Abstract
There is no accurate contemporary global map of the distribution of malaria. We show how guidelines formulated to advise travellers on appropriate chemoprophylaxis for areas of reported Plasmodium falciparum and Plasmodium vivax malaria risk can be used to generate crude spatial limits. We first review and amalgamate information on these guidelines to define malaria risk at national and sub-national administrative boundary levels globally. We then adopt an iterative approach to reduce these extents by applying a series of biological limits imposed by altitude, climate and population density to malaria transmission, specific to the local dominant vector species. Global areas of, and population at risk from, P. falciparum and often-neglected P. vivax malaria are presented for 2005 for all malaria endemic countries. These results reveal that more than 3 billion people were at risk of malaria in 2005.
1. INTRODUCTION
During the halcyon days of global malaria eradication, mapping the precise spatial extent of the disease was central to the control efforts of the World Health Organization (WHO). Between the 1940s and 1970s, a huge investment was made in synthesising available information on the distribution of risk using various combinations of expert opinion, elevation, climate, presence/absence records of the disease and vectors, spleen rates, parasite rates, sporozoite rates, biting rates and haemoglobinopathy prevalence (Boyd, 1949; Pampana and Russell, 1955; WHO, 1966; Lysenko and Semashko, 1968; Dutta and Dutt, 1978). Since the 1970s, as the world’s public health focus shifted from malaria eradication, an interest in mapping global malaria risk waned (Carter and Mendis, 2002; Hay et al., 2004a).
Following a renewed commitment to financing comprehensive malaria control at a global scale, the significance of defining the disease burden has re-emerged as a priority (Hay et al., 2004a; Snow et al., 2005). This will allow regional and national requirements for drugs, insecticides, bed nets and other commodities to be assessed more accurately, and so malaria to be rolled back more effectively (Snow, 2004; Sachs, 2005). Despite an obvious need to map risk (Snow et al., 1996), there remains no comprehensive definition of the spatial limits of malaria. Previous studies (Rogers and Randolph, 2000; Hay et al., 2004b) have used information provided by the WHO on the extent of risks due to P. falciparum and P. vivax from advice to travellers (WHO, 2003a, b). Precise details of how the WHO constructed these limits are difficult to obtain and there are several other public-domain sources of travel advice (CDC, 2003; IAMAT, 2004), which have not been harmonised with the WHO data (WHO, 2003b). Here we use geographic information systems (GIS) to triangulate and standardise international travel health guideline (ITHG) information and refine these limits with country-specific altitudinal exclusions, climate suitability criteria and population density, to make a new map of global malaria risk. The result is a species-specific estimate of the limits of malaria transmission in 2005.
2. THE DISTRIBUTION OF MALARIA RISK FROM TRAVEL GUIDELINES
ITHGs have been developed to advise travellers on appropriate malaria chemoprophylaxis. These guidelines are the only contemporary, global source of information on national and sub-national malaria risk. Three primary sources are available in the public-domain: the WHO’s International Travel and Health guidelines (WHO-ITH) (WHO, 2005), the World Malaria Risk Chart of the International Association for Medical Assistance to Travellers (IAMAT-WMRC) (IAMAT, 2004) and the Health Information for International Travel (“Yellow Book”) of the Centres for Disease Control and Prevention (CDC-YB) (CDC, 2003). These guidelines provide country-specific information that variously include (i) sub-national risk distribution; (ii) altitude-based transmission limits; (iii) risk definitions in specific urban and rural areas; (iv) P. falciparum to P. vivax ratios; (v) dominant vector species; (vi) anti-malarial drug resistance status; and (vii) prophylaxis regimens recommended. An example entry for Ecuador is as follows: “Malaria risk—P. falciparum (34%), P. vivax (66%)—exists throughout the year below 1500 m, with some risk in Cotopaxi, Loja and Los Ríós. Higher transmission risk is found in El Oro, Esmeraldas and Manabí. There is no risk in Guayaquil or Quito.” (WHO, 2005). Despite sometimes being incomplete, as in this example, it should be acknowledged that ITHGs are inclusive, rather than exclusive of geographic areas of malaria risk, so that information given to potentially non-immune travellers is risk-averse. The empirical data used as input to these national entries are rarely detailed.
To map these data we focussed on the three main criteria: administrative boundaries, altitudinal limits and urban centres (Table 1). We combined the sub-national description of malaria with databases of administrative areas within countries to define crude spatial limits. We obtained first- and, occasionally, second-level sub-national administrative boundaries for all malarious countries from the Food and Agriculture Organization’s GeoNetwork portal (http://www.fao.org/geonetwork/) (n = 94), the International Centre for Tropical Agriculture (CIAT) (http://www.ciat.cgiar.org/) (n = 6), and the Environmental Systems Research Institute (ArcView Data & Maps CD, ESRI, Redlands, California, USA) (n = 4).
Table 1.
Comprehensiveness of the information provided by international travel and health guidelines. Figures indicate the number of entries per category that prove useful for mapping.
| Source | Administrative | Altitude | Urban | All |
|---|---|---|---|---|
| WHO-ITHa | 27 | 20 | 14 | 61 |
| IAMAT-WMRCb | 30 | 41 | 31 | 102 |
| CDC-YBc | 30 | 14 | 26 | 70 |
| Uniqued | 42 | 42 | 37 | 121 |
International Travel and Health Guidelines of the WHO (WHO, 2005).
International Association for Medical Assistance to Travellers’ World Malaria Risk Chart (IAMAT, 2004).
Centres for Disease Control and Prevention’s Yellow Book (CDC, 2003).
The maximum number of entries per criteria regardless of source.
We defined all classifications of malaria risk in the ITHG entries as malaria presence, except those of “no risk”, “negligible risk” and “sporadic cases”, which we classified as absent. Descriptions of sub-national malaria risk that were not geographically specific were impossible to map and were ignored. An exception was made if malaria risk was described as present in ≥50% of the administrative area, in which case transmission was considered possible throughout that administrative unit. Where data were available from more than one source (Table 1), we used the finest spatial resolution and most comprehensive information. A digital elevation model (DEM) at approximately 1 × 1 km spatial resolution (Hastings and Dunbar, 1998) was used to implement national altitudinal transmission limits, reported in the ITHGs in metres above sea level. We could not map non-specific “highland” or “lowland” descriptions. If the ITHG sources provided conflicting limits, we used the higher altitude threshold. Finally, the ITHGs reported 70 cities as malaria free. These were geo-referenced using electronic geographic databases (Microsoft Corporation, 2005; The Getty Research Institute, 2005; University of California, 2005), co-located to their urban extents as defined by the Global Rural-Urban Mapping Project (GRUMP) (CIESIN/IFPRI/WB/CIAT, 2004) and then excluded.
