Abstract
Alpha-synuclein is intimately involved in the pathogenesis of Parkinson’s disease, and has been implicated in the regulation of synthesis, release and reuptake of dopamine. However, mice lacking members of the synuclein family have been reported to display no overt behavioural phenotype. This may be a result of compensatory upregulation of other synucleins during development. Here we report on behaviour and dopamine synapse function of alpha-synuclein null, gamma-synuclein null and alpha-gamma-synuclein double-null knockout mice. Double-null mice were hyperactive in a novel environment and alternated at a lower rate in a T-maze spontaneous alternation task, a phenotype reminiscent of mice expressing reduced levels of the dopamine transporter. To investigate a possible hyperdopaminergic phenotype in alpha-gamma-synuclein double-null mice, we used fast-scan cyclic voltammetry at carbon-fiber microelectrodes to assess dopamine release and reuptake in striatal slices from wild-type, alpha-null, gamma-null and double-null mice in real time. Double-null mice were found to have a two-fold increase in the extracellular concentration of dopamine detected after discrete electrical stimuli in the striatum. By measuring the rate of reuptake of dopamine and tissue dopamine content in these animals, we showed that the observed increase in size of striatal dopamine transients was not attributable to a decrease in reuptake of dopamine via the dopamine transporter, and can not be attributed to an increase in tissue dopamine levels in the striatum. Rather, we propose that loss of both alpha and gamma-synuclein causes an increase in release probability from dopaminergic synapses.
Keywords: cocaine, dopamine, knockout mice, Parkinson’s disease, synuclein, voltammetry
Introduction
The synucleins are a family of small proteins of unknown function, which localise to synaptic terminals (Nakajo et al., 1993; Totterdell et al., 2004; Totterdell and Meredith, 2005). The three members, alpha, beta and gamma-synuclein share considerable N-terminal amino acid sequence homology (Nakajo et al., 1993; Jakes et al., 1994), and are each capable of binding to lipid vesicles (Fortin et al., 2004; Fortin et al., 2005; Kubo et al., 2005). To date, mice with targeted deletions of alpha-synuclein, beta-synuclein and gamma-synuclein, as well as mice lacking both alpha and beta-synuclein and mice lacking both alpha and gamma-synuclein have been reported to present no overt behavioural phenotype in the absence of a drug challenge (Abeliovich et al., 2000; Cabin et al., 2002; Ninkina et al., 2003; Chandra et al., 2004; Robertson et al., 2004). Recently, Kuhn et al (Kuhn et al., 2007) demonstrated that similar changes in gene regulation occur in response to targeted deletion of alpha-synuclein or gamma-synuclein, suggesting a degree of functional overlap. Furthermore, beta-synuclein is up-regulated in the midbrain of mice lacking alpha-synuclein, gamma-synuclein or both alpha and gamma-synuclein (Robertson et al., 2004). Similarly, gamma-synuclein is up-regulated in mice lacking both alpha and beta-synuclein (Chandra et al., 2004). These apparently compensatory changes suggest that there is a degree of functional redundancy in the synuclein gene family.
Alpha-synuclein has been shown to be intimately involved in the pathogenesis of Parkinson’s Disease (PD) (Polymeropoulos et al., 1997; Spillantini et al., 1998; Kruger et al., 2001; Singleton et al., 2003; Chartier-Harlin et al., 2004; Zarranz et al., 2004). This has led several groups to suggest that alpha-synuclein is involved in regulating dopamine (DA) homeostasis. In tissue culture models, alpha-synuclein has been shown to inhibit DA synthesis by regulating the activity of tyrosine hydroxylase, protein phosphotase 2A, and aromatic amino acid decarboxylase (Perez et al., 2002; Peng et al., 2005; Tehranian et al., 2006). Other groups have shown that alpha-synuclein regulates the trafficking of the dopamine transporter (DAT) to the cell surface (Lee et al., 2001; Wersinger et al., 2003; Wersinger and Sidhu, 2003; Fountaine and Wade-Martins, 2007) and thus regulates the rate of reuptake of DA. Furthermore, mice lacking alpha-synuclein, gamma-synuclein or alpha and gamma-synuclein are resistant to the Parkinsonian neurotoxin MPTP, the active form of which (MPP+) gains entry into dopaminergic cells via the DAT (Abeliovich et al., 2000; Dauer et al., 2002; Robertson et al., 2004). It has also been shown that alpha and gamma-synuclein expression is altered by chronic exposure to the DAT antagonist cocaine (Brenz Verca et al., 2003; Mash et al., 2003; Qin et al., 2005). Other evidence suggests that alpha-synuclein may be involved in synaptic vesicle fusion or regulation at dopaminergic synapses. Mice lacking alpha-synuclein have been shown to have increased recovery from paired-pulse depression (PPD) at DA synapses in the striatum (Abeliovich et al., 2000), and to have an increased rate of filling of the DA vesicle pool (Yavich et al., 2004). Mice overexpressing mutated alpha-synuclein exhibit reduced DA release without a corresponding loss in DA neurons (Yavich et al., 2005). Likewise, over-expression of alpha-synuclein inhibited vesicle fusion in rat pheochromocytoma (PC12) cells (Larsen et al., 2006). Therefore, alpha-synuclein may be a negative regulator of DA release.
Given that alpha-synuclein seems to inhibit DA synthesis, as well as facilitating the reuptake of DA from the synapse by the DAT, and that recovery from synaptic depression is more rapid in alpha-synuclein-null mice, we hypothesised that mice lacking synucleins may exhibit increased extracellular levels of DA. However, this effect may be obscured if beta and gamma-synuclein are able to compensate for the absence of alpha-synuclein in the nigrostriatal DA system, which is possible given their homology with alpha-synuclein, and increase in expression in the absence of alpha-synuclein. Here, we undertook experiments to investigate the effect of deleting one or both of alpha and gamma-synuclein on behaviour and dopamine neurotransmission in mice.
Materials and Methods
Subjects
Female mice lacking alpha-synuclein (alpha-null), gamma-synuclein (gamma-null) or both alpha-synuclein and gamma-synuclein (double-null) were derived from homozygous breeding colonies described previously (Robertson et al., 2004). Wild-type controls were generated from a C57BL/6 breeding colony derived from the original intercross that produced the double-null mice. All mice were 12-20 weeks old. The same cohort of mice was used for all behavioural tests (wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15). All procedures were carried out in accordance with UK Home Office and institutional guidelines.
Rotating rod test
Performance on the rotating rod test was measured as described elsewhere, (Guenther et al., 2001). Briefly, mice were tested on the accelerating rotating rod every 80 minutes, four times a day for three consecutive days. The rod was set to rotate at 4 rpm and the mouse was placed onto the rod nose first. After 10 seconds, the rod accelerated at 20rpm−2, to a maximum speed of 40 rpm. The speed at which the mouse fell was recorded and averaged for each day; if the mouse did not fall then the speed was recorded as 40 rpm. Performance was compared by 2-way ANOVA, comparing performance across days.
