Abstract
Objective
To determine whether chronic occupational exposure to organophosphates (OP) pesticides leads to cognitive impairment using event-related potentials (ERPs).
Methods
ERPs of 38 vegetable farmers applying OP pesticides and 35 controls were recorded using an auditory oddball paradigm. The N1, P2, N2 and P300 ERP components and the number of counting errors were compared between the groups.
Results
The farmers made significantly more counting errors than controls in the oddball task. The mixed model ANOVA of component latencies revealed a significant component × group interaction, suggesting farmers had a greater delay in later ERP components. Intergroup comparisons of individual components showed significant delays in N2 and P300 latencies. Subsequent ANCOVA showed significant P300 delay even after adjusting for the latency of the preceding component, N2. Intergroup differences of P300 amplitudes were not significant, although there was limited evidence of a difference in scalp topography.
Conclusion
Our findings indicate that chronic low-level occupational exposure to OP pesticides is associated with progressively increasing delay in successive ERP components, particularly P300.
Significance
Chronic exposure to OP pesticides may delay the neurophysiological processes underlying early stages of selective attention and late stages of sensory information processing that include stimulus evaluation and updating of working memory.
Keywords: organophosphate pesticides, chronic exposure, cognitive effects, P300, event-related potentials
Introduction
Long-term neurotoxicity of organophosphate (OP) compounds have been investigated over several decades under two broad areas viz. the effects of acute high-dose poisoning and the effects of chronic low-level exposure (Jamal, 1997; Jamal et al., 2002; Kamel and Hoppin, 2004). The latter is of particular importance as OP compounds have been the principal means of agricultural pest control throughout the world since 1980s (Stephens et al., 1995). Neurocognitive effects of long-term exposure to OPs have been studied in many occupational groups including farmers (Fiedler et al., 1997; Stallones and Beseler, 2002), farm-workers (Daniell et al., 1992; Farahat et al., 2003; Catano et al., 2007), sheep-dippers (Stephens et al., 1995; Jamal et al., 2002; Tahmaz et al., 2003) and termiticide applicators (Steenland et al., 2000).
Symptom inquiries, clinical criteria and neuropsychological tests have been used in assessment of possible neurocognitive deficits. These outcomes show inconsistencies among different studies, and some workers have found no change in cognitive functions in exposed groups (Rodnitzky, 1975; Daniell et al., 1992; Ames et al., 1995; Fiedler et al., 1997; Steenland et al., 2000). In this context, some authors have questioned the objectivity of the testing methods and the sensitivity of the existing objective tools in identifying any subtle cognitive impairment (Steenland et al., 2000; Kamel & Hoppin, 2004). However, despite having numerous inconsistencies, studies on chronic low-dose exposure have reported that OP exposed individuals have poor performance in tests of attention (Stephens et al., 1995; Fiedler et al., 1997; Bazylewicz-Walczak et al., 1999; Srivastava et al., 2000).
Event-related potentials (ERPs) have been used to study the neurophysiological processes and neuroanatomical correlates underlying different cognitive functions. In this context, successive ERP components generated during a particular cognitive task are interpreted in relation to different stages of information processing operating in the cognitive task (Hillyard and Kutas, 1983). ERPs appear to be sensitive in detecting long-term cognitive impairment caused by acute OP poisoning. P300 latency prolongation has been reported following acute OP insecticide poisoning (Dassanayake et al., 2008) and after acute exposure to the OP chemical warfare agent sarin (Murata et al., 1997). According to the context-updating theory (Donchin and Coles, 1988; Polich and Criado, 2006; Polich, 2007), P300 reflects neural processes underlying attention and updating of working memory. However, theories that presume ERP components represent stages of information processing argue that any delay in late ERP components (such as P300) may not necessarily be due to the effects of external factors on the processes underlying that ERP component. If the preceding ERP component (in this instance N2) also shows an equal latency delay it can be deduced that the external factor influences the neural processes underlying this preceding ERP component (i.e. N2) or even an earlier stage of processing (Meyer et al., 1988).
In the present study, our aim was to determine whether long-term occupational exposure to OP pesticides leads to any impairment in neurocognitive processes as reflected in ERPs generated in an auditory oddball task. We mainly focused on the P300 component and the behavioural outcome of the oddball task, but also examined earlier ERP components (viz. N1, P2 and N2) which represent preceding stages of attention-mediated information processing. In a cross-sectional study, we compared a group of vegetable farmers with long-term occupational exposure to OP pesticides with a group of control subjects.
