Abstract
Objective
To determine prolonged effects of organophosphorus (OP) insecticide poisoning on cognitive event-related potentials (ERPs).
Method
ERPs of a group of 32 patients recovered from cholinergic phase of OP insecticide poisoning were compared with those of two matched control groups: 32 healthy volunteers and nine patients hospitalised with paracetamol overdose. A follow-up assessment was done in 21 patients (66% of the initial sample) six months after OP intoxication and the findings were compared with their initial ERP data.
Results
Patients showed highly significant prolongation of P300 latency, compared to healthy controls (p=0.003) and the controls with paracetamol overdose (p=0.016). Follow-up ERP findings of the patients revealed that this impairment remained unchanged even six months after OP poisoning (p=0.790). There was no significant difference in N100, P200 and N200 latencies or P300 amplitude either among the groups or between the two assessments of the patients with OP poisoning.
Conclusion
Our results suggest that acute OP poisoning causes a delay in cognitive processes involved in stimulus classification, lasting at least for six months.
Significance
These findings highlight the possibility of development of long-lasting cognitive deficits following OP insecticide poisoning, and warrant longer-term prospective studies to determine whether this impairment is permanent.
Keywords: organophosphorus insecticide poisoning, cognitive effects, P300, event-related potentials
Introduction
Long-term central nervous system effects of organophosphorus (OP) poisoning have been studied for over four decades (Gerson and Shaw, 1961; Dille and Smith, 1964; Metcalf and Homes; 1969; Savage et al., 1988; Rosenstock et al., 1991; Steenland et al., 1994; Wesseling et al., 2002). Many studies report neurological symptoms among those exposed, without demonstrating significant impairment in more objective tests which quantify the deficits of different components of cognitive functions. The results of the four large scale epidemiological studies on chronic neuropsychological effects of acute OP pesticide poisoning are inconclusive (Savage et al., 1988; Rosenstock et al., 1991; Steenland et al., 1994; Wesseling et al., 2002). Two of these studies have reported significantly impaired sustained attention in the patients (Rosenstock et al., 1991; Steenland et al., 1994) whilst one showed no difference between the patients and the controls (Wesseling et al., 2002). Simple reaction time, which is an indicator of attention (Lezak, 1995), was not significantly impaired in the patients in all three studies where simple reaction time was measured (Rosenstock et al., 1991; Steenland et al., 1994; Wesseling et al., 2002). Some subsequent workers have questioned the validity of the symptomatology, objectivity of the testing methods, and the sensitivity of the existing objective testing tools in identifying any subtle impairment in cognitive functions (Steenland et al., 2000; Kamel & Hoppin, 2004).
Psychophysiological correlates of cognitive functions, such as cognitive event-related potentials (ERPs), have the advantage over symptomatic and behavioural data, as ERPs reflect the actual neural activity operating in cognitive processing of information. We selected the P300 component of the auditory ERP for assessment of cognitive deficits in patients with OP insecticide poisoning. According to the context-updating theory (Donchin and Coles, 1988; Polich and Criado, 2006; Polich, 2007), P300 reflects neural processes underlying attention and updating of working memory. Thus if OP insecticide poisoning leads to attention deficits as suggested by neuropsychological findings of previous studies (Rosenstock et al., 1991; Steenland et al., 1994; Wesseling et al., 2002), the alterations of the attentional processes should be reflected as changes in the P300 component. In fact, delayed P300 latency was found after exposure to the OP chemical warfare agent sarin (Murata et al., 1997). However, to date there are no data on the long-term auditory ERP changes following acute poisoning with OP insecticides, which is much commoner than exposure to OP chemical warfare agents (Eddleston, 2000).
We conducted a case-control study to determine whether there are changes in ERPs after clinical recovery from the acute cholinergic phase of poisoning, and a follow up assessment to determine whether OP insecticide poisoning leads to long-term changes of auditory P300 ERPs.
Methods
Participants
We tested patients with OP insecticide poisoning, admitted from January 2005 to September 2006 to the Teaching Hospital, Peradeniya, Sri Lanka. These patients were clinically assessed on admission and while inpatients. A reliable history of acute OP insecticide intake and clinical features of cholinergic over-activity (fasciculation, miosis, bradycardia, excessive sweating / secretions, dyspnea / lung signs, impaired consciousness) were the inclusion criteria.
