Abstract
A mechanistic understanding of the relationship between the chemistry of drug antigen formation and immune function is lacking. Thus, mass spectrometric methods were employed to detect and fully characterize circulating antigens derived from piperacillin in patients undergoing therapy and the nature of the drug derived-epitopes on protein which can function as an antigen to stimulate T-cells. Albumin modification with piperacillin in vitro resulted in the formation of two distinct haptens, one formed directly from piperacillin and a second in which the dioxopiperazine ring had undergone hydrolysis. Modification was time- and concentration-dependent, with selective modification of Lys541 observed at low concentrations, whereas at higher concentrations up to 13/59 lysine residues were modified, four of which (Lys190, 195, 432 and 541) were detected in patients’ plasma. Piperacillin-specific T-lymphocyte responses (proliferation, cytokines and granzyme-B release) were detected ex vivo with cells from hypersensitive patients, and analysis of incubation medium showed that modification of the same lysine residues in albumin occurred in situ. The antigenicity of piperacillin-modified albumin was confirmed by stimulation of T-cells with characterized synthetic conjugates. Analysis of minimally-modified T-cell stimulatory albumin conjugates revealed peptide sequences incorporating Lys190, 432 and 541 as principal functional epitopes for T-cells. This study has characterized the multiple haptenic structures on albumin in patients, and showed that they constitute functional antigenic determinants for T-cells.
INTRODUCTION
The presence of antigen-specific T-cells in blood and target organs of drug hypersensitive patients provides a robust case for their involvement in the pathogenesis of a reaction (1-6). It is thought that drugs activate T-cells by covalent modification of protein generating novel antigenic determinants (2,3,7-9). However, the paucity of studies that define the chemistry of drug-protein binding in patients has severely restricted mechanistic studies that relate the chemistry of antigen formation to immune function. Indeed, the simple concept of the hapten hypothesis of drug hypersensitivity has been brought into question by studies which have demonstrated that drugs may activate T cells through non-covalent interactions (4,5,10-16).
Hypersensitivity reactions to β-lactam antibiotics remain an important clinical problem. For antigen formation, the β-lactam ring is targeted by nucleophilic lysine residues, leading to ring opening and binding of the penicilloyl group (17-19). We have developed novel mass spectrometric techniques to define unequivocally the chemistry of drug–protein conjugation in patients under physiological conditions (20-23). In this manuscript we report on the methods we have developed to detect and fully characterize circulating antigens derived from piperacillin and its metabolite in patients undergoing therapy. Using the same mass spectrometry methods, it was possible to characterize the nature of the drug derived-epitopes on a protein which can function as an antigen and as a potential immunogen to stimulate T-cells from patients with clinically characterized drug hypersensitivity. For this purpose, we have studied piperacillin hypersensitivity reactions in patients with cystic fibrosis. In these patients, intravenous antibiotics provide the cornerstone of treatment for recurrent respiratory infections and help reduce the rate of decline in lung function and overall health. The overall prevalence of clinically relevant β-lactam reactions in patients with cystic fibrosis is 26 – 50 % (24-26). We found that the frequency of drug-specific T-cells in such patients was greater than 75 %. It was therefore possible to investigate the chemistry of functional antigens formed from piperacillin and albumin not only in patients’ blood, but also in ex vivo incubations with patients T-cells in order to relate the chemistry of protein modification to drug antigenicity and immunogenicity.
MATERIALS AND METHODS
Reagents
A sterile intravenous preparation of Tazocin (Wyeth Pharmaceuticals) was purchased for skin testing. Histamine and saline controls, together with lancets for skin prick testing, were purchased from ALK Abello (Hørsholm, Denmark). The following products were purchased from Sigma-Aldrich (Gillingham, UK): Hanks balanced salt solution; penicillin-streptomycin; L-glutamine; HEPES; RPMI 1640; human AB serum; and piperacillin. Invitrogen (Paisley, UK) provided fetal bovine serum (FBS). Radiolabeled thymidine was obtained from Moravek International Limited (CA, USA).
Preparation/isolation of modified human serum albumin
The time and concentration dependent modification of human serum albumin was investigated in vitro. Human serum albumin (66 mg/mL, 1 mM) in phosphate buffer (KH2PO4, 13.08 mM; K2HPO4, 62.27 mM, pH 7.4) was incubated at 37°C piperacillin at molar ratios of piperacillin to human serum albumin of 0.01:1, 0.1:1, 1:1, 10:1 and 50:1 for 24 h, and at 50:1 for 1 h, 24 h, 48 h, 72 h and 96 h. The protein was precipitated by the addition of nine volumes of ice-cold methanol followed by centrifugation at 14,000 g and 4°C for 15 min. The precipitation was repeated in order to ensure the removal of non-covalently bound drug. The concentration of human serum albumin was determined by Bradford assay (27) and aliquots were prepared in serum-free RPMI for application in T-cell assays, in 50 mM ammonium bicarbonate for mass spectrometric analysis and in Laemmli sample buffer for Western blotting. Prior to mass spectrometry, all samples were incubated with dithiothreitol (10 mM) at room temperature for 15 min, and with iodoacetamide (166 mM) for a further 15 min at room temperature before again being subjected to methanol precipitation. They were reconstituted in ammonium bicarbonate buffer (50 mM), digested with trypsin overnight at 37°C and then desalted using C18 Zip-Tips (Millipore).
Human serum albumin was recovered from 100 μL aliquots of clarified culture supernatants using the same protocol as above. For higher sensitivity detection of adducts in human plasma, samples from piperacillin-exposed patients were processed individually for 3D-LC-MS/MS analysis. Human serum albumin was first isolated from plasma by affinity chromatography using a POROS anti-human serum albumin column (ABSciex, Foster City, CA, USA) (21). Aliquots of 400 μg of affinity isolated human serum albumin were precipitated and digested as described above, and the digests were fractionated on a Polysulfoethyl A strong cation-exchange column (200 × 4.6 mm, 5 μm, 300Å; Poly LC, Columbia, MD). Fractions of 2 mL were collected and were dried by centrifugation under vacuum (SpeedVac, Eppendorf). All samples were analysed by reversed phase LC-MS.
Mass spectrometric characterization of β-lactam albumin binding
Samples were reconstituted in 2 % ACN/0.1% formic acid (v/v), and aliquots of 2.4-5 pmole were delivered into a QTRAP 5500 hybrid quadrupole-linear ion trap mass spectrometer (ABSciex) by automated in-line liquid chromatography (U3000 HPLC System, 5 mm C18 nano-precolumn and 75 μm × 15 cm C18 PepMap column [Dionex, California, USA]) via a 10 μm inner diameter PicoTip (New Objective, Massachusetts, USA). A gradient from 2 % ACN/0.1 % FA (v/v) to 50 % ACN/0.1 % FA (v/v) in 70 min was applied at a flow rate of 280 nL/min. The ionspray potential was set to 2,200-3,500 V, the nebuliser gas to 18 and the interface heater to 150°C.
Multiple reaction monitoring (MRM) transitions specific for drug modified peptides were selected as follows: the m/z values were calculated for all possible peptides with a missed cleavage at a lysine residue; to these were added the mass of the appropriate hapten (cyclized 517 amu, hydrolyzed 535 amu, desethyl cyclized 489 amu and desethyl hydrolyzed 507 amu); the parent ion masses were then paired with a fragment mass of 160 ([M+H]+ of cleaved thiazolidine ring present in all of the haptens) and/or a fragment mass of 106 ([M+H]+ of cleaved benzylamine group of hydrolyzed haptens). MRM transitions were acquired at unit resolution in both the Q1 and Q3 quadrupoles to maximize specificity, they were optimised for collision energy and collision cell exit potential, and dwell time was 20 ms. MRM survey scans were used to trigger enhanced product ion MS/MS scans of drug-modified peptides, with Q1 set to unit resolution, dynamic fill selected and dynamic exclusion for 20 s. Total ion counts were determined from a second aliquot of each sample analyzed by conventional LC-MS/MS and were used to normalize sample loading on column. MRM peak areas were determined by MultiQuant 1.2 software (ABSciex). Epitope profiles were constructed by comparing the relative intensity of MRM peaks for each of the modified lysine residues within a sample and normalization of those signals across samples.
Western blotting
Aliquots of 5μg protein were separated by electrophoresis on a 10 % SDS-polyacrylamide gel, and electroblotted onto nitrocellulose membrane. Non-specific binding was blocked using Tris/saline/Tween buffer (TST: NaCl, 150 mM; Tris-HCl, 10 mM; Tween 20, 0.05 %; pH 8.0) containing 10 % non-fat dry milk for 16 h at 4°C. The blot was incubated with primary anti-penicillin antibody (mouse anti-penicillin monoclonal antibody; AbD Serotec) diluted 1:20,000 in 5 % milk/TST for 1 h, followed by incubation with horse radish peroxidise conjugated anti-mouse IgG antibody (Abcam) diluted 1:10,000 in 5 % milk/TST for a further 1 h. Signal was detected by enhanced chemiluminescence (Western Lightning, PerkinElmer, Boston, USA) using autoradiography film and a GS800 calibrated scanning densitometer (BioRad, Hemel Hempstead, UK).