Methods
Participants
This study was carried out in Sri Lanka at the Agriculture Instructor’s Department of the Marassana Divisional Secretariat of the Central Province, and the Clinical Neurophysiology Unit, Teaching Hospital, Peradeniya, from September 2007 to February 2008. We tested vegetable farmers engaged in applying OP pesticides for more than five years. All of them were men working in their own fields. The control group consisted of male hospital labourers who were not handling any agrochemicals.
Exclusion criteria
Farmers and the controls who had any history of acute OP poisoning that required medical care, hearing impairment, pre-existing illnesses or medication that may predispose to cognitive dysfunction or weekly consumption of more than 21units of alcohol (one unit: equivalent of 10 ml of alcohol) were excluded (IPCS, 2001). Individuals who had random blood glucose levels more than 140mg/dl were also excluded.
The test group was recruited from a larger group of farmers who attended educational programmes held in the Agriculture Instructor’s Department of the Marassana Divisional Secretariat of the Central Province of Sri Lanka. Fifty-two vegetable farmers who had been applying OP pesticides for more than five years were interviewed for eligibility. Of those we excluded 14: nine on account of excessive consumption of alcohol, four because of diagnosed diabetes mellitus / hyperglycaemia detected on blood testing, and one due to ongoing treatment for a psychiatric illness. Accordingly 38 men underwent the ERP testing. Of these, ERP recordings of three participants were discarded due to their poor quality. These exclusions resulted in 35 men aged between 35 and 64 years in the farmer group. One participant was 64 years old and the remainder aged between 35 to 58 years. Among the farmers, the duration of pesticide use was 26.0 ± 10.4 years. During the six months preceding the time of data collection they had sprayed OP compounds at least three times. Sixteen (46%) of them had used only one type of OP formulation during this period, whereas the rest has used two or more. The OP formulations used by these 38 farmers include dimethoate (30 farmers), chlorpyrifos (13), quinalphos (6), profenophos (2), phenthoate (2), fenitrothion (1), fenthion (1). All are cholinesterase inhibitors.
The potential participants for the control group were initially informed using an open recruitment notice that indicated the nature of the study and eligibility criteria. A trained health care worker from the Clinical Neurophysiology Unit provided further details. Forty-two hospital labourers contacted the researchers and 40 individuals attended the initial interview and clinical examination. Of those 40, one consumed more than 21 units of alcohol per week and one had a random blood glucose level more than 140mg/dl. These two individuals were excluded. Consequently, the control group comprised 38 male hospital labourers who fulfilled all the eligibility criteria. Their age ranged from 25 years to 58 years. Except two controls who were 25 and 27 years, ages ranged between 38 and 58 years.
Ethical Considerations
Ethical clearance for the study was granted by the Research and Ethical Review Committee, Faculty of Medicine, University of Peradeniya, Sri Lanka. Informed written consent was obtained from all study participants.
Procedures
For all subjects, general information and the details of any illnesses were recorded as well as the details of the duration, frequency, and types of OP use for the farmers. A complete clinical examination was performed to exclude any clinically evident but previously undiagnosed neurological conditions. Once these data were collected, the subjects were given an appointment about one week ahead for ERP recordings. In order to minimize the effect of short-term factors modifying information processing speed, the subjects were instructed to abstain from alcohol, tobacco and coffee in the 24 hours prior to the test and to have at least six hours of sleep the night before testing (IPCS, 2001). In order to avoid the acute short-lasting cholinergic effects of OPs on the nervous system, the farmers were also instructed to abstain from applying / handling agrochemicals during for four days preceding ERP assessment. In the instances where non-compliance was detected on the day of ERP testing, the sessions were rescheduled.
