figure 5). Simvastatin plus ezetimibe was associated with non-significantly more deaths from any non-vascular cause (668 [14·4%] vs 612 [13·2%]; RR 1·09, 95% CI 0·98–1·21, p=0·13), but was not associated with a significant excess of deaths from any particular non-vascular cause considered separately or with excess deaths from any unknown cause (113 [2·4%] vs 115 [2·5%]; RR 0·98, 0·76–1·27, p=0·87). There was no significant effect on deaths from any cause (1142 [24·6%] vs 1115 [24·1%]; RR 1·02, 95% CI 0·94–1·11; p=0·63).
Figure 5.
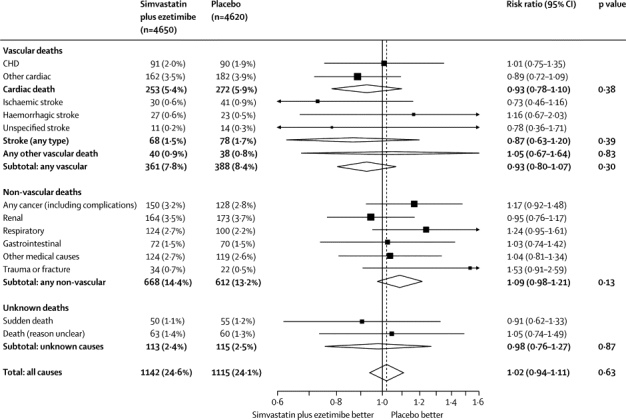
Cause-specific and overall mortality
CHD=coronary heart disease.
First post-randomisation cancers occurred in 877 (9%) patients during the scheduled follow-up period. There was no evidence that simvastatin plus ezetimibe increased the incidence of cancer (438 [9·4%] vs 439 [9·5%], RR 0·99, 95% CI 0·87–1·13, p=0·89; table 3) or of increasing hazard with length of follow-up (webappendix p 4). There was also no significantly increased incidence of, or mortality from, cancer at any particular site (table 3).
Table 3.
Cancer incidence and cancer mortality by site
|
Cancer incidence |
Cancer mortality |
|||||
|---|---|---|---|---|---|---|
| Simvastatin plus ezetimibe (n=4650) | Placebo (n=4620) | p value | Simvastatin plus ezetimibe (n=4650) | Placebo (n=4620) | p value | |
| Lip/mouth/pharynx/oesophagus | 14 (0·3%) | 16 (0·3%) | 0·84 | 9 (0·2%) | 8 (0·2%) | 1·0 |
| Stomach | 11 (0·2%) | 14 (0·3%) | 0·68 | 10 (0·2%) | 11 (0·2%) | 1·0 |
| Large bowel or intestine | 53 (1·1%) | 35 (0·8%) | 0·07 | 20 (0·4%) | 15 (0·3%) | 0·51 |
| Pancreas | 9 (0·2%) | 10 (0·2%) | 1·0 | 7 (0·2%) | 10 (0·2%) | 0·62 |
| Liver/gallbladder/bile ducts | 8 (0·2%) | 4 (0·1%) | 0·39 | 4 (0·1%) | 4 (0·1%) | 1·0 |
| Lung | 42 (0·9%) | 35 (0·8%) | 0·51 | 32 (0·7%) | 22 (0·5%) | 0·23 |
| Other respiratory | 3 (0·1%) | 4 (0·1%) | 1·0 | 2 (0·0%) | 3 (0·1%) | 1·0 |
| Skin | 136 (2·9%) | 153 (3·3%) | 0·32 | 4 (0·1%) | 4 (0·1%) | 1·0 |
| Breast | 29 (0·6%) | 21 (0·5%) | 0·33 | 1 (0·0%) | 1 (0·0%) | 1·0 |
| Prostate | 39 (0·8%) | 52 (1·1%) | 0·20 | 6 (0·1%) | 2 (0·0%) | 0·27 |
| Kidney* | 31 (0·7%) | 23 (0·5%) | 0·35 | 5 (0·1%) | 1 (0·0%) | 0·22 |
| Bladder and urinary tract (not kidney) | 26 (0·6%) | 32 (0·7%) | 0·50 | 8 (0·2%) | 7 (0·2%) | 1·0 |
| Genital site | 12 (0·3%) | 14 (0·3%) | 0·84 | 4 (0·1%) | 2 (0·0%) | 0·69 |
| Haematological | 26 (0·6%) | 27 (0·6%) | 1·0 | 6 (0·1%) | 14 (0·3%) | 0·12 |
| Other known site | 9 (0·2%) | 12 (0·3%) | 0·65 | 3 (0·1%) | 5 (0·1%) | 0·72 |
| Unspecified cancer | 13 (0·3%) | 7 (0·2%) | 0·27 | 11 (0·2%) | 5 (0·1%) | 0·21 |
| Any incident cancer† | 438 (9·4%) | 439 (9·5%) | 0·89 | 132 (2·8%) | 114 (2·5%) | 0·26 |
For the individual sites, multiple continuity corrected p values are reported; any value that is based on data from more than five patients could have yielded a value less than 0·05 by chance. Uncorrected p values that are less than the inverse of the number of such tests were therefore corrected by multiplying by the number of such tests to correct for this multiplicity of comparisons. In all cases, this yielded p values of 1·0.