Patients
The medical records of the 350 patients with cystic fibrosis attending the Regional Adult Cystic Fibrosis Unit in Leeds were reviewed. Eighty eight (25 %) had a previous non-immediate reaction to piperacillin. Reactions were defined as an adverse reaction occurring after at least 48 hours of intravenous piperacillin. This study enrolled 28 hypersensitive patients; the reactions mainly consisted of maculopapular exanthema, fevers, urticarial eruptions and flu-like symptoms. In every case treatment had to be discontinued, and patients received anti-histamines and/or oral steroids, when clinically indicated. Detailed information on each of the hypersensitive patients is presented in Table I.
Table I.
Clinical characteristics of the hypersensitive patients
| Age (years)/ Gender |
Drug | Reaction | Time to reaction (days) |
Time since reaction (years) |
No. of courses prior to reaction |
LTT | |
|---|---|---|---|---|---|---|---|
| 1 | 18/M | Tazocin | MPE/Fevers | 9 | 7 | 3 | +++ |
| 2 | 29/F | Piperacillin | MPE | 7 | 12 | 11 | ++++ |
| 3 | 29/F | Tazocin | Fever/eosinophilia | 7 | 0.5 | 14 | ++ |
| 4 | 26/M | Tazocin | MPE | 5 | 2 | 3 | ++++ |
| 5 | 29/M | Tazocin | MPE | 3 | 5 | 4 | ++ |
| 6 | 24/F | Tazocin | Delayed angioedema | 6 | 3 | 4 | − |
| 7 | 17/M | Piperacillin | Flu-like illness | 2 | 5 | 3 | ++++ |
| 8 | 24/M | Piperacillin | MPE | 2 | >5* | NA | +++ |
| 9 | 23/M | Tazocin | MPE | 11 | 4 | 9 | +++ |
| 10 | 30/F | Tazocin | Flu-like illness | 3 | 1 | 9 | ++ |
| 11 | 45/F | Tazocin | Fevers | 11 | 5 | 14 | − |
| 12 | 19/M | Tazocin | MPE/fever | 2 | 0.5 | 4 | ++++ |
| 13 | 22/F | Tazocin | MPE | 7 | 5 | 6 | ++ |
| 14 | 21/F | Tazocin | Arthralgia | 5 | 4 | 4 | ++++ |
| 15 | 17/M | Tazocin | MPE | 4 | 6 | 11 | − |
| 16 | 24/F | Tazocin | Arthralgia/MPE | 7 | 2 | 7 | + |
| 17 | 22/M | Tazocin | Pruritis | 5 | 4 | 11 | + |
| 18 | 28/F | Tazocin | Fever/arthralgia | 9 | 6 | 7 | +++ |
| 19 | 31/F | Tazocin | Flu-like illness | 9 | 6 | 12 | + |
| 20 | 34/M | Piperacillin | MPE | 2 | 10 | 11 | − |
| 21 | 32/M | Piperacillin | MPE | 5 | 10 | 8 | + |
| 22 | 23/F | Tazocin | Fevers/unwell | 5 | 3 | 12 | + |
| 23 | 29/M | Tazocin | Tight chest | 4 | 6 | 9 | − |
| 24 | 18/M | Piperacillin | Urticarial rash | 2 | >5* | NA | − |
| 25 | 40/M | Piperacillin | Fevers | 10 | 12 | 11 | − |
| 26 # | 29/F | Piperacillin | MPE | 4 | 5 | 9 | − |
| 27 # | 34/F | Tazocin | MPE | 3 | 1 | 17 | − |
| 28 # | 35/F | Piperacillin | MPE | 7 | 10 | 17 | − |
F: female; M: male; MPE: maculopapular exanthema; NT: not tested; ID: intradermal; LTT: lymphocyte transformation test.
+: SI 2-5; ++: SI 5-10; +++: SI 10-20; ++++: >20
Indicates that the exact time period since reaction is not known due to missing medical records or occurrence at another cystic fibrosis centre.
Indicates that patient on long-term immunosuppressive therapy. Patients 26 and 27 were receiving hydroxychloroquine for cystic fibrosis related arthritis. Patient 28 received long term low dose steroids as prophylaxis against hypersensitive bronchopulmonary aspergillosis.
Nine patients (5 males and 4 females) identified as “tolerant” had received piperacillin without any adverse event. Blood samples were also collected from 11 healthy naïve volunteers who had never received piperacillin. There were no significant differences when the tolerant and hypersensitive groups were compared for age, lung function, and sputum classification. Dose and treatment duration were identical in both groups. Skin and biological tests were performed when patients were clinically well and had not received intravenous antibiotics for at least 6 weeks. Further blood samples were taken from the tolerant group during a course of piperacillin to characterize albumin conjugates in vivo. Written informed consent was obtained from all patients and the study was approved by The Leeds East Ethics Committee.
Lymphocyte transformation test and the generation of drug-specific T-cell lines and clones
Freshly isolated peripheral blood mononuclear cells (PBMC) from heparinized venous blood were dispensed into a 96-well U-bottom culture plate (0.15×106 cells per well in 200 μl cell culture medium [RPMI 1640 supplemented with penicillin (100 μg/ml), streptomycin (100 μg/ml), HEPES (25 mM), L-glutamine (2 mM), 10 % pooled human AB serum and transferrin (12.5 mg)]). Piperacillin was first tested from 7.5 μM to 4 mM. Tetanus toxoid (0.5 μg/ml) was used as a positive control. Cell cultures were incubated in a CO2 ventilated (5 %) incubator at 37 °C for 6 days. On the fifth day 0.5 μCi of [3H]thymidine was added to each well. Cells were finally harvested onto filter membranes, and the amount of incorporated radioactivity was measured (counts per minute, cpm) using a β-counter (MicroBeta Trilux, PerkinElmer, Cambridge, UK). Thereafter, the results were expressed as stimulation index (SI = average counts per minute in drug replicates / average counts per minute in medium replicates). An SI above 2 was considered positive.
T-cell lines were generated by culturing purified CD3+ T-cells (4×106; 1 ml) with piperacillin (2 mM) and autologous irradiated PBMC (1×106). IL-2 was added on day 3 to sustain the drug-specific proliferative response. Lines were restimulated with piperacillin and autologus irradiated PBMC weekly for 4 weeks prior to analysis of drug-specific proliferation and IFN-γ secretion by ELIspot (see below). Antigen-specific T-cell clones were generated by serial dilution using established methodology (15,16). To test the specificity of the clones, T-cells (0.5 × 105) were incubated with autologous EBV-transformed B-cells (0.1 × 105) and piperacillin (2 mM). After 48 h, [3H] thymidine (0.5 μCi) was added, and 16 h later proliferation was measured by scintillation counting.
Analysis of the relationship between piperacillin albumin conjugate formation and the proliferative response of peripheral blood mononuclear cells from hypersensitive patients
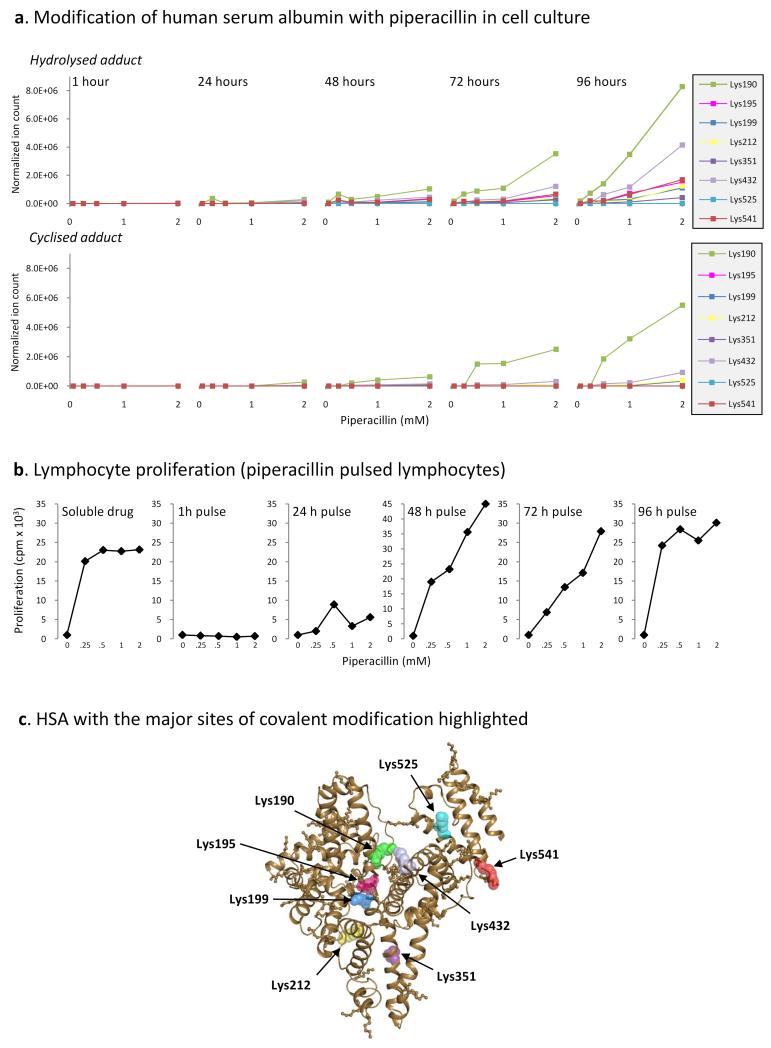
PBMC (1.5×106; 200 μl) were pulsed with piperacillin (0.25-2 mM) for 4 days. After 1 h, 1 day, 2 days, 3 days and 4 days, supernatant was collected for mass spectrometric analysis of piperacillin-albumin binding. At each time-point, cells were washed to remove unbound drug, suspended in drug-free medium and dispensed into a second culture plate. On the fifth day, 0.5 μCi of [3H]thymidine was added to each well for the analysis of lymphocyte proliferation.
