Abstract
Objective
There is wide variation and lack of evidence in current recommendations for atropine dosing schedules leading to subsequent variation in clinical practice. Therefore we sought to examine the safety and effectiveness of a titrated versus ‘ad hoc’ atropine treatment regimen in a cohort of patients with acute cholinesterase inhibitor pesticide poisoning.
Design
A prospective cohort study was conducted in 3 district secondary referral hospitals in Sri Lanka using a structured data collection form that collected details of clinical symptoms and outcomes of cholinesterase inhibitor pesticide poisoning, atropine doses and signs of atropinisation. We compared two hospitals that used a titrated dosing protocol based on a structured monitoring sheet for atropine infusion with another hospital using an ‘ad hoc’ regime.
Findings
During the study 272 symptomatic patients with anticholinesterase poisoning requiring atropine were admitted to the three hospitals. Outcomes of death and ventilation were analyzed for all patients, 226 patients were prospectively assessed for atropine toxicity. At baseline patients in the titrated dose cohort had clinical signs consistent with greater toxicity. This in part may be due to ingestion of more toxic OPs. They received less pralidoxime and atropine and were less likely to develop features of atropine toxicity such as delirium (1% vs 17%), hallucinations (1% vs 35%) or either (1% vs 35%) and need for patient restraint (3% vs 48%) compared with the ‘ad hoc’ dose regime. After adjusting for the pesticides ingested, there was no difference in mortality and ventilatory rates between protocols.
Conclusions
‘Ad hoc’ high dose atropine regimens are associated with more frequent atropine toxicity without any obvious improvement in patient outcome compared with doses titrated to clinical effect. Atropine doses should be titrated against response and toxicity. Further education and the use of a structured monitoring sheet may assist in more appropriate atropine use in anticholinesterase pesticide poisoning.
Keywords: Atropine toxicity, Organophosphorous poisoning, atropinisation protocols
Introduction
In developing countries, the majority of deaths from self-poisoning are due to cholinesterase-inhibiting insecticides such as organophosphorus (OP) and carbamate compounds[1]. The number of intoxications is estimated at 3,000,000 per year and the casualties around 300,000 per year[2]. Despite anti-muscarinic agents such as atropine being universally accepted as the cornerstone of treatment, there are no standard treatment protocols and there are over 31 different recommendations in 38 texts [3]. Despite this variation, there is consensus that adequate atropinisation is achieved when the peripheral muscarinic symptoms of bronchial hypersecretion is controlled and cardiovascular function stabilized. Other endpoints include control of bradycardia, absence of sweating and reversal of miosis. Atropine also has central therapeutic effects - animal studies suggest that atropine may reduce centrally mediated respiratory failure[4].
Comparative studies of different protocols have not been done to guide clinicians. South Asian Clinical Toxicology Research Collaboration (SACTRC) works in partnership with local physicians in Sri Lanka to conduct clinical trials in a number of clinical research centers. In this context we have observed considerable variation in the use of atropine within Sri Lanka.
We were requested to assess the atropine use in a hospital where we had recently commenced clinical research. Atropine use in this center was without a fixed protocol. At other research centers established over the last 4 years, SACTRC has promoted an atropine protocol that is tailored to clinical response using a structured monitoring sheet [5]. We aimed to compare effectiveness and adverse effects in patients treated with a titrated atropine protocol with those treated with an ‘ad hoc’ dosing regime.
Methods
This study was part of a larger observational poisoning cohort study that has ethical approval from the Sri Lanka Medical Association and the Australian National University Human Research Ethics Committees.
This was a prospective observational cohort study of symptomatic anticholinesterase insecticide poisoned patients undertaken from February to May 2006. Two hospitals have had a titrated dose atropine protocol with a monitoring sheet actively promoted since 2002. Patients from these hospitals are analyzed together as the ‘titrated cohort’. The other hospital had no standard promoted protocol but the doctors frequently gave boluses and high fixed dose infusions of atropine – this group will be referred to as the ‘ad hoc cohort’.
Patients who were prescribed pralidoxime chloride typically received a 1 g bolus followed by further bolus doses of 1 g every 6 h for 1–3 days. The decision to use pralidoxime was made by the treating physician; pralidoxime was not routinely used by one treating physician in the titrated group. Patients known to have taken carbamates received no pralidoxime.
The recommended dosing protocol for symptomatic patients in the titrated atropine cohort has been described more fully [5]. It consists of an initial bolus of 1.5 to 3 mg of atropine with doses doubling every 5 minutes until atropinisation is achieved. Clearing of chest on auscultation was used as the primary endpoint of atropinization. Following that an infusion is given with a rate that is estimated from the size of the initial dose required to achieve atropinisation. This is typically in the range of 1 to 2 mg/hour. The infusion dose is subsequently adjusted up or down depending on the presence of signs of under atropinisation (chest signs, sweating, bradycardia and miosis) or over atropinisation (absent bowel sounds, fever, tachycardia, mydriasis and confusion). The ‘ad hoc’ dose cohort received intermittent boluses, infusion or a combination of bolus and infusion as decided by the treating doctors.
Only patients with a history of pesticide ingestion who had cholinergic features who received atropine were included in the study. The decision to atropinise patients who presented was made by the treating medical team without involvement of the research team. Where possible the type of ingested pesticide was considered confirmed if the ingested pesticide could be named or if the bottle was presented during the admission. Previous studies have shown this approach to be reliable for identification of the ingested pesticide [6].
Patient demographics, the type and amount of anti-cholinesterase insecticide, alcohol co-ingestion and other co-morbid conditions, and the major clinical outcomes of death and ventilation were prospectively recorded for all patients.