Includes two versus one cases and one versus zero deaths due to cancer in a transplanted kidney.
Excludes 18 (0·4%) versus 14 (0·3%) deaths from cancers diagnosed before randomisation.
Allocation to simvastatin plus ezetimibe did not yield significant excesses of creatine kinase concentrations of 10–40 times the upper limit of normal (ULN) or greater than 40 times the ULN (table 4). There were very few cases of myopathy of any severity (defined as creatine kinase greater than ten times the ULN with muscle symptoms: 9 [0·2%] simvastatin plus ezetimibe vs 5 [0·1%] placebo) or of more severe cases with rhabdomyolysis (defined as myopathy with creatine kinase greater than 40 times the ULN: 4 [0·1%] vs 1 [0·0%]). Nor were there significant excesses of persistently raised transaminases to greater than three times the ULN, hepatitis, or gallstones (with or without complications). Among patients allocated simvastatin plus ezetimibe, there were fewer cases of pancreatitis without gallstones (table 4).
Table 4.
Effects of allocation to simvastatin plus ezetimibe on muscle and hepatobiliary system
| Simvastatin plus ezetimibe (n=4650) | Placebo (n=4620) | p value | ||
|---|---|---|---|---|
| Muscle pain | ||||
| Any report | 992 (21·3%) | 960 (20·8%) | 0·53 | |
| Study treatment stopped | 49 (1·1%) | 28 (0·6%) | 0·02 | |
| Increased creatine kinase* | ||||
| >5 to ≤10 times ULN | 50 (1·1%) | 47 (1·0%) | 0·86 | |
| >10 to ≤40 times ULN | 17 (0·4%) | 16 (0·3%) | 1·00 | |
| >40 times ULN | 4 (0·1%) | 5 (0·1%) | 0·99 | |
| Persistently increased transaminases† | 30 (0·6%) | 26 (0·6%) | 0·71 | |
| Hepatitis | ||||
| Infective | 12 (0·3%) | 12 (0·3%) | 1·00 | |
| Non-infective | 6 (0·1%) | 4 (0·1%) | 0·76 | |
| No cause identified | 3 (0·1%) | 3 (0·1%) | 1·00 | |
| Any hepatitis | 21 (0·5%) | 18 (0·4%) | 0·76 | |
| Gallstones | ||||
| Complicated | 85 (1·8%) | 76 (1·6%) | 0·55 | |
| Uncomplicated | 21 (0·5%) | 30 (0·6%) | 0·25 | |
| Pancreatitis (without gallstones) | 12 (0·3%) | 27 (0·6%) | 0·02 | |
ULN=upper limit of normal.
Myopathy, defined as creatine kinase greater than ten times the ULN with muscle symptoms, occurred in nine (0·19%) versus five (0·11%) patients, of whom eight (0·17%) versus three (0·06%) were taking allocated treatment (and not taking any non-study statin) at the time of the event (both p=NS); for rhabdomyolysis, defined as myopathy with creatine kinase greater than 40 times the ULN (and hence included in counts of myopathies), the corresponding numbers were four (0·09%) versus one (0·02%) and four (0·09%) versus none, again both p=NS.