Multiple static rods
Performance on the multiple static rods test was measured as described elsewhere (Deacon et al., 2002a). Briefly, mice were placed on a rod suspended approximately 30 cm above a cushioned surface, facing the end of the rod. The time taken for the mouse to turn through 180° was recorded, as was the time taken to traverse the rod and step onto a platform at the end. The experiment was carried out using three rods of decreasing diameter (rod 1 - 35 mm, rod 2 - 22 mm, and rod 3 - 9 mm). The data were analysed by one-way ANOVA.
Glucose consumption
Glucose-induced polydipsia was measured as described previously (Guenther et al., 2001). Mice were housed individually overnight with free access to food and either water or glucose solution at 4%, 5%, 8%, or 12% (wt./vol.). Water bottles were weighed to the nearest 0.1 g before the start of the test and again the next day. The amount of water/glucose consumed was calculated from the difference between start and end weight. The data were analysed by two-way ANOVA comparing weight of glucose solution drunk as a proportion of body weight by concentration of glucose.
Open field test
The non-anxiogenic open field test was performed as described previously (Deacon et al., 2002b). A mouse was placed in one corner of a grey open field measuring 50 cm by 30 cm, divided into 10 cm squares, and behaviour was observed for 3 minutes. The time taken for the mouse to leave the corner square and the time taken to the first rear were recorded. The total number of squares entered with all four paws was recorded, as was the total number of rears.
Home cage activity
Mice were housed individually overnight with free access to food and water. Activity was monitored using pressure sensors over a 15-hour period from 5:30 pm until 8:30 am using the Threshold activity monitoring system (Med Associates, Georgia, Vermont). The number of times a pressure threshold was crossed was used as an index of activity. Total number of threshold crossings was analysed.
Spontaneous alternation in a T-maze
Spontaneous alternation in a T-maze is typically viewed as a measure of spatial working memory. When given a choice between two goal arms, one of which has been explored before, wild-type mice will prefer to explore the novel arm. (Deacon and Rawlins, 2006). Rates of alternation in wild-type mice are typically 70-90% depending on strain. Spontaneous alternation in a T-maze is sensitive to hippocampal lesions, genetic ablation of glutamate receptor subunits (Deacon et al., 2002b; Bannerman et al., 2004; Sanderson et al., 2007), as well as dopamine agonists (Kokkinidis and Anisman, 1976a, 1976b; Kokkinidis, 1987). Spontaneous alternation in a T-maze was assessed as described previously (Deacon and Rawlins, 2006). Briefly, a mouse was placed in the start-arm of the T-maze and allowed to explore. Once the mouse entered a goal-arm a sliding door was closed preventing the mouse leaving the goal arm. The arm entered (left or right) was recorded and the mouse was allowed to explore that goal-arm for 25 seconds. The mouse was then returned to the start-arm, with all doors open again, and allowed to explore again. The goal-arm entered on the second run was recorded and the mouse was returned to its home cage. Each mouse was tested twice each day for a total of 20 trials. The percentage of trials on which the mouse entered a different goal arm on the second run (i.e. alternated) was calculated.
Fast-scan cyclic voltammetry
Mice were killed by an overdose of halothane by inhalation, decapitated, and their brains rapidly removed on to ice. Coronal striatal slices, 350 μm thick, were prepared from the brains of mice in ice-cold, HEPES-buffered physiological saline saturated with 95% O2/5% CO2 and maintained in a bicarbonate-buffered artificial cerebrospinal fluid (aCSF, containing 2.4 mM Ca2+) as described previously (Cragg, 2003; Rice and Cragg, 2004). Extracellular dopamine concentration ([DA]o) was monitored and quantified in dorsal striatum at 32 °C using fast-scan cyclic voltammetry (FCV) as described previously (Cragg, 2003) with 10 μm-diameter carbon-fiber microelectrodes (exposed tip length, ~ 100 μm; WPI, UK) and a Millar Voltammeter (PD Systems, UK). The applied voltage was a triangular waveform, with a voltage range of −0.7 V to 1.3 V to −0.7 V vs. Ag/AgCl at a scan rate of 800 V/s. The sampling frequency was 8 Hz. Data were acquired and analyzed using Strathclyde Whole Cell Program (University of Strathclyde, UK). Electrodes were positioned in striatal slices to a minimum depth of 100 μm. The substance monitored in every evoked release signal was identified as DA by comparison of the potentials for peak oxidation and reduction currents in the signal voltammogram with those of DA in calibration media (typically +500-600 and −200 mV vs. Ag/AgCl respectively). Current sampled at the oxidation peak potential was measured from the baseline of each voltammogram to provide accurate profiles of [DA]o versus time. This procedure minimizes inclusion of contributions from other electroactive and non-electroactive species to the DA oxidation current. Electrodes were calibrated in 2 μM DA in experimental media.
Electrical stimulation was applied using a surface, concentric bipolar Pt/Ir electrode (25 μm diameter, FHC, USA) as described previously (Rice and Cragg, 2004) at stimulation currents previously found to be peri-maximal for release by a single pulse in the mouse dorsal striatum (650 μA, 200 μsec pulse duration).Where stimulus protocols were repeated at a given recording site, a minimum of 3-minutes were allowed to ensure consistent release, as previously (Rice and Cragg, 2004)
DA release from synapses in the dorsal striatum typically exhibit pronounced depression following exocytosis (Abeliovich et al., 2000; Cragg, 2003). To investigate the recovery from such paired-pulse depression (PPD) we applied two stimulus pulses at fixed intervals of 1, 2, 3, 5, 7 or 15 seconds. Recovery from PPD is given by the ratio of peak [DA]o following the second stimulus to that following the first stimulus. Stimuli pairs consisted of either two single pulses, a single pulse followed by a burst (four pulses at 100 Hz), or a pair of two bursts.
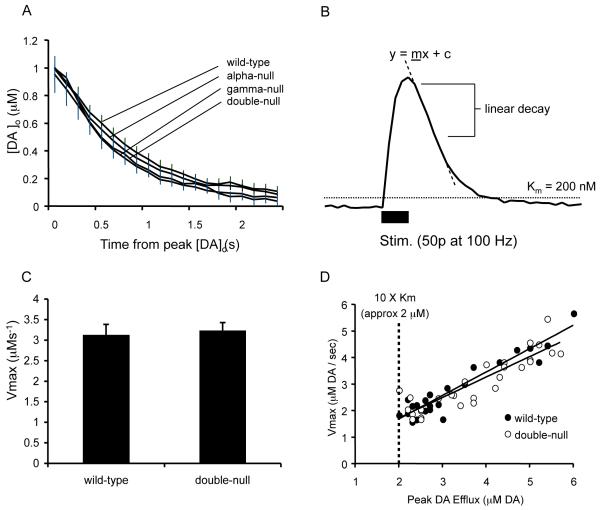
DA uptake was assessed using FCV data in two ways. Firstly, the overall decay phases of DA transients (evoked by 1 pulse) were compared between genotypes. DA uptake by the DAT is the main factor governing DA decay in these evoked transients (Giros et al., 1996). The rate of DA uptake by the DAT obeys Michaelis-Menten kinetics and therefore is proportional to Vmax and varies with [DA]o; by comparing the decay phase of DA transients matched for similar peak [DA]o, large differences in uptake rate due to differences in [DA]o are eliminated and prevailing differences in Vmax should be apparent (Cragg et al., 2000). DA transients were concentration-matched by sub-selecting a sample of two records each with a peak [DA]o of 1.0 ± 0.05 μM from each of four animals for each genotype (n = 8 for each genotype).