The test group of patients with OP poisoning was compared with two control groups: (1) healthy control group: healthy volunteers from the community and (2) hospitalised control group: patients hospitalised with paracetamol overdose. This second group was added to match the general wellbeing and the psychological status of the test group, at the time of neurophysiological assessment. The drug paracetamol was chosen as it has no direct action on cognitive functions and thus does not affect the electrophysiological outcome measures by itself. All the controls were individually matched for age (nearest 5 years) and sex with the subjects in the test group. An equal number of matching healthy volunteers was enrolled from the community as the healthy control group. However, the patients admitted to the hospital with paracetamol poisoning were enrolled in the hospitalised control group only if they could be matched for sex and age with the test group on one-to-one basis.
Exclusion criteria: Patients and controls who had chronic exposure to OP compounds, hearing impairment, pre-existing illnesses or medication that may predispose to cognitive dysfunction or consumed more than the recommended upper levels of alcohol (men > 21Units/wk, women > 14Units/wk. one unit: equivalent of 10ml of alcohol) were excluded (International Programme on Chemical Safety, 2001). Acute OP poisoning in the past was also an exclusion criterion for the controls.
The test group and the healthy control group had 32 participants each (20 men and 12 women). The age of the test subjects ranged from 14 to 56 years, while that of the healthy control group ranged from 15 to 52 years. The paracetamol poisoned control group had nine participants (two men and seven women) ranging from 14 to 27 years of age.
Ethical Considerations
Ethical clearance was granted by the Research and Ethical Review Committee, Faculty of Medicine, University of Peradeniya, Sri Lanka. Informed written consent was obtained from the patients and the controls.
Procedures
Details of the episode of poisoning, clinical features, management measures and any major complications with the potential to cause brain damage were entered in a structured data sheet, in OP exposed patients and hospitalised controls while they were in the hospital. Presence of systemic intoxication was clinically confirmed by the presence of the signs of cholinergic crisis. All patients had ingested the OP insecticides as a single bolus, within few minutes. In the instances where reliable information was available, the amount of OP ingested was roughly estimated using the information given by the patient/other informants and the concentration of known OP formulations. There were no facilities to assess the levels of OP insecticides in blood. Auditory P300 ERPs were initially recorded after clinical recovery from the acute cholinergic phase, usually on the day of discharge from the hospital. Recovery from acute poisoning was considered complete when the patient was free of all signs and symptoms of the acute cholinergic syndrome and free of any medication (such as atropine) given to manage the acute phase of poisoning. The controls with paracetamol overdose were tested on the day of discharge from the ward and healthy controls were tested on an appointment basis. In the test group, a follow-up ERP assessment was done on an outpatient basis about 6 months after OP poisoning. Twenty-one patients (13 men and 8 women) attended the follow up assessment.
In order to minimize the effect of short-term factors modifying the information processing speed, It was ensured that the subjects abstained from alcohol, tobacco and coffee in the 24 hours prior to the test and advised them to have at least six hours of sleep the night before testing (International Programme on Chemical Safety, 2001).
ERP assessment was done in the Clinical Neurophysiology Laboratory, Teaching Hospital, Peradeniya. Auditory oddball paradigm (standard tone: 70dB, 1000Hz, probability 80%. target tone: 70dB, 2000Hz, probability 20%) was applied to elicit auditory P300 ERP, conforming to the IFCN (International Federation of Clinical Neurophysiologists) guidelines (Heinze et al., 1999). Stimuli were presented binaurally through headphones at random intervals ranging from one to two seconds, in a random order. The duration of each stimulus was 120 ms with rise and fall times of 10ms each. ERPs were recorded over the vertex (Cz position), while placing combined reference electrodes over mastoids and the ground electrode on Fp position, conforming to international 10-20 scalp electrode placement standards (Klem et al., 1999). The high frequency filter was set at 50Hz and the low frequency filter was set at 0.2Hz. The recording epoch was 1000ms and the sensitivity was 10μV per division. The sampling rate was 48kHz. Each subject was exposed to 250 stimuli (~ 200 standard tones and ~50 target tones) and the ERP waveforms were recorded and averaged using a Medtronic Keypoint Portable™ signal averaging machine. Targets were covertly counted by the subjects and the totals were accurate to within +/− 5 of the 50 targets given. Electro-oculogram was not recorded, but subjects were asked to keep their eyes closed during testing. ERP measurements were made by an unblinded experimenter. At the end of signal acquisition, N100, P200, N200 and P300 components were quantified. In each recording, the positive waveform between 250 and 750ms was identified as P300. The peak of this waveform was marked as P300 when there was a single peak. In the instances where this positive waveform was bifid, the latter peak was marked as P300. P300 amplitude was obtained by measuring the difference between N200 and the P300 peaks.
