Abstract
For more than three decades, the venom of the black widow spider and its principal active components, latrotoxins, have been used to induce release of neurotransmitters and hormones and to study the mechanisms of exocytosis. Given the complex nature of α-latrotoxin actions, this research has been continuously overshadowed by many enigmas, misconceptions and perpetual changes of the underlying hypotheses. Some of the toxin’s mechanisms of action are still not completely understood. Despite all these difficulties, the extensive work of several generations of neurobiologists has brought about a great deal of fascinating insights into presynaptic processes and has led to the discovery of several novel proteins and synaptic systems. For example, α-latrotoxin studies have contributed to the widespread acceptance of the vesicular theory of transmitter release. Presynaptic receptors for α-latrotoxin – neurexins, latrophilins and protein tyrosine phosphatase σ – and their endogenous ligands have now become centerpieces of their own areas of research, with a potential of uncovering new mechanisms of synapse formation and regulation that may have medical implications. However, any future success of α-latrotoxin research will require a better understanding of this unusual natural tool and a more precise dissection of its multiple mechanisms.
Keywords: α-latrotoxin, neurexin, latrophilin, protein tyrosine phosphatase, synapse, exocytosis
Neurons communicate with each other and with target cells at synapses. Given the very small size of most synapses, their direct analysis is difficult. Fortunately for scientists, synapses are a target of multiple natural neurotoxins that can inhibit (or activate) synaptic mechanisms. By using these poisons as molecular tools, scientists have been able to scrutinize many molecules and processes involved in synapse formation, functioning and regulation. However, the conversion of nature’s powerful weapons into specific research tools has proved difficult because most neurotoxins have evolved to cause multiple effects, ensuring universal and robust responses in targeted neurons. Therefore, the use of unmodified natural toxins has often led to contradictory results and conflicting conclusions.
This problem has been vividly illustrated by the history of research based on the application of α-latrotoxin (α-LTX) from black widow spider venom (BWSV). α-LTX, a potent stimulant of secretion in neurons and endocrine cells, has been used to stimulate nerves since the 1930’s (D’Amour et al. 1936). The effect of α-LTX is very strong and apparently straightforward. However, like so many other toxins, α-LTX acts through multiple mechanisms, which have been obscuring its molecular targets. The deeper researchers delved into the mode of action of α-LTX, the more complex it appeared, with every new finding leading to a thorough revision of the previous, so painfully built and seemingly so fitting, theory. In this sense, α-LTX studies resemble the myth of Penelope, the wife of Odysseus, who weaved a robe (web) by day, only to undo the work by night. Despite the clear connotations with this proverbial description of something being perpetually done but never finished, α-LTX research has actually brought about a great deal of important new molecules and concepts.
This review will examine how, in a long series of rigorous studies conducted by various laboratories, α-LTX and other black widow spider latrotoxins (LTXs) have not only improved our understanding of the complex mechanisms of neurotransmitter release, but also led to the discovery of a number of novel proteins, which, in turn, have since commanded their own lines of investigation.
Spider venom
The black widow spider (genus Lactrodectus) has horrified humans for millennia – mostly by its very painful (sometimes fatal; Bogen and Loomis 1936) bite, but not least by its striking coloration and enigmatic behavior. A more scientific study of the venom, and its exploitation in research, began in the 1930s, when it was first discovered that the active ingredients of the venom were proteins (D’Amour et al. 1936), acting upon neurons (Sampayo 1944). However, it was not until its direct effects on presynaptic neurotransmitter release were clearly demonstrated (Longenecker et al. 1970), that several generations of neurobiologists – with Penelope’s fidelity – dedicated their research to untangling the web of intrigue surrounding this infamous spider and the cellular effects of its potent venom.
Black widow spider venom contains a plethora of unique proteins (Duan et al. 2006), of which the large LTXs are the principal toxic components. There are at least seven highly homologous LTXs. The best characterized of these is the vertebrate-specific toxin, α-LTX (Grasso 1976; Frontali et al. 1976). The venom also contains five insect-specific toxins, called latroinsectotoxins α, β, γ, δ and ε (Grishin 1998), and one crustacean-specific protein, α-latrocrustatoxin (Krasnoperov et al. 1990; Volynskii et al. 1999). Although these toxins have been employed in research much less, their application has largely confirmed, in respective animal taxa, the data obtained with α-LTX.
Actions in secretory cells
From the pioneering study of the toxin’s action at the frog neuromuscular junction (NMJ) (Longenecker et al. 1970), it emerged that the venom causes exhaustive neurotransmitter release by acting specifically at the presynaptic nerve terminal. Later, in numerous experiments, this effect was unequivocally attributed to α-LTX. At low, subnanomolar concentrations, the toxin has no morphological effect on the nerve terminal; however, its action can be detected electrophysiologically as an increase in the frequency of miniature end-plate potentials (mepps) (Longenecker et al. 1970). At high, nanomolar concentrations, the toxin causes massive neurotransmitter release (Ceccarelli et al. 1979), which is sustained for a considerable amount of time and followed by substantial morphological changes in nerve terminals and even neuronal cell death. There is a concomitant dramatic drop in ATP levels and disintegration of the plasma membrane (McMahon et al. 1990), which is most strongly manifested in central synapses (Davletov et al. 1998; Ashton et al. 2001).
The effects of α-LTX on release of various neurotransmitters and peptide hormones have also been studied in synaptosomes from rat (Grasso et al. 1978), dog (Tzeng and Siekevitz 1979) and guinea pig (Nicholls et al. 1982) brain, rat and mouse brain slices (Frontali et al. 1972), primary cerebellar granule cell cultures (Grasso and Mercanti-Ciotti 1993), organotypic hippocampal cultures (Capogna et al. 1996) and neurohypophysis (Hlubek et al. 2003). The toxin has also been shown to stimulate exocytosis form non-neuronal excitable cells, such as PC12 cells (Grasso et al. 1982), chromaffin cells (Picotti et al. 1982), pancreatic β-cells and cell lines (Lang et al. 1998) and mast cells (Zhou and Misler 1995). However, α-LTX-induced release of glutamate in cultured astrocytes has also been reported (Parpura et al. 1995).
The potency of α-LTX as a secretagogue is quite remarkable: it causes complete depletion of millions of synaptic vesicles in frog NMJs (Ceccarelli et al. 1979; Fesce et al. 1986) and can even stimulate exocytosis at central synapses when the vesicle fusion machinery has been perturbed by knockout of one or another SNARE protein (Deák et al. 2009).
In general, LTXs from the spider venom have similar mechanisms of action, which are, however, limited to specific organisms. The reason for such a specialization of these highly homologous toxins is that, in order to act, they must bind to cell surface receptors, which appear to be distinct in different animal taxa (reviewed in Rohou et al. 2007).
Transmitter release and Ca2+
One of the most intriguing aspects of α-LTX action, discovered early on at the frog NMJ (Clark et al. 1970; Longenecker et al. 1970; Ceccarelli et al. 1979), was the toxin’s ability to cause transmitter release in both the presence and absence of extracellular calcium ions (Ca2+e). This distinguished α-LTX action from Ca2+-dependent depolarization-induced exocytosis (Gorio et al. 1978a) and made the toxin’s effect extremely interesting.
It was established by the initial work (Ceccarelli et al. 1979) and many subsequent experiments that the Ca2+e-dependent and -independent mechanisms of α-LTX action are distinct by many criteria:
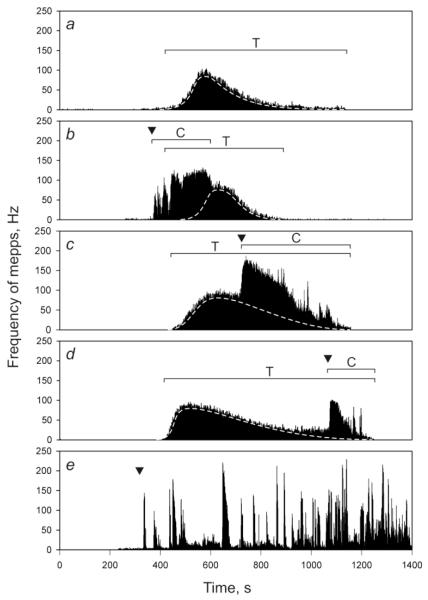
1. The characteristics of exocytosis evoked by α-LTX with and without Ca2+e are very different (Ceccarelli and Hurlbut 1980; Tsang et al. 2000). In the absence of Ca2+e, secretion is tonic: the frequency of spontaneous exocytotic events rises slowly and, on reaching a broad peak, decreases gradually (Fig. 1a), consistent with quasi-stationary tonic exocytosis (Fesce et al. 1986). By contrast, in the presence of 1-2 mM Ca2+e, α-LTX causes clonic release, appearing as fast bursts of acetylcholine exocytosis. These are later overlaid by a continual tonic rise in mepps frequency, which subsequently also declines (Fig. 1b) (Longenecker et al. 1970; Ceccarelli et al. 1979; Fesce et al. 1986; Volynski et al. 1999; Lelianova et al. 2009).
Fig. 1.
Dissection of the two distinct mechanisms of α-LTX action in the presence and absence of 2 mM Ca2+e. (a) A representative recording at the mouse NMJ of mepps induced by 1 nM wild-type α-LTX in the absence of Ca2+e. (b-d) Similar recordings using wild-type α-LTX, with Ca2+e added at different times (arrowheads). Two phases are clearly visible: clonic (C) and tonic (T). Only the tonic phase occurs in the absence of Ca2+; it is characterized by a smooth, slowly rising and falling pattern (dotted lines) and always results in the cessation of spontaneous release events, even after the subsequent addition of Ca2+. Clonic exocytosis only occurs in the presence of Ca2+ and is additive with tonic release. (e) In a similar recording, on addition of Ca2+ (arrowhead), 1 nM LTXN4C only triggers the clonic phase. This burst-like spontaneous exocytosis continued for 5 hr. (From (Lelianova et al. 2009); modified).
2. When α-LTX-evoked stimulation is submaximal, the Ca2+-dependent and -independent actions are additive and both occur in the presence of Ca2+e. Therefore, the frequency of transmitter exocytosis and the total number of quanta released are much higher in the presence of Ca2+e than in its absence (Fesce et al. 1986; Valtorta et al. 1988; Auger and Marty 1997; Davletov et al. 1998; Ashton et al. 2001). This is most vivdly illustrated in mouse NMJs (Fig. 1b-d): when Ca2+e is added to terminals already responding to wild-type toxin, the frequency of exocytosis increases 2-20 times, depending on the underlying frequency of Ca2+e-independent release.
It must be pointed out that because both LTX actions have a common target – the vesicles present in a synapse, the size of the vesicular pool can affect this comparison. Thus, the toxin-evoked exocytosis in excitatory central synapses is often similar with or without Ca2+ (Capogna et al. 1996; Deák et al. 2009). Yet, the mechanisms of the Ca2+-dependent and -independent components of α-LTX action are very different (Deák et al. 2009).
3. The Ca2+-independent toxin action also depends on divalent cations and can be fully supported by Mg2+ (Misler and Hurlbut 1979). As will be discussed below, Mg2+ promotes α-LTX tetramerization and pore formation, suggesting that tonic exocytosis is caused by α-LTX forming cation pores in the membrane.
Thus, α-LTX appears to induce two forms of secretion: clonic exocytosis that occurs only in the presence of Ca2+e and tonic exocytosis that can be recorded both in the presence and absence of Ca2+e. Given the additivity of tonic and clonic actions (Fig. 1b-d), the toxin is thought to trigger both types of transmitter release in the presence of Ca2+e (Davletov et al. 1998; Ashton et al. 2001).