ERPs were recorded and averaged using a Medtronic Keypoint Portable™ 2-channel signal averaging machine. An auditory oddball paradigm (standard tone: 75 dB, 1000 Hz, probability 80%; target tone: 75 dB, 2000 Hz, probability 20%) was applied to elicit auditory ERPs, conforming to the IFCN (International Federation of Clinical Neurophysiologists) guidelines (Heinze et al., 1999). Tones were presented binaurally through headphones at random intervals ranging from 1 to 2 seconds, in a random order. The duration of each stimulus was 120 ms with rise and fall times of 10 ms each. ERPs were recorded over the Cz and Pz positions, while placing combined reference electrodes over mastoids and the ground electrode on Fp position, conforming to international 10–20 scalp electrode placement standards (Klem et al., 1999). The low-pass filter was set at 50 Hz and the high-pass filter was set at 0.2 Hz with roll-off slopes of 6dB/octave. A 50Hz notch filter was not used. The recording epoch was 1000 ms and the sensitivity was 10 μV per division. The sampling rate was 48 kHz. Each subject was exposed to 250 stimuli (200 standard tones and 50 target tones) and the ERP waveforms were recorded and averaged. The subjects covertly counted the target tones and the total count was noted down at the end of the session. The electro-oculogram was not recorded, but subjects were asked to keep their eyes closed during testing. At the end of signal acquisition, ERP measurements were made and N1, P2, N2 and P300 components were quantified by one of the authors (VW) who was blind to the subjects’ exposure status. For each component, N1, P2, N2, and P300, the maximum negative or positive peak in fixed latency windows was determined. The search latency windows for each component were for N1, 60 - 160ms, for P2, 120 - 250ms, for N2, 170 - 340ms and for P300, 250 – 600ms. P300 amplitude was obtained by measuring the difference between N2 and the P300 peaks.
Data Analysis
The difference between the target tone count made by the subject and the actual target count (i.e. 50) was calculated, and this counting error was compared between groups as a behavioural correlate of the accuracy of test performance. As all components were readily distinguished at the Cz site, between group and component comparisons were conducted on component latency measures at this site only. The data were analyzed using Statistical Package for the Social Sciences (SPSS™) for Windows version 17.0. ERP component latencies between the groups were compared initially with a mixed model analysis of variance (ANOVA) with one within-subjects factor of component (N1, P2, N2 and P300) and one between-subjects factor of group. Greenhouse-Geisser corrected degrees of freedom were used where appropriate. Subsequent comparisons of group differences of latencies of each component were made using Student’s t tests. A stepwise Bonferroni correction procedure described by Larzerele and Mulaik was used for multiple comparisons (Howell, 2001). Secondly, analysis of covariance (ANCOVA) was performed on P2, N2 and P300 components separately, taking into account the preceding component as a covariate, to partial out variance in the component of interest associated with variance in the immediately preceding component. Amplitude data for P300 were measured at both Cz and Pz sites and analysed using mixed model ANOVA with within-subjects factor of site (Cz and Pz) and between subjects of group.
Results
The age distributions of the two groups were similar (farmers: 50.1 ± 7.6 years, controls: 49.0 ± 7.2 years, p = 0.511). There was a significant positive correlation between age and P300 latency in the control group (r = 0.328, p = 0.045) and the farmers (r = 0.380, p = 0.032). None of the other components of the control group were significantly correlated with age. Among the farmers, N2 latency (r = 0.347, p = 0.048) was also significantly correlated with age.