Secondly, experiments with FCV were also used to estimate Vmax of DA uptake as described previously (Cragg et al., 2000). By applying a high-intensity stimulation, [DA]o can become sufficiently high (>>Km of the DAT, Km ≈ 200 nM, Ross, 1991) that DATs become saturated i.e. the Michaelis-Menten clearance rate, V, for DA approaches a constant, Vmax (when uptake kinetics become zero order, independent of [DA]o and constant with time). In turn, at these high [DA]o (> 2 μM), the decay phase of extracellular DA transients becomes linear, with a slope that approaches Vmax. Other factors such as electrode response time may also contribute to this absolute decay rate, which is therefore only an approximation or a minimum estimate of Vmax. In the experiments conducted here, possible contributions to this clearance slope of variable electrode response times were standardized by using the same electrode in paired wild-type and null striata. High [DA]o were evoked by a train of 50 pulses at 100 Hz, in aCSF with elevated Ca2+ (4.8 mM) and containing 1 μM dihydro-beta-erythroidine (DHβE) to block nicotinic acetylcholine receptors in order to overcome the pronounced synaptic depression typically observed in the dorsal striatum (Cragg et al., 2002; Cragg, 2003; Rice and Cragg, 2004).
HPLC analysis of tissue DA content
Following FCV experiments, the caudate putamen (CPu) was dissected from two coronal slices per animal and stored at −20°C in 0.5 ml of 0.1 M HClO4. On the day of analysis, the samples were thawed and homogenized and centrifuged at 16,000 g for 15 min at 4°C. The supernatant was analysed for DA and 3,4-dihydroxyphenylacetic acid (DOPAC) using HPLC with electrochemical detection. Analytes were separated on a 100mm Microsorb C18 reverse-phase column and detected using a LC-4B electrochemical detector and a carbon working electrode held at + 0.7 V vs a Ag/AgCl reference electrode. The mobile phase consisted of 14.5% methanol, 0.1 M NaH2PO4, 0.8 mM EDTA, and 3.2 mM sodium octane sulfonate, pH 3.35, and flow rate was fixed at 1 ml/min. The size of the tissue sample was estimated by protein quantification using a BCA protein quantification kit and analyte measurements were normalised to protein measurements for each sample.
Statistics and data analysis
All data are expressed as mean ± SEM. Statistical analysis was performed using SPSS 13 (SPSS Inc., Chicago, Illinois) and GraphPad (GraphPad Software, San Diego, California).
Results
Mice lacking both alpha-synuclein and gamma-synuclein exhibit a hyperdopaminergic-like behavioural phenotype
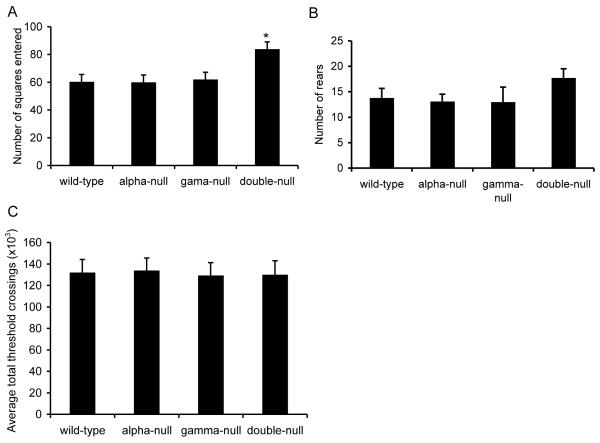
In order to assess the effect of deleting one or both of alpha-synuclein and gamma-synuclein, we performed a broad behavioural screen using mice deleted for alpha-synuclein (alpha-null), gamma-synuclein (gamma-null) or both alpha-synuclein and gamma-synuclein (double-null), as well as wild-type control mice. We assessed performance on the rotating rod, multiple static rods, activity in a non-anxiogenic open field, home cage activity, glucose consumption and performance on a T-maze test of spontaneous alternation. One-way analysis of variance (ANOVA) revealed a significant main effect of genotype on activity in the non-anxiogenic open field (Fig. 1A, wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15, F3,41, = 3.56, P = 0.022). Post-hoc comparison showed that double-null mice were significantly hyperactive compared to wild-type mice (Bonferroni post-tests, *P = 0.039). There were no statistically significant differences between any other lines (Bonferroni post-tests, P > 0.05 in all cases). Although it appeared that double-null mice reared more often in the open field (Fig. 1B) this result was not statistically significant (wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15, one-way ANOVA, F3,41 = 1.42 P = 0.251). Deletion of synuclein genes was found to have no effect on activity in the home cage (Fig. 1C, one-way ANOVA, wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15, F3,41 = 0.026, P = 0.994).
Fig. 1.
Activity of wild-type, alpha-null, gamma-null and double-null mice in the non-anxiogenic open field and home cage. (A) Double-null mice were significantly hyperactive in the non-anxiogenic open field compared to wild-type mice (one-way ANOVA, wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15 F3,41, = 3.56, P = 0.022; Bonferroni post-tests *P = 0.039 compared to wild-type). (B) There was no significant effect of genotype on the rate of rearing in the non-anxiogenic open field (one-way ANOVA, wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15 F3,41 = 1.42 P = 0.251). (C) There were no significant differences between any of the lines of mice in activity in the home-cage (one-way ANOVA, wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15 F3,41 = 0.026, P = 0.994).
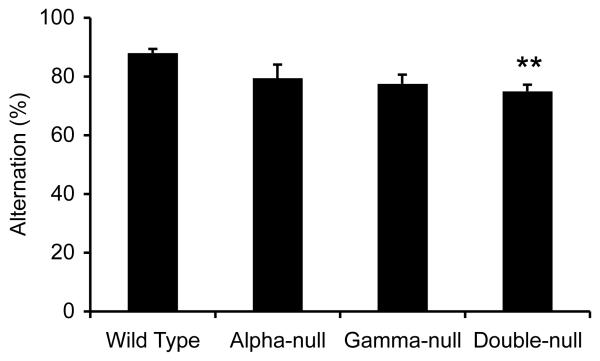
Double-null, but not alpha-null or gamma-null mice, performed significantly worse than wild-type mice on a test of spontaneous alternation (Fig. 2, one-way ANOVA, wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15 F3,41 = 5.716, P = 0.002, Bonferroni post-tests **P = 0.002). No statistically significant differences were found between any of the lines of mice on the rotating rod test, the multiple static rods test or glucose consumption (data not shown).