Therefore, one of the major problems encountered when α-LTX is used to study synaptic transmission has been the difficulty of measuring the Ca2+-dependent α-LTX action in isolation. This conundrum was addressed by subtracting the secretion in the absence of Ca2+e from the total secretion in its presence (Fesce et al. 1986; Davletov et al. 1998). Although this brought about some important insights into the Ca2+-dependent toxin’s actions (Davletov et al. 1998; Rahman et al. 1999; Ashton et al. 2000; Ashton et al. 2001), it was clearly inadequate. An alternative approach to selectively inhibiting the Ca2+-independent component, was based on the application of micromolar La3+ together with α-LTX (Capogna et al. 2003; see also below).
However, because La3+ can stimulate transmitter release in its own right (Angaut-Petit et al. 1998), by far the best results have been obtained with mutant α-LTX, LTXN4C (Ichtchenko et al. 1998) (see below for details). This mutant does not form pores (Ashton et al. 2001; Volynski et al. 2003; Capogna et al. 2003) and consequently only causes high-frequency clonic secretion and no tonic release (Fig. 1e). Similar to the clonic exocytosis caused by wild-type α-LTX, the effect of LTXN4C critically requires Ca2+.
A characteristic feature of α-LTX action in the absence of Ca2+e is that it causes eventual cessation of all exocytosis. This has been attributed to the Ca2+e requirement for endocytosis and recycling of synaptic vesicles, meaning that in Ca2+e-free media vesicles fusing with the plasma membrane become stranded in it (Ceccarelli and Hurlbut 1980; Valtorta et al. 1988). As vesicle recycling is not impeded in the presence of Ca2+, multiple rounds of exocytosis can occur and toxin’s effect continues for much longer, as shown in the frog NMJ (Ceccarelli and Hurlbut 1980; Fesce et al. 1986). However, exocytosis induced by wild-type toxin eventually stops even in the presence of Ca2+e (Fig. 1a-d) (Longenecker et al. 1970; Ceccarelli and Hurlbut 1980; Lelianova et al. 2009). The most likely reason for this is that the tonic, high-frequency exocytosis gradually overwhelms vesicle recycling (J. Suckling, K. Volynski, V. Lelianova, R. Ribchester, Y. Ushkaryov, paper in preparation).
It would follow then that if the Ca2+-independent α-LTX mechanism is blocked, the Ca2+-dependent toxin action might continue indefinitely. Indeed, it does when nerve terminals are stimulated by LTXN4C (Fig. 1e). Interestingly, a similar Ca2+-dependent clonic release (without the tonic component) has been described at the frog NMJ stimulated by brown widow spider venom in saline containing Ca2+ but not Mg2+ (del Castillo and Pumplin 1975). Using focal extracellular recordings, these bursts were attributed to independent activation of single active zones (del Castillo and Pumplin 1975). The addition of Mg2+ in these experiments led to the appearance of both clonic and tonic phases. Therefore, true dissection of the Ca2+-dependent and independent mechanisms of α-LTX action can only be accomplished using α-LTX mutants similar to LTXN4C.
Finally, the two α-LTX actions target distinct types of synapses. Experiments in different neuronal preparations have demonstrated that α-LTX stimulates Ca2+-independent exocytosis of glutamate, γ-aminobutyric acid or acetylcholine, but not catecholamines or peptides (Matteoli et al. 1988; Davletov et al. 1998; Khvotchev et al. 2000). Endocrine cells, containing large dense-cored granules, are also insensitive to α-LTX in the absence of Ca2+ (Grasso et al. 1982; Picotti et al. 1982). On the other hand, all neurotransmitters can be released by the Ca2+e-dependent action, which is specifically induced by LTXN4C (Ashton et al. 2001; Volynski et al. 2003; Capogna et al. 2003; Lelianova et al. 2009). As we shall see later in this review, LTXN4C has proved to be very useful and has helped to change many prevailing concepts about the actions of wild-type α-LTX and physiological synaptic mechanisms. The possible contribution of the toxin pore to the Ca2+-independent α-LTX action will also be discussed below.
The data described above establish the following important facts: (1) α-LTX evokes exocytosis by two mechanisms, Ca2+-dependent and -independent; (2) these two mechanisms are distinct; (3) the tonic Ca2+-independent action develops slowly but ultimately leads to a total block of neurotransmitter release; (4) the Ca2+-dependent bursts of high frequency exocytosis appear abruptly and periodically but occur over a long time; (5) the Ca2+-independent exocytosis requires the presence of Mg2+; (6) synaptic vesicles carrying distinct neurotransmitters have different sensitivities to the two toxin actions.
Contribution to the exocytosis debate
One extremely important outcome of the first phase of toxin studies was its contribution to the debate regarding the nature of neurotransmitter release. At the time, transmitter exocytosis (as opposed to transporter-mediated outflow) was still a controversial idea, and the unique ability of α-LTX to cause sustained release of transmitters in the absence of Ca2+e played a decisive role in this argument. As mentioned above, synaptic vesicles, fused with the plasma membrane, require Ca2+e for their endocytosis (Ceccarelli and Hurlbut 1980). If α-LTX is applied in a Ca2+-free medium, fused vesicles become stranded in the membrane and eventually depleted from the terminal. This elegant paradigm, based on the peculiar features of α-LTX, was successfully employed in a series of cornerstone works (Ceccarelli et al. 1973; Gorio et al. 1978a). Finally, using electron microscopy and electrophysiological recordings, the authors concluded that quantal release at the NMJ occured as long as vesicles were present in the nerve terminal (Ceccarelli and Hurlbut 1980). This lent enormous support to the exocytotic theory of transmitter secretion (Hurlbut et al. 1990).
On the other hand, α-LTX never seizes to bring surprises, and the toxin has been also shown to cause non-vesicular release of neurotransmitters (McMahon et al. 1990; Deri and Adam-Vizi 1993; Davletov et al. 1998; Volynski et al. 2000). This Ca2+-independent action has been attributed to the large pore made by the toxin in the plasma membrane (as described below), and the relative amount of this non-vesicular leakage depends on the particular experimental approach used (see Ashton et al. 2000).
It is uncontestable, however, that α-LTX causes mostly vesicular secretion. This is clearly demonstrated by the wealth of electrophysiological recordings of individual quantal events (mepps). In addition, as a truly vesicular process, the toxin’s action requires SNARE proteins and is blocked by clostridial neurotoxins which cleave different SNAREs (Dreyer et al. 1987; Janicki and Habermann 1983; Stahl et al. 1994; Capogna et al. 1996; Aguado et al. 1997; Capogna et al. 1997; Davletov et al. 1998; Rahman et al. 1999; Ashton et al. 2001). Only botulinum toxin A (BoNT/A) may not fully inhibit α-LTX-induced secretion because Ca2+ influx via the toxin pore rescues SNAP-25 cleaved by BoNT/A (Cull-Candy et al. 1976; Dreyer et al. 1987; Angaut-Petit et al. 1998).
Both Ca2+-dependent and -independent effects of α-LTX were found to need intact synaptobrevin, syntaxin and SNAP-25 (Capogna et al. 1996; Capogna et al. 1997; Davletov et al. 1998; Rahman et al. 1999; Ashton et al. 2001). The requirement of SNARE proteins and unperturbed exocytotic machinery for the Ca2+-independent α-LTX-induced exocytosis at central synapses has been recently confirmed using mice with knocked out synaptobrevin, SNAP-25 or Munc13-1 (Deák et al. 2009).
However, in a new twist of Penelope’s tale, the Ca2+-dependent α-LTX action still occurred in neurons lacking synaptobrevin 2, SNAP-25 or Munc13-1 (Deák et al. 2009). This effect was mimicked by the Ca2+ ionophore ionomycin and was fully attributed to the pore-forming activity of α-LTX, suggesting that a novel pathway of vesicular fusion exists that does not require the presence of all SNARE proteins and that can be activated by a large ingress of Ca2+ into the terminal (Deák et al. 2009). Given the well-documented sensitivity of the Ca2+-dependent α-LTX action in wild-type nerve terminals to acute removal of SNAREs by cleavage with clostridial toxins, the striking results in SNARE knockout mice probably reflect an unusual assembly of the exocytotic machinery. It is tempting to speculate that in the continuous absence of synaptobrevin or SNAP-25, different synaptotagmins might take over the role of the missing SNARE, giving rise to this atypical Ca2+-dependent, but action potential-insensitive, pathway of exocytosis.
Membrane pore formation
The initial findings using α-LTX suggested that it could be a perfect molecular tool to study intrinsic presynaptic processes underlying neurotransmitter exocytosis. However, early into α-LTX research, a fascinating discovery appeared to solve the mystery of α-LTX action (and made it suddenly less attractive to many researchers): α-LTX was shown to spontaneously insert itself into artificial lipid bilayers and form cation-selective channels/pores (Finkelstein et al. 1976). Clearly, influx of Ca2+ through such pores could induce vesicular exocytosis, making the toxin receptors mere passive acceptors and, therefore, not worth in-depth studies. It was perhaps only due to the Ca2+-independent action of α-LTX, which could not be adequately explained by the effect of the pore and might arguably be mediated by the receptors, that the α-LTX research continued.
Inevitably, much interest also focused on the nature of α-LTX-induced cation channels. In particular, it was unclear how this large hydrophilic protein could penetrate the lipid bilayer and what the role of receptors in pore formation was.
These channels are permeable to cations (especially divalent), but not to anions (Finkelstein et al. 1976; Mironov et al. 1986). The toxin pores are insensitive to specific blockers of Na+, K+ and Ca2+ channels (Wanke et al. 1986; Scheer 1990). Only trivalent cations (e.g. La3+) and Cd2+ block the toxin pore (or its formation) (Scheer 1989; Rosenthal et al. 1990; Ashton et al. 2001; Van Renterghem et al. 2000). When forming the pore, the toxin inserts itself into the membrane, at least partially, as it becomes protected against trypsinization at 37°C but not at 0°C, when the membrane is impervious to protein insertion (Khvotchev et al. 2000). The involvement of any proteins/peptides, usually contaminating α-LTX preparations (Volkova et al. 1995; Pescatori et al. 1995), has been ruled out, as even highly purified α-LTX (Ashton et al. 2000) still forms cation permeable pores (albeit inefficiently) in lipid bilayers devoid of any receptors (Volynski et al. 2000). The α-LTX pores can be blocked by anti-toxin antibodies applied extracellularly (Cattaneo and Grasso 1986; Chanturiya et al. 1996), indicating that the toxin does not cross the lipid bilayer entirely.
Some researchers dismissed the relevance of toxin insertion into lipid membranes because α-LTX could not penetrate the plasma membrane of cells lacking its receptors. Therefore, it was proposed that biological membranes are generally refractory to toxin and that receptors allow α-LTX to reach the lipid bilayer and insert itself into it. Indeed, when α-LTX receptors were cloned (see later), their expression on the surface of non-neuronal cells made the latter a target for efficient α-LTX pore formation (Volynski et al. 2000). Moreover, the features of the toxin channels in such receptor-expressing cells were similar to those in pure lipid bilayers and did not depend on the nature of the receptor (Hlubek et al. 2000; Van Renterghem et al. 2000).
The major question was (and still remains) what role do toxin pores play in α-LTX-induced exocytosis? Superficially, Ca2+e influx through the pores induced by the toxin in the plasma membrane can account for Ca2+-dependent neurotransmitter release and/or membrane depolarization (Hurlbut et al. 1994). Indeed, the effect of α-LTX in neurons lacking SNARE proteins is fully explained by the toxin pore (Deák et al. 2009).