Of the 107 countries reporting some degree of malaria risk, we mapped 104 according to our ITHG exclusion criteria (Table 2). Uzbekistan reported only “sporadic cases” and was not mapped as a malaria endemic country (MEC) in this paper. For Algeria, no corresponding administrative data could be obtained, and for North Korea there was insufficient detail in the sub-national description of risk. Despite the ITHGs being independent documents, there was relatively little complementary information: of a potential 318 entries (106 MECs × 3, i.e. risk information defined by administrative boundaries, altitude or urbanisation), there were only 121 unique reports, with IAMAT-WMRC the most comprehensive and WHO-ITH the least (Table 1).
Table 2.
Country summary data of area and population at risk (PAR) extractions
| Countrya | ITHGs criteriab |
Pfrc | Ad1d | Areae |
Populationf |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad | Alt | Urb | Total | WHO 2002 |
ITHG | ALT- MASK |
POP- MASK |
Pf | Pv | Pf+Pv | |||
| AFRO | |||||||||||||
| Algeria | Yes | No | No | 0.610 | 48 | 2.32 | 0.02 | n/a | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Angola | No | No | No | 1.000 | 18 | 1.25 | 1.25 | 1.25 | 1.24 | 0.93 | 12.92 | 0.00 | 0.00 |
| Benin | No | No | No | 1.000 | 12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.11 | 6.29 | 0.00 | 0.00 |
| Botswana | Yes | No | No | 1.000 | 10 | 0.58 | 0.17 | 0.18 | 0.18 | 0.04 | 0.16 | 0.00 | 0.00 |
| Burkina Faso | No | No | No | 1.000 | 45 | 0.27 | 0.27 | 0.27 | 0.27 | 0.27 | 13.49 | 0.00 | 0.00 |
| Burundi | No | No | No | 1.000 | 17 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 6.03 | 0.00 | 0.00 |
| Cameroon | No | No | No | 1.000 | 10 | 0.47 | 0.47 | 0.47 | 0.46 | 0.44 | 12.56 | 0.00 | 0.00 |
| Cape Verde | Yes | No | No | 1.000 | 17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 0.00 |
| Central Af. Republic | No | No | No | 1.000 | 17 | 0.62 | 0.62 | 0.62 | 0.62 | 0.27 | 3.22 | 0.00 | 0.00 |
| Chad | No | No | No | 1.000 | 14 | 1.26 | 0.81 | 1.26 | 0.89 | 0.52 | 9.05 | 0.00 | 0.00 |
| Comoros | No | No | No | 0.950 | 3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.61 | 0.00 | 0.00 |
| Congo | No | No | No | 1.000 | 10 | 0.34 | 0.34 | 0.34 | 0.34 | 0.24 | 3.43 | 0.00 | 0.00 |
| Côte d’Ivoire | No | No | No | 1.000 | 16 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | 14.10 | 0.00 | 0.00 |
| Dem. Rep. Congo | No | No | No | 1.000 | 11 | 2.34 | 2.33 | 2.34 | 2.31 | 2.06 | 46.88 | 0.00 | 0.00 |
| Equatorial Guinea | No | No | No | 1.000 | 7 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.50 | 0.00 | 0.00 |
| Eritrea | No | Yes | Yes | 0.644 | 9 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.00 | 0.00 | 4.21 |
| Ethiopia | No | Yes | Yes | 0.622 | 11 | 1.13 | 0.94 | 0.94 | 0.89 | 0.79 | 0.00 | 0.62 | 44.09 |
| Gabon | No | No | No | 1.000 | 9 | 0.27 | 0.27 | 0.27 | 0.27 | 0.06 | 1.30 | 0.00 | 0.00 |
| Gambia | No | No | No | 1.000 | 7 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 1.08 | 0.00 | 0.00 |
| Ghana | No | No | No | 1.000 | 10 | 0.24 | 0.24 | 0.24 | 0.24 | 0.24 | 18.38 | 0.00 | 0.00 |
| Guinea | No | No | No | 1.000 | 8 | 0.25 | 0.25 | 0.25 | 0.25 | 0.23 | 8.02 | 0.00 | 0.00 |
| Guinea-Bissau | No | No | No | 1.000 | 9 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 1.39 | 0.00 | 0.00 |
| Kenya | No | Yes | Yes | 1.000 | 8 | 0.57 | 0.53 | 0.52 | 0.51 | 0.42 | 23.67 | 0.00 | 0.00 |
| Liberia | No | No | No | 1.000 | 9 | 0.10 | 0.10 | 0.10 | 0.10 | 0.09 | 2.39 | 0.00 | 0.00 |
| Madagascar | No | No | No | 1.000 | 6 | 0.59 | 0.59 | 0.59 | 0.59 | 0.59 | 15.75 | 0.00 | 0.00 |
| Malawi | No | No | No | 1.000 | 3 | 0.11 | 0.11 | 0.11 | 0.11 | 0.10 | 11.78 | 0.00 | 0.00 |
| Mali | No | No | No | 1.000 | 9 | 1.26 | 0.66 | 1.26 | 0.99 | 0.60 | 11.82 | 0.00 | 0.00 |
| Mauritania | Yes | No | No | 1.000 | 13 | 1.04 | 0.28 | 0.76 | 0.59 | 0.23 | 1.08 | 0.00 | 0.00 |
| Mauritius | Yes | No | No | 0.000 | 9 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.57 | 0.00 |
| Mayotte | No | No | No | 1.000 | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.31 | 0.00 | 0.00 |
| Mozambique | No | No | No | 1.000 | 11 | 0.78 | 0.78 | 0.78 | 0.78 | 0.71 | 17.97 | 0.00 | 0.00 |
| Namibia | Yes | No | No | 1.000 | 13 | 0.83 | 0.15 | 0.45 | 0.34 | 0.17 | 1.14 | 0.00 | 0.00 |
| Niger | No | No | No | 1.000 | 8 | 1.19 | 0.57 | 1.19 | 0.85 | 0.36 | 12.20 | 0.00 | 0.00 |
| Nigeria | No | No | No | 1.000 | 37 | 0.91 | 0.91 | 0.91 | 0.91 | 0.89 | 108.52 | 0.00 | 0.00 |
| Rwanda | No | No | No | 1.000 | 12 | 0.03 | 0.03 | 0.03 | 0.02 | 0.02 | 5.50 | 0.00 | 0.00 |
| S. Tome & Principe | No | No | No | 0.950 | 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 |