Data analysis
The data were analyzed using Statistical Package for the Social Sciences (SPSS™) for Windows version 10.0. ERP measurements are presented as means and standard deviations, and the values of the test and the healthy control groups were compared with Student’s non-paired t-test. The ERP data of the subgroup of patients with OP poisoning, the matched controls with paracetamol poisoning and the matched healthy controls were initially compared with one-way ANOVA and the post-hoc analysis was done with Bonferroni test. The outcome measures of OP poisoned group in the two occasions were compared using paired t-test. All tests were two-sided and assessed at the 5% significance level.
Results
Of the patients admitted with suspected OP insecticide poisoning from January 2005 to September 2006, 44 fulfilled the inclusion criteria of the study. Of those we excluded nine patients: seven on account of excessive intake of alcohol and two because of previous chronic exposure to OP insecticides. Of the 35 eligible patients, three could not be tested, because they left the hospital prematurely. Accordingly, 32 patients were enrolled.
The OP insecticide formulations taken by the 32 patients included chlorpyrifos (14 patients), dimethoate (4 patients), phenthoate (4 patients), fenthion (2 patients), malathion (2 patients), coumaphos (1 patient), oxydemeton methyl (1 patient) and profenofos (1 patient). The type of OP was not known in 3 instances. All 32 patients had ingested an OP insecticide, 31 intentionally and one accidentally. The 31 patients with intentional ingestion were seen by a psychiatrist. In 25 of them, poisonings were categorized as impulsive acts of self-harm, and they were not on any medication on the day of testing. Six patients (i.e. 19% of the total) were diagnosed as having depression and were on antidepressant fluoxetine at the time of testing. Patients were first tested at a median duration of 12 days after poisoning (range: from 5 to 45 days), depending on the time for recovery from the acute stage of poisoning. The duration of hospital stay in this group ranged from 5 to 31 days, with a median of 7 days. All the test subjects received atropine during their hospital stay. The median duration between the last dose of atropine and the date of neurophysiological assessment was six days (range: 1 to 42 days). All of them, but two, were treated with pralidoxime. They were tested after a median duration of seven days (range: 1 to 42 days) after their last dose of pralidoxime. Of the 32 patients enrolled in the study, 21 (i.e. 66%) attended the follow up assessment, after a median duration of 182 days (range: from 124 to 402 days) of poisoning.
All nine control subjects hospitalised with paracetamol overdose were seen by a psychiatrist. Eight poisonings were categorized as impulsive acts of self-harm, and those patients were not on any drugs on the day of testing. One subject was diagnosed as having depression, and was on fluoxetine at the time of testing. None of the patients with paracetamol overdose developed liver failure or hepatic encephalopathy that could affect cognitive functions. These patients were tested at a median duration of 3 days after poisoning (range: from 2 to 6 days). There were 32 age- and sex- matched individuals in the healthy control group.