However, the toxin does not always cause depolarization (Lang et al. 1998), and the channel formation cannot easily explain the Ca2+-independent release. Many different mechanisms of how the toxin pore could mediate secretion in the absence of Ca2+ have been proposed. For example, the pore passes Na+, which might replace Ca2+ in some of its reactions (Adam-Vizi et al. 1993). In addition, influx-efflux of other cations and influx of water (Krasilnikov and Sabirov 1992) could tip the cell’s homeostasis.
To complicate the story further, the pores were found to also pass large substances, such as fluorescent dyes and neurotransmitters and possibly ATP (McMahon et al. 1990; Deri and Adam-Vizi 1993; Davletov et al. 1998; Volynski et al. 2000). This suggested (Ashton et al. 2000) that biochemically measured neurotransmitter discharge triggered by α-LTX could often represent an outflow of cytosolic neurotransmitter through the toxin pore.
Some of the hypothetical mechanisms of Ca2+-dependent exocytosis have been dismissed by direct experiments. For instance, the influx of Na+ has been found to be inconsequential because, when extracellular Na+ was replaced with glucosamine or choline-Cl, the Ca2+-independent effect of α-LTX persisted (Gorio et al. 1978b; Tsang et al. 2000). However, other mechanisms were more difficult to test. From this point of view, it is interesting to recall that Ca2+-independent α-LTX-induced transmitter release is specifically blocked by La3+ (Scheer 1989; Ashton et al. 2001), while some (but not all) Ca2+-dependent secretion is resistant to trivalent cations (Ashton et al. 2001; Capogna et al. 2003). This is remarkably consistent with the α-LTX pore (but not the receptor-mediated action) being blocked (or disrupted) by La3+ (Scheer 1989; Hurlbut et al. 1994; Ashton et al. 2001). It is tempting to suggest that the Ca2+-independent toxin’s action and some Ca2+-dependent mechanism are mediated by the toxin pore (or at least coincide with it), while a certain part of the specific Ca2+-dependent action is actually pore-independent and must be mediated by toxin’s interaction with receptors or associated proteins (Ashton et al. 2001).
Perhaps the most plausible hypothesis as to how the toxin pore can mediate its Ca2+-independent action is that an inflow of water through the toxin pores could cause swelling of nerve terminals, exerting some mechanical effect on the presynaptic plasma membrane. In this sense, the action of α-LTX pore in the absence of Ca2+ is similar to the effect of hypertonic sucrose: hypertonicity also acts by creating a tension in the presynaptic membrane (Rosenmund and Stevens, 1996), while the lack of Ca2+e prevents recycling of synaptic vesicles during application of hypertonic sucrose solutions (Ashton and Ushkaryov, 2005).
It can be concluded from the toxin pore studies, which resulted in nearly a hundred publications from many different laboratories, that (1) α-LTX indeed has an ability to form membrane pores; (2) these pores are permeable to cations (especially Ca2+) but are blocked by trivalent cations; (3) the toxin makes such pores in the membrane of any cells as long as they expresses, naturally or artificially, any of its receptors; (4) when inserted into the lipid membrane, the α-LTX molecule faces both the cytosolic and the extracellular side of the membrane; (5) the role of the pore in the toxin’s action remains controversial.
α-LTX structure
Given the toxin’s complex action, much hope was initially placed in the cloning and sequencing of α-LTX. However, the structural studies, too, have not been without controversies.
Sequence analysis (Kiyatkin et al. 1990) and immunocytochemistry (Cavalieri et al. 1990) shows that α-LTX is synthesized on free cytosolic ribosomes in the spider’s venom glands as a large pro-toxin, which is proteolytically processed in the lumen of the venom gland to produce the mature toxin with a molecular mass of ~130 kDa (see Ushkaryov 2002). The latter contains two domains: the N-terminal one-third of the molecule has no significant sequence homology to known proteins, while the C-terminal two-thirds contain 22 ankyrin-like repeats (ALRs), the most prominent feature of α-LTX (Kiyatkin et al. 1995). ALRs are found in a wide variety of unrelated proteins with diverse functions, and are thought to mediate intra- and/or inter-molecular interactions (Li et al. 2006). Consistent with its obligatory processing in the venom gland, the pro-toxin is inactive (Kiyatkin et al. 1995). However, it becomes fully active if a stop codon is placed after the ALR-containing domain. This recombinant construct has been subsequently used in many studies.
Unfortunately, the primary structure of α-LTX did not provide any clear clues as to how it could stimulate transmitter release or form membrane pores, and researchers became interested in the spatial organization of the toxin. Several attempts at crystallization have so far been unsuccessful. However, cryo-electron microscopy, a method that allows one to observe individual molecules frozen in their native conformation, has proved extremely useful (Orlova et al. 2000). As can be seen from the three-dimensional (3D) reconstructions (Orlova et al. 2000), α-LTX in solution is not a monomer, but rather a stable dimer, which can undergo further oligomerization into tetrameric complexes. The four toxin monomers create a central channel that fully pierces the structure. Intriguingly, this tetramerization is facilitated by physiological concentrations of divalent cations (Mg2+ and Ca2+) (Ashton et al. 2000), and the presence of at least one of these cations is absolutely required for α-LTX action (Misler and Hurlbut 1979; Rosenthal et al. 1990). It follows then that when the toxin is active (i.e. in the presence of Mg2+ or Ca2+), it exists almost exclusively in its tetrameric form.
The 3D structure of α-LTX has provided perhaps the best evidence so far to support the ability of α-LTX to make membrane pores. The toxin tetramer has been shown to spontaneously insert itself into the lipid bilayer of liposomes (Orlova et al. 2000), with the central hole connecting the outside and inside of the vesicle. This insertion is thought to be possible due to the hydrophobic nature of the tetramer’s base. The hole in the middle of the tetramer has a sufficiently large internal diameter of (10 – 25 Å) (similar to that estimated by Krasilnikov and Sabirov 1992) to permit the influx of Na+ and Ca2+, but also the efflux of neurotransmitters and cytosolic components in a Ca2+-independent non-quantal fashion (as proposed by (McMahon et al. 1990; Deri and Adam-Vizi 1993; Davletov et al. 1998).
Electron microscopy has also uncovered the remarkable ability of α-LTX tetramers to form two-dimensional crystals (Lunev et al. 1991); A. Rohou, M. van Heel and Y. Ushkaryov, paper in preparation). If the toxin arranged into such lattices on the cell surface, this could explain the synchronous opening of multiple channels demonstrated in several systems (Robello et al. 1987; Krasilnikov and Sabirov 1992; Filippov et al. 1994) and the detrimental effects of large toxin concentrations on neuronal cells mentioned above.
These studies provided a spectacular insight into the overall structure of α-LTX and the likely mechanism of its pore formation, but the significance of the toxin pore is still disputed. The main reasons for that is the ability of toxin to induce Ca2+e-independent transmitter release (not easily explained by the pore) and the importance of its presynaptic receptors.
Non-pore forming α-LTX mutant
Even before any receptors were isolated and studied, some experiments suggested that α-LTX was able to trigger intracellular signaling. In particular, it was shown that the toxin led to the activation of phospholipase C (PLC) and production of inositol-1,4,5-trisphosphate (IP3), causing subsequent release of Ca2+ from intracellular Ca2+ stores (Vicentini and Meldolesi 1984). However, persistent pore formation by the cell-bound toxin has obviously complicated studies of the receptors’ functions (Volynski et al. 2000). For this reason, a toxin mutant retaining the high affinity for the receptors and lacking the ability to form pores would be an ideal tool to study the effect of receptor activation only.
Serendipitously, a mutant recombinant toxin has been produced (termed LTXN4C) (Ichtchenko et al. 1998), containing a thrombin recognition site inserted upstream of the ALR-containing domain. This insert perturbs the molecule folding but in a very peculiar way: LTXN4C retains the high affinity for α-LTX receptors and the capacity to activate hydrolysis of phosphoinositides, but lacks the natural ability of α-LTX to form membrane pores (Ashton et al. 2001). This is because LTXN4C can assemble into dimers but not tetramers (Volynski et al. 2003) which are required for pore formation (Orlova et al. 2000). In full agreement with this theory, the mutant toxin does not support ion fluxes in cell membrane, but it does, in fact, elicit neurotransmitter release (Ashton et al. 2001; Volynski et al. 2003; Capogna et al. 2003). Therefore, LTXN4C has become the first tool that allows one to study neurotransmitter release induced exclusively by the activation of α-LTX receptors.
To summarize the actions and molecular mechanisms of α-LTX, we believe that the Ca2+-independent effect of LTX (1) is based on its tetramerization, membrane insertion and pore formation. The pore may pass water, leading to a mechanical perturbation of the presynaptic plasma membrane, which causes vesicular exocytosis in a manner similar to that of sucrose. The pore also mediates non-vesicular leakage of neurotransmitters and other molecules from the cytosol, leading to rundown of terminals. The Ca2+-dependent effect (2) consists of at least two actions: (a) Ca2+ influx through the toxin pore, which is induced by wild-type α-LTX and leads to vesicular exocytosis (in some cases even in the absence of SNARE proteins, and (b) receptor stimulation, which is induced by both wild-type α-LTX and the mutant LTXN4C and requires both Ca2+e and stored Ca2+. When wild-type α-LTX is used in the presence of Ca2+e, all these actions appear to take place.
α-LTX receptors
From all the evidence provided by massive research into α-LTX action, it followed that toxin receptors could hold the key to presynaptic regulatory mechanisms hijacked by α-LTX, and so several laboratories began a hot pursuit of the receptors. However, a long period of “web weaving and undoing” lay ahead.
Essentially the same method was applied in several studies aimed at isolating α-LTX receptors: affinity chromatography of solubilized brain extracts on a column with immobilized toxin. In hindsight, the outcome of these studies was very surprising: to date, three structurally unrelated receptors have been isolated by this procedure. In chronological order these are: (1) neurexin (Petrenko et al. 1990); (2) latrophilin, or CIRL (Davletov et al. 1996; Krasnoperov et al. 1996); and (3) receptor-like protein tyrosine phosphatase σ (PTPσ) (Krasnoperov et al. 2002b). The significance of this diversity of toxin’s targets is still unclear. Perhaps, the evolutionary pressure on α-LTX has ensured its universal ability to attack all secretory cells that could be reached by the toxin but differed in receptor composition. Otherwise, α-LTX could evolve in parallel with evolutionary changes in the repertoire of receptors in the vertebrate nervous and endocrine systems. Whatever the reason for this versatility is, it means that the toxin can cause distinct effects on binding (1) the same type of receptor in different cell types, or (2) different types of receptors in the same cell type. This, obviously, has important implications for any attempts at deciphering the mechanism of toxin’s action.
Neurexin
Neurexin Iα was initially purified together with a set of other proteins, considered to be subunits of the receptor complex (Petrenko et al. 1990). Through cloning and sequencing of the genes for the largest constituents of this “receptor complex”, a novel family of highly polymorphic neuronal cell-surface proteins was discovered and called neurexins (Ushkaryov et al. 1992). Neurexin Iα, a neuronal cell-surface protein, binds α-LTX with high affinity (Kd ~ 4 nM) (Petrenko 1993), in a strictly Ca2+-dependent manner (Davletov et al. 1995).