The proliferative response of PBMC and T-cell clones to piperacillin (0.25-2 mM) and piperacillin-modified albumin (0.25-4 mg/ml) was also evaluated. The protocols used were essentially the same as described above for the parent drug, with the exception that unconjugated albumin subjected to the same extraction protocol as piperacillin-conjugated albumin was used as a control.
Cytokine/chemokine profiling
ELIspot (IFNγ, IL13 and granzyme B) was used to monitor secretory profiles from piperacillin hypersensitive patients. PBMC (0.5×106; 0.5 ml) were incubated with or without piperacillin (0.5-2 mM) for 48h in antibody coated plates prior to development.
Supernatants (25 μl) were also collected prior to the addition of [3H]thymidine, for the analysis of cytokine/chemokine secretion using a Millipore multiplex assay kit (Millipore, Watford, UK). Cytokine and chemokine concentrations (IL1α, IL1β, IL4, IL5, IL6, IL8, IL10, IL13, IL17, TNFα, IFNγ, eotaxin, MIP-1α, MIP-1β) were measured using a Bio-Plex Suspension Array System (model Luminex100), and its BioRad Bio-Plex Manager 3.0 Software (BioRad, Hertfordshire, UK).
Skin Testing
Skin prick tests were performed using a previously published protocol (28), against Tazocin (piperacillin-tazobactam), histamine (10mg/ml positive control) and 0.9 % saline (negative control) to exclude a diagnosis of immediate hypersensitivity. Intravenous piperacillin preparations were used at final concentrations of 2 mg/ml and 20 mg/ml in 0.9 % saline. The reagents were prepared under sterile conditions and tested on the volar forearm. Readings were performed at 20 min. A wheal 3 mm or greater in diameter than the negative control was considered positive. Intradermal injections were also performed with delayed readings at 48 and 72 hours for the diagnosis of non-immediate reactions. An infiltrated erythema greater than 5 mm in diameter was considered a positive reaction.
Statistics
Results were analyzed using a Mann Whitney test or a Wilcoxon test for paired data sets when comparing lymphocyte proliferation and cytokine concentrations. A Fisher exact test was used to compare frequencies among and between groups, and a Spearmann analysis allowed non-parametric correlation analysis. A p value below 0.05 was considered statistically significant.
RESULTS
Identification and characterization of piperacillin hapten formation in vitro
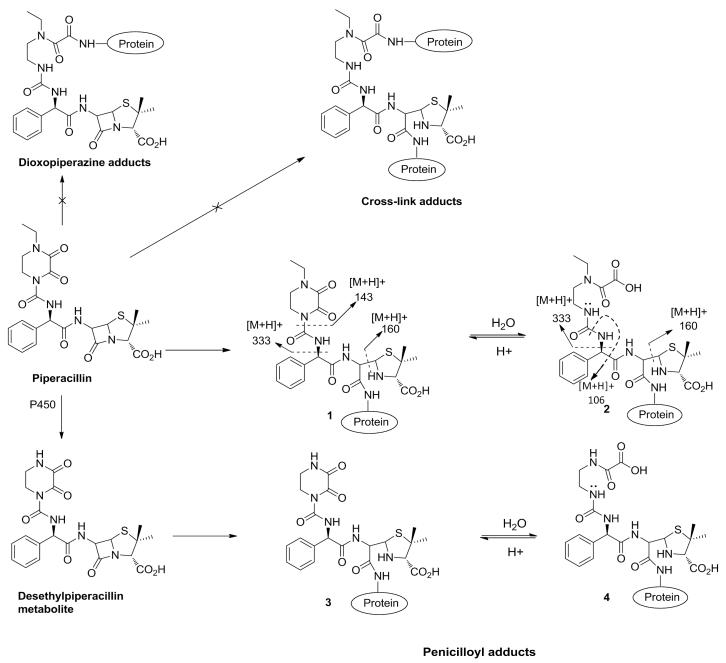
Mass spectrometric analysis of piperacillin-albumin conjugates formed in phosphate buffer revealed a hapten of the predicted mass of 517 amu, which was formed from direct adduction of piperacillin (cyclized hapten [1]), but also a second hapten of mass 535 amu hypothesized to be formed through hydrolysis of the 2,3-dioxopiperazine ring (hydrolyzed hapten [2]; Figure 1). As shown in the MS/MS spectra of the peptide 182LDELRDEGK*ASSAK195 modified with the cyclized hapten (Figure 2A), the presence of the most characteristic fragmentation ions of m/z at 160 and 143 indicated the incorporation of piperacillin in this peptide. In addition, the presence of an abundant ion of m/z at 868, corresponding to the doubly charged peptide mass plus 216 (a portion associated with penicilloyl adducts of the piperacillin structure) provided further evidence that the hapten of mass of 517 amu was formed by the addition to β-lactam ring rather than the dioxopiperazine ring. In the mass spectrum of the hydrolyzed hapten, the most characteristic fragment ions were detected at m/z of 106 and 160 (Figure 2A). Similarly, the ion at m/z of 867.8 confirmed that the nucleophilic addition took place at the β-lactam ring whereas the hydrolysis occurred at the 2,3-dioxopiperazine ring. In addition, an equilibration between cyclized and hydrolyzed haptens was observed (results not shown), further confirming that the hydrolysis occurred at the 2,3-dioxopiperazine ring as the hydrolysis of β-lactam ring is irreversible.
Figure 1. Chemical structures of cyclized and hydrolyzed piperacillin and desethyl piperacillin haptens showing the dominant mass spectrometry-induced fragmentation sites.
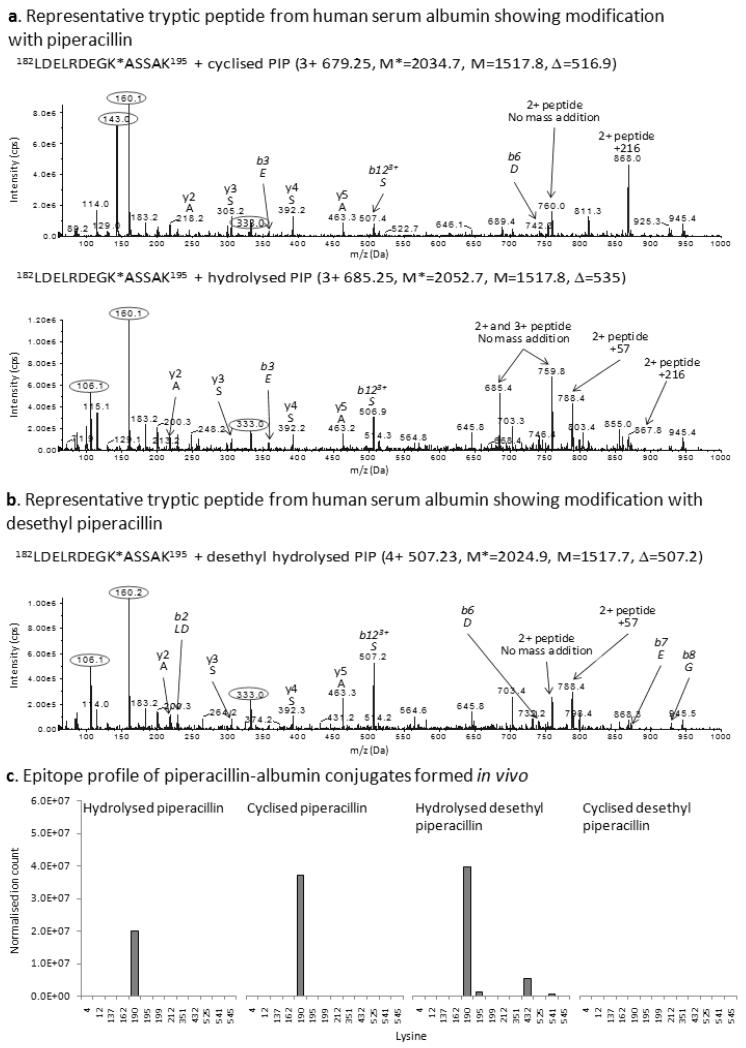
Figure 2. Mass spectrometric characterization of piperacillin haptens formed on albumin in vitro and isolated from plasma of patients undergoing piperacillin treatment.