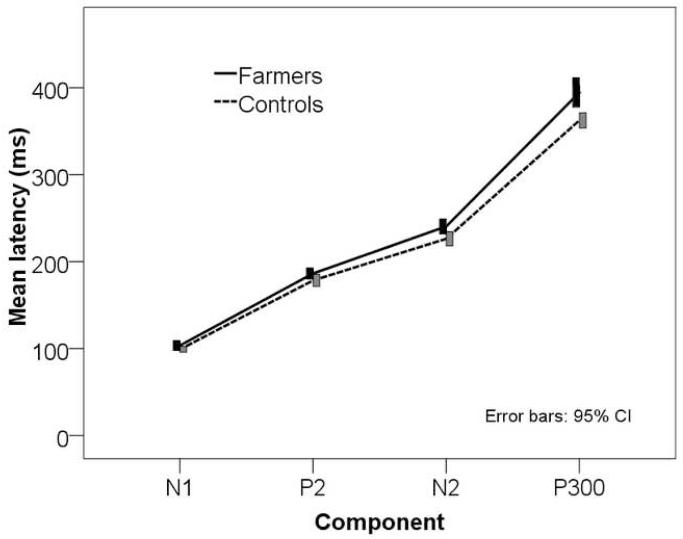
The farmers made significantly more counting errors compared to the controls in the oddball task (table 1). Figure 1 shows sample ERP waveforms from one subject from each group. Grand average waveforms of the two groups are shown in figure 2. In addition to significant main effects of group [F (1, 66) = 9.707, p = 0.003] and component [F (1.934, 127.6) = 2115.0, p < 0.001], the mixed model ANOVA of component latencies revealed a significant component × group interaction [F (1.934, 127.6) = 5.739, p = 0.005], suggesting greater intergroup differences in later ERP components (Figure 3). According to Larzelere and Mulaik procedure, the comparison of intergroup differences on individual components was started with Bonferroni correction for four groups at a significance level of 0.0125 (i.e. 0.05/4), and this showed a significant delay in P300 latency in the farmers. Then the N2 latencies of the two groups were compared at a significance level of 0.0167 (i.e. 0.05/3) and once again farmers showed a significant delay in the N2 latency. Intergroup comparison of latencies at the next step at a significance level of 0.025 did not show significant intergroup differences in either P2 or N1 latencies (table 1). The outcomes of ANCOVAs conducted on each of the P2, N2 and P300 latencies with the latency of the preceding component as a covariate are shown in table 2. When delay in the preceding N2 component was taken into account, the delay in farmers’ P300 latency was still significant [F (1, 66) = 7.107, p = 0.010]. Neither N2 nor P2 group differences were significant after adjustment for the preceding component latency.
Table 1.
Comparison of the vegetable farmers and control group (Counting error: the difference between the target tone count made by the subject and the actual target count.)
| Farmers (n=35) mean (SD) |
Controls (n=38) mean (SD) |
Significance (p value) |
Mean difference (95% CI) |
|
|---|---|---|---|---|
| N100 latency (ms) | 103.5 (16.5) | 99.1 (10.5) | 0.173 | 4.4 (−2.0 – 10.8) |
| P200 latency (ms) | 186.4 (18.0) | 178.4 (21.4) | 0.094 | 8.0 (1.4 – 17.4) |
| N200 latency (ms) | 240.5 (24.4) | 226.3 (25.4) | 0.019 | 14.2 (2.4 – 25.9) |
| P300 latency (ms) | 394.5 (47.0) | 362.4 (27.0) | 0.0006 | 32.1 (14.1 – 50.0) |
| P300 amplitude (μV), Cz | 14.9 (8.6) | 12.1 (5.7) | 0.108 | 2.8 (−0.6 – 6.2) |
| P300 amplitude (μV), Pz | 12.4 (5.1) | 11.9 (5.2) | 0.670 | 0.5 (−1.9 – 3.0) |
| Counting errors | 4.8 (3.6) | 3.2 (2.7) | 0.044 | 1.5 (0.4 – 3.0) |
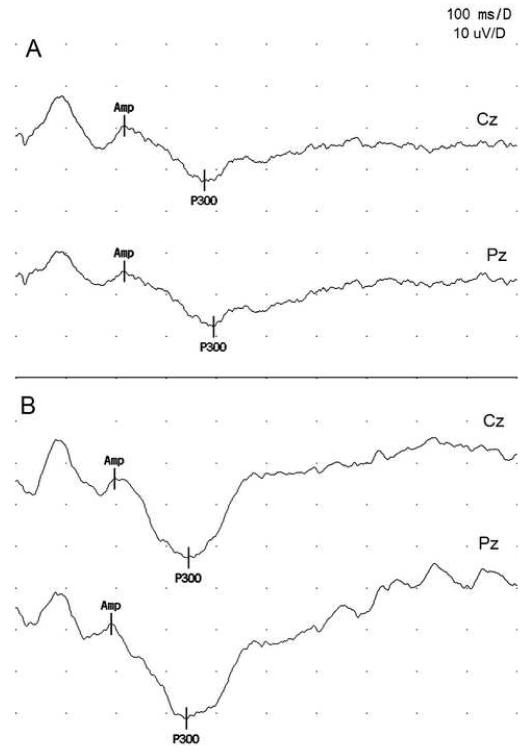
Figure 1.
Sample ERP waveforms for target tones: (A) a vegetable farmer and (B) a control subject.