Neurexins contain large extracellular domains (composed of epidermal growth factor-like repeats and LNS repeats that are also present in laminin and sex hormone-binding globulin), one transmembrane region (TMR) and a very short cytoplasmic tail. They exhibit high but regimented heterogeneity based on the existence of three homologous genes, each of which can be transcribed from two independent promoters and alternatively spliced (Ushkaryov et al. 1992; Ushkaryov and Sudhof 1993). The two promoters direct the synthesis of the longer α-neurexins and the shorter β-neurexins (Ushkaryov et al. 1994) (Fig. 2). This generates six principle neurexins: Iα, Iβ, IIα, IIβ, IIIα and IIIβ. In addition, α-neurexins are alternatively spliced at five canonical positions and β-neurexins at two, with the splice sites used independently and containing variable inserts. These combinations can potentially create more than 1000 distinct neurexins in the brain (Ullrich et al. 1995).
Fig. 2.
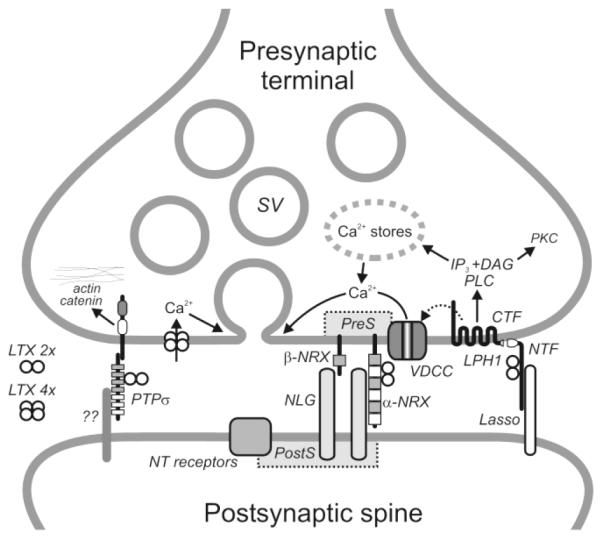
Generalized scheme of the α-LTX receptors and some of its mechanisms of action at the synapse. The wild-type toxin is dimeric, but it assembles into tetramers in the presence of millimolar Mg2+. After binding to its receptors, tetrameric α-LTX inserts itself into the plasma membrane and forms non-selective cation channels. The subsequent influx of Ca2+ is able to mediate Ca2+-dependent α-LTX evoked neurotransmitter release. Mutant LTXN4C (dimeric only) is able to stimulate receptor-mediated neurotransmitter release which requires both intracellular and extracellular Ca2+. This mode of action may involve voltage-dependent Ca2+ channels and is probably mediated by latrophilin 1, which, upon binding the toxin, signals via the PLC cascade. Both neurexin and latrophilin 1 appear to organize VDCCs. Abbreviations used here are: DAG, diacyl glycerol; LPH1, latrophilin 1; LTX 4x, α-LTX tetramers; LTX 2x, α-LTX dimers; NLG, neuroligin; NRX, neurexin; NT, neurotransmitter; PKC, protein kinase C; PreS, presynaptic scaffolding; PostS, postsynaptic scaffolding; SV, synaptic vesicles; VDCC; voltage-dependent Ca2+ channels.
Neurexins and synapse formation
The polymorphic structure of the neurexins, their specific differential neural distribution (Ullrich et al. 1995; Missler et al. 1998), and their sequence similarity to extracellular matrix proteins (laminin, slit, and agrin) suggest a function in cell recognition and cell-adhesion at the nerve terminal (Fig. 2). Indeed, neurexins are expressed almost entirely presynaptically in the brain (Berninghausen et al. 2007). The role for neurexins as presynaptic cell-adhesion molecules is supported by the discovery of their endogenous ligands, neurexophilin and neuroligins (Nguyen and Sudhof 1997; Missler et al. 1998). Neurexophilin is a small (29 kDa) secreted peptide which binds to the extracellular domain of neurexin Iα (Petrenko et al. 1996). Neuroligin is a post-synaptic transmembrane protein that (in a splicing-dependent manner) binds β- or α-neurexins and facilitates synapse formation (Scheiffele et al. 2000) (Fig. 2).
Neuroligins are involved in the maturation of synapses by validating excitatory versus inhibitory synapses (Chubykin et al. 2007). Mice lacking neuroligin or neurexin genes show improper synapse function and are not viable, although synapse formation itself is not affected (Missler et al. 2003; Varoqueaux et al. 2006).
An intriguing link to neurodevelopmental disorders was made when genetic screens revealed that mutations in the neurexin and neuroligin genes, although infrequent and not prerequisite, are among the numerous genetic causes that may contribute to susceptibility towards autism spectrum disorders and mental retardation (Szatmari et al. 2007; Glessner et al. 2009; Maestrini et al. 2009). Studies on one particular NL3 mutation associated with autism shows enhanced inhibition and impaired social interactions in mice (Tabuchi et al. 2007). Therefore, gaining a better understanding of the molecular mechanism of these proteins may prove useful in treating or even preventing certain disorders of the central nervous system.
Intracellularly, neurexins interact with scaffolding proteins (Biederer and Sudhof 2000), the synaptic vesicle protein synaptotagmin (Petrenko et al. 1991) and mediate functional coupling of voltage-gated Ca2+ channels to the presynaptic machinery (Missler et al. 2003). These interactions constitute a link from neurexins to both synaptic vesicles and the vesicle fusion apparatus. Thus, neurexins participate in the structural organization, maturation and functional maintenance of presynaptic terminals.
Latrophilin
After α-neurexin was shown to strictly require Ca2+ for α-LTX binding (Davletov et al. 1995), it became obvious that the Ca2+-independent α-LTX actions must be mediated by a different receptor. Investigators started searching for neuronal receptors able to bind α-LTX in the absence of Ca2+. After a number of attempts, a brain-specific protein was isolated that bound α-LTX specifically. Based on its high affinity for α-LTX (0.5 – 0.7 nM) the new receptor was termed latrophilin (Davletov et al. 1996). The same protein was independently purified by another laboratory and named CIRL (calcium-independent receptor of α-LTX) (Krasnoperov et al. 1996). In the literature, this protein is sometimes referred to as CL, which stands for CIRL/latrophilin (Sugita et al. 1998).
Unusual structure/function of latrophilin
Latrophilin comprises a long extracellular domain, seven hydrophobic TMRs and a long cytoplasmic tail (Lelianova et al. 1997; Krasnoperov et al. 1997) (Fig. 2). The extracellular domain is connected to the first TMR via a short GPS motif (for G protein-coupled receptor proteolysis site; Krasnoperov et al. 1997). This motif contains four conserved cysteine residues and a cleavage site that divides the protein into non-covalently bound N- and C-terminal fragments (NTF and CTF, respectively).
This constitutive cleavage, occurring in the endoplasmic reticulum, is required for latrophilin delivery to the cell surface (Krasnoperov et al. 2002a; Volynski et al. 2004). Here, the two subunits can dissociate and behave as independent cell-surface proteins that are delivered to distinct parts of the cell membrane and are recycled separately. To explain this, NTF has been proposed to anchor in the membrane independently of the CTF (Volynski et al. 2004).
Under certain conditions (e.g. the binding of agonist, α-LTX), the latrophilin fragments are able to reassociate. Treatment of latrophilin expressing cells with the non-pore-forming LTXN4C, results in the formation of large ternary α-LTX-NTF-CTF complexes on the plasma membrane which are able to mediate signal transduction.
Latrophilin has two very similar homologues, latrophilin 2 and latrophilin 3 (Sugita et al. 1998; Matsushita et al. 1999; Ichtchenko et al. 1999). Despite their high sequence homology, latrophilins are differentially distributed in mammalian tissues: latrophilin 1 and latrophilin 3 are brain-specific, while latrophilin2 is ubiquitously expressed. Similar to neurexin Iα (Ushkaryov et al. 1992; Occhi et al. 2002), very small levels of latrophilin 1 mRNA can be detected outside brain, in the kidneys and lung, and both receptors reside in endocrine cells, such as pancreatic β-cells (Lang et al. 1998). Although all three latrophilins are present in the brain, at least latrophilin 1 is strictly expressed in neurons but not glial cells (Kreienkamp et al. 2000). There have been suggestions that latrophilin 1 may be postsynaptic because its CTF can interact in a yeast two-hybrid system with the PDZ domain of Shank 1 (Tobaben et al. 2000; Kreienkamp et al. 2000), a postsynaptic scaffolding protein (Sheng and Kim 2000). However, this result may reflect the normal interaction of the ubiquitous latrophilin 2 rather than the neuronal latrophilin 1 because this binding occurred at the C-terminus (Kreienkamp et al. 2000), which has the same sequence in both latrophilin isoforms. In contrast, the dramatic inhibition of α-LTX binding and action in the latrophilin knockout mouse (Tobaben et al. 2002), clearly indicate that latrophilin 1 is localized in presynaptic terminals. Moreover, a series of comprehensive studies (H. Matsushita, O. Berninghausen, M. A. Rahman, J.-P. Silva, A. Tonevitsky, Y. Ushkaryov, submitted for publication) has demonstrated unequivocally that latrophilin 1 is not only presynaptic but also enriched at the active zones.
Recent experiments have shown an interaction of CTF of latrophilin with TRIP8b, a cytosolic protein that binds clathrin and subunits of the AP2 complex, suggesting a role for latrophilin and TRIP8b in receptor-mediated endocytosis (Popova et al. 2007; Popova et al. 2008).
Latrophilin as a GPCR
Latrophilin has become one of the founding members of a new family of GPCRs, known as “adhesion GPCRs” (Fredriksson et al. 2003). These receptors have relatively conserved CTFs and entirely unrelated NTFs. The NTFs are unusually large and complex and contain various motifs likely to participate in cell adhesion. CTFs comprise seven TMRs and are expected to function as GPCRs. Thus, this group of receptors represents natural chimeras of adhesion and signaling receptors (Krasnoperov et al. 2002a). Latrophilin is the first receptor of this group that has been shown to bind specifically to G proteins, namely, Gαo and Gαq/11 (Rahman et al. 1999; Serova et al. 2008). This interaction is strong, but also functional because it is disrupted by conditions that allow G-protein activation and dissociation from the receptor (Rahman et al. 1999). Coupling to Gαq/11 can activate PLC, with subsequent signaling to intracellular Ca2+ stores and Ca2+i-mediated Ca2+e influx, ultimately resulting in neurotransmitter release.
Many questions still remain in the latrophilin story. The identification of its endogenous ligand(s) would greatly aid in understanding its function. In an effort to find such a candidate, we have recently identified a postsynaptic protein, called Lasso (for latrophilin associated synaptic surface protein), that binds latrophilin with high affinity (J.-P. Silva, V. Lelianova, P. Hitchin, M. A. Rahman, A. Dell and Y. Ushkaryov; paper in preparation). The structure of Lasso and the role of its interaction with latrophilin (Fig. 2) are currently being investigated.
PTPσ
When α-LTX affinity chromatography of brain extract was conducted in the absence of Ca2+, in addition to latrophilin, a small amount of receptor-like protein tyrosine phosphatase σ (PTPσ) was also identified (Krasnoperov et al. 2002b). In contrast to neurexin and latrophilin which were discovered as a result of toxin chromatography, PTPσ had been known before this work and in this respect is not, strictly speaking, an outcome of α-LTX research.
PTPσ is a member of the family of receptor-like PTPs that contain cell adhesion molecule-like extracellular domains, a single TMR and one or two cytoplasmic phosphatase domains (reviewed by Tonks 2006). The extracellular domain contains N-terminal immunoglobulin-like modules and from four to eight fibronectin type III-like domains (Fig. 2). The cytosolic portion of the protein contains a catalytically active PTP phosphatase domain and a pseudo-phosphatase. Similar to latrophilin, PTPσ is proteolytically cleaved into two fragments, but the resulting subunits remain non-covalently associated.