| Senegal | No | No | No | 1.000 | 10 | 0.20 | 0.20 | 0.20 | 0.20 | 0.17 | 8.24 | 0.00 | 0.00 |
| Sierra Leone | No | No | No | 1.000 | 4 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 4.41 | 0.00 | 0.00 |
| South Africa | Yes | No | No | 1.000 | 9 | 1.22 | 0.08 | 0.30 | 0.17 | 0.10 | 11.12 | 0.00 | 0.00 |
| Swaziland | No | No | No | 1.000 | 4 | 0.02 | 0.00 | 0.02 | 0.02 | 0.02 | 0.92 | 0.00 | 0.00 |
| Togo | No | No | No | 1.000 | 5 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 5.17 | 0.00 | 0.00 |
| Uganda | No | No | Yes | 1.000 | 56 | 0.21 | 0.21 | 0.21 | 0.20 | 0.19 | 24.44 | 0.00 | 0.00 |
| U. Rep. of Tanzania | No | Yes | No | 1.000 | 25 | 0.88 | 0.88 | 0.86 | 0.86 | 0.56 | 28.97 | 0.00 | 0.00 |
| Zambia | No | No | No | 1.000 | 9 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 11.25 | 0.00 | 0.00 |
| Zimbabwe | No | Yes | Yes | 1.000 | 10 | 0.39 | 0.39 | 0.30 | 0.39 | 0.35 | 10.68 | 0.00 | 0.00 |
| 23.19 | 16.01 | 18.54 | 17.13 | 13.17 | 477.15 | 1.19 | 48.31 | ||||||
| AMRO | |||||||||||||
| Argentina | Yes | Yes | No | 0.000 | 23 | 2.78 | 0.01 | 0.14 | 0.14 | 0.10 | 0.00 | 2.19 | 0.00 |
| Belize | No | Yes | Yes | 0.000 | 6 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.00 | 0.23 | 0.00 |
| Bolivia | Yes | Yes | Yes | 0.046 | 9 | 1.08 | 0.27 | 0.76 | 0.76 | 0.38 | 0.00 | 1.71 | 0.53 |
| Brazil | Yes | Yes | Yes | 0.214 | 27 | 8.52 | 4.82 | 5.08 | 5.08 | 1.61 | 0.00 | 2.40 | 14.94 |
| Colombia | Yes | Yes | Yes | 0.384 | 32 | 1.14 | 0.95 | 0.90 | 0.90 | 0.48 | 0.00 | 0.87 | 12.37 |
| Costa Rica | Yes | Yes | Yes | 0.002 | 7 | 0.05 | 0.05 | 0.03 | 0.03 | 0.03 | 0.00 | 0.70 | 0.00 |
| Dominican Republic | Yes | Yes | No | 0.997 | 31 | 0.05 | 0.05 | 0.01 | 0.01 | 0.01 | 0.68 | 0.00 | 0.00 |
| Ecuador | Yes | Yes | Yes | 0.212 | 22 | 0.26 | 0.11 | 0.12 | 0.12 | 0.09 | 0.00 | 0.00 | 3.35 |
| El Salvador | Yes | Yes | No | 0.000 | 14 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 1.09 | 0.00 |
| French Guiana | No | No | No | 0.696 | 2 | 0.08 | 0.02 | 0.04 | 0.04 | 0.00 | 0.00 | 0.00 | 0.05 |
| Guatemala | Yes | Yes | Yes | 0.051 | 22 | 0.11 | 0.04 | 0.07 | 0.07 | 0.06 | 0.00 | 0.00 | 3.80 |
| Guyana | No | No | Yes | 0.470 | 10 | 0.21 | 0.20 | 0.21 | 0.20 | 0.08 | 0.00 | 0.00 | 0.64 |
| Haiti | Yes | Yes | No | 1.000 | 9 | 0.03 | 0.03 | 0.01 | 0.01 | 0.01 | 3.15 | 0.00 | 0.00 |
| Honduras | No | Yes | Yes | 0.035 | 18 | 0.11 | 0.11 | 0.09 | 0.09 | 0.08 | 0.00 | 4.10 | 0.00 |
| Mexico | Yes | Yes | No | 0.004 | 32 | 1.96 | 0.82 | 0.41 | 0.41 | 0.40 | 0.00 | 15.79 | 0.00 |
| Nicaragua | No | Yes | Yes | 0.133 | 16 | 0.12 | 0.13 | 0.09 | 0.09 | 0.06 | 0.00 | 0.00 | 1.83 |
| Panama | Yes | Yes | Yes | 0.150 | 10 | 0.08 | 0.03 | 0.02 | 0.02 | 0.02 | 0.00 | 0.00 | 0.19 |
| Paraguay | Yes | No | No | 0.000 | 18 | 0.40 | 0.02 | 0.05 | 0.05 | 0.05 | 0.00 | 1.26 | 0.00 |
| Peru | Yes | Yes | Yes | 0.171 | 25 | 1.29 | 0.54 | 0.76 | 0.76 | 0.38 | 0.00 | 0.40 | 5.23 |
| Suriname | Yes | Yes | Yes | 0.787 | 10 | 0.15 | 0.11 | 0.13 | 0.13 | 0.02 | 0.01 | 0.00 | 0.02 |
| Venezuela | Yes | Yes | Yes | 0.088 | 23 | 0.92 | 0.20 | 0.38 | 0.38 | 0.15 | 0.00 | 1.04 | 3.02 |
| 19.39 | 8.56 | 9.33 | 9.32 | 4.03 | 3.83 | 31.78 | 45.98 | ||||||
| EMRO | |||||||||||||
| Afghanistan | No | Yes | No | 0.385 | 32 | 0.64 | 0.51 | 0.38 | 0.38 | 0.37 | 1.77 | 0.14 | 12.44 |
| Djibouti | No | No | No | 0.980 | 5 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.22 | 0.00 | 0.00 |
| Egypt | Yes | No | No | 4 | 0.98 | 0.01 | 0.01 | n/a | n/a | n/a | n/a | n/a | |
| Iran | No | Yes | No | 0.200 | 24 | 1.61 | 0.56 | 1.02 | 1.02 | 0.98 | 0.00 | 0.00 | 38.83 |
| Iraq | Yes | Yes | Yes | 0.000 | 19 | 0.44 | 0.29 | 0.10 | 0.10 | 0.10 | 0.00 | 7.95 | 0.00 |
| Morocco | Yes | No | Yes | 0.000 | 15 | 0.41 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 |
| Oman | Yes | Yes | No | 0.000 | 8 | 0.31 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 |
| Pakistan | No | Yes | No | 0.365 | 5 | 0.88 | 0.85 | 0.74 | 0.74 | 0.68 | 0.00 | 0.00 | 122.99 |
| Saudi Arabia | Yes | No | Yes | 0.471 | 14 | 1.93 | 0.18 | 0.86 | 0.85 | 0.31 | 1.20 | 0.13 | 12.21 |
| Somalia | No | No | No | 0.722 | 18 | 0.64 | 0.64 | 0.64 | 0.58 | 0.54 | 0.00 | 0.00 | 7.52 |
| Sudan | No | No | No | 0.851 | 18 | 2.49 | 1.86 | 2.49 | 2.13 | 1.57 | 26.31 | 0.00 | 2.83 |
| Syrian Arab Rep. | No | Yes | No | 0.540 | 12 | 0.19 | 0.03 | 0.04 | 0.04 | 0.04 | 0.00 | 0.00 | 4.02 |
| Yemen | No | Yes | Yes | 0.956 | 21 | 0.46 | 0.34 | 0.43 | 0.43 | 0.25 | 13.20 | 0.00 | 1.78 |
| 10.99 | 5.30 | 6.75 | 6.29 | 4.86 | 42.71 | 8.32 | 202.62 | ||||||
| EURO | |||||||||||||
| Armenia | Yes | No | No | 0.000 | 11 | 0.03 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.27 | 0.00 |