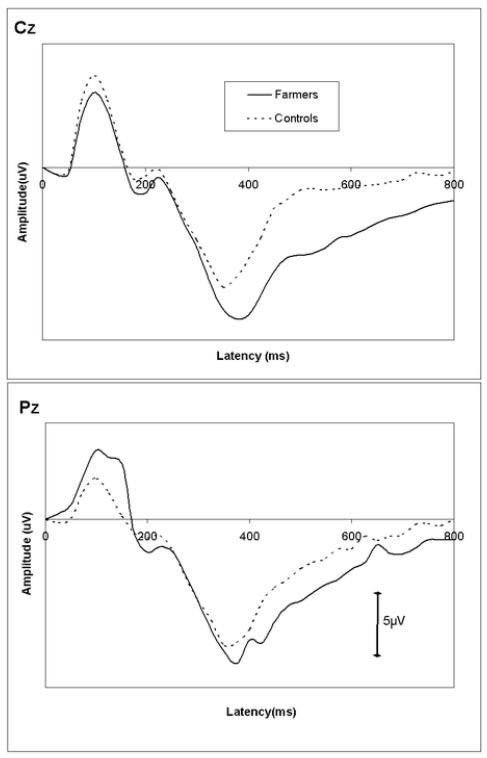
Figure 2.
Grand average waveforms for target tones in farmers and the controls in Cz and Pz positions.
Figure 3.
Mean ERP component latencies of farmers and the control group
Table 2.
Intergroup differences of ERP latencies, adjusted for the differences in preceding ERP components.
| ERP component | covariate component |
F | Significance (p) |
|---|---|---|---|
| P200 | N100 | 0.835 | 0.364 |
| N200 | P200 | 2.749 | 0.102 |
| P300 | N200 | 7.107 | 0.010 |
ANOVA of P300 amplitudes of Cz and Pz sites did not show a significant group main effect [F (1, 68) = 1.426, p > 0.237)] but there was a significant site × group interaction [F (1, 68) = 5.113, p = 0.009]. Testing of simple group effects did not show significant intergroup differences at either site (table 1). However, testing simple effects of the site revealed that farmers had a significantly larger P300 at Cz than that at Pz (t = 2.631, p = 0.013). Such difference was not observed in the control group.
Discussion
We studied cognitive sequelae of long-term occupational exposure to OP pesticides, using auditory oddball ERPs, which provide a neurophysiological correlate of cognitive processing of information. The test group, i.e., the farmers who are exposed to OP pesticides, made more counting errors compared to the control group suggesting impaired accuracy of stimulus classification. The main analysis of absolute ERP latencies in our data also shows a trend of progressively increasing delay in successive ERP components in the vegetable farmers with long-term exposure to OP pesticides (Table 1 and figure 3). Intergroup comparison of individual components showed significantly delayed N2 and P300 latencies in the farmers compared to the control group. The latencies of the earlier ERP components N1 and P2 were similar between the groups and there was no significant overall intergroup difference in P300 amplitudes, although there was a suggestion of a more anterior distribution of P300 in farmers.
Two previous studies report long-lasting ERP changes following acute large-dose OP intoxication (Murata et al. 1997, Dassanayake et al. 2008). Murata et al. (1997) tested a group of victims 6 months after the Tokyo subway sarin attack and Dassanayake et al. (2008) tested the patients with acute OP pesticide poisoning showed prolongation of P300 latency 6 months after the episode, and in line with the present study, both these previous studies showed delayed P300 latencies in the exposed groups. N1 latency was similar between the test and the control groups in both studies. P2 and N2 component latencies were not reported by Murata et al. (1997) and were found to be similar in the patients and the controls in the Dassanayake et al. (2008) acute study.
From a behavioural perspective, our results suggest that different levels of attentional processing may be susceptible to chronic OP exposure. ERP components N1, P2 and N2 are elicited both by attended and unattended stimuli, and represent early stages of attention-related processing, although there are controversies as to how much attentional resources are actively utilised at these stages (Näätänen, 1992, Rugg and Coles, 1995; Crowley and Colrain, 2004). There was an increasing delay in the successive ERP component latencies (as evident in group × component interaction) and this was significant at the stage of N2. However, ANCOVA that considered the latency of the preceding component as a covariate, did not show a significant difference in N2 latencies when the latency of the preceding component was eliminated. Thus the N2 delay in our test group is more likely to suggest a cumulative slowing of early stages of attentional processing. In contrast, farmers showed a significant delay in P300 latency, even when the effect of the immediately preceding ERP component latency was eliminated through ANCOVA. That is, the delay in P300 latency in farmers cannot be accounted for solely by the delay in N2 and therefore, there are effects of OP exposure on P300 latency over and above any detrimental OP effects on earlier components. This effect on P300 component reflects significant impairment in active processing of information.