PTPσ has two splice variants: the large variant is found in most tissues, while the shorter one, which binds α-LTX, is mostly brain-specific (Yan et al. 1993; Pulido et al. 1995) but, similar to neurexin Iα and latrophilin 1, is also found in small amounts in some other tissues (Yan et al. 1993). There have been no suggestions that PTPσ is involved in regulation of exocytosis, but and its primary function may be the regulation of axonal growth and synapse formation. PTPσ interacts with extracellular matrix, EGF receptors and N-cadherin. By dephosphorylating N-cadherin, PTPσ regulates its interaction with the cytoskeleton and inhibits growth of axons (McLean et al. 2002; Siu et al. 2006) (Fig. 2). In addition, deposphorylation of proteins localized in focal adhesions may also be important for the regulation of cell-cell contacts.
Which receptor transduces an α-LTX signal?
Thus, in defiance of all expectations, the search for α-LTX receptors has only complicated the α-LTX field. The toxin has been found to interact with three structurally and functionally unrelated receptors, making its mechanism of action very hard to understand, unless one assumes that toxin’s effect is limited to pore formation. However, do the three receptors contribute equally to the toxin’s effect at the synapse?
α-Neurexin can only bind to α-LTX in the presence of Ca2+e (Davletov et al. 1995), while the toxin can act in Ca2+-free buffers, indicating that one or both of its Ca2+-independent receptors (latrophilin and PTPσ) must be involved in this action of α-LTX. Knockout of α-neurexin in mice has demonstrated that it definitely contributes to α-LTX binding in the presence of Ca2+ (Geppert et al. 1998). However, the secretory effect of α-LTX – both in the presence and absence of Ca2+ – is only moderately affected in the knockout mice. Thus, α-neurexin, is non-critical for both Ca2+-dependent and -independent mechanisms of α-LTX.
There is no doubt, however, that neurexins can serve as functional α-LTX receptors, by providing toxin binding sites. This, for instance, explains the increase in sensitivity of PC12 cells to α-LTX when such cells are transfected neurexin (Sugita et al. 1999). Pore formation by the wild-type toxin used in these experiments, explains this effect. In addition, neurexins apparently play a structural (rather than signaling) role in the synapse and are unlikely to mediate the fast exocytotic signal induced by α-LTX.
More compelling evidence that rules out the necessity of neurexins in α-LTX action has perhaps come from the observation based on the use of LTXN4C (Volynski et al. 2003). LTXN4C-evoked neurotransmitter release is strictly Ca2+-dependent (Ashton et al. 2001; Capogna et al. 2003). However, Sr2+, which does not aid toxin binding to α-neurexin, fully supports the stimulatory effect of LTXN4C (Volynski et al. 2003).
These considerations make it difficult to assign neurexins a role in the α-LTX action other than that of passive toxin acceptors. Of course, this does not in the least diminish their role in synaptic physiology.
As shown above, latrophilin binds α-LTX with the highest affinity, compared to the other two receptors. The effect of α-LTX at nerve terminals has been associated with an increased breakdown of phosphoinositides and cytosolic concentration of Ca2+ (reviewed in Scheer et al. 1984). Moreover, the toxin’s effect on release of norepinephrine and amino acid neurotransmitters is inhibited by thapsigargin (which depletes intracellular Ca2+ stores), 2-aminoethoxydiphenyl borate (which blocks IP3-induced Ca2+ release), or U73122 (which inhibits PLC) (Davletov et al. 1998; Rahman et al. 1999; Ashton et al. 2001; Capogna et al. 2003). It is not surprising then that latrophilin is not only structurally capable of mediating these effects, but has also been shown to interact with G proteins (see above). In fact, even in receptor-transfected non-neuronal cells, LTXN4C was able to stimulate release of intracellular Ca2+ and this effect was fully dependent on the presence of functional latrophilin (Volynski et al. 2004; Silva et al. 2009). This indicates that coupling of latrophilin to Ca2+ stores is responsible for at least some transmitter exocytosis induced by α-LTX. Finally, knockout of latrophilin in mice (Tobaben et al. 2002) leads to a 50-70 % decrease in toxin binding but, at the same time, to a much greater inhibition of toxin’s effect.
The structure of PTPσ is consistent with its role in binding ligands on the cell surface. Similar to latrophilin, it is also capable of signaling. PTPσ has been shown to interact with heparin sulphate proteoglycan in the basement membrane (Sajnani-Perez et al. 2003) and some cell adhesion molecules. However, the binding of the toxin dimer (or tetramer) to PTP, should lead to its dimerization and inhibition (Tonks 2006), rather than activation, of phosphatase activity. In addition, α-LTX binds PTPσ within the fibronectin-like domains 2 and 3 (Krasnoperov et al. 2002b). These two domains are present in both splice variants of PTPσ, but only the shorter variant mediates the secretory action of wild-type α-LTX in pancreatic β-cells (Lajus and Lang 2006), suggesting that it simply facilitates toxin pore formation by bringing α-LTX closer to the plasma membrane.
Taken together, these considerations establish latrophilin as the most likely receptor that mediates an exocytotic signal from the toxin. However, α-LTX causes many different effects, and it is quite possible that some of the long-term signals are mediated specifically by neurexin or PTPσ. It is also likely that some toxin’s actions – in particular its pore formation in the cell membrane – bypass any signaling that can be triggered by the receptors.
What are the results of α-LTX research?
So, after about forty years of modern research using α-LTX, what do we know about mechanisms of action of this fascinating neurotoxin? An overview of α-LTX knowledge base, with all its uncertainties and complex logical links, is provided in Fig. 3.
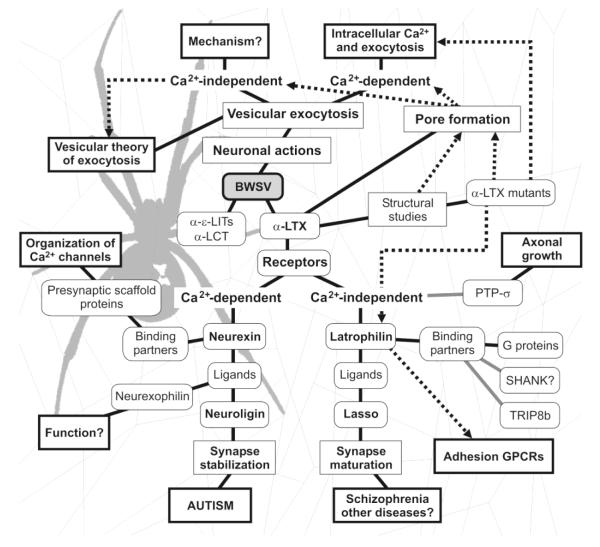
Fig. 3.
A web of intrigue. A summary of processes (rectangles) and molecules (rounded rectangles) revealed using BWSV and α-LTX. Key proteins, concepts and actions are shown in bold. Solid black lines indicate causal links between findings; solid gray lines show interactions of unknown significance; dotted arrows signify contributions to the understanding of respective phenomena. Other toxins from BWSV, α-ε-latroinsectotoxins (α-ε-LITs) and α-LCT, have largely confirmed the findings made using α-LTX (Rohou et al. 2007).
Even though the effect of toxin application in various systems is obvious and well-documented, the molecular machinery underlying its mode of action are still highly debated and controversial. α-LTX is a naturally occurring highly poisonous agent which is used by black widow spiders to inflict excruciating pain and scare away its enemy (a vertebrate animal). It is, therefore, not surprising that the toxin’s effects are so powerful and diverse, while its mechanisms (including its targeting of multiple receptors and several modes of actions) are so complex. Scientists have tried to utilize this natural toxin to answer their questions regarding exocytosis, but perhaps often interpreted the results in a rather simplistic manner.
Nevertheless, the α-LTX studies have led to the formulation of extremely important scientific concepts. It now appears obvious that neurotransmitter release occurs via exocytosis, but in the early days of α-LTX research, it was a hotly debated topic. Toxin studies have also led to the discovery of several presynaptic receptors and their ligands (in particular neurexins/neuroligins and latrophilin/Lasso) (Fig. 2). The heterophilic trans-synaptic interactions involving these receptor systems have been shown to control synapse formation, maturation and/or activity.
There is also a much greater understanding of the toxin itself. It is now generally accepted that the wild-type α-LTX can form non-selective cation channels and that this ability stems from the tetramerization of toxin molecules. Based on these insights, researchers have tried to move away from using this rather blunt tool and, instead, have engineered various recombinant toxin mutants which could be used with more precision and less uncertainty. For example, the toxin mutant LTXN4C has become a new tool enabling researchers to specifically study α-LTX-induced receptor-mediated exocytosis. While extremely beneficial, LTXN4C is still not an ideal tool, leaving many questions unanswered. Indeed, although LTXN4C does not form membrane pores and acts via receptors (Volynski et al. 2003), it still requires Ca2+ to evoke neurotransmitter release (Capogna et al. 2003). So it remains to be investigated whether Ca2+e enters via latrophilin-activated Ca2+ channels (Lajus and Lang 2006), or whether this cation serves as a co-factor for latrophilin. In addition, LTXN4C binds both latrophilin and neurexin (and possibly PTPσ), making it impossible to unequivocally assign its signaling pathways.
For these reasons, a series of remarkable deletion mutants of α-LTX (Li et al. 2005) seem to offer a great potential. These authors showed that the removal of just one C-terminal ALR makes the toxin pore enormously conductive, while further C-terminal truncations lead to the total loss of pore forming ability. It would be very interesting to compare the ability of these truncation mutants to tetramerize and induce transmitter exocytosis in neurons.
Finally, α-LTX has also been hypothesized to penetrate partially through the cell membrane and interact with exocytotic proteins on the cytosolic side and thus stimulate receptor-independent effects (Khvotchev et al. 2000). This promises to open yet other novel aspects of toxin’s action and – more importantly – tell us more about the intricate mechanisms of synaptic function.
Even though much controversy still exists, it is clear that the “web” (Fig. 3) covering α-LTX will be eventually completely removed.
Remaining questions and new avenues
Surprisingly, after all the years of α-LTX use in synaptic research, the mechanism/s of its Ca2+-independent action are still unknown. Novel toxin mutants and unconventional approaches will have to be employed to address this question.
α-LTX receptors are now being studied in-depth. Several questions in this field that require more work: What are the physiological functions of these receptors? What are the consequences of their interactions with their respective endogenous ligands? How do the different toxin receptors interact with each other? Why is the structure of latrophilin so unusual and why can this receptor exchange fragments with other aGPCRs? Finally, what are the signaling mechanisms of each α-LTX receptor?
There is no doubt that studies of α-LTX and its receptors should continue, but at a new, modern level.
Acknowledgements
This work was supported by a Wellcome Project grant (083199) to Y.U.