| Azerbaijan | No | No | No | 0.000 | 63 | 0.08 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 |
| Georgia | Yes | No | Yes | 0.000 | 14 | 0.07 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.53 | 0.00 |
| Kyrgyzstan | Yes | No | No | 0.000 | 7 | 0.19 | n/a | 0.08 | 0.03 | 0.03 | 0.00 | 1.25 | 0.00 |
| Tajikistan | Yes | No | No | 0.003 | 6 | 0.14 | 0.01 | 0.11 | 0.04 | 0.04 | 0.00 | 3.23 | 0.00 |
| Turkey | Yes | No | No | 0.000 | 81 | 0.78 | 0.13 | 0.19 | 0.13 | 0.13 | 0.00 | 13.64 | 0.00 |
| Turkmenistan | Yes | No | No | 0.000 | 6 | 0.46 | 0.02 | 0.09 | 0.09 | 0.09 | 0.00 | 1.16 | 0.00 |
| Uzbekistan | No | No | No | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| 1.76 | 0.19 | 0.48 | 0.29 | 0.30 | 0.00 | 20.26 | 0.00 | ||||||
| SEARO | |||||||||||||
| Bangladesh | No | No | Yes | 0.407 | 6 | 0.14 | 0.14 | 0.14 | 0.14 | 0.13 | 0.00 | 0.00 | 124.61 |
| Bhutan | Yes | Yes | No | 0.387 | 18 | 0.04 | 0.02 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.91 |
| India | Yes | Yes | Yes | 0.278 | 34 | 3.09 | 2.93 | 2.94 | 2.94 | 2.86 | 0.00 | 88.26 | 857.93 |
| Indonesia | No | Yes | Yes | 0.385 | 27 | 1.90 | 1.71 | 1.75 | 1.75 | 1.44 | 0.00 | 0.00 | 151.08 |
| Korea, D. P. R. | No | No | No | 12 | 0.12 | 0.00 | n/a | n/a | n/a | n/a | n/a | n/a | |
| Myanmar | Yes | Yes | Yes | 0.788 | 16 | 0.67 | 0.66 | 0.53 | 0.53 | 0.47 | 0.00 | 0.00 | 38.36 |
| Nepal | Yes | Yes | Yes | 0.090 | 5 | 0.15 | 0.08 | 0.06 | 0.06 | 0.06 | 0.00 | 7.66 | 9.64 |
| Sri Lanka | Yes | Yes | Yes | 0.222 | 9 | 0.07 | 0.06 | 0.06 | 0.06 | 0.06 | 0.00 | 0.00 | 10.32 |
| Thailand | No | No | Yes | 0.469 | 76 | 0.52 | 0.35 | 0.51 | 0.49 | 0.50 | 0.00 | 0.00 | 58.08 |
| Timor-Leste | No | No | No | 0.534 | 14 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.62 |
| 6.70 | 5.97 | 6.01 | 5.99 | 5.54 | 0.00 | 95.93 | 1 251.55 | ||||||
| WPRO | |||||||||||||
| Cambodia | No | No | Yes | 0.870 | 24 | 0.18 | 0.18 | 0.18 | 0.18 | 0.16 | 2.03 | 0.00 | 10.79 |
| China | Yes | Yes | Yes | 0.100 | 32 | 9.44 | 1.24 | 2.36 | 2.36 | 2.32 | 0.00 | 453.90 | 287.79 |
| Lao P. D. R. | No | No | Yes | 0.960 | 18 | 0.23 | 0.23 | 0.23 | 0.23 | 0.22 | 3.56 | 0.00 | 2.30 |
| Malaysia | No | Yes | No | 0.565 | 14 | 0.33 | 0.33 | 0.27 | 0.27 | 0.26 | 0.00 | 0.00 | 9.69 |
| Papua New Guinea | No | Yes | No | 0.727 | 20 | 0.46 | 0.38 | 0.41 | 0.41 | 0.41 | 0.47 | 0.00 | 3.69 |
| Philippines | Yes | Yes | Yes | 0.605 | 16 | 0.30 | 0.30 | 0.21 | 0.21 | 0.21 | 0.00 | 0.00 | 46.95 |
| Republic of Korea | No | No | No | 0.000 | 15 | 0.10 | 0.00 | 0.03 | 0.03 | 0.02 | 0.00 | 4.86 | 0.00 |
| Solomon Islands | Yes | Yes | No | 0.646 | 9 | 0.03 | 0.03 | 0.02 | 0.02 | 0.02 | 0.00 | 0.00 | 0.31 |
| Vanuatu | No | No | Yes | 0.525 | 6 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | 0.18 |
| Viet Nam | No | Yes | Yes | 0.590 | 61 | 0.33 | 0.33 | 0.32 | 0.32 | 0.32 | 0.00 | 0.00 | 69.76 |
| 11.42 | 3.03 | 4.05 | 4.04 | 3.95 | 6.06 | 458.76 | 431.47 | ||||||
| Global | 73.44 | 39.06 | 45.15 | 43.06 | 31.85 | 529.75 | 616.25 | 1 979.93 | |||||
The data are presented alphabetically by WHO regional office and country name with totals shown in bold at the end of each section and at the end of the table for the World.
Refers to presence or absence of any of the main three mapping criteria used (Ad, administrative, Alt, altitude and Urb, urban).
The number of administrative one level divisions per country.
Area totals are presented for each country as per the WHO 2002 boundaries, the ITHGs and the ITHGs with the altitudinal mask (ALT-MASK) and the population mask (POP-MASK) exclusions in millions of km2.
Populations in 2005 living predominantly under P. falciparum (Pf) and P. vivax (Pv) and mixed (Pf+Pv) risk are also presented in millions. Populations were projected to 2005 from GRUMP at ~1 × 1km2 spatial resolution (CIESIN/IFPRI/WB/CIAT, 2004).
3. THE BIOLOGICAL LIMITS OF TRANSMISSION
3.1. Altitudinal Mask
Temperature is inversely related to altitude, dropping by approximately 0.98°C for every 100-metre increase above absolute sea level (Henderson-Sellers and Robinson, 1991). Mosquitoes and malaria transmission are thus sensitive to altitude (Cox et al., 1999). Altitudinal limits from the ITHGs were available for 42 countries. The majority of the countries (44/62) for which no information was available were in Africa and we assumed no altitudinal limits for most of these (see Section 3.2). For the 18 remaining non-African MECs, we defined limits by those of neighbouring countries with similar dominant vector species. To identify the latter, we used a global map developed by Kiszewski et al. (2004) and mapped the altitudinal limits using the same 1 × 1 km spatial resolution DEM (Hastings and Dunbar, 1998).