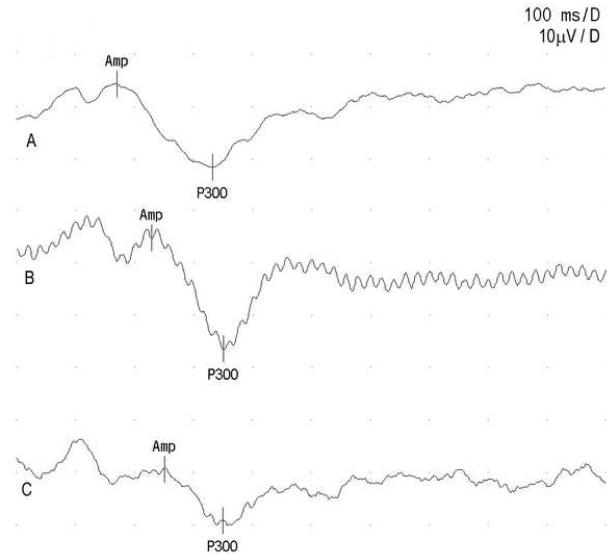
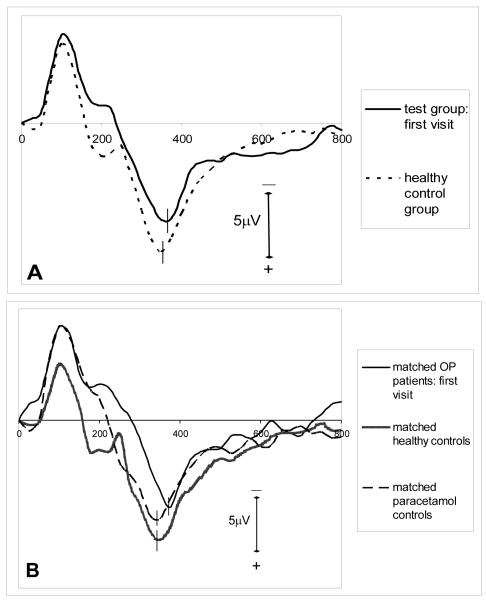
Three sample ERP waveforms are shown in figure 1. Initial ERP measurements in the test group are compared with those of the healthy control group in table 1, and the grand average ERP waveforms for target tones of these two groups are shown in figure 2A. The test group had a significantly prolonged P300 latency compared to healthy control group, but there was no significant difference in P300 amplitude or the latencies of early ERP components. Comparison of the outcome measures of the subgroup of OP intoxicated patients, matched controls with paracetamol overdose and the subgroup of matched healthy controls using one-way ANOVA showed significant intergroup differences in P200 and P300 latencies (table 2). N100 and N200 latencies and the P300 amplitudes were similar among the groups. Subsequent multiple comparisons with Bonferroni test revealed a significant delay in P300 latency in the patients with OP poisoning in comparison to both control groups (table 3). The ERP grand averages of these groups are depicted in figure 2B.
Figure 1.
Sample ERP waveforms. A) a patient with OP poisoning, B) a healthy control subject, C) a control subjects with paracetamol overdose
Table 1.
Comparison of the patients with OP poisoning (first visit) and matched healthy controls.
| Test group (n=32) mean (SD) |
Healthy controls (n=32) mean (SD) |
Significance (p value) |
Mean difference (95% CI) |
|
|---|---|---|---|---|
| Age (years) | 24.2 (9.0) | 23.7 (8.8) | 0.82 | −0.50 (−4.0 – 5.0) |
| N100 latency (ms) | 109.9 (12.2) | 105.0 (12.7) | 0.17 | 3.6 (−2.3 – 12.2) |
| P200 latency (ms) | 180.2 (27.3) | 185.9 (21.2) | 0.46 | −5.7 (−20.9 – 9.6) |
| N200 latency (ms) | 230.9 (29.1) | 236.6 (28.7) | 0.50 | −5.7 (−22.9 – 11.4) |
| P300 latency (ms) | 387.0 (65.3) | 347.1(34.9) | 0.003 | 39.9 (13.7 – 66.0) |
| P300 amplitude (μV) | 11.35 (6.81) | 11.23 (5.38) | 0.94 | 0.12 (−3.11 – 3.35) |
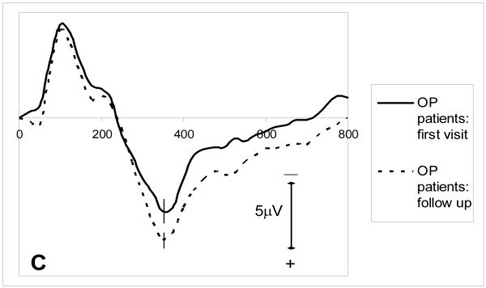
Figure 2.
grand average ERP waveforms for target tones: (A) test group (first visit) (n=32) and healthy control group (n=32) (B) subgroup of OP poisoned patients (first visit) (n=9), matched healthy controls (n=9) and matched paracetamol controls (n=9) (C) first visit and the follow up recordings of the OP poisoned patients who were followed up (n=21).