Representative MS/MS spectrum of the albumin peptide 182LDELRDEGKASSAK195 modified at Lys190 with (A) the cyclized and hydrolyzed piperacillin haptens and (B) the hydrolyzed desethyl piperacillin hapten. Characteristic fragment ions derived from partial cleavage of the hapten are circled. (C) Epitope profile showing the lysine residues of albumin modified in vivo with the cyclized and hydrolyzed piperacillin and desethyl piperacillin haptens.
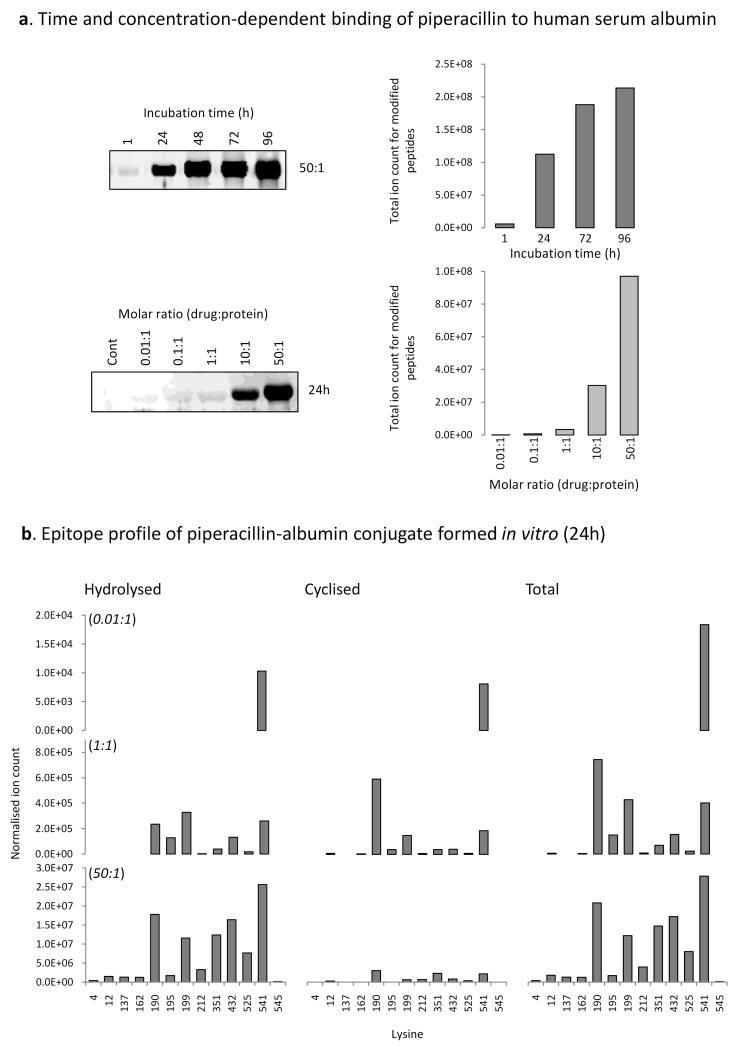
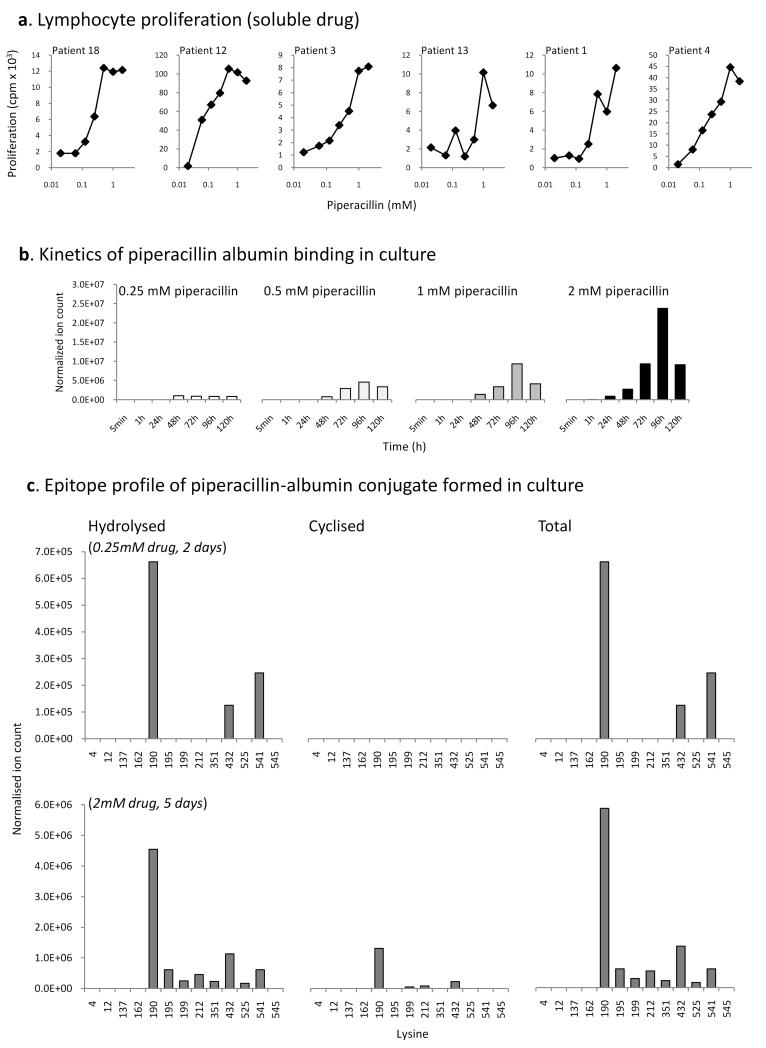
The masses of the modified peptides in combination with masses of the signature fragment ions enabled high sensitivity detection of modified peptides in human serum albumin exposed to piperacillin in vitro. The cyclized and hydrolyzed forms of the piperacillin hapten were detected after 24h at a drug:protein ratio of 50:1 at 8/59 and 13/59 lysine residues in human serum albumin, respectively. Modification at Lys190, Lys432 and Lys541 resulted in the strongest MRM signals and that, notwithstanding the differences in ionization efficiency, the hydrolyzed hapten was more prevalent than the cyclized (Figure 3B; Table II shows the amino acid sequence of the modified peptides). The time and concentration-dependency of the modification revealed by Western blotting was confirmed by MRM-MS, whilst the exquisite sensitivity of the mass spectrometric approach revealed modification on Lys541 at a molar ratio of drug to protein of 0.01:1 (Figure 3).
Figure 3. Time- and concentration-dependent binding of piperacillin to albumin in vitro.
(A) Western blotting with an anti-drug antibody and mass spectrometric analysis of the time- and concentration-dependent binding of piperacillin to albumin. (B) Epitope profile showing the lysine residues of albumin modified in vitro with the cyclized and hydrolyzed piperacillin haptens.
Table II.
Human serum albumin derived tryptic peptides containing piperacillin-modified lysine residues
| Lysine | Peptide | Cyclized hapten | Hydrolyzed hapten |
|---|---|---|---|
| 4 | DAHK*SEVAHR | X | √ |
| 12 | FK*DLGEENFK | √ | √ |
| 137 | K*YLYEIAR | X | √ |
| 162 | YK*AAFTECCQAADK | X | √ |
| 190 | LDELRDEGK*ASSAK | √ | √ |
| 195 | ASSAK*QR | X | √ |
| 199 | LK*CASLQK | √ | √ |
| 212 | AFK*AWAVAR | √ | √ |
| 351 | LAK*TYETTLEK | √ | √ |
| 432 | NLGK*VGSK | √ | √ |
| 525 | K*QTALVELVK | √ | √ |
| 541 | ATK*EQLK | √ | √ |
| 545 | EQLK*AVMDDFAAFVEK | X | √ |
Piperacillin:albumin ratio 50:1; incubation time 24 h.
indicates site of piperacillin modification
Modification of human serum albumin with piperacillin in vivo
Albumin was isolated from 4 piperacillin-exposed patients with cystic fibrosis to characterize antigen formation in vivo. In order to enhance the sensitivity of detection of the modified peptides, a 3D-LC approach was adopted which enabled the detection of the cyclized [1] and hydrolyzed [2] forms of the piperacillin hapten at Lys190. A further mass addition of 507 amu which was associated with fragment ions of 106 and 160 amu was detected at Lys190 (Figure 2B), Lys195, Lys432 and Lys541 (Figure 2C), and we propose that this is the hapten formed from the desethyl metabolite of piperacillin (29) with hydrolysis of the dioxopiperazine ring. Figure 1 shows the structure of desethyl piperacillin and the cyclized [3] and hydrolyzed [4] forms of the piperacillin metabolite-derived hapten. No desethyl cyclized hapten was detected. The possibility that the desethyl structure was formed by in-source fragmentation was ruled out as the retention times of the peptide containing hydrolyzed and desethyl hydrolyzed Lys190 differed by 1-2 mins during both cation exchange and reversed phase chromatography (data not shown).