3.2. Climate Suitability Mask
Since the information in ITHG reports for African countries was so sparse (Table 2), we used the MARA/ARMA climate suitability model to further adjust the malaria transmission limits on this continent (Craig et al., 1999). The MARA model describes climatic conditions (or fuzzy climate suitability, FCS) that range from unsuitable (0) to completely suitable (1) for stable P. falciparum transmission. Since the MARA model includes climatology-derived temperature limits, this can also be used as a surrogate altitudinal mask. To do this we have assumed that FCS values of zero are incompatible with malaria risk, as supported by a recent analysis of parasite prevalence and FCS values in East Africa (Omumbo et al., 2004). The spatial resolution of the MARA model was too coarse to apply exclusions to the territories of Cape Verde, Comoros, Mauritius, Mayotte and Sao Tome and Principe. For Comoros, we assumed the same altitudinal limit as that of Ethiopia (2000 m), based on their similar dominant vectors. Altitude masks were unnecessary for the remaining low-lying island states as they have no areas above 1800 m, which is at or below the lowest altitude threshold reported elsewhere in Africa for the same dominant vector species compositions (Kiszewski et al., 2004).
3.3. Population Density Mask
Two population density extremes were applied to refine the spatial limits of transmission further. First masked, are those areas where environmental conditions may support malaria, but where there are too few people, so the human malaria parasites cannot complete their life-cycle and do not pose any public health concern; these include, for example, dense forests and true deserts. We therefore used the 1 × 1 km resolution GRUMP population density surface, which allows for equal area corrections (CIESIN/IFPRI/WB/CIAT, 2004) (see below), to exclude all areas in the remaining distribution with a population density of <1 person per km2.
Second are those areas where population density is so high that conditions become unsuitable for transmission through the process of urbanisation (Hay et al., 2000; Robert et al., 2003; Omumbo et al., 2005). Urbanisation has been shown to reduce malaria transmission on average by an order of magnitude across Africa (Hay et al., 2005). There is no reason to think that the same fundamental processes of reduced (i) Anopheline diversity; (ii) biting rates; (iii) sporozoite rates; (iv) transmission; and thus (v) human malaria infections in urban versus rural areas do not apply globally. This is certainly true for the 70 cities cited as malaria free in ITHGs. Moreover, the 24 cities that report urban malaria often refer to infection risk on their peripheries. A potential confounder to this global trend could be the presence of the urban malaria vector Anopheles stephensi in the Indian sub-continent (Rowland et al., 2002). A detailed look at the evidence indicates that vector densities and sporozoite rates show similar declines from rural, through peri-urban, to urban localities in Delhi (Sharma et al., 1993), Gurgaon (Sharma, 1995) and Karachi (Nalin et al., 1985) to those in Africa.
We projected population counts for the year 2000 (CIESIN/IFPRI/WB/CIAT, 2004) to 2005 by applying national, medium variant, intercensal growth rates by country (UNPD, 2004) before deriving contemporary population densities using an area-by-pixel surface (CIESIN/IFPRI/WB/CIAT, 2004). We geo-referenced (Microsoft Corporation, 2005; The Getty Research Institute, 2005; University of California, 2005) cities with populations equal to or greater than one million people (UNSD, 2001) in MECs and identified their urban extents in GRUMP (n = 204). We then investigated population density frequency statistics within each of these urban extents. Significant regional differences in population density were apparent, so a conservative threshold of intensity of urbanisation was used, corresponding to the median of population density means associated with the urban extents by region. The medians were 4218, 1533 and 2513 persons per km2 for Africa, the Americas and Asia-Europe, respectively. The same median as Asia-Europe was used in Oceania MECs, where no cities of ≥1 million people currently exist. We thus masked as no malaria risk all those areas that could be unambiguously classified as intensely urban relative to their corresponding region.
The resulting map, after applying the three masks described here, is shown in Figure 1 (Figure 1 is Plate 5.1 in the Separate Color Plate Section).
Plate 5.
Malaria distribution in 2005 after altitudinal and population exclusions indicating areas at risk according to species of Plasmodium.
4. DISTINGUISHING P. FALCIPARUM AND P. VIVAX RISK
Global information of the distribution of P. falciparum and P. vivax is not comprehensively detailed in the ITHGs. Sub-national statistics are available from other sources (FDRE, 2002; PAHO, 2003; Hay et al., 2004a; Sintasath, 2004; Kolaczinski et al., 2005). For countries or areas within countries where no sub-national data were available, national average data were used from ITHGs or other sources (PAHO, 2003; Korenromp, 2005)(Table 2). Specific data for Mayotte were not available, so we assumed 100% P. falciparum risk based on ITHG descriptions. We defined areas reporting ≥95% P. falciparum cases as predominantly P. falciparum and those ≤5% P. falciparum as predominantly P. vivax endemic. The remainder of the distribution is of mixed (P. falciparum and P. vivax) endemicity. P. ovale and P. malariae were not considered here, as these are relatively rare malaria parasites and infrequently reported in national statistics. These divisions are shown globally (Figure 1) and in greater detail by WHO geographic region (Figures 2A–R) (Figures 2A–R are Plate 5.2A–R in the Separate Color Plate section). We now discuss the implications of the biological limits to transmission and the parasite species distributions by region and highlight some known anomalies.
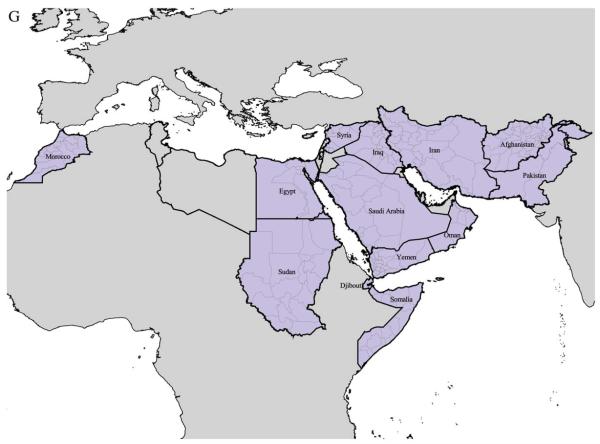
Plate 5.
A–R First level administrative division boundaries and P. falciparum (Pf) and P. vivax (Pv) ratios by WHO region (A–C: AFRO; D–F: AMRO; G–I: EMRO; J–L: EURO; M–O: SEARO; P–R: WPRO). Outlined in thick black line are countries belonging to each region. Malarious countries are filled in light blue with a light grey thin line representing sub-national boundaries. Dark grey areas represent malaria distribution outside the given region and light grey ones are malaria-free areas.