Abbreviations used
- 3D
three-dimensional
- ALR
ankyrin-like repeat
- BWSV
black widow spider venom
- Ca2+e
extracellular calcium ions
- CIRL
Ca2+-independent receptor of α-latrotoxin
- CTF
C-terminal fragment
- GPCR
G-protein-coupled receptor
- IP3
inositol-1,4,5-trisphosphate
- α-LTX
α-latrotoxin
- LTXs
latrotoxins
- mepps
miniature end-plate potentials
- NMJ
neuromuscular junction
- NTF
N-terminal fragment
- PLC
phospholipase C
- PTPσ
receptor-like protein tyrosine phosphatase σ
- TMR
transmembrane region
References
- Adam-Vizi V, Deri Z, Bors P, Tretter L. Lack of involvement of [Ca2+]i in the external Ca2+-independent release of acetylcholine evoked by veratridine, ouabain and α-latrotoxin: possible role of [Na+]i. J. Physiol. Paris. 1993;87:43–50. doi: 10.1016/0928-4257(93)90023-m. [DOI] [PubMed] [Google Scholar]
- Aguado F, Gombau L, Majo G, Marsal J, Blanco J, Blasi J. Regulated secretion is impaired in AtT-20 endocrine cells stably transfected with botulinum neurotoxin type A light chain. J. Biol. Chem. 1997;272:26005–26008. doi: 10.1074/jbc.272.41.26005. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D, Molgo J, Faille L, Juzans P, Takahashi M. Incorporation of synaptotagmin II to the axolemma of botulinum type-A poisoned mouse motor endings during enhanced quantal acetylcholine release. Brain Res. 1998;797:357–360. doi: 10.1016/s0006-8993(98)00475-2. [DOI] [PubMed] [Google Scholar]
- Ashton AC, et al. Tetramerisation of α-latrotoxin by divalent cations is responsible for toxin-induced non-vesicular release and contributes to the Ca2+-dependent vesicular exocytosis from synaptosomes. Biochimie. 2000;82:453–468. doi: 10.1016/s0300-9084(00)00199-1. [DOI] [PubMed] [Google Scholar]
- Ashton AC, Ushkaryov YA. Properties of synaptic vesicle pools in mature central nerve terminals. J. Biol. Chem. 2005;280:37278–37288. doi: 10.1074/jbc.M504137200. [DOI] [PubMed] [Google Scholar]
- Ashton AC, Volynski KE, Lelianova VG, Orlova EV, Van Renterghem C, Canepari M, Seagar M, Ushkaryov YA. α-Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J. Biol. Chem. 2001;276:44695–44703. doi: 10.1074/jbc.M108088200. [DOI] [PubMed] [Google Scholar]
- Auger C, Marty A. Heterogeneity of functional synaptic parameters among single release sites. Neuron. 1997;19:139–150. doi: 10.1016/s0896-6273(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Berninghausen O, Rahman MA, Silva JP, Davletov B, Hopkins C, Ushkaryov YA. Neurexin Iβ and neuroligin are localized on opposite membranes in mature central synapses. J Neurochem. 2007;103:1855–1863. doi: 10.1111/j.1471-4159.2007.04918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederer T, Sudhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J. Biol. Chem. 2000;275:39803–39806. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- Bogen E, Loomis R. Poisoning poisonous spiders: an experimental investigation in the control of the black widow spider (Latrodectus mactans) Cal. West Med. 1936;45:31–38. [PMC free article] [PubMed] [Google Scholar]
- Capogna M, Gahwiler BH, Thompson SM. Calcium-independent actions of α-latrotoxin on spontaneous and evoked synaptic transmission in the hippocampus. J. Neurophysiol. 1996;76:3149–3158. doi: 10.1152/jn.1996.76.5.3149. [DOI] [PubMed] [Google Scholar]
- Capogna M, McKinney RA, O’Connor V, Gahwiler BH, Thompson SM. Ca2+ or Sr2+ partially rescues synaptic transmission in hippocampal cultures treated with botulinum toxin A and C, but not tetanus toxin. J. Neurosci. 1997;17:7190–7202. doi: 10.1523/JNEUROSCI.17-19-07190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogna M, Volynski KE, Emptage NJ, Ushkaryov YA. The α-latrotoxin mutant LTXN4C enhances spontaneous and evoked transmitter release in CA3 pyramidal neurons. J. Neurosci. 2003;23:4044–4053. doi: 10.1523/JNEUROSCI.23-10-04044.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Grasso A. A functional domain on the α-latrotoxin molecule, distinct from the binding site, involved in catecholamine secretion from PC12 cells: identification with monoclonal antibodies. Biochemistry. 1986;25:2730–2736. doi: 10.1021/bi00357a068. [DOI] [PubMed] [Google Scholar]
- Cavalieri M, Corvaja N, Grasso A. Immunocytological localization by monoclonal antibodies of α-latrotoxin in the venom gland of the spider Latrodectus tredecimguttatus. Toxicon. 1990;28:341–346. doi: 10.1016/0041-0101(90)90069-j. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B, Grohovaz F, Hurlbut WP. Freeze-fracture studies of frog neuromuscular junctions during intense release of neurotransmitter. I. Effects of black widow spider venom and Ca2+-free solutions on the structure of the active zone. J. Cell Biol. 1979;81:163–177. doi: 10.1083/jcb.81.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J. Cell Biol. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J. Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanturiya AN, Nikolaenko AN, Shatursky OY, Lishko VK. Probing the structure-function relationship of α-latrotoxin- formed channels with antibodies and pronase. Toxicon. 1996;34:1157–1164. doi: 10.1016/0041-0101(96)00053-0. [DOI] [PubMed] [Google Scholar]
- Chubykin AA, Atasoy D, Etherton MR, Brose N, Kavalali ET, Gibson JR, Sudhof TC. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–931. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AW, Mauro A, Longenecker HE, Jr., Hurlbut WP. Effects of black widow spider venom on the frog neuromuscular junction. Effects on the fine structure of the frog neuromuscular junction. Nature. 1970;225:703–705. doi: 10.1038/225703a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Lundh H, Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J. Physiol. (Lond. ) 1976;260:177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour F, Becker FE, van Riper W. The black widow spider. Q. Rev. Biol. 1936;11:123–160. [Google Scholar]
- Davletov BA, Krasnoperov V, Hata Y, Petrenko AG, Sudhof TC. High affinity binding of α-latrotoxin to recombinant neurexin Iα. J. Biol. Chem. 1995;270:23903–23905. doi: 10.1074/jbc.270.41.23903. [DOI] [PubMed] [Google Scholar]
- Davletov BA, Meunier FA, Ashton AC, Matsushita H, Hirst WD, Lelianova VG, Wilkin GP, Dolly JO, Ushkaryov YA. Vesicle exocytosis stimulated by α-latrotoxin is mediated by latrophilin and requires both external and stored Ca2+ EMBO J. 1998;17:3909–3920. doi: 10.1093/emboj/17.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Shamotienko OG, Lelianova VG, Grishin EV, Ushkaryov YA. Isolation and biochemical characterization of a Ca2+-independent α-latrotoxin-binding protein. J. Biol. Chem. 1996;271:23239–23245. doi: 10.1074/jbc.271.38.23239. [DOI] [PubMed] [Google Scholar]
- Deák F, Liu X, Khvochtev M, Li G, Kavalali ET, Sugita S, Südhof TC. α-Latrotoxin stimulates a novel pathway of Ca2+-dependent synaptic exocytosis independent of the classical synaptic fusion machinery. 2009. pp. 8639–8648. [DOI] [PMC free article] [PubMed]
- del Castillo J, Pumplin DW. Discrete and discontinuous action of brown widow spider venom on the presynaptic nerve terminals of frog muscle. J. Physiol. (Lond. ) 1975;252:491–508. doi: 10.1113/jphysiol.1975.sp011154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deri Z, Adam-Vizi V. Detection of intracellular free Na+ concentration of synaptosomes by a fluorescent indicator, Na+-binding benzofuran isophthalate: the effect of veratridine, ouabain, and α-latrotoxin. J. Neurochem. 1993;61:818–825. doi: 10.1111/j.1471-4159.1993.tb03592.x. [DOI] [PubMed] [Google Scholar]
- Dreyer F, Rosenberg F, Becker C, Bigalke H, Penner R. Differential effects of various secretagogues on quantal transmitter release from mouse motor nerve terminals treated with botulinum A and tetanus toxin. Naunyn Schmiedebergs Arch. Pharmacol. 1987;335:1–7. doi: 10.1007/BF00165027. [DOI] [PubMed] [Google Scholar]
- Duan ZG, et al. Extraction and protein component analysis of venom from the dissected venom glands of Latrodectus tredecimguttatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006;145:350–357. doi: 10.1016/j.cbpb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Fesce R, Segal JR, Ceccarelli B, Hurlbut WP. Effects of black widow spider venom and Ca2+ on quantal secretion at the frog neuromuscular junction. J. Gen. Physiol. 1986;88:59–81. doi: 10.1085/jgp.88.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Tertishnikova SM, Alekseev AE, Tsurupa GP, Pashkov VN, Grishin EV. Mechanism of α-latrotoxin action as revealed by patch-clamp experiments on Xenopus oocytes injected with rat brain messenger RNA. Neuroscience. 1994;61:179–189. doi: 10.1016/0306-4522(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Finkelstein A, Rubin LL, Tzeng M-C. Black widow spider venom: effect of purified toxin on lipid bilayer membranes. Science. 1976;193:1009–1011. doi: 10.1126/science.948756. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Frontali N, Ceccarelli B, Gorio A, Mauro A, Siekevitz P, Tzeng MC, Hurlbut WP. Purification from black widow spider venom of a protein factor causing the depletion of synaptic vesicles at neuromuscular junctions. J. Cell Biol. 1976;68:462–479. doi: 10.1083/jcb.68.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontali N, Granata F, Parisi P. Effects of black widow spider venom on acetylcholine release from rat cerebral cortex slices in vitro. Biochem. Pharmacol. 1972;21:969–974. doi: 10.1016/0006-2952(72)90401-7. [DOI] [PubMed] [Google Scholar]
- Geppert M, Khvotchev M, Krasnoperov V, Goda Y, Missler M, Hammer RE, Ichtchenko K, Petrenko AG, Sudhof TC. Neurexin Iα is a major α-latrotoxin receptor that cooperates in α-latrotoxin action. J. Biol. Chem. 1998;273:1705–1710. doi: 10.1074/jbc.273.3.1705. [DOI] [PubMed] [Google Scholar]
- Glessner JT, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A, Hurlbut WP, Ceccarelli B. Acetylcholine compartments in mouse diaphragm. Comparison of the effects of black widow spider venom, electrical stimulation, and high concentrations of potassium. J. Cell Biol. 1978a;78:716–733. doi: 10.1083/jcb.78.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A, Rubin LL, Mauro A. Double mode of action of black widow spider venom on frog neuromuscular junction. J. Neurocytol. 1978b;7:193–202. doi: 10.1007/BF01217918. [DOI] [PubMed] [Google Scholar]
- Grasso A. Preparation and properties of a neurotoxin purified from the venom of black widow spider (Latrodectus mactans tredecimguttatus) Biochim. Biophys. Acta. 1976;439:406–412. doi: 10.1016/0005-2795(76)90077-5. [DOI] [PubMed] [Google Scholar]
- Grasso A, Mercanti-Ciotti MT. The secretion of amino acid transmitters from cerebellar primary cultures probed by α-latrotoxin. Neuroscience. 1993;54:595–604. doi: 10.1016/0306-4522(93)90231-4. [DOI] [PubMed] [Google Scholar]