Fig. 2.
Performance of wild-type, alpha-null, gamma-null and double-null mice on spontaneous alternation in a T-maze. Double-null mice alternated at a significantly lower rate in a test of spontaneous alternation (one-way ANOVA, wild-type n = 10, alpha-null n = 10, gamma-null n = 10 and double-null n = 15, F3,41 = 5.716, P = 0.002, Bonferroni post-tests **P = 0.002 compared with wild-type).
Elevated levels of electrically evoked DA in the caudate putamen of mice lacking both alpha-synuclein and gamma-synuclein
It has been previously shown that hyperdopaminergic mice also exhibit hyperactivity in a novel environment but not in the home cage, and show a reduced preference for novelty in a spontaneous alternation paradigm (Zhuang et al., 2001). Furthermore, mice rendered hyperdopaminergic by treatment with amphetamine also exhibit impaired spontaneous alternation. (Kokkinidis and Anisman, 1976a, 1976b; Kokkinidis, 1987). In both cases, these mice have elevated levels of extracellular DA. Therefore an increase in DA release may underlie the behavioural phenotype that we have observed in mice lacking both alpha and gamma-synuclein.
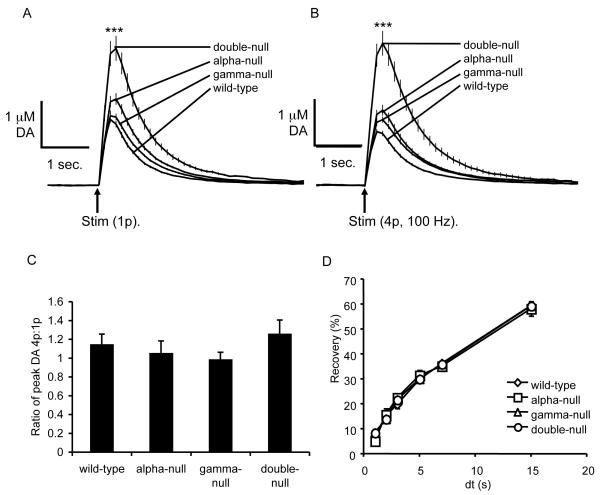
We therefore undertook experiments using fast-cyclic voltammetry (FCV) at a carbon fibre microelectrode to examine whether dopaminergic neurotransmission is affected in the caudate putamen in wild-type and knockout mice. We firstly explored fundamental characteristics of DA release in striatum, in particular, DA transients released by single pulses (200 μs) as well as by burst stimuli (4 pulses at 100 Hz). Typically, dorsal striatal DA synapses in vitro readily show short-term synaptic depression after prior release by a single pulse that limits re-release during a burst (Cragg, 2003; Rice and Cragg, 2004). Notably, peak [DA]o evoked by a single pulse were approximately two-fold greater in double-null mice than wild-type control mice (Fig. 3A, wild-type n = 16, alpha-null n = 22, gamma-null = 37, double-null n = 24 (from four animals in each genotype), one-way ANOVA, F3,95, = 21.19, P < 0.001, Bonferroni post-tests ***P < 0.001) whereas [DA]o evoked in alpha-null and gamma-null mice were not different from wild-type mice (Bonferroni post-tests P > 0.05 in all cases). Peak [DA]o evoked by burst stimuli were similarly modified with genotype (Fig. 3B, one-way ANOVA, wild-type n = 21, alpha-null n = 24, gamma-null = 28, double-null n = 23 (representing four animals in each genotype), F3,92 = 18.73, P < 0.001, Bonferroni post-tests ***P<0.001) and therefore the relative release probability of DA by bursts versus non-burst stimuli was not significantly different between genotypes (Fig. 3C, one-way ANOVA, n values as for 3B and C, F3,95 = 1.6, P = 0.194).
Fig. 3.
Electrically evoked DA transients in wild-type, alpha-null, gamma-null and double-null mice measured by fast-scan cyclic voltammetry. (A) Mean profiles of [DA]o versus time (mean ± s.e.m.) after a single stimulus pulse (0.2 μs, arrow). Mice lacking both alpha-synuclein and gamma-synuclein, but not mice lacking only one of the synucleins, show increased levels of electrically evoked DA transients at synapses in the CPu relative to wild-type mice (one-way ANOVA, wild-type n = 16, alpha-null n = 22, gamma-null = 37, double-null n = 24 (from four animals in each genotype), F3,95, = 21.19, P < 0.001, Bonferroni post-tests ***P < 0.001). (B) DA transients evoked by 4 pulses at 100 Hz are similarly modified by genotype (one-way ANOVA, wild-type n = 21, alpha-null n = 24, gamma-null = 28, double-null n = 23 (from four animals in each genotype), F3,92 = 18.73, P < 0.001, Bonferroni post-tests ***P < 0.001). The low ratios of DA evoked by a burst stimuli (4 pulses, 100 Hz) compared to a single pulse (4p:1p ratio) show that the limited release by a burst after prior release by a single pulse that is typical of dorsal striatal DA synapses (Cragg, 2003) occurs in all genotypes in a manner that did not differ between genotypes (C, one-way ANOVA, n values as for 3A and B, F3,95 = 1.6, P = 0.194). (D) Recovery from paired-pulse depression in the striatum was not significantly different between strains (two-way ANOVA, n = 6-9 for each time point, representing 4 animals from each genotype. main effect of genotype F3,155 = 0.068, P = 0.977, genotype × time interaction F15,155 = 0.48, P = 0.947).
Previous studies have reported an increase in recovery from short-term paired pulse depression (PPD) at DA synapses in the caudate putamen in mice lacking alpha-synuclein (Abeliovich et al., 2000). We did not resolve an increase in recovery from PPD in any of the three knockout mouse lines compared with wild-type mice (Fig. 3D, two-way ANOVA, n = 6-9 for each time point, representing 4 animals from each genotype. Main effect of genotype F3,155 = 0.068, P = 0.977, genotype × time interaction F15,155 = 0.48, P = 0.947).
Loss of alpha-synuclein or gamma-synuclein or both alpha-synuclein and gamma-synuclein does not affect dopamine content in the striatum
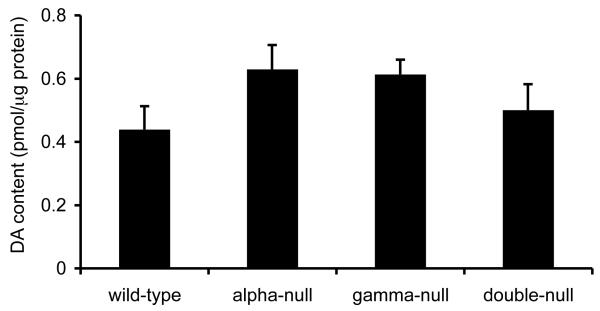
We next investigated whether the observed increase in electrically evoked [DA]o in double-null mice was due to an increase in DA content of the striatum of double-null mice by measuring DA content of the caudate putamen using HPLC. We found no significant difference in DA content between genotypes (Fig. 4, one-way ANOVA each group n = 16 (4 samples from 4 animals), F3,62 = 1.89, P = 0.141), which suggests that the observed increase in size of the evoked DA transients can not be attributed to an increase in tissue DA levels. These data do not exclude the possibility that the density of DA release sites or axons has increased with concomitant proportional decrease in DA content per release site.