5. REGIONAL ANALYSIS
Before the biological exclusions were applied, the global malaria area at risk of malaria was 45.15 million km2, considerably higher than the 39.06 million km2 derived from the WHO 2002 boundaries (Figure 3A,Table 2). The altitude and climate mask reduced this slightly to 43.06 million km2. The largest percentage reductions were in countries of the European Regional Office (EURO) (39.9%) and African Regional Office (AFRO) (7.5%) due to the mountainous areas and large expanses of zero climate suitability in the northern Sahel, respectively (Figure 3B; Table 2). The population mask had a very considerable effect in reducing the global area at risk further, to 31.85 million km2 (43.4% of the MECs total land area) (Figures 1 and 3A, Table 2). This incremental reduction was most noticeable in countries of the American Regional Office (AMRO) (56.8%), AFRO (23.1%) and the Eastern Mediterranean Regional Office (EMRO) (22.8%), mainly due to the exclusion of large, relatively unpopulated areas of forest and desert land. In the final coverage, the rank of regions by area at risk was AFRO (13.17 million km2, 56.8% of the regional office MEC land area), the South East Asian Regional Office (SEARO) (5.54 million km2, 82.7% of the regional office MEC land area), EMRO (4.86 million km2, 44.2% of the regional office MEC land area), AMRO (4.03 million km2, 20.8% of the regional office MEC land area), the Western Pacific Regional Office (WPRO) (3.95 km2, 34.6% of the region’s land area) and EURO (0.30 million km2, 17.0% of the regional office MEC land area) (Figure 3B, Table 2). The global malarious area was thus progressively reduced by iterations of biological exclusions with our final distribution model 18.5% smaller (7.21 million km2) in area than that which would be derived from using the WHO 2002 limits (WHO, 2003b)(Figure 3A).
Figure 3.
A–D Bar charts showing area (A and B) and population at risk (PAR) (C and D) of malaria according to the WHO 2002 limits (white), ITHGs (grey), and the progressive implementation of our altitudinal (dark grey) population (black) masks, globally and stratified by WHO regional office. The scales of area (km2) and population are in millions. Figures were calculated using ArcView 3.2 (ESRI, Redlands, California, USA). Area extractions were undertaken on an equal area projection.
The global heterogeneity in the distribution of human population (Cohen and Small, 1998) generated some rather striking contrasts in population at risk (PAR) extractions. Global PAR derived from the WHO 2002 boundaries is 3.133 billion persons, much less than the 3.616 billion suggested by the crude ITHG limits (Figure 3C). Our altitude and climate mask marginally reduced this figure further to 3.596 billion people. After implementing the population mask, the final global PAR is estimated at 3.126 billion. This was almost entirely due to the removal of the highly populous urban areas of low to zero malarial risk. This final PAR estimate is very similar to that extracted from the WHO 2002 boundaries (only 0.22% lower) but critically different in regional distribution of PAR estimates (Figure 3D, Table 2). Extractions from the final coverage show the malarious regions by rank PAR were SEARO (1.347 billion persons), WPRO (0.896 billion persons), AFRO (0.527 billion persons), EMRO (0.254 billion persons), AMRO (0.082 billion persons) and EURO (0.020 billion persons). These changes in rank show the important contribution that the very large population concentrations in SEARO and WPRO make to the PAR estimates. It is essential to note at this stage that all risk is not equal, and a more detailed discussion of the P. falciparum and P. vivax partitioning by region follows.
In AFRO (Figures 2A–C), we can see how the various iterations have refined the transmission limits for malaria infection particularly to the arid north and south of the continent, although significant areas have been excised from the mid-latitude tropical forest range. A small focus of malaria risk emerged in Illizi department, Algeria, after our climate masking, which we assumed to correspond to the focus reported by ITHGs in Ihrir (CDC, 2003). Hence, our final map allows for a small area of malaria risk in Algeria. The distribution of almost exclusively P. falciparum on the African continent is remarkable, and fits with received wisdom about the evolution to fixation of the Duffy negativity blood group allele, making these populations refractory to P. vivax infection (Livingstone, 1984; Zimmerman, 2004). Information on parasite species ratio is poor in AFRO, however, and the stark transitions between southern Ethiopia and neighbouring countries (as well as Sudan and Somalia from EMRO (Figures 2G–I)), suggest that a more detailed investigation of the distribution of P. vivax is warranted in these areas on the latitidudinal margins of risk.
In AMRO (Figures 2D–F), by contrast, P. vivax is by far the dominant parasite (Roberts et al., 2002). The most obvious exclusions are those of the low-populated tropical forests. The regional distribution of risk compares favourably with other sources (Roberts et al., 2002; PAHO, 2003), but the biological exclusions failed to capture completely the situation in Argentina and Paraguay, where incidence of malaria is reportedly low (PAHO, 2003), yet our map indicates non-negligible areas and PARs for these countries (Table 2).
EMRO (Figures 2G–I) is a truly heterogeneous region encompassing Morocco, Somalia and Sudan, as well as the Middle Eastern block through to Pakistan (Beljaev, 2002). Summary is therefore difficult but P. falciparum is highest in the south-west with risk declining as one travels north and east. Areas of “very limited risk” reported by ITHGs in Egypt (CDC, 2003; WHO, 2005) are not captured by our climate mask and were excluded. Our final map, therefore, does not allow for malaria risk in Egypt, where no cases have been reported since 1998 (WHO, 2005) despite high malaria risk being documented in Fayoum governorate (Hassan et al., 2003).
The EURO MECs (Figures 2J–L) are essentially Turkey and the southern states newly independent from the former Soviet Union (Sabitinelli, 2002). Their small global fraction of malaria PAR is largely due to P. vivax infections.
SEARO (Figures 2M–O) is best characterised as endemic for predominantly P. vivax, but with significant foci of P. falciparum transmission (Sharma, 2002). A known anomaly in our coverage is evident from the fact that malaria has long been endemic in the Korean peninsula (Feighner et al., 1998). After a period of decline following the Korean War, malaria re-emerged in the Demilitarised Zone, probably due to an epidemic in North Korea since 1993 (Lee et al., 2002). ITHGs, however, are ambiguous about the areas of risk in this country, making them impossible to map.
In WPRO (Figures 2P–R), P. vivax dominance gives way to P. falciparum as one moves south and east (Schapira, 2002). China dominates the PAR extractions for this region and introduces a warming. An overestimation in area, and hence PAR, by our map is possible in this country, where the average spatial resolution of heavily populated first administrative units is poor and ITHG sub-national descriptions fail to capture lower-level administrative detail.
6. DISCUSSION
The most widely cited map of the current global malaria distribution is WHO 2002 (WHO, 2003b). The information source is cited as (WHO, 2003a) but there are important discrepancies between the map (WHO, 2003b) and suggested source data. For example, the spatial limits according to ITHGs are 6.09 million km2 larger than the WHO 2002 boundary (Table 2). These geographical inconsistencies and a lack of detailed information of their origins make them of unknown fidelity in risk mapping. In contrast, the methods presented here are implemented with public-domain data and are hence easily reproducible, the maps used to generate individual country data are presented in detail, and the PAR numbers are made available for scrutiny. In addition, and for the first time, parasite species’ distributions are defined globally (including the often neglected P. vivax) and are suggested as a more comprehensive map against which to measure PAR of malaria in 2005.