The context-updating model describes P300 component elicited by a two-stimulus ‘oddball’ paradigm as a reflection of attentional processing and updating of working memory (Donchin and Coles, 1988; Polich and Criado, 2006; Polich, 2007). According to this model, the sensory information of the stimuli enters the processing systems, and the working memory systems compare the current signal with the neural representation of the previous stimulus. If the incoming signal is the same, the representation is unchanged and the signal elicits only the ERP components of early cortical processing. If the new stimulus is different, the attentional resources are selectively allocated to the novel stimulus and the existing neural representation in the working memory system is updated. P300 amplitude correlates with the amount of neural resources in the attentional system that is allocated for the task performance, and P300 latency reflects the speed of allocation of the available neural resources. Thus according to this model, P300 latency is considered a measure of stimulus classification speed which reflects the time required to detect and evaluate the target stimulus (Kutas et al., 1977; McCarthy and Donchin, 1981). In this cognitive framework, our findings suggest that chronic subclinical exposure to OP pesticides may adversely affect updating of working memory and to delayed stimulus evaluation. These findings corroborate the results of several neurobehavioral studies that report impaired selective attention and reaction time in chronic occupational exposure to OP pesticides (Stephens et al., 1995; Fiedler et al., 1997; Bazylewicz-Walczak et al., 1999; Srivastava et al., 2000).
All the OP pesticides to which the farmers in the test group were exposed are cholinesterase inhibitors. However, none of the farmers in our sample handled OP pesticides during four days preceding the test and none of them showed cholinergic features of OP intoxication, thus at the time of testing they were not having at least clinically evident cholinergic effects of any OP exposure. It is the anticholinergic agents such as scopolamine that are known to reduce the amplitude and increase the latency of P300 (Callaway et al., 1985; Hammond et al., 1987; Meador et al., 1987) rather than cholinergic agents such as OPs. Therefore, in chronic low-level exposure, the OP compounds do not appear to affect the cognitive functions by simply increasing acetylcholine levels through acetylcholinesterase inhibition in cerebral cholinergic neural circuits.
Similar to our findings, delay in N2 and P300 latencies have been observed in diseases classified as subcortical dementias including Parkinson’s disease and Huntington’s disease (Goodin and Aminoff, 1986) HIV encephalopathy (Messenheimer et al., 1992, Arendt et al., 1993), corticobasal degeneration (Takeda et al., 1998) and progressive supranuclear palsy (Johnson et al., 1991, Takeda, et al., 1998). However, we did not find a significant amplitude reduction which was observed in some of the above studies (Johnson et al., 1991, Arendt et al., 1993). Johnson et al. also observed that there were no topographic differences in P300 amplitude reduction in patients, and propose that the neural structures responsible for generating these potentials were intact in these patients. In contrast, we found a group × site interaction of P300 amplitude, and significantly greater amplitude of P300 at Cz compared to that at Pz only in farmers. This enhancement of P300 in relatively anterior site in OP-exposed group may be interpreted either as an interference by an anteriorly generated P3a (novelty P3) component or as anterior shift of P300 sources, although the validity of these interpretations are severely restricted as recordings are available only at two scalp sites.
The ERP findings of our study are restricted by some technical limitations. The ERP components were identified based on the waveforms recorded at only two scalp locations. EOG was not recorded and there was no method of artefact rejection following data acquisition. Thus there is a possibility of data being contaminated, mainly with subvocalisation artefacts and eye movement artefacts. However, blinking artefacts were eliminated by instructing subjects to keep their eyes closed during the task.
In conclusion, the ERP findings of the present study suggest that chronic exposure to OP pesticides may delay the neurophysiological processes underlying early stages of selective attention and late stages of sensory information processing that include stimulus evaluation and updating of working memory and the behavioural findings suggest that they also impair the accuracy of stimulus classification. These findings corroborate the neurobehavioural findings of humans chronically exposed to OP pesticides. Our findings warrant further research on the role of ERPs as an electrophysiological marker of subclinical neurocognitive deficits and a predictor of neuropsychiatric sequelae of chronic low-level exp osure to OP compounds.