- Grasso A, Pelliccia M, Alema S. Characterization of α-latrotoxin interaction with rat brain synaptosomes and PC12 cells. Toxicon. 1982;20:149–156. doi: 10.1016/0041-0101(82)90184-2. [DOI] [PubMed] [Google Scholar]
- Grasso A, Rufini S, Senni I. Concanavalin A blocks black widow spider toxin stimulation of transmitter release from synaptosomes. FEBS Lett. 1978;85:241–244. doi: 10.1016/0014-5793(78)80464-5. [DOI] [PubMed] [Google Scholar]
- Grishin EV. Black widow spider toxins: the present and the future. Toxicon. 1998;36:1693–1701. doi: 10.1016/s0041-0101(98)00162-7. [DOI] [PubMed] [Google Scholar]
- Hlubek M, Tian D, Stuenkel EL. Mechanism of α-latrotoxin action at nerve endings of neurohypophysis. Brain Res. 2003;992:30–42. doi: 10.1016/j.brainres.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Hlubek MD, Stuenkel EL, Krasnoperov VG, Petrenko AG, Holz RW. Calcium-independent receptor for α-latrotoxin and neurexin Iα facilitate toxin-induced channel formation: evidence that channel formation results from tethering of toxin to membrane. Mol. Pharmacol. 2000;57:519–528. doi: 10.1124/mol.57.3.519. [DOI] [PubMed] [Google Scholar]
- Hurlbut WP, Chieregatti E, Valtorta F, Haimann C. α-Latrotoxin channels in neuroblastoma cells. J. Membr. Biol. 1994;138:91–102. doi: 10.1007/BF00211072. [DOI] [PubMed] [Google Scholar]
- Hurlbut WP, Iezzi N, Fesce R, Ceccarelli B. Correlation between quantal secretion and vesicle loss at the frog neuromuscular junction. J. Physiol. (Lond. ) 1990;425:501–526. doi: 10.1113/jphysiol.1990.sp018115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Bittner MA, Krasnoperov V, Little AR, Chepurny O, Holz RW, Petrenko AG. A novel ubiquitously expressed α-latrotoxin receptor is a member of the CIRL family of G-protein-coupled receptors. J. Biol. Chem. 1999;274:5491–5498. doi: 10.1074/jbc.274.9.5491. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Khvotchev M, Kiyatkin N, Simpson L, Sugita S, Sudhof TC. α-Latrotoxin action probed with recombinant toxin: receptors recruit α-latrotoxin but do not transduce an exocytotic signal. EMBO J. 1998;17:6188–6199. doi: 10.1093/emboj/17.21.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki PK, Habermann E. Tetanus and botulinum toxins inhibit, and black widow spider venom stimulates the release of methionine-enkephalin-like material in vitro. J. Neurochem. 1983;41:395–402. doi: 10.1111/j.1471-4159.1983.tb04755.x. [DOI] [PubMed] [Google Scholar]
- Khvotchev M, Lonart G, Sudhof TC. Role of calcium in neurotransmitter release evoked by α-latrotoxin or hypertonic sucrose. Neuroscience. 2000;101:793–802. doi: 10.1016/s0306-4522(00)00378-x. [DOI] [PubMed] [Google Scholar]
- Kiyatkin NI, Dulubova IE, Chekhovskaya IA, Grishin EV. Cloning and structure of cDNA encoding α-latrotoxin from black widow spider venom. FEBS Lett. 1990;270:127–131. doi: 10.1016/0014-5793(90)81250-r. [DOI] [PubMed] [Google Scholar]
- Kiyatkin NI, Kulikovskaya IM, Grishin EV, Beadle DJ, King LA. Functional characterization of black widow spider neurotoxins synthesised in insect cells. Eur. J. Biochem. 1995;230:854–859. doi: 10.1111/j.1432-1033.1995.tb20628.x. [DOI] [PubMed] [Google Scholar]
- Krasilnikov OV, Sabirov RZ. Comparative analysis of latrotoxin channels of different conductance in planar lipid bilayers. Evidence for cluster organization. Biochim. Biophys. Acta. 1992;1112:124–128. doi: 10.1016/0005-2736(92)90262-k. [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA, Ichtchenko K, Petrenko AG. Post-translational proteolytic processing of the calcium-independent receptor of α-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J. Biol. Chem. 2002a;277:46518–46526. doi: 10.1074/jbc.M206415200. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Beavis R, Chepurny OG, Little AR, Plotnikov AN, Petrenko AG. The calcium-independent receptor of α-latrotoxin is not a neurexin. Biochem. Biophys. Res. Commun. 1996;227:868–875. doi: 10.1006/bbrc.1996.1598. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, et al. α-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron. 1997;18:925–937. doi: 10.1016/s0896-6273(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Bittner MA, Mo W, Buryanovsky L, Neubert TA, Holz RW, Ichtchenko K, Petrenko AG. Protein tyrosine phosphatase-σ is a novel member of the functional family of α-latrotoxin receptors. J. Biol. Chem. 2002b;277:35887–35895. doi: 10.1074/jbc.M205478200. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Shamotienko OG, Grishin EV. Isolation and properties of insect-specific neurotoxins from venoms of the spider Lactodectus mactans tredecimguttatus (letter) Bioorg. Khim. 1990;16:1138–1140. [PubMed] [Google Scholar]
- Kreienkamp HJ, Zitzer H, Gundelfinger ED, Richter D, Bockers TM. The calcium-independent receptor for α-latrotoxin from human and rodent brains interacts with members of the ProSAP/SSTRIP/Shank family of multidomain proteins. J. Biol. Chem. 2000;275:32387–32390. doi: 10.1074/jbc.C000490200. [DOI] [PubMed] [Google Scholar]
- Lajus S, Lang J. Splice variant 3, but not 2 of receptor protein-tyrosine phosphatase σ can mediate stimulation of insulin-secretion by α-latrotoxin. J. Cell Biochem. 2006;98:1552–1559. doi: 10.1002/jcb.20871. [DOI] [PubMed] [Google Scholar]
- Lang J, Ushkaryov Y, Grasso A, Wollheim CB. Ca2+-independent insulin exocytosis induced by α-latrotoxin requires latrophilin, a G protein-coupled receptor. EMBO J. 1998;17:648–657. doi: 10.1093/emboj/17.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelianova V, Thomson D, Ribchester RR, Tonevitsky AG, Ushkaryov YA. Activation of α-latrotoxin receptors at neuromuscular nerve terminals leads to a prolonged burst-like release of acetylcholine. Bull. Exp. Biol. Med. 2009;147 doi: 10.1007/s10517-009-0600-5. in press. [DOI] [PubMed] [Google Scholar]
- Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA. α-Latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J. Biol. Chem. 1997;272:21504–21508. doi: 10.1074/jbc.272.34.21504. [DOI] [PubMed] [Google Scholar]
- Li G, Lee D, Wang L, Khvotchev M, Chiew SK, Arunachalam L, Collins T, Feng ZP, Sugita S. N-terminal insertion and C-terminal ankyrin-like repeats of α-latrotoxin are critical for Ca2+-dependent exocytosis. J. Neurosci. 2005;25:10188–10197. doi: 10.1523/JNEUROSCI.3560-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mahajan A, Tsai MD. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- Longenecker HE, Hurlbut WP, Mauro A, Clark AW. Effects of black widow spider venom on the frog neuromuscular junction. Effects on end-plate potential, miniature end-plate potential and nerve terminal spike. Nature. 1970;225:701–703. doi: 10.1038/225701a0. [DOI] [PubMed] [Google Scholar]
- Lunev AV, Demin VV, Zaitsev OI, Spadir SI, Grishin EV. Electron microscopy of α-latrotoxin from the venom of the black widow spider Latrodectus mactans tredecimguttatus. Bioorg. Khim. 1991;17:1021–1026. [PubMed] [Google Scholar]
- Maestrini E, et al. High-density SNP association studyand copy number variation analysis of the AUTS1 and AUTS5 loci implicate IMMP2L-DOCK4 gene region in autism susceptibility. Mol. Psychiatry. 2009:1–15. doi: 10.1038/mp.2009.34. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita H, Lelianova VG, Ushkaryov YA. The latrophilin family: multiply spliced G protein-coupled receptors with differential tissue distribution. FEBS Lett. 1999;443:348–352. doi: 10.1016/s0014-5793(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Matteoli M, Haimann C, Torri-Tarelli F, Polak JM, Ceccarelli B, De Camilli P. Differential effect of α-latrotoxin on exocytosis from small synaptic vesicles and from large dense-core vesicles containing calcitonin gene-related peptide at the frog neuromuscular junction. Proc. Natl. Acad. Sci. U. S. A. 1988;85:7366–7370. doi: 10.1073/pnas.85.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J, Batt J, Doering LC, Rotin D, Bain JR. Enhanced rate of nerve regeneration and directional errors after sciatic nerve injury in receptor protein tyrosine phosphatase σ knockout mice. J. Neurosci. 2002;22:5481–5491. doi: 10.1523/JNEUROSCI.22-13-05481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Rosenthal L, Meldolesi J, Nicholls DG. α-Latrotoxin releases both vesicular and cytoplasmic glutamate from isolated nerve terminals. J. Neurochem. 1990;55:2039–2047. doi: 10.1111/j.1471-4159.1990.tb05793.x. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Sokolov Y, Chanturiya AN, Lishko VK. Channels produced by spider venoms in bilayer lipid membrane: mechanisms of ion transport and toxic action. Biochim. Biophys. Acta. 1986;862:185–198. doi: 10.1016/0005-2736(86)90482-7. [DOI] [PubMed] [Google Scholar]
- Misler S, Hurlbut WP. Action of black widow spider venom on quantized release of acetylcholine at the frog neuromuscular junction: dependence upon external Mg2+ Proc. Natl. Acad. Sci. U. S. A. 1979;76:991–995. doi: 10.1073/pnas.76.2.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M, Fernandez-Chacon R, Sudhof TC. The making of neurexins. J. Neurochem. 1998;71:1339–1347. doi: 10.1046/j.1471-4159.1998.71041339.x. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. α-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1β reveal function as heterophilic cell adhesion molecules. J. Biol. Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Rugolo M, Scott IG, Meldolesi J. α-Latrotoxin of black widow spider venom depolarizes the plasma membrane, induces massive calcium influx, and stimulates transmitter release in guinea pig brain synaptosomes. Proc. Natl. Acad. Sci. U. S. A. 1982;79:7924–7928. doi: 10.1073/pnas.79.24.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhi G, Rampazzo A, Beffagna G, Antonio DG. Identification and characterization of heart-specific splicing of human neurexin 3 mRNA (NRXN3) Biochem. Biophys. Res. Commun. 2002;298:151–155. doi: 10.1016/s0006-291x(02)02403-8. [DOI] [PubMed] [Google Scholar]
- Orlova EV, Rahman MA, Gowen B, Volynski KE, Ashton AC, Manser C, van Heel M, Ushkaryov YA. Structure of α-latrotoxin oligomers reveals that divalent cation-dependent tetramers form membrane pores. Nat. Struct. Biol. 2000;7:48–53. doi: 10.1038/71247. [DOI] [PubMed] [Google Scholar]
- Parpura V, Liu F, Brethorst S, Jeftinija K, Jeftinija S, Haydon PG. α-latrotoxin stimulates glutamate release from cortical astrocytes in cell culture. FEBS Lett. 1995;360:266–270. doi: 10.1016/0014-5793(95)00121-o. [DOI] [PubMed] [Google Scholar]
- Pescatori M, Bradbury A, Bouet F, Gargano N, Mastrogiacomo A, Grasso A. The cloning of a cDNA encoding a protein (latrodectin) which co-purifies with the α-latrotoxin from the black widow spider Latrodectus tredecimguttatus (Theridiidae) Eur. J. Biochem. 1995;230:322–328. doi: 10.1111/j.1432-1033.1995.tb20566.x. [DOI] [PubMed] [Google Scholar]
- Petrenko AG. α-Latrotoxin receptor. Implications in nerve terminal function. FEBS Lett. 1993;325:81–85. doi: 10.1016/0014-5793(93)81418-y. [DOI] [PubMed] [Google Scholar]