Our map comes with obvious caveats, since we have implemented crude rules at the global scale. Noticeable anomalies (e.g. Argentina, Egypt, Morocco, North Korea, Paraguay, and possibly China) have been highlighted and it is clear that, at a sub-national level, discrepancies will be found as, because risk is not a static phenomenon, and global information is incomplete. We therefore propose these maps as a working template for future refinement.
Reconnaissance of global malaria data is required to refine distributions at the margins and this can only be done with malaria-risk data at higher spatial resolution. These data probably exist at the country level and need to be collated at the global scale within a GIS framework. In terms of PAR, the top ten countries globally are India, China, Indonesia, Bangladesh, Pakistan, Nigeria, Vietnam, Thailand, Democratic Republic of the Congo and the Philippines (Table 2). Pragmatically, Error in PAR estimates globally would be reduced most substantially by focussing on improving distribution limits and P. falciparum ratios for these territories.
Modelling risks within these margins is also critical and is the subject of planned future work. Local endemicity within these transmission limits will be substantially mediated by the influences of land-use changes such as deforestation (Walsh et al., 1993) and urbanisation (Hay et al., 2005), prevalence of other conditions such as HIV/AIDS, tuberculosis and malnutrition (Bates et al., 2004), as well as local control and intervention efforts (Korenromp, 2005). Our immediate goal is to validate historical malaria endemicity maps (Lysenko and Semashko, 1968) using empirical data and to generate plausible scenarios of some of the above-mentioned confounding influences to satisfactorily adjust endemicity to modern day risk. This will fill pressing needs to estimate global incidence of malaria and commodity burdens. Our longer-term goal is to construct an independent global map of modern day endemicity within the boundaries we have defined and will continue to refine.
7. CONCLUSIONS
While there will remain uncertainties about the precise global extent of malaria, we have reduced these as far as possible without systematic country-specific surveys and information. Urgent attention is required to reduce the uncertainty surrounding factors that affect the spatial extent of malaria on a global scale and these would sensibly target the most populous malarious nations. This will allow the international community to better define the needs for therapeutic and disease prevention commodities so that well-intentioned governments and UN agencies can make requests for sufficient financial resources.
ACKNOWLEDGEMENTS
We thank Simon Brooker, Alastair Graham, Sarah Hay, Eline Korenromp, Abdisalan Noor, Sarah Randolph and Andy Tatem for comments on earlier drafts of the manuscript. The WHO/Roll Back Malaria Department Monitoring and Evaluation team and staff from the WHO regional office provided passive case detection data from which some P. falciparum and P. vivax ratios were derived. Lorena Lucioparedes is thanked for geo-referencing UN cities. CAG is partially funded by the Fundación para la Ciencia y Tecnología (FUND-ACYT), Quito, Ecuador. RWS is a Wellcome Trust Senior Research Fellow (#058992) and acknowledges the support of the Kenyan Medical Research Institute (KEMRI). SIH is funded by a Research Career Development Fellowship from the Wellcome Trust (#069045). This paper is published with the permission of the director of KEMRI.
REFERENCES
- Bates I, Fenton C, Gruber J, Lalloo D, Lara A. Medina, Squire SB, Theobald S, Thomson R, Tolhurst R. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infectious Diseases. 2004;4:267–277. doi: 10.1016/S1473-3099(04)01002-3. [DOI] [PubMed] [Google Scholar]
- Beljaev AE. Determinants of malaria in the Middle East and North Africa. In: Casman EA, Dowlatabadi H, editors. The Contextual Determinants of Malaria. Resources for the Future Press; Washington, DC: 2002. pp. 137–166. [Google Scholar]
- Boyd MF. Malariology. W.B. Saunders; Philadelphia: 1949. [Google Scholar]
- Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clinical Microbiology Reviews. 2002;15:564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Health information for international travel 2003– 2004. Centers for Disease Control and Prevention; Atlanta: 2003. [Google Scholar]
- CIESIN/IFPRI/WB/CIAT . Global Rural Urban Mapping Project (GRUMP): Gridded Population of the World, version 3, with urban re-allocation (GPW-UR) Center for International Earth Science Information Network, Columbia University; International Food Policy Research Institute; The World Bank; and Centro Internacional de Agricultura Tropical; Socioeconomic Data and Applications center (SEDAC), Columbia University; Palisades, NY: 2004. Available at http://sedac.ciesin.columbia.edu/gpw. (downloaded October 2004) [Google Scholar]
- Cohen JE, Small C. Hypsographic demography: the distribution of human population by altitude. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14009–14014. doi: 10.1073/pnas.95.24.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Craig M, Le Sueur D, Sharp B. Mapping malaria risk in the highlands of Africa. Technical report. MARA/HIMAL; Durban: 1999. [Google Scholar]
- Craig MH, Snow RW, le Sueur D. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitology Today. 1999;15:105–111. doi: 10.1016/s0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- Dutta HM, Dutt AK. Malarial ecology: a global perspective. Social Science & Medicine. 1978;12:69–84. [PubMed] [Google Scholar]
- FDRE . Health and Health Related Indicators. Planning and Programming Department, Ministry of Health, Federal Democratic Republic of Ethiopia; Addis Ababa, Ethiopia: 2002. [Google Scholar]
- Feighner BH, Pak SI, Novakoski WL, Kelsey LL, Strickman D. Reemergence of Plasmodium vivax malaria in the republic of Korea. Emerging Infectious Diseases. 1998;4:295–297. doi: 10.3201/eid0402.980219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan AN, Kenawy MA, Kamal H, Sattar A.A. Abdel, Sowilem MM. GIS-based prediction of malaria risk in Egypt. Eastern Mediterranean Health Journal. 2003;9:548–558. [PubMed] [Google Scholar]
- Hastings DA, Dunbar PK. Development and assessment of the global land 1 km base elevation digital elevation model (GLOBE) International Archives of Photogrammetry and Remote Sensing. 1998;32:218–221. [Google Scholar]
- Hay SI, Guerra CA, Snow RW. Report on Agreement to Perform Work, M50/370/19. World Health Organization; Geneva: 2004b. Determination of populations at malaria risk. [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infectious Diseases. 2004a;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nature Reviews Microbiology. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rate (EIR) across Africa: literature survey, internet access and review. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Sellers A, Robinson PJ. Contemporary Climatology. Longman Scientific and Technical; London, UK: 1991. [Google Scholar]