Table 2.
Intergroup comparison (one-way ANOVA) of ERP components.
| Patients with OP poisoning (n=9) mean (SD) |
Matched controls with paracetamol poisoning (n=9) mean (SD) |
Matched healthy controls (n = 9) mean (SD) |
F | Significance (p) |
|
|---|---|---|---|---|---|
|
N100 latency
(ms) |
104.9 (11.9) | 105.7 (8.4) | 104.4 (16.6) | 0.21 | 0.979 |
|
P200 latency
(ms) |
167.7 (15.5) | 160.8 (16.8) | 183.8 (17.9) | 3.65 | 0.47 |
|
N200 latency
(ms) |
236.0 (27.4) | 214.0 (26.4) | 232.7 (22.4) | 1.55 | 0.238 |
|
P300 latency
(ms) |
385.3 (34.7) | 341.0 (22.4) | 344.9 (25.4) | 6.93 | 0.004 |
|
P300 amplitude
(μV) |
11.81 (5.40) | 12.79 (6.99) | 11.1 (5.96) | 0.17 | 0.842 |
Table 3.
Significance levels (p values) of multiple comparisons of ERP component (post-hoc Bonferroni test results).
| Comparison | Patients with OP poisoning vs. matched paracetamol controls |
Patients with OP poisoning vs. matched healthy controls |
Paracetamol controls vs. matched healthy controls |
|---|---|---|---|
|
P200 latency
(ms) |
0.265 | 0.468 | 0.058 |
|
P300 latency
(ms) |
0.016 | 0.008 | 1.000 |
The results of the 21 OP poisoned patients who participated in the follow up assessment are compared with their initial ERP findings in table 4, and the grand averages are shown in figure 2C. The latencies of the early ERP components and the P300 amplitude remained normal after six months. The mean P300 latency six months after poisoning was similar to the initial latency, showing no significant improvement.
Table 4.
Comparison of initial and follow-up P300 data of the patients with OP poisoning.
| 1st assessment (n=21) |
Follow-up (n=21) |
Significance (p value) |
Mean difference (95% CI) |
|
|---|---|---|---|---|
|
N100 latency, mean
(SD), (ms) |
108.6 (12.2) | 106.2 (13.3) | 0.29 | 2.4 (−2.2 – 7.0) |
|
P200 latency, mean
(SD), (ms) |
176.2 (27.5) | 174.3 (21.6) | 0.70 | 1.9 (−8.5 – 12.2) |
|
N200 latency, mean
(SD), (ms) |
230.0 (36.3) | 225.5 (28.6) | 0.60 | 4.5 (−13.4 – 22.3) |
|
P300 latency, mean
(SD), (ms) |
366.1 (37.1) | 364.0 (34.9) | 0.79 | 2.2 (−15.1 – 19.4) |
|
P300 amplitude, mean
(SD), (μV) |
12.60 (7.48) | 13.23 (7.47) | 0.73 | −0.62 (−4.32 – 3.07) |
Discussion
We assessed the psychophysiological correlates of auditory information processing using auditory ERPs in patients with acute exposure to OP insecticides. The results indicate that, in comparison to matched controls, P300 latency is prolonged in the patients even after clinical recovery from the acute cholinergic phase of intoxication, and this impairment does not improve even after six months. However, normal N100, P200 and N200 components suggest that there is no effect of OP on the early stages of auditory information processing. These findings are consistent with those of Murata et al. who found prolonged P300 latency but no difference in N100 latency among the victims of the Tokyo subway sarin attack, six months after the incident (Murata et al., 1997). Similar results have been reported by Misra et al., who demonstrated prolonged P300 latency and unchanged P300 amplitude in workers engaged in spraying fenthion, an OP pesticide (Misra et al., 1994). However, the present study has demonstrated, for the first time, long-lasting P300 changes following acute OP insecticide poisoning.