Stimulation of patient peripheral blood mononuclear cells with piperacillin, skin testing and characterization of the major antigen formed in cell culture
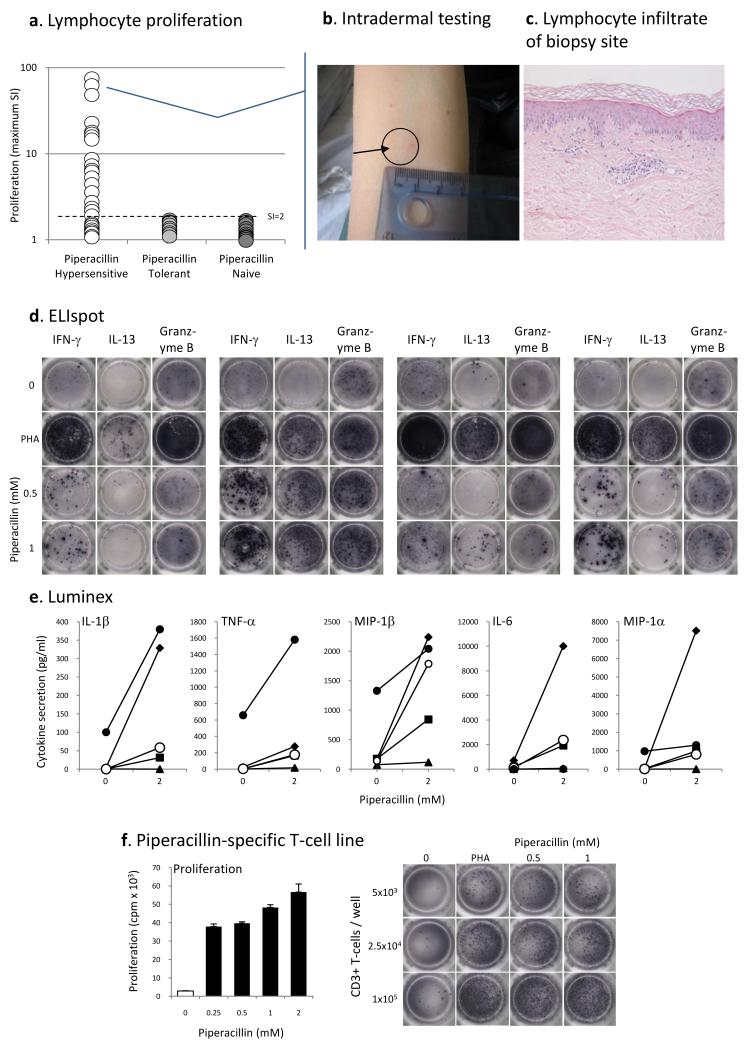
PBMC from 19 out of the 28 piperacillin hypersensitive patients (68 % sensitivity) were found to proliferate in the presence of piperacillin (Table I; Figure 4A). The sensitivity of the assay was increased to 76 % when excluding the 3 patients receiving oral steroids at the time of blood sampling (patients 26-28; Table I). The number of positive patients did not differ when those with and without cutaneous manifestation were compared. The lymphocyte transformation test was repeated on ten hypersensitive patients with at least a 1 month interval between assays. Although the strength of the proliferative response varied slightly, number of lymphocyte transformation test positive patients remained the same (results not shown). PBMC from patients hypersensitive to piperacillin alone were not stimulated with other structurally-related β-lactam antibiotics.
Figure 4. Piperacillin-specific stimulation of peripheral blood mononuclear cells and T-cell lines from hypersensitive patients.
(A) PBMC from 19 hypersensitive patients were specifically stimulated with piperacillin (SI above 2). (B) Positive intradermal skin test from 1 of 4 patients presenting with cutaneous signs and a strong in vitro proliferative response against piperacillin. (C) A biopsy of the maculopapular reaction site. Immune-stain confirmed the lymphocytic infiltrate as being almost entirely T-cell in character, with a CD3+, CD45RO+ phenotype. Both CD4+ and CD8+ subsets were present. (D) Piperacillin-specific-specific IFN-γ, IL13 and granzyme B ELIspot. The figure shows PBMC from 4 hypersensitive patients stimulated with piperacillin for 48 h. (E) Multiplex analysis of cytokines/chemokines secreted from hypersensitive patient PBMC (n=5) incubated with stimulatory concentrations of piperacillin. (F) Concentration-dependent proliferation and IFNγ secretion by a piperacillin-responsive T-cell line. T-cell lines were generated by repetitive stimulation of blood lymphocytes with piperacillin and irradiated autologous PBMC in IL-2 containing medium.
PBMC from tolerant patients with cystic fibrosis and drug naïve volunteers were not stimulated to proliferate with piperacillin.
Skin prick tests, which are traditionally used in patients with immediate hypersensitivity (28), did not generate positive readings with piperacillin in any of the patients tested. Four out of the 28 piperacillin hypersensitive patients had positive intradermal readings to piperacillin. Positive readings were only detected in patients with cutaneous signs and a positive lymphocyte transformation test (Figure 4B). Patients 2 and 9 developed marked erythema and induration 24 hours following injection. A biopsy of the skin reaction from patient 2 revealed epidermal basal layer vacuolation, necrotic keratinocytes and a dermal perivascular infiltration of T-lymphocytes (Figure 4C). Patients 4 and 21 showed significant induration at 48 hours.
The drug-specific proliferative response was associated with the secretion of IFN-γ, IL-13 and the granulation molecule granzyme B - a key mediator of cell killing (PBMC, Figure 4D; T-cell line, Figure 4F). Muliplex analysis of cell culture supernatant was performed to obtain a more global analysis of cytokines secreted from hypersensitive and tolerant patients PBMC. In addition to the cytokines IFN-γ and IL-13, significantly higher levels of IL-1β (p=0.031), IL-6 (p=0.016), TNF-α (p=0.012), and MIP-1α (p=0.031) were found in cell cultures containing piperacillin-treated PBMC from hypersensitive patients when compared to the tolerant and naïve controls (Figure 4E).
The response of PBMC from hypersensitive patients was concentration-dependent and detectable over a wide range of piperacillin concentrations (Figure 5A). Therefore, the lymphocyte transformation test was used to explore the relationship between drug albumin binding and drug immunogencitiy. Cyclized [1] and hydrolyzed [2] forms of the piperacillin hapten were detectable on albumin and the levels of albumin binding increased progressively over 96 h (Figure 5B). Binding was concentration-dependent and was observed at piperacillin concentrations that stimulate the proliferation of PBMC from hypersensitive patients. Both piperacillin haptens (hydrolyzed and cyclized forms) were found to bind preferentially to Lys190, followed by Lys432 and Lys541 on albumin. Drug modifications were also detected at an additional 5 lysine residues (Lys195, 199, 212, 351, 525) (Figure 5C).
Figure 5. Concentration-dependent stimulation of patient peripheral blood mononuclear cells with piperacillin and characterization of the major antigen formed in cell culture.
(A) Concentration-dependent piperacillin-specific proliferation of PBMC from hypersensitive patients. Proliferative responses were analyzed by incorporation of [3H]thymidine of the final 16 h of the experiment. (B) Analysis of total levels of albumin binding with piperacillin concentrations associated with a significant lymphocyte proliferative response after 1-120 h. (C) Epitope profile showing the lysine residues of albumin modified with the cyclized and hydrolyzed piperacillin haptens in culture medium.
Identification of the key piperacillin modified lysine residues in albumin involved in a lymphocyte proliferative response
To determine which piperacillin-modified lysine residues in albumin are the key epitopes involved in the stimulation of a lymphocyte proliferative response, PBMC from hypersensitive patients were cultured with piperacillin (0.25-2 mM). After 1-96 h, PBMC were washed repeatedly to remove soluble drug, suspended in culture medium, and dispensed into fresh culture plates for the remainder of the assay. Modified Lys190 and Lys432 were detectable at 2 mM after a 1 h incubation; however, the level of modification was extremely low, coinciding with a negative lymphocyte proliferative response (Figure 6). An increase in the level of modification at Lys190 and Lys432 and an increase in the number of sites modified (Lys199 and Lys541) at 24 h was associated with a weak proliferative response of patient PBMC (Figure 6). At intermediate time-points (48-72 h), there was a further increase in the number of sites modified and a concentration-dependent increase in the level of modification at each site. At 72 h, piperacillin modifications were detectable at 6 lysine residues (Lys190, Lys199, Lys212, Lys351, Lys432 and Lys541). This correlated with the concentration-dependent proliferation of T-cells from hypersensitive patients, reaching a maximum response at 2mM. Finally, at 96 h, when the highest levels of albumin binding were detected, a maximal proliferative response was seen with each stimulatory concentration of piperacillin (0.25-2 mM). Hydrolyzed [1] and/or cyclized [2] forms if the piperacillin hapten were detected on eight lysine residues (Figure 6C), including Lys190, Lys195, Lys432 and Lys541, which were modified with piperacillin in patients.