Fig. 4.
Striatal DA content in wild-type and double-null mice measured by HPLC. No statistically significant differences in striatal DA content were observed between wild-type and double-null mice (one-way ANOVA each group n = 16 (4 samples from 4 animals), F3,62 = 1.89, P = 0.141).
Uptake of DA in synuclein knockout mice is not significantly different from control mice
Several groups have reported functional interactions between alpha-synuclein and the DAT (Lee et al., 2001; Wersinger et al., 2003; Wersinger and Sidhu, 2003; Fountaine and Wade-Martins, 2007). We investigated whether the increase in evoked [DA]o detected in the double-null mice was caused by a reduction in DA uptake using two approaches. Comparison of transients from each of the four strains of mice matched for peak [DA]o (1.0 ± 0.05 μM) allowed ready comparison of the decay phase of the extracellular DA transient which is primarily governed by uptake with this method (Giros et al., 1996). However, the overall rate of decay was similar across genotypes (Fig. 5A, two-way ANOVA, n = 8 for each genotype, F117,1120 = 0.76, P = 0.9706) suggesting no difference between any of the mouse lines in the rate of reuptake of DA. As a second method of assessing uptake of DA, we used a stimulus train of 50 pulses at 100 Hz to force release of DA to peak [DA]o that were at least 10-fold the Km of the DAT (Km of the DAT ≈ 200 nM, (Ross, 1991)). The subsequent decay of [DA]o includes an initial decay phase that is linear (Fig. 5B) with a gradient proportional to Vmax (gradient equals a minimum estimate for Vmax, see Methods). Using this approach, we were able to estimate Vmax for DA uptake in wild-type and double-null mice. Average Vmax values were 3.13 μMsec−1 ± 0.25 in wild-types and 3.23 μMsec−1 ± 0.19 in double-null mice which were not significantly different (Fig. 5C, two-sample t-test, wild-type n = 18, double-null n = 25, P = 0.49), nor was the relationship between Vmax and the peak [DA]o released at contributing recording sites different between genotypes (Fig. 5D, type II linear regression, F1,39 = 1.164, P < 0.0001). This suggests that the relationship between the relative densities of DA-releasing fibres and corresponding DATs is similar in wild-type and synuclein-knockout striatum.
Fig. 5.
Comparison between genotypes of rates of uptake of evoked DA transients as measured by FCV. (A) Mean profiles of [DA]o versus time (mean ± s.e.m.) after a single stimulus pulse (0.2 μs, arrow). Comparison of the rates of decay of concentration-matched DA transients suggest that DA uptake rates are not different between the four genotypes (two-way ANOVA, n = 8 for each genotype, F117,1120 = 0.76, P = 0.9706). (B) A sample profile of [DA]o versus time after a 50 pulse, 100 Hz stimulus (solid bar). This stimulus permits synapses to release [DA]o reaching concentrations reaching at least 10-fold the Km of the DAT, when DA uptake rate becomes independent of [DA]o i.e. approaches Vmax. This produces an initial period of linear decay (dashed line), the gradient of which is a minimum estimate of Vmax at that site. (C) Mean Vmax estimates were not significantly different between genotypes. (two-sample t-test, wild-type n = 18, double-null n = 25, P = 0.489). (D) Observed Vmax calculations co-varied with peak [DA]o. (type II linear regression, wild-type n = 18, F1,16 = 178.4, P < 0.0001; double-null n = 25, F 1,23 = 68.76, P < 0.0001) in keeping with locally variable packing density of dopaminergic terminals. To control for possible differences in packing density at subpopulation of recording sites sampled and corresponding co-variation in Vmax, we used the gradient of the regression line of Vmax against peak [DA]o in panel (D) as a modified measure of Vmax. There is no difference between this modified Vmax constant in wild-type and double-null mice (F1,39 = 1.164, P = 0.287).
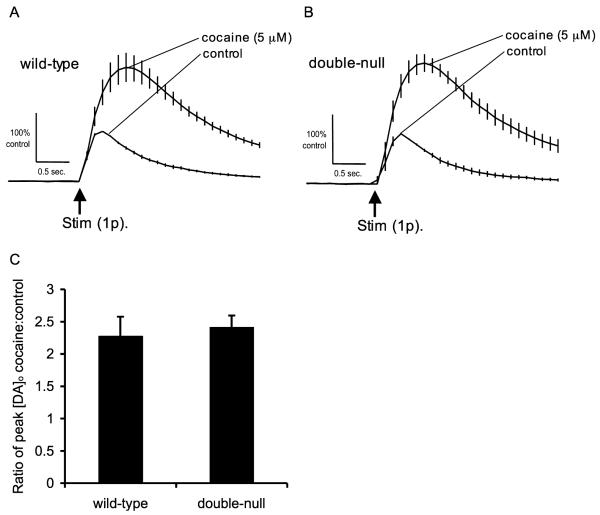
We also assessed pharmacologically whether the different apparent properties of DA release seen in wild-type and double-null mice (Fig. 3) could nonetheless be due to differences in DA uptake that could be reversed by blocking DA uptake with cocaine (5 μM). Addition of cocaine increased peak [DA]o evoked by a single pulse (Fig. 6A, B, [DA]o values are normalized to peak [DA]o in the absence of cocaine) and extended consequent extracellular lifetimes of DA in both genotypes consistent with uptake inhibition (wild-type, Fig. 6A, one sample t-test, t = 4.393 df = 2, P = 0.0481; double-null, Fig. 6B, one sample t-test, t = 8.015 df = 2, P = 0.0041) but did not eliminate the difference in evoked DA release (mean [DA]o in the presence of 5 μM cocaine = 1.55 μM +/− 0.08 for wild-type mice and 2.26 μM +/− 0.15 for double-null mice) or significantly affect the difference in evoked DA transients between wild-type and double-null mice (Fig. 6C, two-sample t-test, t = 0.4215 df = 5, P = 0.6909).
Fig. 6.
Effect of DA uptake inhibition by cocaine on extracellular DA transients in wild-type and double-null mice. Mean profiles of [DA]o versus time after a single stimulus pulse before and after addition of cocaine (5 μM) in (A) wild type and (B) double-null mice (normalised to peak [DA]o in the absence of cocaine for each genotype). Cocaine, by inhibiting DA uptake, increased peak [DA]o and extended extracellular lifetimes of DA in both wild-type and double-null mice. (C) Evoked [DA]o transients were enhanced significantly by cocaine in both wild-type (one sample t-test, t = 4.393 df = 2, P = 0.0481) and double-null mice (one sample t-test, t = 8.015 df = 2, P = 0.0041), however the relative increase in evoked [DA]o did not differ between genotypes.