The context-updating model describes P300 component elicited by a simple two-stimulus ‘oddball’ paradigm as a reflection of attentional processing and updating of working memory (Donchin and Coles, 1988; Polich and Criado, 2006; Polich, 2007). According to this theory, the sensory signals of the stimuli enter the processing systems and the working memory systems compare the current stimulus with the neural representation of the previous stimulus. If the incoming stimulus is the same, the representation is unchanged and only the initial sensory processing is reflected as N100, P200 and N200 components of the ERPs. If the new stimulus is different, the attentional resources are selectively allocated to the novel stimulus and the existing neural representation in the working memory system is updated. P300 amplitude correlates with the amount of neural resources in the attentional system that is allocated for the task performance. This is quite distinct from the P300 latency which reflects the speed of allocation of whatever the neural resources available. According to this model, P300 latency is considered a measure of stimulus classification speed which reflects the time required to detect and evaluate the target stimulus (Kutas et al., 1977; McCarthy and Donchin, 1981).
The results of this study provide objective and quantitative psychophysiological evidence to corroborate the positive neuropsychological findings of some previous cross-sectional studies that have reported impaired sustained attention among the patients with history of OP poisoning (Rosenstock et al., 1991; Steenland et al., 1994). However, simple reaction time, which is also an indicator of attention (Lezak, 1995), was not significantly impaired in the patients in all three previous studies where simple reaction time was measured (Rosenstock et al., 1991; Steenland et al., 1994; Wesseling et al., 2002). However, the mean duration between exposure and cognitive assessment was much longer in those studies (Rosenstock et al., 1991: 24 months. Steenland et al., 1994: 7 years. Wesseling et al., 2002: 27 months). Thus it is possible that the attentional impairment caused by acute OP intoxication may last for several months or so and patients recover gradually over a period of few years. Longer follow up of the OP poisoned patients with repeated ERP measurements would help to test this hypothesis.
Neuropathological findings of animal experiments corroborate the association between OP poisoning and ERP changes. Neuronal necrosis has been observed in multiple cortical and subcortical regions in experimental rats exposed to large acute doses of OP chemical warfare agents (Petras, 1981; Kadar et al., 1992, 1995) and pesticides (Veronesi et al., 1990). Damage to hippocampal formation and medial temporal lobe structures (Petras, 1981, Veronesi et al, 1990) is of particular importance as this region is thought to be one of the neural generators of P300 (Halgren et al.. 1980, 1995; Okada et al., 1983; McCarthey et al., 1989). It is not conclusive whether the brain cell necrosis is due to hypoxia following respiratory failure and seizure induced by OP compounds, as the histopathological changes observed in some studies are different from those caused by hypoxia (Petras, 1981). None of the patients in the present study developed clinically detectable seizures.
Although our study indicates that OP insecticides may lead to long-lasting impairment of cognitive processes, the variations in the type and amount of OP compound, comorbidities and the treatment measures may have confounded our results to a certain extent. Six patients (i.e. 19%) in the test group and one control (11%) with paracetamol overdose were clinically depressed and were on fluoxetine at the time of ERP assessment. Depression is known to reduce P300 amplitude (Hurby, 2003). P300 latency changes in the studies adopted two-tone discrimination task report inconsistent results. Kraiuhin et al., (1990) found no significant difference in P300 latency between depressed patients and controls whereas Karaaslan et al., (2003) reported a study where P300 latency was prolonged in patients with major depression in the pre-treatment phase and normalized after antidepressant treatment. Fluoxetine 20mg/day was also found to reduce P300 amplitude in healthy volunteers (d’Ardhuy et al. 1999).