Figure 6. Identification of the key piperacillin-modified lysine residues in albumin involved in a lymphocyte proliferative response.
(A) Kinetic profile of the hydrolyzed and cyclized haptens of piperacillin bound to each modified lysine residue on albumin in cell culture. Profiles derive from MRM-MS analysis of the modified tryptic peptides. (B) Proliferative response of PBMC from hypersensitive patients pulsed with piperacillin for 1-96 h. Proliferative responses were analyzed by incorporation of [3H]thymidine of the final 16 h of the experiment. (C) Model of albumin showing piperacillin binding sites at positions Lys190, Lys195, Lys199, Lys212, Lys351, Lys432, Lys525 and Lys541.
Stimulation of patient peripheral blood mononuclear cells and T-cell clones with a synthetic piperacillin albumin conjugate
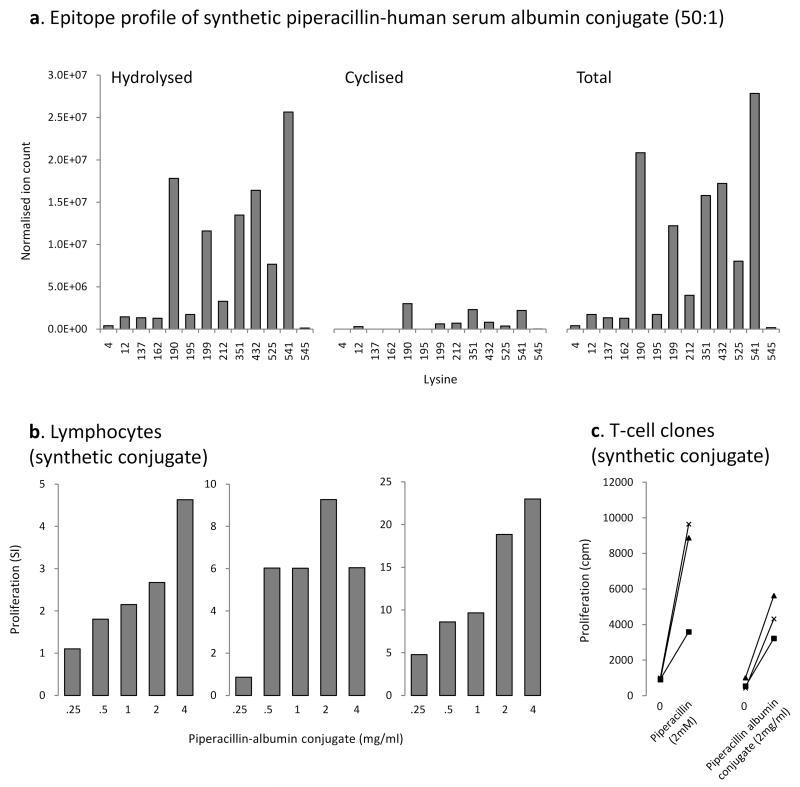
To generate a synthetic piperacillin albumin conjugate for use as an antigen in in vitro assays, human serum albumin was modified with piperacillin at a molar ratio of drug to protein of 50:1 for 24 h in phosphate buffer. The epitope profile is shown in Figure 7A; this largely mirrored the profile of albumin binding detected in culture supernatant in that (1) hydrolyzed and cyclized forms of the piperacillin hapten were detected on the 8 lysine residues modified in culture and (2) the 3 sites most readily detected by MRM-MS were Lys190, Lys432 and Lys541.
Figure 7. Stimulation of patient peripheral blood mononuclear cells and T-cell clones with a synthetic piperacillin albumin conjugate.
(A) Epitope profile of the piperacillin-albumin conjugate, derived from MRM-MS analysis of modified tryptic peptides. Specific proliferation of (B) PBMC and (C) T-cell clones from hypersensitive patients with the synthetic albumin conjugate. Unconjugated albumin subjected to the same extraction protocol as the piperacillin albumin conjugate was used as a control in each experiment. Proliferation in cultures containing unconjugated albumin were consistently less than 2000 cpm and no significant difference was observed when unconjugated albumin and medium controls were compared.
The synthetic piperacillin albumin conjugate stimulated the proliferation of PBMC and CD4+ T-cell clones from hypersensitive patients (Figure 7B).
DISCUSSION
Although little is known about the mechanisms that lead to drug hypersensitivity reactions, several hypotheses to explain drug immunogenicity have been postulated and one of the most popular is the hapten hypothesis. This is based on the concept that drugs form haptenic structures – compounds with a propensity to bind covalently to biological macromolecules – that modify endogenous protein to stimulate an immune response (30-32). Processed peptides derived from the modified protein are presumed to interact with MHC molecules prior to stimulating T-cells through the T-cell receptor. Many drugs associated with a high prevalence of hypersensitivity reactions in humans form protein adducts; however, the absence of sufficiently sensitive bioanalytical methods to characterize functional drug protein conjugates has effectively prohibited any attempt to study the relationship between antigen formation and immunogenicity.
We have recently developed and employed mass spectrometry methods to qualify the site(s) of drug protein conjugation (20-23). Drugs and drug metabolites bind in a dose-dependent manner to proteins such as albumin and can display different preferences for the sites of modification. In the current study, we sought to exploit these methods to investigate piperacillin hypersensitivity in patients with cystic fibrosis. Specific objectives of the project were to characterize haptenic structures on albumin and the relationship between drug modification of protein and drug-specific lymphocyte responses, and thereby determine the fundamental relationship between the chemistry of antigen formation and drug hypersensitivity.
Mass spectrometric analysis, after protein digestion, revealed that piperacillin forms multiple haptenic structures on human serum albumin in vitro. Consistent with the known chemistry of β-lactam antibiotics, we have shown that piperacillin can form adducts with lysine residues by direct opening of the β-lactam ring. However, the chemistry of antigen formation is complex. There is also a hapten formed in which the 2,3-dioxopiperazine ring has undergone hydrolysis. Thus, two distinct haptens can be formed. In principle, adducts resulting from opening of the 2,3-dioxopiperazine ring or cross linking adducts could also be generated. However, these adducts were not detected indicating that the β-lactam ring is more susceptible to nucleophiles than 2,3-dioxopiperazine ring. No evidence of modification at other amino acid residues could be found. This might be because adducts resulting from piperacillin binding to other nucleophilic amino acids such as serine, histidine and cysteine are not formed. However, such adducts might be too labile to be detected under current mass spectrometry conditions. The extent of piperacillin albumin binding was dependent on incubation time at each drug concentration studied. This is important as cystic fibrosis patients are treated by rapid infusion with high doses of drug (4 g over 20 min, 3 times a day), leading to a plasma Cmax of 0.4-0.65 mM and plasma clearance of the drug after 4.5 h (33). The concentration of human serum albumin in plasma is approximately 35 g/L (0.53 mM) (34) and the molar ratio of drug to protein at Cmax is 0.75-1.22:1. These data indicate that conjugates would be formed in vivo and, because the half-life of human serum albumin is approximately 19 days (35), the modified protein is likely to accumulate over the course of the therapeutic intervention which is usually 14 days in duration and the average time to the reaction occurring (average time 9 days).
To characterize haptens formed in vivo, albumin was isolated from patients receiving piperacillin and subjected to affinity, cation exchange and reverse phase chromatography, after trypsin digestion and prior to mass spectrometry. In addition to the hydrolyzed and cyclized haptens of piperacillin, a further haptenic structure was detected that was derived from the desethyl metabolite of piperacillin with a hydrolyzed piperazine ring. This reveals that not all of the products of piperacillin metabolism in the liver are excreted in the bile or urine, but a significant amount is released back into the circulation.
A restricted profile of piperacillin albumin binding was detected in vitro and in vivo. At the lowest drug concentration investigated (drug to protein ratio of 0.01:1) modification of a single lysine (Lys541) could be detected (Figure 3B). Modified Lys541 was also detected on albumin isolated from patients, alongside modification of Lys190, Lys195 and Lys432. The selective modification of lysine residues on albumin was not simply related to the pKa of individual lysine residues and therefore the reactivity of the side-chain amino group. Instead, the majority of the sites of modification were associated with Sudlow sites I and II, hydrophobic pockets in human serum albumin involved in the non-covalent interaction with endogenous ligands and drugs (21,36,37). This indicates that the orientation of the non-covalent interaction of drug with protein and the subsequent stabilization by hydrogen bonding influences which lysine residues are in close proximity with the drug and are therefore preferentially covalently modified.