- IAMAT . World Malaria Risk Chart. International Association for Medical Assistance to Travellers; Guelph, Canada: 2004. [Google Scholar]
- Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J. A global index representing the stability of malaria transmission. American Journal of Tropical Medicine and Hygiene. 2004;70:486–498. [PubMed] [Google Scholar]
- Kolaczinski J, Graham K, Fahim A, Brooker S, Rowland M. Malaria control in Afghanistan: progress and challenges. Lancet. 2005;365:1506–1512. doi: 10.1016/S0140-6736(05)66423-9. [DOI] [PubMed] [Google Scholar]
- Korenromp E. Malaria incidence estimates at country level for the year 2004 – proposed estimates and draft report. World Health Organization (WHO); Geneva, Switzerland: 2005. [Google Scholar]
- Lee JS, Lee WJ, Cho SH, Ree HI. Outbreak of vivax malaria in areas adjacent to the demilitarized zone, South Korea, 1998. American Journal of Tropical Medicine and Hygiene. 2002;66:13–17. doi: 10.4269/ajtmh.2002.66.13. [DOI] [PubMed] [Google Scholar]
- Livingstone FB. The Duffy blood-groups, vivax malaria, and malaria selection in human-populations—a review. Human Biology. 1984;56:413–425. [PubMed] [Google Scholar]
- Lysenko AY, Semashko IN. Geography of malaria: a medico-geographic profile of an ancient disease [in Russian] In: Lebedew AW, editor. Medicinskaja Geografija. Academy of Sciences; Moscow: 1968. pp. 25–146. [Google Scholar]
- Microsoft Corporation . Encarta 2005 Premium Suite. Microsoft Corporation; Seattle: 2005. [Google Scholar]
- Nalin DR, Mahood F, Rathor H, Muttalib A, Sakai R, Chowdhry MA, Safdar G, ul, Haq I, Munir M, Suleiman M, Bahir M, Mujtaba SM. A point survey of periurban and urban malaria in Karachi. Journal of Tropical Medicine and Hygiene. 1985;88:7–15. [PubMed] [Google Scholar]
- Omumbo JA, Guerra CA, Hay SI, Snow RW. The influence of urbanisation on measures of Plasmodium falciparum infection prevalence in East Africa. Acta Tropica. 2005;93:11–21. doi: 10.1016/j.actatropica.2004.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omumbo JA, Hay SI, Guerra CA, Snow RW. The relationship between the Plasmodium falciparum parasite ratio in childhood and climate estimates of malaria transmission in Kenya. Malaria Journal. 2004;3:17. doi: 10.1186/1475-2875-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAHO . Report on the status of malaria programs in the Americas (based on 2002 data) CD44/INF/3—44th Directing Council/55th Session of the Regional Committee. Pan American Health Organization; Washinghton, DC: 2003. [Google Scholar]
- Pampana EJ, Russell PF. Le Paludisme: problème mondial. Vol. 69. World Health Organization; Geneva: 1955. pp. 317–321. [Google Scholar]
- Robert V, Macintyre K, Keating J, Trape JF, Duchemin JB, Warren M, Beier JC. Malaria transmission in urban sub-Saharan Africa. American Journal of Tropical Medicine and Hygiene. 2003;68:169–176. [PubMed] [Google Scholar]
- Roberts DR, Masuoka P, Au AY. Determinants of malaria in the Americas. In: Casman EA, Dowlatabadi H, editors. The Contextual Determinants of Malaria. Resources for the Future Press; Washington, DC: 2002. pp. 35–58. [Google Scholar]
- Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–1766. doi: 10.1126/science.289.5485.1763. [DOI] [PubMed] [Google Scholar]
- Rowland M, Mohammed N, Rehman H, Hewitt S, Mendis C, Ahmad M, Kamal M, Wirtz R. Anopheline vectors and malaria transmission in eastern Afghanistan. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:620–626. doi: 10.1016/s0035-9203(02)90331-7. [DOI] [PubMed] [Google Scholar]
- Sabitinelli G. Determinants of malaria in WHO European region. In: Casman EA, Dowlatabadi H, editors. The Contextual Determinants of Malaria. Resources for the Future Press; Washington, DC: 2002. pp. 66–92. [Google Scholar]
- Sachs JD. Achieving the millennium development goals—the case of malaria. The New England Journal of Medicine. 2005;352:115–117. doi: 10.1056/NEJMp048319. [DOI] [PubMed] [Google Scholar]
- Schapira A. Determinants of malaria in Oceania and East Asia. In: Casman EA, Dowlatabadi H, editors. The Contextual Determinants of Malaria. Resources for the Future Press; Washington, DC: 2002. pp. 93–109. [Google Scholar]
- Sharma RS. Urban malaria and its vectors Anopheles stephensi and Anopheles culicifacies (Diptera: Culicidae) in Gurgaon, India. Southeast Asian Journal of Tropical Medicine and Public Health. 1995;26:172–176. [PubMed] [Google Scholar]
- Sharma SN, Subbarao SK, Choudhury DS, Pandey KC. Role of An. culicifacies and An. stephensi in malaria transmission in urban Delhi. Indian Journal of Malariology. 1993;30:155–168. [PubMed] [Google Scholar]
- Sharma VP. Determinants of malaria in South Asia. In: Casman EA, Dowlatabadi H, editors. The Contextual Determinants of Malaria. Resources for the Future Press; Washington, DC: 2002. pp. 110–132. [Google Scholar]
- Sintasath D. National malaria prevalence survey (2000–2001) Environmental Health Project, Ministry of Health of Eritrea and the Office of Health, Infectious Diseases and Nutrition, Bureau of Global Health, U.S. Agency for International Development; Washington DC: 2004. [Google Scholar]
- Snow RW. The invisible victims. Nature. 2004;430:934–935. doi: 10.1038/430934a. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Marsh K, le Sueur D. The need for maps of transmission intensity to guide malaria control in Africa. Parasitology Today. 1996;12:455–457. [Google Scholar]
- The Getty Research Institute . Getty Thesaurus of Geographic Names Online. The Getty Research Institute; Los Angeles, CA: 2005. [Google Scholar]
- University of California . Alexandria Digital Library Project. University of California; Santa Barbara: 2005. [Google Scholar]
- UNPD . World Population Prospects: population database. United Nations Population Division; New York: 2004. http://esa.un.org/unpp/ [Google Scholar]
- UNSD . Demographic Yearbook. United Nations Statistics Division; New York: 2001. [Google Scholar]
- Walsh JF, Molyneux DH, Birley MH. Deforestation: effects on vector-borne disease. Parasitology. 1993;106:S55–S75. doi: 10.1017/s0031182000086121. [DOI] [PubMed] [Google Scholar]
- WHO Malaria eradication in 1965. World Health Organization Chronicle. 1966;20:286–300. [Google Scholar]
- WHO . International Travel and Health: Situation as on 1 January 2003. World Health Organization; Geneva: 2003a. [Google Scholar]
- WHO . Worldwide Malaria Distribution in 2002. Public Health Mapping Group, World Health Organizaion; Geneva: 2003b. [Google Scholar]
- WHO . International Travel and Health: Situation as on 1 January 2005. World Health Organization; Geneva: 2005. [Google Scholar]
- Zimmerman PA. The enigma of Plasmodium vivax malaria and erythrocyte Duffy negativity. In: Dronamraju KR, editor. Infectious Disease and Host-Pathogen Evolution. Cambridge University Press; Cambridge: 2004. pp. 141–172. [Google Scholar]