Consecutive increases of alanine or aspartate transaminase greater than three times the ULN.
Among the 6247 patients not on dialysis at randomisation, allocation to simvastatin plus ezetimibe did not produce significant reductions in any of the prespecified measures of renal disease progression: end-stage renal disease defined as commencement of maintenance dialysis or transplantation (1057 [33·9%] vs 1084 [34·6%]; RR 0·97, 95% CI 0·89–1·05, p=0·41); end-stage renal disease or death (1477 [47·4%] vs 1513 [48·3%]; RR 0·97, 0·90–1·04, p=0·34); and end-stage renal disease or doubling of baseline creatinine (1190 [38·2%] vs 1257 [40·2%]; RR 0·93, 0·86–1·01; p=0·09).
Discussion
The SHARP results show that lowering LDL cholesterol with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with chronic kidney disease. As in people without kidney disease, the proportional reduction in major atherosclerotic events produced by a given absolute reduction in LDL cholesterol is broadly similar irrespective of age, sex, diabetes, history of vascular disease, and presenting lipid profile. The SHARP results are relevant, therefore, to most patients with chronic kidney disease (panel).
Panel. Research in context.
Systematic review
The Cholesterol Treatment Trialists' collaborative meta-analysis of individual participant data from 26 randomised trials5 has shown that lowering LDL cholesterol with a statin regimen reduces the risk of myocardial infarction, coronary death, ischaemic stroke, and coronary revascularisation procedures by about a fifth per 1 mmol/L LDL cholesterol reduction in a wide range of people. However, none of the three trials19–21 in patients with chronic kidney disease included in that meta-analysis reported a significant reduction in its primary vascular disease outcome, leading to uncertainty about whether lowering of LDL cholesterol is effective in renal patients.
Interpretation
The SHARP randomised trial has now shown that lowering of LDL cholesterol with simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with chronic kidney disease. When the SHARP results are compared with those of the previous statin trials in renal patients, it appears that the absence of significant reductions in earlier trials could have been due both to the much smaller number and the much smaller proportion of vascular events in their primary outcomes that were related to atherosclerosis and, hence, preventable by lowering of LDL cholesterol.
The effects of lowering LDL cholesterol with a statin in populations without chronic kidney disease have been described by the Cholesterol Treatment Trialists' (CTT) Collaboration, and show that statin therapy reduces the risk of myocardial infarction or coronary death, stroke, or coronary revascularisation by about a fifth per 1 mmol/L LDL cholesterol reduction.5 In the SHARP trial, an average reduction of 0·85 mmol/L yielded a significant 17% reduction in major atherosclerotic events, which is similar to the effects seen in the CTT with statin regimens of equivalent LDL-lowering efficacy. The reduction in non-fatal myocardial infarction or coronary death (RR 0·92, 95% CI 0·76–1·11) in SHARP was not statistically significant, but the trial lacked power for separate assessment of components of major atherosclerotic events, and the confidence interval is consistent with the results of the CTT meta-analysis.5 The significant one-quarter reduction in coronary revascularisation procedures (p=0·0027) in SHARP suggests that the reduction in coronary disease reflects a real benefit. Similarly, the significant one-quarter reduction in ischaemic strokes (p=0·0073) is consistent with the one-fifth reduction reported in previous statin trials.5 There was no overall reduction in the risk of vascular mortality in SHARP, but this finding is again what would be expected if, as seen in the CTT analyses, reduction of LDL cholesterol reduces coronary mortality but has little effect on other vascular causes of death.5 Only 181 (24%) of 749 vascular deaths in SHARP were regarded as definitely attributable to coronary disease, so the expected effect of treatment on vascular mortality was very small and would have needed a much larger trial for its detection.