Anticholinergic drug scopolamine is known to reduce the amplitude and increase P300 latency, which may last for several hours after administration (Callaway et al., 1985; Hammond et al., 1987; Meador et al., 1989). However, in contrast to atropine, scopolamine has depressant effects on the central nervous system. Further, atropine has a half life of about four hours and these patients were tested a median duration of six days after discontinuation of atropine. Thus there would not have been any effective concentrations of atropine in the blood or brain and it is very unlikely for the drug to affect P300 measurements in our study group. Pralidoxime does not easily cross the blood-brain barrier and has no reported cognitive effects. Furthermore, the ERPs of the OP poisoned patients were initially recorded a median duration of seven days after their last dose of pralidoxime.
In conclusion, the ERP findings of this study suggest that acute OP insecticide poisoning may lead to a delay in stimulus classification, which in turn depends on attentional resources and the working memory systems of the brain. This impairment appears to persist even six months after poisoning. Although these deficits may not influence routine clinical assessments of cognition, sensory information processing speed may play a key role in critical situations where the circumstances demand rapid stimulus evaluation and response. Despite possible contamination by some confounding factors, our findings warrant longer-term follow up studies to determine the natural course of the impairment of cognitive processing of information following OP poisoning.
Acknowledgements
Financial support for this study was partly provided by South Asian Clinical Toxicology Research Collaboration (SACTRC), Faculty of Medicine, University of Peradeniya, Sri Lanka. SACTRC is funded by the Wellcome Trust and the Australian National Health and Medical Research Committee (Grant no: GR071669MA).
Footnotes
This study was carried out in Teaching Hospital, Peradeniya, Sri Lanka.
The authors have no conflicts of interest to disclose.
References
- Callaway E, Halliday R, Naylor H, Schechter G. Effects of oral scopolamine on human stimulus evaluation. Psychopharmacology (Berl) 1985;85(2):133–138. doi: 10.1007/BF00428401. [DOI] [PubMed] [Google Scholar]
- d’Ardhuy XL, Boeijinga PH, Renault B, Luthringer R, Rinaudo G, Soufflet L, Toussaint M, Macher JP. Effects of Serotonin-Selective and Classical Antidepressants on the Auditory P300 Cognitive Potential. Neuropsychobiology. 1999;40:207–213. doi: 10.1159/000026621. [DOI] [PubMed] [Google Scholar]
- Dille JR, Smith PW. Central nervous system effects of chronic exposure to organophosphate insecticides. Aerosp Med. 1964;35:325–334. [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 manifestation of context updating? Behav Brain Sci. 1988;11:357–374. [Google Scholar]
- Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–731. doi: 10.1093/qjmed/93.11.715. (2000) [DOI] [PubMed] [Google Scholar]
- Gerson S, Shaw FB. Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet. 1961;1:1371–1374. doi: 10.1016/s0140-6736(61)92004-9. (1961) [DOI] [PubMed] [Google Scholar]
- Halgren E, Squires NK, Wilson CL, Rohrbaugh JW, Babb TL, Crandall PH. Endogenous potentials generated in the human hippocampal formation and amygdala by infrequent events. Science. 1980;210:803–805. doi: 10.1126/science.7434000. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Marinkovic K, Devaux B, Vignal JP, Biraben A. Intracerebral potentials to rare target and distractor auditory and visual stimuli: II. Medial, lateral and posterior temporal lobe. Electroenceph clin Neurophysiol. 1995;94:229–250. doi: 10.1016/0013-4694(95)98475-n. [DOI] [PubMed] [Google Scholar]
- Hammond EJ, Meador KJ, Aung-Din R, Wilder BJ. Cholinergic modulation of human P3 event related potentials. Neurology. 1987;37:346–350. doi: 10.1212/wnl.37.2.346. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Munte TF, Kutas M, Butler SR, Näätänen R, Goodin DS. Cognitive event-related potentials. Electroenceph clin Neurophysiol Suppl. 1999;52:91–95. [PubMed] [Google Scholar]