- Petrenko AG, Kovalenko VA, Shamotienko OG, Surkova IN, Tarasyuk TA, Ushkaryov YuA, Grishin EV. Isolation and properties of the α-latrotoxin receptor. EMBO J. 1990;9:2023–2027. doi: 10.1002/j.1460-2075.1990.tb08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko AG, Perin MS, Davletov BA, Ushkaryov YA, Geppert M, Sudhof TC. Binding of synaptotagmin to the α-latrotoxin receptor implicates both in synaptic vesicle exocytosis. Nature. 1991;353:65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- Petrenko AG, Ullrich B, Missler M, Krasnoperov V, Rosahl TW, Sudhof TC. Structure and evolution of neurexophilin. J. Neurosci. 1996;16:4360–4369. doi: 10.1523/JNEUROSCI.16-14-04360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picotti GB, Bondiolotti GP, Meldolesi J. Peripheral catecholamine release by α-latrotoxin in the rat. Naunyn Schmiedebergs Arch. Pharmacol. 1982;320:224–229. doi: 10.1007/BF00510132. [DOI] [PubMed] [Google Scholar]
- Popova NV, Plotnikov A, Deev IE, Petrenko AG. Interaction of calcium-independent latrotoxin receptor with intracellular adapter protein TRIP8b. Dokl. Biochem. Biophys. 2007;414:149–151. doi: 10.1134/s1607672907030155. [DOI] [PubMed] [Google Scholar]
- Popova NV, Plotnikov AN, Ziganshin RK, Deyev IE, Petrenko AG. Analysis of proteins interacting with TRIP8b adapter. Biochemistry (Mosc. ) 2008;73:644–651. doi: 10.1134/s0006297908060035. [DOI] [PubMed] [Google Scholar]
- Pulido R, Serra-Pages C, Tang M, Streuli M. The LAR/PTPδ/PTPσ subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTPδ, and PTPσ isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP.1. Proc. Natl. Acad. Sci. U. S. A. 1995;92:11686–11690. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MA, Ashton AC, Meunier FA, Davletov BA, Dolly JO, Ushkaryov YA. Norepinephrine exocytosis stimulated by α-latrotoxin requires both external and stored Ca2+ and is mediated by latrophilin, G proteins and phospholipase C. Phil. Trans. R. Soc. Lond. B. 1999;354:379–386. doi: 10.1098/rstb.1999.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robello M, Fresia M, Maga L, Grasso A, Ciani S. Permeation of divalent cations through α-latrotoxin channels in lipid bilayers: steady-state current-voltage relationships. J. Membr. Biol. 1987;95:55–62. doi: 10.1007/BF01869630. [DOI] [PubMed] [Google Scholar]
- Rohou A, Nield J, Ushkaryov YA. Insecticidal toxins from black widow spider venom. Toxicon. 2007;49:531–549. doi: 10.1016/j.toxicon.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. 1996. pp. 1197–1207. [DOI] [PubMed]
- Rosenthal L, Zacchetti D, Madeddu L, Meldolesi J. Mode of action of α-latrotoxin: role of divalent cations in Ca2+- dependent and Ca2+-independent effects mediated by the toxin. Mol. Pharmacol. 1990;38:917–923. [PubMed] [Google Scholar]
- Sajnani-Perez G, Chilton JK, Aricescu AR, Haj F, Stoker AW. Isoform-specific binding of the tyrosine phosphatase PTPσ to a ligand in developing muscle. Mol. Cell Neurosci. 2003;22:37–48. doi: 10.1016/s1044-7431(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Sampayo RRL. Pharmacological action of the venom of Latrodectus mactans and other Latrodectus spiders. J. Pharmacol. Exp. Ther. 1944;80:309–322. [Google Scholar]
- Scheer H, Madeddu L, Dozio N, Gatti G, Vicentini LM, Meldolesi J. α-Latrotoxin of black widow spider venom: an interesting neurotoxin and a tool for investigating the process of neurotransmitter release. J. Physiol. (Paris) 1984;79:216–221. [PubMed] [Google Scholar]
- Scheer HW. Interactions between α-latrotoxin and trivalent cations in rat striatal synaptosomal preparations. J. Neurochem. 1989;52:1590–1597. doi: 10.1111/j.1471-4159.1989.tb09213.x. [DOI] [PubMed] [Google Scholar]
- Scheer HW. Interactions between the presynaptically active neurotoxins α-latrotoxin and ω-conotoxin GVIA: studies on calcium fluxes and binding parameters in rat and chicken synaptosomes. Can. J. Physiol. Pharmacol. 1990;68:1049–1054. doi: 10.1139/y90-158. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Serova OV, Popova NV, Deev IE, Petrenko AG. Identification of proteins in complexes with α-latrotoxin receptors. Bioorg. Khim. 2008;34:747–753. doi: 10.1134/s1068162008060046. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim E. The Shank family of scaffold proteins. J. Cell Sci. 2000;113(Pt 11):1851–1856. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- Silva JP, Lelianova V, Hopkins C, Volynski KE, Ushkaryov Y. Functional Cross-interaction of the Fragments Produced by the Cleavage of Distinct Adhesion G-protein-coupled Receptors. J. Biol. Chem. 2009;284:6495–6506. doi: 10.1074/jbc.M806979200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu R, Fladd C, Rotin D. N-cadherin is an in vivo substrate for PTPσ and participates in PTPσ-mediated inhibition of axon growth. Mol. Cell Biol. 2006;27:208–219. doi: 10.1128/MCB.00707-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl B, von Mollard GF, Walch-Solimena C, Jahn R. GTP cleavage by the small GTP-binding protein Rab3A is associated with exocytosis of synaptic vesicles induced by α-latrotoxin. J. Biol. Chem. 1994;269:24770–24776. [PubMed] [Google Scholar]
- Sugita S, Ichtchenko K, Khvotchev M, Südhof TC. α-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors. G-protein coupling not required for triggering exocytosis. J. Biol. Chem. 1998;273:32715–32724. doi: 10.1074/jbc.273.49.32715. [DOI] [PubMed] [Google Scholar]
- Sugita S, Khvochtev M, Südhof TC. Neurexins are functional α-latrotoxin receptors. Neuron. 1999;22:489–496. doi: 10.1016/s0896-6273(00)80704-7. [DOI] [PubMed] [Google Scholar]
- Szatmari P, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genetics. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, Sudhof TC. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaben S, Sudhof TC, Stahl B. The G Protein-coupled Receptor CL1 Interacts Directly with Proteins of the Shank Family. J. Biol. Chem. 2000;275:36204–36210. doi: 10.1074/jbc.M006448200. [DOI] [PubMed] [Google Scholar]
- Tobaben S, Sudhof TC, Stahl B. Genetic analysis of α-latrotoxin receptors reveals functional interdependence of CIRL/Latrophilin 1 and neurexin Iα. J. Biol. Chem. 2002;277:6359–6365. doi: 10.1074/jbc.M111231200. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- Tsang CW, Elrick DB, Charlton MP. α-Latrotoxin releases calcium in frog motor nerve terminals. J. Neurosci. 2000;20:8685–8692. doi: 10.1523/JNEUROSCI.20-23-08685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng MC, Siekevitz P. The binding interaction between α-latrotoxin from black widow spider venom and a dog cerebral cortex synaptosomal membrane preparation. J. Neurochem. 1979;33:263–274. doi: 10.1111/j.1471-4159.1979.tb11728.x. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Sudhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- Ushkaryov Y. α-Latrotoxin: from structure to some functions. Toxicon. 2002;40:1–5. doi: 10.1016/s0041-0101(01)00204-5. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Hata Y, Ichtchenko K, Moomaw C, Afendis S, Slaughter CA, Sudhof TC. Conserved domain structure of β-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J. Biol. Chem. 1994;269:11987–11992. [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the α-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Sudhof TC. Neurexin IIIα: extensive alternative splicing generates membrane-bound and soluble forms. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6410–6414. doi: 10.1073/pnas.90.14.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtorta F, Jahn R, Fesce R, Greengard P, Ceccarelli B. Synaptophysin (p38) at the frog neuromuscular junction: its incorporation into the axolemma and recycling after intense quantal secretion. J. Cell Biol. 1988;107:2717–2727. doi: 10.1083/jcb.107.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Renterghem C, Iborra C, Martin-Moutot N, Lelianova V, Ushkaryov Y, Seagar M. α-Latrotoxin forms calcium-permeable membrane pores via interactions with latrophilin or neurexin. Eur. J. Neurosci. 2000;12:3953–3962. doi: 10.1046/j.1460-9568.2000.00282.x. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Vicentini LM, Meldolesi J. α-Latrotoxin of black widow spider venom binds to a specific receptor coupled to phosphoinositide breakdown in PC12 cells. Biochem. Biophys. Res. Commun. 1984;121:538–544. doi: 10.1016/0006-291x(84)90215-8. [DOI] [PubMed] [Google Scholar]
- Volkova TM, Pluzhnikov KA, Woll PG, Grishin EV. Low-molecular-weight components from black-widow spider venom. Toxicon. 1995;33:483–489. doi: 10.1016/0041-0101(94)00166-6. [DOI] [PubMed] [Google Scholar]
- Volynski KE, Capogna M, Ashton AC, Thomson D, Orlova EV, Manser CF, Ribchester RR, Ushkaryov YA. Mutant α-latrotoxin (LTXN4C) does not form pores and causes secretion by receptor stimulation. This action does not require neurexins. J. Biol. Chem. 2003;278:31058–31066. doi: 10.1074/jbc.M210395200. [DOI] [PubMed] [Google Scholar]
- Volynski KE, Nosyreva ED, Ushkaryov YA, Grishin EV. Functional expression of α-latrotoxin in baculovirus system. FEBS Lett. 1999;442:25–28. doi: 10.1016/s0014-5793(98)01624-x. [DOI] [PubMed] [Google Scholar]
- Volynski KE, Silva JP, Lelianova VG, Atiqur RM, Hopkins C, Ushkaryov YA. Latrophilin fragments behave as independent proteins that associate and signal on binding of LTXN4C. EMBO J. 2004;23:4423–4433. doi: 10.1038/sj.emboj.7600443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volynski KV, et al. Latrophilin, neurexin and their signaling-deficient mutants facilitate α-latrotoxin insertion into membranes but are not involved in pore formation. J. Biol. Chem. 2000;275:41175–41183. doi: 10.1074/jbc.M005857200. [DOI] [PubMed] [Google Scholar]
- Volynskii KE, Volkova TM, Galkina TG, Krasnoperov VG, Pluzhnikov KA, Khvoshchev MV, Grishin EV. Molecular cloning and primary structure of cDNA fragment for α-latrocrustatoxin from black widow spider venom. Bioorg. Khim. 1999;25:25–30. [PubMed] [Google Scholar]
- Wanke E, Ferroni A, Gattanini P, Meldolesi J. α-Latrotoxin of the black widow spider venom opens a small, non-closing cation channel. Biochem. Biophys. Res. Commun. 1986;134:320–325. doi: 10.1016/0006-291x(86)90565-6. [DOI] [PubMed] [Google Scholar]
- Yan H, Grossman A, Wang H, D’Eustachio P, Mossie K, Musacchio JM, Silvennoinen O, Schlessinger J. A novel receptor tyrosine phosphatase-σ that is highly expressed in the nervous system. J. Biol. Chem. 1993;268:24880–24886. [PubMed] [Google Scholar]
- Zhou Z, Misler S. Amperometric detection of stimulus-induced quantal release of catecholamines from cultured superior cervical ganglion neurons. Proc. Natl. Acad. Sci. U. S. A. 1995;92:6938–6942. doi: 10.1073/pnas.92.15.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]