Discussion
We have described the results of a broad behavioural screen using mice lacking alpha-synuclein, gamma-synuclein or both alpha and gamma-synuclein. The mice lacking both alpha and gamma-synuclein (double-null) were found to be significantly hyperactive in a novel environment, but not in the home cage, and to alternate at a lower rate in a spontaneous alternation paradigm. Most significantly, double-null mice were shown to have a two-fold increase in electrically evoked DA release in the striatum. Consistent with previous reports showing that targeted deletion of alpha-synuclein does not cause deficits in motor performance (Abeliovich et al. 2000), no differences between any of the genotypes were observed in tests of motor performance. We also included a test of glucose-induced polydipsia, as sugar-induced polydipsia correlates with alcohol preference in both humans and mice (Overstreet et al., 1993; Kampov-Polevoy et al., 1995; Kampov-Polevoy et al., 1999), and alpha-synuclein has been linked to craving in alcoholic patients (Bonsch et al., 2004; Bonsch et al., 2005b; Bonsch et al., 2005a). However, there was no significant difference between any of the genotypes in the quantity of glucose consumed.
The lack of either a behavioural phenotype or an increase in evoked DA transients in mice lacking either alpha-synuclein (alpha-null) or gamma-synuclein (gamma-null) alone suggests that there is considerable functional redundancy in the synuclein gene family, and that functional complementation may occur between members of the synuclein family in the nigrostriatal DA system. This is consistent with reports that when one or more synucleins are deleted, the remaining members of the family are up-regulated in some brain regions (Chandra et al., 2004; Robertson et al., 2004), and that there is considerable overlap in the pools of genes whose expression is changed in the absence of alpha-synuclein or gamma-synuclein (Kuhn et al., 2007).
The behavioural phenotype observed in the double-null mice is broadly similar to that observed in hyperdopaminergic mice expressing reduced levels of the DAT (Zhuang et al., 2001) suggesting changes in the DA system may also have occurred in mice lacking both alpha and gamma-synuclein. The hypothesis that mice lacking synucleins may have functional dysregulation of the DA system is corroborated by previous studies which have shown alpha-synuclein knockout mice to exhibit a decrease in locomotor response to amphetamine (Abeliovich et al., 2000) and an increase in acquisition of an intracranial self-stimulation reward (Oksman et al., 2006). Furthermore, there is a growing literature suggesting that alpha-synuclein is involved in reward and addiction (Li et al., 2002; Brenz Verca et al., 2003; Mash et al., 2003; Bonsch et al., 2004; Bonsch et al., 2005b; Bonsch et al., 2005a; Qin et al., 2005), which implies a role for alpha-synuclein in DA regulation.
A previous report showed that mice lacking alpha-synuclein display an increased rate of recovery from paired-pulse depression (PPD) in the striatum (Abeliovich et al., 2000). We measured recovery from PPD in the dorsal striatum, but were unable to detect an effect of the deletion of alpha-synuclein, gamma-synuclein, or both alpha and gamma-synuclein on the rate of recovery from PPD at dopaminergic synapses in the dorsal striatum. This apparent discrepancy may be attributed to several factors. First, the genetic background of the mice is different in our experiments (pure C57BL6) than in the Abeliovich et al study (F1 C57BL6 × 129SV/j hybrid), and it has been shown that the effect of targeted deletion of genes involved in regulation of DA varies depending on strain background (Morice et al., 2004). Second, the mice used in our study were older (approximately 6 months old) than those used in the Abeliovich et al study (6-8 weeks). It is possible that over time, the nigrostriatal DA system is able to compensate for the loss of alpha-synuclein on recovery from PPD. Third, our experiments were carried out at 32°C, while those of Abeliovich et al were carried out at 36°C. While subphysiological temperatures are commonly used in electrophysiological experiments to limit ex vivo decline in slice viability, temperature differences may expose different limiting factors in release probability.
Importantly, we have shown that DA release evoked by single action potentials as well as short burst stimuli in the striatum is significantly greater in double-null mice than in wild-type controls or in alpha-null or gamma-null mice. This supports the hypothesis that double-null mice, but not alpha-null or gamma-null mice, are hyperdopaminergic. Three separate approaches used here to explore the regulation of extracellular DA transients by DA uptake suggest that the increase size in evoked DA transients is not readily attributable to a decrease in the rate of reuptake of DA via the DAT: Firstly, concentration-matched DA transients had identical decay phases. Secondly, estimates of Vmax were similar in wild-types and double-null mice; and thirdly, inhibition of DA uptake did not eliminate differences in extracellular DA behaviour between wild-types and double-null mice. Thus we were not able to detect an effect of inactivation of both alpha and gamma-synuclein on reuptake of DA. This result is consistent with previous measurements of DA uptake in synuclein-null mice (Robertson et al., 2004; Yavich et al., 2004), but inconsistent with data obtained in tissue culture systems suggesting that alpha-synuclein acts to facilitate or inhibit movement of DAT to the cell surface (Lee et al., 2001; Wersinger et al., 2003; Fountaine and Wade-Martins, 2007). While Mash et al (2003) and Qin et al (2005) showed that alpha-synuclein and DAT are upregulated in response to chronic cocaine use, it is not clear from these studies whether the increase in alpha-synuclein is tightly linked to an increase in DAT at the cell surface, or whether the change in alpha-synuclein levels is part of a wider set of synaptic alterations. We can only speculate that there may be differences in the way that DAT is handled in mice in vivo compared to in a cell culture system that make DAT trafficking less sensitive to the loss of synucleins.
Based on our observations of increased DA release with no change in DAT activity and no change in tissue dopamine content, we propose that mice lacking both alpha and gamma-synuclein, but not mice lacking only alpha or gamma-synuclein, have an increase in the probability of release of DA from synapses in response to a given stimulation. By measuring DA release and content in the same slices we have been able to show that the increase in evoked DA release is not caused by any simple increase in the average density of dopaminergic synapses in the striatum, or in the DA content of each synapse. However, we cannot rule out an increase in terminal density coupled to a corresponding decrease in synaptic DA content, or vice versa. That being said, the changes in terminal density that would be required for these effects are not supported by the histological or ultrastructural studies to date of axon terminal density or vesicular pools which do not report detectable changes after alpha-synuclein single and double (alpha/beta or alpha/gamma) deletion (Abeliovich et al., 2000; Chandra et al., 2004; Robertson et al., 2004).