- International Programme on Chemical Safety (IPCS) Neurotoxicity risk assessment for human health: principles and approaches. World Health Organization (WHO); Geneva: 2001. Environmental Health Criteria 223. [Google Scholar]
- Kadar T, Cohen G, Sarah R, et al. Long-term study of brain lesions following soman, in comparison to DFP and metrazol poisoning. Hum Exp Toxicol. 1992;11:517–523. doi: 10.1177/096032719201100613. (1992) [DOI] [PubMed] [Google Scholar]
- Kadar T, Shapira S, Cohen G, et al. Sarin induced neuropathology in rats. Hum Exp Toxicol. 1995;14:252–259. doi: 10.1177/096032719501400304. [DOI] [PubMed] [Google Scholar]
- Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112(9):950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. Electroenceph clin Neurophysiol Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed Oxford University Press; 1995. [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: A comparison of P300 latency and reaction time. Science. 1981;211:77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood C, Williamson P, Spencer D. Task-dependent field potentials in human hippocampal formation. J Neurosci. 1989;9:4253–4266. doi: 10.1523/JNEUROSCI.09-12-04253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ, Loring DW, Adams RJ, Platel BR, Davis HC, Hammond EJ. Central cholinergic systems and the auditory P3 evoked potential. Int J Neurosci. 1987;33:199–205. doi: 10.3109/00207458708987404. [DOI] [PubMed] [Google Scholar]
- Metcalf DR, Homes JH. EEG, psychological, and neurological alterations in humans with organophosphorous exposure. Ann NY Acad Sci. 1969;160:357–365. doi: 10.1111/j.1749-6632.1969.tb15857.x. [DOI] [PubMed] [Google Scholar]
- Misra UK, Prasad M, Pandey CM. A study of cognitive functions and event related potentials following organophosphate exposure. Electromyogr clin Neurophysiol. 1994;34(4):197–203. [PubMed] [Google Scholar]
- Murata K, Araki S, Yokoyama K, Okumura T, Ishimatsu S, Takasu N, White RF. Asymptomatic sequelae to acute sarin poisoning in the central and autonomic nervous system 6 months after the Tokyo subway attack. J Neurol. 1997;244:601–606. doi: 10.1007/s004150050153. [DOI] [PubMed] [Google Scholar]
- Okada YC, Kaufman L, Williamson SJ. The hippocampal formation as a source of the slow endogenous potentials. Electroencephal clin Neurophysiol. 1983;55:417–426. doi: 10.1016/0013-4694(83)90130-x. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007 doi: 10.1016/j.clinph.2007.04.019. doi:10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rosenstock L, Keifer M, Daniell WE, McConnell R, Claypoole K. Chronic central nervous system effects of acute OP pesticide intoxication. Lancet. 1991;338:223–227. doi: 10.1016/0140-6736(91)90356-t. [DOI] [PubMed] [Google Scholar]
- Savage EP, Keefe TJ, Mounce LM, Heaton RK, Lewis JA, Burcar PJ. Chronic neurological sequelae of acute OP pesticide poisoning. Arch Environ Health. 1988;43:38–45. doi: 10.1080/00039896.1988.9934372. [DOI] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14(3-4):199–224. [PubMed] [Google Scholar]
- Steenland K, Jenkins B, Ames RG, O’Malley M, Chrislip D, Russo J. Chronic neurological sequelae to OP pesticide poisoning. Am J Public Health. 1994;84:731–736. doi: 10.2105/ajph.84.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Dick RB, Howell RJ, Chrislip DW, Hines CJ, Reid TM, Lehman E, Laber P, Kreig EF, Jr., Knott C. Neurologic function among termiticide applicators exposed to chlorpyrifos. Environ Health Perspect. 2000;108:293–300. doi: 10.1289/ehp.00108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi B, Jones K, Pope C. The neurotoxicity of subchronic acetylcholinesterase (AChE) inhibition in rat hippocampus. Toxicol Appl Pharmacol. 1990;104:440–56. doi: 10.1016/0041-008x(90)90166-r. [DOI] [PubMed] [Google Scholar]
- Wesseling C, Keifer M, Ahlbom A, McConnell R, Moon J, Rosenstock L, Hogstedt C. Long-term neurobehavioral effects of mild poisonings with organophosphates and N-methyl carabamate pesticides in banana workers. Int J Occup Environ Health. 2002;8:27–34. doi: 10.1179/oeh.2002.8.1.27. [DOI] [PubMed] [Google Scholar]