SHARP did not have sufficient power to assess the effects on major atherosclerotic events separately in dialysis and non-dialysis patients, but there was not good statistical evidence that the proportional effects in dialysis patients differed to those seen in patients not on dialysis. Moreover, since about a third of the patients who were not on dialysis at baseline began dialysis during the trial (with about one third of those doing so within the first year), the effects of simvastatin plus ezetimibe in the dialysis subgroup are reinforced by the favourable results in the non-dialysis subgroup. It is also important in any comparison of the effects on vascular outcomes in different circumstances to make allowance for any differences in the achieved absolute LDL cholesterol reduction (which meta-analyses suggest is the chief determinant of the proportional reduction in atherosclerotic events4,5). Lower baseline LDL cholesterol concentrations and less average use of LDL-cholesterol-lowering therapy led to absolute reductions in LDL cholesterol that were about a third smaller in those on dialysis (0·60 mmol/L) than in those not on dialysis (0·96 mmol/L). After weighting for these LDL cholesterol reductions, the proportional reductions in major atherosclerotic events per 1 mmol/L LDL cholesterol reduction were similar: 25% (RR 0·75, 95% CI 0·56–1·00) for stage 3; 21% (RR 0·79 95% CI 0·63–0·98) for stage 4; 24% (RR 0·76, 95% CI 0·48–1·18) for stage 5; and 16% (RR 0·84, 95% CI 0·62–1·13) in patients on dialysis, respectively, with a test for trend across these four groups that was not significant (χ12=0·23, p=0·63; webappendix p 3). The absence of any trend towards larger benefit in patients with less severe renal impairment is also consistent with the finding that a quarter of cardiac deaths were coronary at each stage of chronic kidney disease. Taken together, the available data suggest that the most appropriate estimate of the reduction in major atherosclerotic events, both in patients on dialysis and those who are not, is the overall 19% reduction per 1 mmol/L LDL cholesterol reduction (webappendix p 3).
Before completion of the SHARP trial, two trials of statin regimens in patients on haemodialysis (4D19 and AURORA20), and one trial in patients who had undergone renal transplantation (ALERT21), had not detected significant benefits in their primary outcomes. At first sight, these findings might appear to be discrepant with our results, but the LDL-cholesterol-weighted proportional effects on particular vascular outcomes in these three trials and SHARP were statistically compatible for non-fatal myocardial infarction, non-fatal haemorrhagic stroke, non-fatal non-haemorrhagic stroke, coronary revascularisation (which was not part of the primary outcomes of the 4D and AURORA trials), and any vascular death (p values non-significant for all heterogeneity tests; figure 6). Moreover, the effects on particular vascular outcomes in these four renal trials were compatible with those recorded in the trials of statin therapy in non-renal populations that were included in the CTT meta-analysis.5 Consequently, the failure to achieve statistical significance in the previous renal trials might derive from both the much smaller number and the much smaller proportion of modifiable vascular events in their primary outcomes: whereas more than half the primary outcomes in 4D and AURORA were vascular deaths (for which there were small, and non-significant, benefits), about three-quarters in SHARP were non-fatal atherosclerotic events (for which there were clear benefits).
Figure 6.
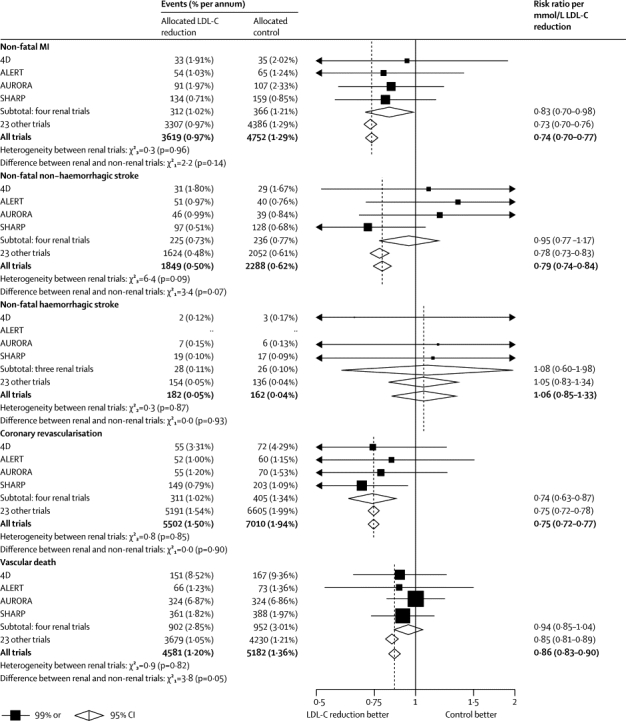
Effects of LDL-lowering therapy on particular vascular outcomes in four trials in patients with chronic kidney disease and 23 trials in other patients
Data from the Cholesterol Treatment Trialists' Collaboration.5 χ2 tests are shown for heterogeneity between rate ratios for each outcome in the four trials (4D,19 ALERT,21 AURORA,20 and SHARP) in patients with chronic kidney disease. MI=myocardial infarction. LDL-C=LDL-cholesterol.