Previous studies have examined the effect of deleting two members of the synuclein family on behaviour and/or DA release in mice (Chandra et al. 2004; Robertson et al. 2004). Our results are broadly consistent with these reports, in that mice lacking two members of the synuclein family do not display severe behavioural phenotypic changes, and that mice lacking both alpha and gamma-synuclein showed no change in striatal tissue DA levels (Robertson et al., 2004). While Chandra et al. observed some decrease in striatal DA content in mice lacking both alpha and beta synuclein, neither we, nor Robertson et al. (2004) have observed this in mice lacking both alpha and gamma-synuclein. This discrepancy may be attributable to the different roles of beta and gamma-synuclein, and their relative functional homology to alpha-synuclein. In mice lacking both alpha and beta-synuclein, Chandra et al. did not find any change in the fraction of DA content that can be released but did not, however, report absolute DA release. In that study, the release of radio-labelled DA was recorded from synaptosomes after a 30-s pulse of high K+ (25 mM) to directly open voltage-gated Ca2+-channels and subsequent hypertonic sucrose (30-s pulse, 0.5 M), that reportedly induces Ca2+-independent exocytosis of docked vesicles from the readily releasable pool (Rosenmund and Stevens, 1996). DA release evoked by these approaches is strongly influenced by the size of the total releasable pool of dopamine and may be insensitive to many discrete factors that could regulate neurotransmitter release probability during physiological action potentials. Such previous studies of dopamine release used methodologies lacking the temporal and spatial resolution of the electrochemical techniques used here. The approach we employ in the current study to detect release of endogenous DA using FCV after discrete sub-millisecond electrical stimuli is highly sensitive to factors that control release probability rather than just DA content (Cragg 2003). Furthermore, Chandra et al. detect release over 1-minute sample windows, whereas we report measurements of [DA]o sampled at 8 Hz. Overall, it is unlikely that the stimulation protocol used by Chandra et al. is able to model release of DA in response to discrete, physiological action potentials within the setting of the intact striatum.
Our results form part of an emerging body of evidence which suggests the synucleins play a role in synaptic vesicle regulation at dopaminergic synapses. Previous studies in mice lacking alpha-synuclein have reported alterations in the regulation of synaptic vesicle pools in hippocampal slices (Cabin et al., 2002; Yavich et al., 2004). Cabin et al (2002) demonstrated a marked reduction in the number of vesicles in the resting pool, but not the readily releasable pool. Yavich et al (2004) showed that mice lacking alpha-synuclein have an increased rate of refilling of the readily releasable pool, and this observation is consistent with the increase in recovery from PPD in alpha-synuclein-null mice observed by Abeliovich et al (2000). Taken together, these data suggest that the absence of alpha-synuclein promotes the transport of vesicles from the reserve storage pool to the readily releasable pools within the synapse. It may be that the increase in electrically evoked DA release in mice lacking both alpha and gamma-synuclein that we report here is attributable to an increase in the size of the readily releasable pool. The data of Cabin et al (2002) suggests that this increase is likely to be matched by a corresponding decrease in the size of the reserve pool, and hence the synaptic content is unchanged.
More recently, Yavich and colleagues (Yavich et al., 2005) have demonstrated that over-expression of human A30P mutant alpha-synuclein in mice results in a decrease in DA release in response to medial forebrain stimulation. This decrease was not caused by a change in the uptake of DA via the DAT or a reduction in the level of DA or its metabolites. Furthermore, it has been shown that overexpression of alpha-synuclein causes a decrease in the rate of release of DA from chromaffin cells, possibly by interfering with a late step in exocytosis (Larsen et al., 2006). Thus it appears that alpha-synuclein may be a negative regulator of neurotransmitter release, by regulating both the rate of transfer of vesicles to the readily releasable pool, and the probability of vesicle fusion at a given synaptic stimulation. Our data are consistent with these results, and provide indirect support for the hypothesis that the synucleins are involved in synaptic vesicle fusion. We suggest that deletion of alpha and gamma-synuclein results in an increase in the probability of DA release, possibly by altering the ability of synaptic vesicles to fuse with the cell membrane in response to a stimulus, but deletion of alpha or gamma-synuclein alone can be compensated for by the remaining synucleins.
Acknowledgements
The authors would like to thank Dr T. Sharp and Dr K. Jennings for their kind assistance with HPLC measurements of DA content in striatal tissue samples. This work was supported by a Wellcome Trust 4-Year D.Phil. Studentship in Neurosciences (SLS), a Wellcome Trust Research Career Development Fellowship (RW-M) and a Wellcome Trust Programme Grant (VLB).
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Brady S, Bruce A, Sprengel R, Seeburg PH, Rawlins JN. A comparison of GluR-A-deficient and wild-type mice on a test battery assessing sensorimotor, affective, and cognitive behaviors. Behav Neurosci. 2004;118:643–647. doi: 10.1037/0735-7044.118.3.643. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Reulbach U, Bayerlein K, Hillemacher T, Kornhuber J, Bleich S. Elevated alpha synuclein mRNA levels are associated with craving in patients with alcoholism. Biol Psychiatry. 2004;56:984–986. doi: 10.1016/j.biopsych.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Lederer T, Reulbach U, Hothorn T, Kornhuber J, Bleich S. Joint analysis of the NACP-REP1 marker within the alpha synuclein gene concludes association with alcohol dependence. Hum Mol Genet. 2005a;14:967–971. doi: 10.1093/hmg/ddi090. [DOI] [PubMed] [Google Scholar]
- Bonsch D, Greifenberg V, Bayerlein K, Biermann T, Reulbach U, Hillemacher T, Kornhuber J, Bleich S. Alpha-synuclein protein levels are increased in alcoholic patients and are linked to craving. Alcohol Clin Exp Res. 2005b;29:763–765. doi: 10.1097/01.alc.0000164360.43907.24. [DOI] [PubMed] [Google Scholar]