Circulating piperacillin-specific lymphocytes were detected in the majority of piperacillin-hypersensitive patients with cystic fibrosis. The piperacillin-specific proliferative response was reproducible on repeated testing and dose-dependent, with the highest response detected with therapeutic piperacillin concentrations that are estimated to be in the range of 0.5–1 mM. Piperacillin-specific lymphocytes were detected in hypersensitive patients with and without cutaneous symptoms, suggesting that drug-specific lymphocytes are involved in other clinical features of the disease pathogenesis (arthralgia, fevers, or flu-like symptoms). The cytokine profile associated with a particular reaction determines the nature of the induced immune response (1,38-42). Antigen stimulation of PBMC from piperacillin hypersensitive patients was associated with the secretion of a mixed panel of cytokines, with high levels of IFN-γ, IL-13, TNF-α, IL-1β, IL-6, and MIP-1α/β detected. IL-5 secretion, a common feature of drug reactions in non-cystic fibrosis patients (43,44), was not seen in piperacillin hypersensitive patients, and this may relate to the absence of eosinophilia as a clinical feature. The detection of IL-1β, TNF-α and IL-6 in culture supernatant containing piperacillin stimulated PBMC from hypersensitive patients and tolerant controls (albeit to a lesser extent) are suggestive of a dendritic cell response against piperacillin, as has recently been described with amoxicillin (45,46). In support of this argument, piperacillin-specific T-cell clones do not secrete IL-1β (unpublished data). Whether this cytokine profile is a function of the chemistry of the drug or the disease status of the patient is not known and is an area of on-going work. A positive granzyme B ELIspot in patients with a positive proliferative response shows that piperacillin stimulates cytotoxic T-cells and that they may play a role in the hypersensitivity reaction.
In view of the fact that the lymphocyte transformation test can be used to confirm the immunological aetiology of piperacillin hypersensitivity reactions, mass spectrometry methods were used to explore the chemical basis of drug antigenicity in PBMC cultures and to define that albumin conjugates are antigenic per se. Piperacillin albumin binding at individual lysine residues was profiled with respect to incubation time, dose and the stimulation of patient PBMC. Hydrolyzed and/or cyclized forms of the piperacillin hapten were detected at each lymphocyte stimulating concentration of piperacillin (Figure 6). Modified Lys190, Lys199, Lys432 and Lys541 were detectable at 2mM piperacillin after a 24 h incubation. The level of modification at each site was low, but coincided with a weak proliferative response. Piperacillin haptens were detectable on 6 lysine residues after 48-72 h and a concentration-dependent increase in the level of modification at each site was observed. This correlated with the concentration-dependent stimulation of patient PBMC. Analysis of minimally-modified, but lymphocyte stimulatory albumin conjugates formed in culture indicate that peptide sequences around positions Lys190, Lys432 and Lys541 may be the principal functional epitopes generated in the lymphocyte transformation test. To confirm that piperacillin albumin conjugates are indeed antigenic, a conjugate with hydrolyzed and cyclized forms of the piperacillin hapten detectable on each lysine residue modified in culture was generated under physiological conditions and shown to stimulate PBMC and T-cell clones to proliferate. These data are consistent with previous studies showing that synthetic penicillin-albumin constructs generated under forced chemical conditions can stimulate T-cells (2), but crucially relate to antigens that are formed under physiological conditions. We are therefore in a position to prepare fully characterized peptide conjugates of piperacillin of physiological relevance and determine their fit into relevant MHC molecules (8).
To conclude, using mass spectrometric methods we have defined piperacillin modifications on albumin, with respect to hapten formation and peptide epitope profile, that are able to stimulate T-cells ex vivo and shown that such modifications can be detected on circulating albumin in patients receiving the drug. Drug-peptide conjugates derived from modified albumin clearly represent functional antigens for T-cells and may indeed function as immunogens in patients with cystic fibrosis. However, it also possible that alternative proteins, which generate similar drug-modified peptide epitopes may constitute the functional immunogen in the patients. A prospective clinical study of piperacillin hypersensitivity is required to explore immunological consequences of antigen formation before, during and after the development of hypersensitivity, utilizing the techniques developed in this investigational study.
ACKNOWLEDGEMENTS
The authors would like to thank the nurses of the Adult Cystic Fibrosis Unit who helped to collect samples, as well as all the volunteers and patients with cystic fibrosis who participated in the project. The authors would also like to thank Prof. Mark Taylor and his postdoctoral fellow Dr. Kelly Johnston (School of Tropical Medicine, University of Liverpool, UK) for their contribution to the cytokine analysis.
Footnotes
This work was funded by a grant from the Wellcome Trust (078598/Z/05/Z) as part of the Centre for Drug Safety Science supported by the Medical Research Council (G0700654). XM is supported by the NIHR Biomedical Research Centre in Microbial Diseases. MP is a NIHR senior Investigator. SEG and MM are PhD students funded by the Egyptian and Saudi Arabian governments, respectively.
The authors have no conflicting financial interests.
REFERENCES
- 1.Beeler A, Engler O, Gerber BO, Pichler WJ. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol. 2006;117:455–462. doi: 10.1016/j.jaci.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Brander C, Mauri-Hellweg D, Bettens F, Rolli H, Goldman M, Pichler WJ. Heterogeneous T cell responses to beta-lactam-modified self-structures are observed in penicillin-allergic individuals. J Immunol. 1995;155:2670–2678. [PubMed] [Google Scholar]
- 3.Castrejon JL, Berry N, El-Ghaiesh S, Gerber B, Pichler WJ, Park BK, Naisbitt DJ. Stimulation of human T cells with sulfonamides and sulfonamide metabolites. J Allergy Clin Immunol. 2010;125:411–418. doi: 10.1016/j.jaci.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 4.Nassif A, Bensussan A, Boumsell L, Deniaud A, Moslehi H, Wolkenstein P, Bagot M, Roujeau JC. Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J Allergy Clin Immunol. 2004;114:1209–1215. doi: 10.1016/j.jaci.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Schnyder B, Burkhart C, Schnyder-Frutig K, von Greyerz S, Naisbitt DJ, Pirmohamed M, Park BK, Pichler WJ. Recognition of sulfamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J Immunol. 2000;164:6647–6654. doi: 10.4049/jimmunol.164.12.6647. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Farrell J, Pirmohamed M, Park BK, Naisbitt DJ. Generation and characterization of antigen-specific CD4+, CD8+, and CD4+CD8+ T-cell clones from patients with carbamazepine hypersensitivity. J Allergy Clin Immunol. 2007;119:973–981. doi: 10.1016/j.jaci.2006.12.617. [DOI] [PubMed] [Google Scholar]
- 7.Elsheikh A, Lavergne SN, Castrejon JL, Farrell J, Wang H, Sathish J, Pichler WJ, Park BK, Naisbitt DJ. Drug antigenicity, immunogenicity, and costimulatory signaling: evidence for formation of a functional antigen through immune cell metabolism. J Immunol. 2010;185:6448–6460. doi: 10.4049/jimmunol.1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padovan E, Bauer T, Tongio MM, Kalbacher H, Weltzien HU. Penicilloyl peptides are recognized as T cell antigenic determinants in penicillin allergy. Eur J Immunol. 1997;27:1303–1307. doi: 10.1002/eji.1830270602. [DOI] [PubMed] [Google Scholar]
- 9.Padovan E, Mauri-Hellweg D, Pichler WJ, Weltzien HU. T cell recognition of penicillin G: structural features determining antigenic specificity. Eur J Immunol. 1996;26:42–48. doi: 10.1002/eji.1830260107. [DOI] [PubMed] [Google Scholar]
- 10.Burkhart C, von Greyerz S, Depta JP, Naisbitt DJ, Britschgi M, Park KB, Pichler WJ. Influence of reduced glutathione on the proliferative response of sulfamethoxazole-specific and sulfamethoxazole-metabolite-specific human CD4+ T-cells. Br J Pharmacol. 2001;132:623–630. doi: 10.1038/sj.bjp.0703845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depta JP, Altznauer F, Gamerdinger K, Burkhart C, Weltzien HU, Pichler WJ. Drug interaction with T-cell receptors: T-cell receptor density determines degree of cross-reactivity. J Allergy Clin Immunol. 2004;113:519–527. doi: 10.1016/j.jaci.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 12.Farrell J, Naisbitt DJ, Drummond NS, Depta JP, Vilar FJ, Pirmohamed M, Park BK. Characterization of sulfamethoxazole and sulfamethoxazole metabolite-specific T-cell responses in animals and humans. J Pharmacol Exp Ther. 2003;306:229–237. doi: 10.1124/jpet.103.050112. [DOI] [PubMed] [Google Scholar]