Acknowledgements
Financial support for this study was provided by South Asian Clinical Toxicology Research Collaboration (SACTRC), Faculty of Medicine, University of Peradeniya, Sri Lanka (Grant no: GR071669MA). The authors thank P. Michie for her invaluable support in data analysis revisions of the manuscript, and S. Bandara for the assistance provided during data collection.
Footnotes
The authors have no conflicts of interest to disclose.
References
- Ames RG, Steenland K, Jenkins B, Chrislip D, Russo J. Chronic neurologic sequelae to cholinesterase inhibition among agricultural pesticide applicators. Arch Environ Health. 1995;50(6):440–444. doi: 10.1080/00039896.1995.9935980. [DOI] [PubMed] [Google Scholar]
- Arendt G, Hefter H, Jablonowski H. Acoustically evoked event-related potentials in HIV-associated dementia. Electroencephalogr Clin Neurophysiol. 1993;86(3):152–60. doi: 10.1016/0013-4694(93)90002-d. [DOI] [PubMed] [Google Scholar]
- Bazylewicz-Walczak B, Majczakowa W, Szymczak M. Behavioral effects of occupational exposure to organophosphorous pesticides in female greenhouse planting workers. Neurotoxicol. 1999;20:819–826. [PubMed] [Google Scholar]
- Callaway E, Halliday R, Naylor H, Schechter G. Effects of oral scopolamine on human stimulus evaluation. Psychopharmacology (Berl) 1985;85(2):133–138. doi: 10.1007/BF00428401. [DOI] [PubMed] [Google Scholar]
- Catano HC, Carranza E, Huamani C, Hernandez AF. Plasma Cholinesterase Levels and Health Symptoms in Peruvian Farm Workers Exposed to Organophosphate Pesticides. Arch Environ Contam Toxicol. 2007;55(1):153–159. doi: 10.1007/s00244-007-9095-0. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115(4):732–44. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Daniell W, Barnhart S, Demers P, Costa LG, Eaton DL, Miller M, Rosenstock L. Neuropsychological performance among agricultural pesticide applicators. Environ Res. 1992;59(1):217–228. doi: 10.1016/s0013-9351(05)80241-5. [DOI] [PubMed] [Google Scholar]
- Dassanayake T, Weerasinghe V, Dangahadeniya U, Kularatne K, Dawson A, Karalliedde L, Senanayake N. Long-term event-related potential changes following organophosphorus insecticide poisoning. Clin Neurophysiol. 2008;119(1):144–150. doi: 10.1016/j.clinph.2007.09.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med. 2003;60:279–286. doi: 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler N, Kipen H, Kelly-McNeil K, Fenske R. Long-term use of organophosphates and neuropsychological performance. Am J Ind Med. 1997;32(5):487–496. doi: 10.1002/(sici)1097-0274(199711)32:5<487::aid-ajim8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Aminoff MJ. Electrophysiological differences between subtypes of dementia. Brain. 1986;109(Pt 6):1103–13. doi: 10.1093/brain/109.6.1103. [DOI] [PubMed] [Google Scholar]
- Hammond EJ, Meador KJ, Aung-Din R, Wilder BJ. Cholinergic modulation of human P3 event related potentials. Neurology. 1987;37:346–350. doi: 10.1212/wnl.37.2.346. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Munte TF, Kutas M, Butler SR, R N, Goodin DS. Cognitive event-related potentials. Electroenceph clin Neurophysiol Suppl. 1999;52:91–95. [PubMed] [Google Scholar]
- Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Annu Rev Psychol. 1983;34:33–61. doi: 10.1146/annurev.ps.34.020183.000341. [DOI] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods for Psychology. 5th ed Wadsworth; 2001. [Google Scholar]
- IPCS. IPoCS . Neurotoxicity risk assessment for human health: principles and approaches. World Health Organization (WHO); Geneva: 2001. Environmental Health Criteria 223. [Google Scholar]
- Jamal GA. Neurological syndromes of organophosphorus compounds. Adverse Drug React Toxicol Rev. 1997;16(3):133–170. [PubMed] [Google Scholar]
- Jamal GA, Hansen S, Julu PO. Low level exposures to organophosphorus esters may cause neurotoxicity. Toxicology. 2002;181-182:23–33. doi: 10.1016/s0300-483x(02)00447-x. [DOI] [PubMed] [Google Scholar]