- Brenz Verca MS, Bahi A, Boyer F, Wagner GC, Dreyer JL. Distribution of alpha- and gamma-synucleins in the adult rat brain and their modification by high-dose cocaine treatment. Eur J Neurosci. 2003;18:1923–1938. doi: 10.1046/j.1460-9568.2003.02913.x. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, Sudhof TC. Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc Natl Acad Sci U S A. 2004;101:14966–14971. doi: 10.1073/pnas.0406283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J Neurosci. 2003;23:4378–4385. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Hille CJ, Greenfield SA. Dopamine release and uptake dynamics within nonhuman primate striatum in vitro. J Neurosci. 2000;20:8209–8217. doi: 10.1523/JNEUROSCI.20-21-08209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Hille CJ, Greenfield SA. Functional domains in dorsal striatum of the nonhuman primate are defined by the dynamic behavior of dopamine. J Neurosci. 2002;22:5705–5712. doi: 10.1523/JNEUROSCI.22-13-05705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Croucher A, Rawlins JN. Hippocampal cytotoxic lesion effects on species-typical behaviours in mice. Behav Brain Res. 2002a;132:203–213. doi: 10.1016/s0166-4328(01)00401-6. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002b;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH. Neural activity controls the synaptic accumulation of alpha-synuclein. J Neurosci. 2005;25:10913–10921. doi: 10.1523/JNEUROSCI.2922-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountaine TM, Wade-Martins R. RNA interference-mediated knockdown of alpha-synuclein protects human dopaminergic neuroblastoma cells from MPP(+) toxicity and reduces dopamine transport. J Neurosci Res. 2007;85:351–363. doi: 10.1002/jnr.21125. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Guenther K, Deacon RM, Perry VH, Rawlins JN. Early behavioural changes in scrapie-affected mice and the influence of dapsone. Eur J Neurosci. 2001;14:401–409. doi: 10.1046/j.0953-816x.2001.01645.x. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Overstreet DH, Rezvani AH, Janowsky DS. Saccharin-induced increase in daily fluid intake as a predictor of voluntary alcohol intake in alcohol-preferring rats. Physiol Behav. 1995;57:791–795. doi: 10.1016/0031-9384(94)00389-0. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L. Amphetamine-elicited perseverative and rotational behavior: evaluation of directional preference. Pharmacol Biochem Behav. 1987;26:527–532. doi: 10.1016/0091-3057(87)90160-2. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L, Anisman H. Dissociation of the effects of scopolamine and d-amphetamine on a spontaneous alternation task. Pharmacol Biochem Behav. 1976a;5:293–297. doi: 10.1016/0091-3057(76)90081-2. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L, Anisman H. Interaction between cholinergic and catecholaminergic agents in a spontaneous alternation task. Psychopharmacology (Berl) 1976b;48:261–270. doi: 10.1007/BF00496859. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Leenders KL, Sprengelmeyer R, Muller T, Woitalla D, Portman AT, Maguire RP, Veenma L, Schroder U, Schols L, Epplen JT, Riess O, Przuntek H. Familial parkinsonism with synuclein pathology: clinical and PET studies of A30P mutation carriers. Neurology. 2001;56:1355–1362. doi: 10.1212/wnl.56.10.1355. [DOI] [PubMed] [Google Scholar]
- Kubo S, Nemani VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, Edwards RH, Fortin DL. A combinatorial code for the interaction of alpha-synuclein with membranes. J Biol Chem. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- Kuhn M, Haebig K, Bonin M, Ninkina N, Buchman VL, Poths S, Riess O. Whole genome expression analyses of single- and double-knock-out mice implicate partially overlapping functions of alpha- and gamma-synuclein. Neurogenetics. 2007;8:71–81. doi: 10.1007/s10048-007-0079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJ, Liu F, Pristupa ZB, Niznik HB. Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. Faseb J. 2001;15:916–926. doi: 10.1096/fj.00-0334com. [DOI] [PubMed] [Google Scholar]
- Li JY, Jensen P Henning, Dahlstrom A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience. 2002;113:463–478. doi: 10.1016/s0306-4522(02)00143-4. [DOI] [PubMed] [Google Scholar]
- Mash DC, Ouyang Q, Pablo J, Basile M, Izenwasser S, Lieberman A, Perrin RJ. Cocaine abusers have an overexpression of alpha-synuclein in dopamine neurons. J Neurosci. 2003;23:2564–2571. doi: 10.1523/JNEUROSCI.23-07-02564.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice E, Denis C, Giros B, Nosten-Bertrand M. Phenotypic expression of the targeted null-mutation in the dopamine transporter gene varies as a function of the genetic background. Eur J Neurosci. 2004;20:120–126. doi: 10.1111/j.1460-9568.2004.03465.x. [DOI] [PubMed] [Google Scholar]
- Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. A new brain-specific 14-kDa protein is a phosphoprotein. Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993;217:1057–1063. doi: 10.1111/j.1432-1033.1993.tb18337.x. [DOI] [PubMed] [Google Scholar]
- Ninkina N, Papachroni K, Robertson DC, Schmidt O, Delaney L, O’Neill F, Court F, Rosenthal A, Fleetwood-Walker SM, Davies AM, Buchman VL. Neurons expressing the highest levels of gamma-synuclein are unaffected by targeted inactivation of the gene. Mol Cell Biol. 2003;23:8233–8245. doi: 10.1128/MCB.23.22.8233-8245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksman M, Tanila H, Yavich L. Brain reward in the absence of alpha-synuclein. Neuroreport. 2006;17:1191–1194. doi: 10.1097/01.wnr.0000230507.70843.51. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murrelle L, Halikas JA, Janowsky DS. Saccharin intake predicts ethanol intake in genetically heterogeneous rats as well as different rat strains. Alcohol Clin Exp Res. 1993;17:366–369. doi: 10.1111/j.1530-0277.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Peng X, Tehranian R, Dietrich P, Stefanis L, Perez RG. Alpha-synuclein activation of protein phosphatase 2A reduces tyrosine hydroxylase phosphorylation in dopaminergic cells. J Cell Sci. 2005;118:3523–3530. doi: 10.1242/jcs.02481. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Qin Y, Ouyang Q, Pablo J, Mash DC. Cocaine abuse elevates alpha-synuclein and dopamine transporter levels in the human striatum. Neuroreport. 2005;16:1489–1493. doi: 10.1097/01.wnr.0000175617.39054.ba. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robertson DC, Schmidt O, Ninkina N, Jones PA, Sharkey J, Buchman VL. Developmental loss and resistance to MPTP toxicity of dopaminergic neurones in substantia nigra pars compacta of gamma-synuclein, alpha-synuclein and double alpha/gamma-synuclein null mutant mice. J Neurochem. 2004;89:1126–1136. doi: 10.1111/j.1471-4159.2004.02378.x. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Ross SB. Synaptic concentration of dopamine in the mouse striatum in relationship to the kinetic properties of the dopamine receptors and uptake mechanism. J Neurochem. 1991;56:22–29. doi: 10.1111/j.1471-4159.1991.tb02557.x. [DOI] [PubMed] [Google Scholar]
- Sanderson DJ, Gray A, Simon A, Taylor AM, Deacon RM, Seeburg PH, Sprengel R, Good MA, Rawlins JN, Bannerman DM. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav Neurosci. 2007;121:559–569. doi: 10.1037/0735-7044.121.3.559. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehranian R, Montoya SE, Van Laar AD, Hastings TG, Perez RG. Alpha-synuclein inhibits aromatic amino acid decarboxylase activity in dopaminergic cells. J Neurochem. 2006;99:1188–1196. doi: 10.1111/j.1471-4159.2006.04146.x. [DOI] [PubMed] [Google Scholar]
- Totterdell S, Meredith GE. Localization of alpha-synuclein to identified fibers and synapses in the normal mouse brain. Neuroscience. 2005;135:907–913. doi: 10.1016/j.neuroscience.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Totterdell S, Hanger D, Meredith GE. The ultrastructural distribution of alpha-synuclein-like protein in normal mouse brain. Brain Res. 2004;1004:61–72. doi: 10.1016/j.brainres.2003.10.072. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci Lett. 2003;340:189–192. doi: 10.1016/s0304-3940(03)00097-1. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. Faseb J. 2003;17:2151–2153. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Oksman M, Tanila H, Kerokoski P, Hiltunen M, van Groen T, Puolivali J, Mannisto PT, Garcia-Horsman A, MacDonald E, Beyreuther K, Hartmann T, Jakala P. Locomotor activity and evoked dopamine release are reduced in mice overexpressing A30P-mutated human alpha-synuclein. Neurobiol Dis. 2005;20:303–313. doi: 10.1016/j.nbd.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Tortosa E Gomez, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]