The previous trials of statin therapy have shown that the proportional reduction in risk is chiefly determined by the absolute reduction in LDL cholesterol, and that more intensive LDL reduction yields further reductions in risk.4,5 Addition of ezetimibe to a statin reduces LDL cholesterol by the equivalent of around three doublings of the statin dose.22 It therefore offers a potentially useful method of increasing benefits in high-risk populations in which raising the statin dose is not desirable, either because the dose is already high or, as in chronic kidney disease, because of concerns about drug toxicity. The design of SHARP incorporated a three-way randomised comparison with a simvastatin-alone group for the first year so that adverse effects of the addition of ezetimibe to simvastatin could be assessed; as reported previously, no serious safety concerns emerged during this period.12 A more reliable assessment of the safety of the simvastatin plus ezetimibe combination is now provided by comparison with placebo among all 9270 patients in SHARP during 4·9 years of follow-up. There was no evidence of any excess risk of persistent increase of hepatic transaminases, of hepatitis, of gallstones, or of pancreatitis. Patients with chronic kidney disease are at increased risk of statin-induced myopathy,8 but in the SHARP trial the excess incidence of myopathy was only about two per 10 000 patients per year of treatment with simvastatin plus ezetimibe.
While the SHARP trial was in progress some investigators had postulated, on the basis of post-hoc analyses of the SEAS trial23 of simvastatin plus ezetimibe versus placebo in patients with aortic stenosis, that ezetimibe might increase the risk of cancer. This hypothesis-generating observation was not supported at the time by a hypothesis-testing meta-analysis24 of interim data for unadjudicated cancers that had occurred by July, 2008, in SHARP and the IMPROVE-IT trial of simvastatin plus ezetimibe versus simvastatin among patients with acute coronary syndromes.25 The current results from SHARP involve substantially larger numbers of cancers than were available for that previous meta-analysis and, again, provide no credible evidence of any excess risk of cancer or of death from cancer, either overall or at any particular site, and no evidence of any trend towards an increased risk with longer exposure to study treatment (webappendix p 4). Nor was there any evidence of any excess risk of death from particular non-vascular causes. Overall, therefore, the combination of simvastatin plus ezetimibe allowed LDL cholesterol to be lowered substantially, but safely, in a group of patients at high risk of vascular disease.
During SHARP, allocation to simvastatin plus ezetimibe reduced LDL cholesterol by an average of 0·85 mmol/L over about 5 years, yielding a reduction of 17% in major atherosclerotic events, which is equivalent to a one-fifth risk reduction per mmol/L reduction in LDL cholesterol. On average, however, only two-thirds of the patients allocated simvastatin plus ezetimibe were taking an LDL-cholesterol-lowering regimen and so, with full compliance, the LDL cholesterol reduction would typically have been about 1·3 (ie, 0·85×3/2) mmol/L. This calculation implies that patients with chronic kidney disease who take simvastatin plus ezetimibe as prescribed would typically reduce their risk of major atherosclerotic events by about a quarter (ie, 17%×3/2). In SHARP, the annual risk of a major atherosclerotic event in both patients on dialysis and those who were not exceeded the 2% threshold that is widely recommended for LDL-cholesterol-lowering therapy26 and a reduction of one quarter in risk in patients similar to those studied would correspond to the prevention of 30–40 major atherosclerotic events per 1000 patients treated for 5 years. Moreover, the absolute benefit would be even larger in renal patients with a previous history of coronary heart disease, who were excluded from SHARP, but whose absolute risk is two-or-three times higher. These benefits are substantial and suggest that widespread use of LDL-cholesterol-lowering therapy in patients with chronic kidney disease would result in a worthwhile reduction in cardiovascular disease complications in this high-risk population.