- 13.Hashizume H, Takigawa M, Tokura Y. Characterization of drug-specific T cells in phenobarbital-induced eruption. J Immunol. 2002;168:5359–5368. doi: 10.4049/jimmunol.168.10.5359. [DOI] [PubMed] [Google Scholar]
- 14.Keller M, Lerch M, Britschgi M, Tache V, Gerber BO, Luthi M, Lochmatter P, Kanny G, Bircher AJ, Christiansen C, Pichler WJ. Processing-dependent and -independent pathways for recognition of iodinated contrast media by specific human T cells. Clin Exp Allergy. 2010;40:257–268. doi: 10.1111/j.1365-2222.2009.03425.x. [DOI] [PubMed] [Google Scholar]
- 15.Naisbitt DJ, Farrell J, Wong G, Depta JP, Dodd CC, Hopkins JE, Gibney CA, Chadwick DW, Pichler WJ, Pirmohamed M, Park BK. Characterization of drug-specific T cells in lamotrigine hypersensitivity. J Allergy Clin Immunol. 2003;111:1393–1403. doi: 10.1067/mai.2003.1507. [DOI] [PubMed] [Google Scholar]
- 16.Schnyder B, Mauri-Hellweg D, Zanni M, Bettens F, Pichler WJ. Direct, MHC-dependent presentation of the drug sulfamethoxazole to human alphabeta T cell clones. J Clin Invest. 1997;100:136–141. doi: 10.1172/JCI119505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batchelor FR, Dewdney JM, Gazzard D. Penicillin allergy: the formation of the penicilloyl determinant. Nature. 1965;206:362–364. doi: 10.1038/206362a0. [DOI] [PubMed] [Google Scholar]
- 18.Levine BB. Studies on the mechanism of the formation of the penicillin antigen. I. Delayed allergic cross-reactions among penicillin G and its degradation products. J Exp Med. 1960;112:1131–1156. doi: 10.1084/jem.112.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine BB, Ovary Z. Studies on the mechanism of the formation of the penicillin antigen. J Exp Med. 1961;114:875–1153. doi: 10.1084/jem.114.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callan HE, Jenkins RE, Maggs JL, Lavergne SN, Clarke SE, Naisbitt DJ, Park BK. Multiple Adduction Reactions of Nitroso Sulfamethoxazole with Cysteinyl Residues of Peptides and Proteins: Implications for Hapten Formation. Chem Res Toxicol. 2009;22:937–948. doi: 10.1021/tx900034r. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins RE, Meng X, Elliott VL, Kitteringham NR, Pirmohamed M, Park BK. Characterisation of flucloxacillin and 5-hydroxymethyl flucloxacillin haptenated HSA in vitro and in vivo. Proteomics Clinical Applications. 2009;3:720–729. doi: 10.1002/prca.200800222. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson C, Jenkins RE, Aleksic M, Pirmohamed M, Naisbitt DJ, Park BK. Characterization of p-phenylenediamine-albumin binding sites and T-cell responses to hapten-modified protein. J Invest Dermatol. 2010;130:732–742. doi: 10.1038/jid.2009.271. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson C, Jenkins RE, Maggs JL, Kitteringham NR, Aleksic M, Park BK, Naisbitt DJ. A mechanistic investigation into the irreversible protein binding and antigenicity of p-phenylenediamine. Chem Res Toxicol. 2009;22:1172–1180. doi: 10.1021/tx900095r. [DOI] [PubMed] [Google Scholar]
- 24.Burrows JA, Nissen LM, Kirkpatrick CM, Bell SC. Beta-lactam allergy in adults with cystic fibrosis. J Cyst Fibros. 2007;6:297–303. doi: 10.1016/j.jcf.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Koch C, Hjelt K, Pedersen SS, Jensen ET, Jensen T, Lanng S, Valerius NH, Pedersen M, Hoiby N. Retrospective clinical study of hypersensitivity reactions to aztreonam and six other beta-lactam antibiotics in cystic fibrosis patients receiving multiple treatment courses. Rev Infect Dis. 1991;13(Suppl 7):S608–611. doi: 10.1093/clinids/13.supplement_7.s608. [DOI] [PubMed] [Google Scholar]
- 26.Pleasants RA, Walker TR, Samuelson WM. Allergic reactions to parenteral beta-lactam antibiotics in patients with cystic fibrosis. Chest. 1994;106:1124–1128. doi: 10.1378/chest.106.4.1124. [DOI] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Torres MJ, Blanca M, Fernandez J, Romano A, Weck A, Aberer W, Brockow K, Pichler WJ, Demoly P. Diagnosis of immediate allergic reactions to beta-lactam antibiotics. Allergy. 2003;58:961–972. doi: 10.1034/j.1398-9995.2003.00280.x. [DOI] [PubMed] [Google Scholar]
- 29.Ghibellini G, Bridges AS, Generaux CN, Brouwer KL. In vitro and in vivo determination of piperacillin metabolism in humans. Drug Metab Dispos. 2007;35:345–349. doi: 10.1124/dmd.106.012278. [DOI] [PubMed] [Google Scholar]
- 30.Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J Exp Med. 1935;61:643–656. doi: 10.1084/jem.61.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavergne SN, Park BK, Naisbitt DJ. The roles of drug metabolism in the pathogenesis of T-cell-mediated drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2008;8:299–307. doi: 10.1097/ACI.0b013e3283079c64. [DOI] [PubMed] [Google Scholar]
- 32.Park BK, Pirmohamed M, Kitteringham NR. Role of drug disposition in drug hypersensitivity: a chemical, molecular, and clinical perspective. Chem Res Toxicol. 1998;11:969–988. doi: 10.1021/tx980058f. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi Y, Roberts JA, Paterson DL, Lipman J. Pharmacokinetic evaluation of piperacillin-tazobactam. Expert Opin Drug Metab Toxicol. 2010;6:1017–1031. doi: 10.1517/17425255.2010.506187. [DOI] [PubMed] [Google Scholar]
- 34.Anderson NL, Anderson NG. The Human Plasma Proteome: History, Character, and Diagnostic Prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 35.Muller N, Schneider B, Pfizenmaier K, Wajant H. Superior serum half life of albumin tagged TNF ligands. Biochem Biophys Res Commun. 2010;396:793–799. doi: 10.1016/j.bbrc.2010.04.134. [DOI] [PubMed] [Google Scholar]
- 36.Fasano M, Curry S, Terreno E, Galliano M, Fanali G, Narciso P, Notari S, Ascenzi P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life. 2005;57:787–796. doi: 10.1080/15216540500404093. [DOI] [PubMed] [Google Scholar]
- 37.Sudlow G, Birkett DJ, Wade DN. Spectroscopic techniques in the study of protein binding. A fluorescence technique for the evaluation of the albumin binding and displacement of warfarin and warfarin-alcohol. Clin Exp Pharmacol Physiol. 1975;2:129–140. doi: 10.1111/j.1440-1681.1975.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 38.Hertl M, Bohlen H, Jugert F, Boecker C, Knaup R, Merk HF. Predominance of epidermal CD8+ T lymphocytes in bullous cutaneous reactions caused by á-lactam antibiotics. J Invest Dermatol. 1993a;101:794–799. doi: 10.1111/1523-1747.ep12371697. [DOI] [PubMed] [Google Scholar]
- 39.Hertl M, Geisel J, Boecker C, Merk HF. Selective generation of CD8+ T-cell clones from the peripheral blood of patients with cutaneous reactions to beta-lactam antibiotics. Br J Dermatol. 1993b;128:619–626. doi: 10.1111/j.1365-2133.1993.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 40.Lochmatter P, Beeler A, Kawabata TT, Gerber BO, Pichler WJ. Drug-specific in vitro release of IL-2, IL-5, IL-13 and IFN-gamma in patients with delayed-type drug hypersensitivity. Allergy. 2009;64:1269–1278. doi: 10.1111/j.1398-9995.2009.01985.x. [DOI] [PubMed] [Google Scholar]
- 41.Rozieres A, Hennino A, Rodet K, Gutowski MC, Gunera-Saad N, Berard F, Cozon G, Bienvenu J, Nicolas JF. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy. 2009;64:534–542. doi: 10.1111/j.1398-9995.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 42.Yawalkar N, Hari Y, Frutig K, Egli F, Wendland T, Braathen LR, Pichler WJ. T cells isolated from positive epicutaneous test reactions to amoxicillin and ceftriaxone are drug specific and cytotoxic. J Invest Dermatol. 2000;115:647–652. doi: 10.1046/j.1523-1747.2000.00105.x. [DOI] [PubMed] [Google Scholar]
- 43.Pichler WJ, Zanni M, von Greyerz S, Schnyder B, Mauri-Hellweg D, Wendland T. High IL-5 production by human drug-specific T cell clones. Int Arch Allergy Immunol. 1997;113:177–180. doi: 10.1159/000237539. [DOI] [PubMed] [Google Scholar]
- 44.Yawalkar N, Shrikhande M, Hari Y, Nievergelt H, Braathen LR, Pichler WJ. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J Allergy Clin Immunol. 2000b;106:1171–1176. doi: 10.1067/mai.2000.110922. [DOI] [PubMed] [Google Scholar]
- 45.Lima CM, Schroeder JT, Galvao CE, Castro FM, Kalil J, Adkinson NF., Jr. Functional changes of dendritic cells in hypersensivity reactions to amoxicillin. Braz J Med Biol Res. 2010;43:964–968. doi: 10.1590/s0100-879x2010007500096. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Pena R, Lopez S, Mayorga C, Antunez C, Fernandez TD, Torres MJ, Blanca M. Potential involvement of dendritic cells in delayed-type hypersensitivity reactions to beta-lactams. J Allergy Clin Immunol. 2006;118:949–956. doi: 10.1016/j.jaci.2006.07.013. [DOI] [PubMed] [Google Scholar]