- Jamal GA, Hansen S, Pilkington A, Buchanan D, Gillham RA, Abdel-Azis M, Julu PO, Al-Rawas SF, Hurley F, Ballantyne JP. A clinical neurological, neurophysiological, and neuropsychological study of sheep farmers and dippers exposed to organophosphate pesticides. Occup Environ Med. 2002;59(7):434–441. doi: 10.1136/oem.59.7.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Litvan I, Grafman J. Progressive supranucler palsy - altered sensory processing leads to degraded cognition. Neurology. 1991;41(8):1257–62. doi: 10.1212/wnl.41.8.1257. [DOI] [PubMed] [Google Scholar]
- Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112(9):950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. Electroenceph clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: A comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Adams RJ, Patel BR, Davis HC, Hammond EJ. Central cholinergic systems and the auditory P3 evoked potential. Int J Neurosci. 1987;33:199–205. doi: 10.3109/00207458708987404. [DOI] [PubMed] [Google Scholar]
- Messenheimer JA, Robertson KR, Wilkins JW, Kalkowski JC, Hall CD. Event-related potentials in human-immunodeficiency-virus infection - a prospective-study. Arch Neurol. 1992;49(4):396–400. doi: 10.1001/archneur.1992.00530280086027. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Osman AM, Irwin DE, Yantis S. Modern mental chronometry. Biol Psychol. 1988;26(1-3):3–67. doi: 10.1016/0301-0511(88)90013-0. [DOI] [PubMed] [Google Scholar]
- Murata K, Araki S, Yokoyama K, Okumura T, Ishimatsu S, Takasu N, White RF. Asymptomatic sequelae to acute sarin poisoning in the central and autonomic nervous system 6 months after the Tokyo subway attack. J Neurol. 1997;244:601–606. doi: 10.1007/s004150050153. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Attention and Brain Function. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rodnitzky RL. Occupational exposure to organophosphate pesticides: a neurobehavioral study. Arch Environ Health. 1975;30(2):98–103. doi: 10.1080/00039896.1975.10666651. [DOI] [PubMed] [Google Scholar]
- Rugg M, Coles MGH. Electrophysiology of mind: Event-related brain potentials and cognition. Oxford University Press; Oxford, England: 1995. [Google Scholar]
- Srivastava AK, Gupta BN, Bihari V, Mathur N, Srivastava LP, Pangtey BS, Bharti RS, Kumar P. Clinical, biochemical and neurobehavioural studies of workers engaged in the manufacture of quinalphos. Food Chem Toxicol. 2000;38(1):65–69. doi: 10.1016/s0278-6915(99)00123-4. [DOI] [PubMed] [Google Scholar]
- Stallones L, Beseler C. Pesticide illness, farm practices, and neurological symptoms among farm residents in Colorado. Environ Res. 2002;90(2):89–97. doi: 10.1006/enrs.2002.4398. [DOI] [PubMed] [Google Scholar]
- Steenland K, Dick RB, Howell RJ, Chrislip DW, Hines CJ, Reid TM, Lehman E, Laber P, Krieg EF, Jr., Knott C. Neurologic function among termiticide applicators exposed to chlorpyrifos. Environ Health Perspect. 2000;108(4):293–300. doi: 10.1289/ehp.00108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Spurgeon A, Calvert IA, Beach J, Levy LS, Berry H, Harrington JM. Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet. 1995;345:1135–1139. doi: 10.1016/s0140-6736(95)90976-1. [DOI] [PubMed] [Google Scholar]
- Tahmaz N, Soutar A, Cherrie JW. Chronic fatigue and organophosphate pesticides in sheep farming: a retrospective study amongst people reporting to a UK pharmacovigilance scheme. Ann Occup Hyg. 2003;47(4):261–267. doi: 10.1093/annhyg/meg042. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tachibana H, Okuda B, Kawabata K, Sugita M. Electrophysiological comparison between corticobasal degeneration and progressive supranuclear palsy. Clin Neurol Neurosurg. 1998;100(2):94–8. doi: 10.1016/s0303-8467(98)00007-9. [DOI] [PubMed] [Google Scholar]