Acknowledgments
Acknowledgments
We thank the participants in SHARP and the local clinical centre staff, regional and national coordinators, steering committee, and data monitoring committee. The study was funded by Merck/Schering-Plough Pharmaceuticals (North Wales, PA, USA), with additional support from the Australian National Health Medical Research Council, the British Heart Foundation, and the UK Medical Research Council. JE acknowledges support from the British Heart Foundation Centre of Research Excellence (Oxford, UK; RE/08/04).
Contributors
All authors contributed to the collection and analysis of the data and to the preparation of the report.
Conflicts of interest
SHARP was initiated, conducted, and interpreted independently of the principal study funder. The Clinical Trial Service Unit and Epidemiological Studies Unit, which is part of the University of Oxford, has a staff policy of not accepting honoraria or consultancy fees.
Web Extra Material
References
- 1.Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(suppl 3):S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation K/DOQI clinical practice guidelines for managing dyslipidemias in chronic kidney disease. Am J Kidney Dis. 2003;41(suppl 3):S1–91. [PubMed] [Google Scholar]
- 4.Cholesterol Treatment Trialists' (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 5.Cholesterol Treatment Trialists' (CTT) Collaboration Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonelli M, Isles C, Curhan GC. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110:1557–1563. doi: 10.1161/01.CIR.0000143892.84582.60. [DOI] [PubMed] [Google Scholar]
- 7.Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12 064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–1669. doi: 10.1016/S0140-6736(10)60310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The SEARCH Collaborative Group SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 9.Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet. 2005;44:467–494. doi: 10.2165/00003088-200544050-00002. [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Landray M, Leaper C. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis. 2005;45:473–484. doi: 10.1053/j.ajkd.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Landray M, Baigent C, Leaper C. The second United Kingdom Heart and Renal Protection (UK-HARP-II) Study: a randomized controlled study of the biochemical safety and efficacy of adding ezetimibe to simvastatin as initial therapy among patients with CKD. Am J Kidney Dis. 2006;47:385–395. doi: 10.1053/j.ajkd.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 12.SHARP Collaborative Group Study of Heart and Renal Protection (SHARP): Randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160:785–794. doi: 10.1016/j.ahj.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Lang JM, Buring JE, Rosner B. Estimating the effect of the run-in on the power of the Physicians' Health Study. Stat Med. 1991;10:1585–1593. doi: 10.1002/sim.4780101010. [DOI] [PubMed] [Google Scholar]
- 14.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 15.Peto R, Pike MC, Armitage P. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part 1: introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peto R, Pike MC, Armitage P. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. Part 2: analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Greene T, Kusek JW, Beck GJ, the MDRD Study Group A simplified equation to predict glomerular filtration from serum creatinine. J Am Soc Nephrol. 2000;11:155A. (abstr A0828). [Google Scholar]
- 18.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Part 4. Definition and classification of stages of chronic kidney disease. Am J Kidney Dis. 2002;39(suppl 1):S46–S75. [PubMed] [Google Scholar]
- 19.Wanner C, Krane V, März W. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 20.Fellström BC, Jardine AG, Schmieder RE, for the AURORA Study Group Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 21.Holdaas H, Fellström B, Jardine AG, on behalf of the Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–2031. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 22.Davidson MH, McGarry T, Bettis R. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 23.Rossebø AB, Pedersen TR, Boman K, for the SEAS Investigators Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 24.Peto R, Emberson J, Landray M. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359:1357–1366. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 25.Cannon CP, Giugliano RP, Blazing MA, for the IMPROVE-IT investigators Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial) Am Heart J. 2008;156:826–832. [Google Scholar]
- 26.NICE Clinical Guideline 67 . Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. National Institute for Health and Clinical Excellence; London, UK: 2008. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