Additional prospective data collection for atropine effects used a structured data form completed at regular intervals by medically qualified and trained research assistants. A minimum of 6 and an average of 9 data records were taken each day for each patient. Atropine bolus and infusion rates were also recorded. Clinical data included blood pressure, heart rate, respiratory rate, pupil size, bowel sounds, pulmonary signs (i.e. crepitations on auscultation) and presence of auditory and visual hallucinations. Patients were also screened for delirium using Confusion Assessment Method (CAM) (Box 1). Atropine toxicity was defined as atropine induced delirium, hallucinations (which are not a feature of delirium defined by CAM), or both.
Box. Confusion Assessment Method (CAM) Diagnostic Algorithm.
Acute onset and fluctuating course
Inattention
Disorganized thinking
Altered level of consciousness
The Diagnosis of Delirium requires a present/abnormal rating for criteria (1) and (2) with either (3) and (4)
Adopted from Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of internal medicine 1990;113(12):941-8.
Data Analysis
Based upon an intention to treat principle, analysis for major clinical outcomes such as death and ventilation was done for all patients admitted to the hospital who met the inclusion criteria. Analysis for adverse effects was restricted to those patients who had prospective sequential observations for atropine effects recorded from the time of admission to atropinisation.
Statistical analysis was performed using STATA v8. Clinical characteristics were summarized using counts (percentages) for categorical data and median (interquartile range [IQR]) for non-normally distributed continuous variables. The data on age, amount of poison ingested, atropine dosing, length of hospital stay, and duration of intubation were analyzed using the Mann Whitney U test. Proportions were compared with the Chi square test.
Results
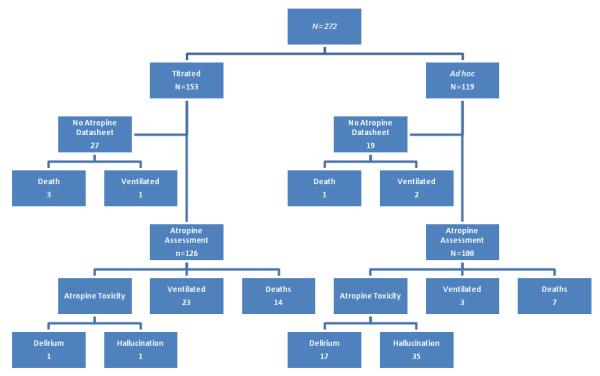
During the study period (flow chart), 272 consecutive symptomatic patients with anticholinesterase poisoning were enrolled. Atropine data were collected for 226/272 (83%).
The baseline characteristics of patients in both groups were different (table 1). There were statistical differences in age, sex, and alcohol co-ingestion, with the ‘ad hoc’ dose group having higher rates of pralidoxime usage and impairment of Glasgow coma scale (GCS) (GCS 9-14) on admission. The median time to admission was 4 hours and similar in both groups.
Table 1.
Summary of baseline characteristics of all patients in the cohort
|
Ad hoc dose N=119 |
Titrated dose N=153 |
P Value | |
|---|---|---|---|
| Males | 85 (71%) | 90 (59%) | 0.031* |
| Age (years)¥ | 25 (20-35) | 26 (22-38) | 0.025* |
| Anti-cholinesterase pesticide OPs |
|||
| Chlorpyrifos | 93 (78%) | 56 (37%) | <0.0001* |
| Dimethioate | 3 (3%) | 28 (18%) | <0.0001* |
| Other Ops£ | 18 (15%) | 38 (25%) | 0.04* |
| Carbamate | 5 (4%) | 13 (8%) | N/S |
| unknown anti-cholinesterase pesticide | 0 (0%) | 18 (12%) | <0.0001* |
| Amount poison ingestion (ml.)¥ | 27 (10-80) | 50 (25-100) | 0.002* |
| Post ingestion time on admission (hrs)¥ | 4 (2-9) | 4 (3-7) | N/S |
| Direct admission | 89 (75%) | 24 (16%) | <0.0001* |
| Atropine given before admission | 20 (17%) | 66 (43%) | <0.0001* |
| Alcohol ingestion | 34 (29%) | 29 (19%) | N/S |
| GCS <15 | 71 (60%) | 110 (72%) | <0.033* |
| GCS 9-14 | 35 (29%) | 20 (13%) | <0.0009* |
| GCS ≤ 9 | 36 (30%) | 90 (59%) | <0.002* |
| Co-morbid illness€ | 15 (13%) | 22 (14%) | N/S |
| Pulmonary signs (crepitations) | 25 (21%) | 55 (36%) | 0.007* |
| Ventilated at baseline | 1 (1%) | 9 (6%) | 0.002* |
| Pralidoxime administered | 48 (40%) | 32 (21%) | 0.0005* |
Data are number (%) unless otherwise indicated.
Median (IQR).
Others: Diazinon, Phenthoate, Acephate, Profenofos, Fenthion, unknown Ops
Co-morbid illness included cardiac, pulmonary, psychiatric, neurologic and undiagnosed disorders
Results that are significantly different and corresponding P value
The severity of poisoning was worse in the titrated group - they were more likely to have been transferred and treated with atropine from a peripheral hospital, to have ingested a higher estimated dose of insecticide and clinically had more signs of anticholinesterase poisoning at presentation manifested by higher rates of pulmonary crepitations on admission. The pattern of pesticide ingestion was dissimilar in that the titrated dose group had a lower proportion taking chlorpyrifos and a higher proportion taking dimethoate (table 1).
The amount of atropine boluses, infusion rates and total daily doses were significantly higher in the ad hoc dose regimen (table 2). As a consequence, atropine toxicity was significantly more common with the ad hoc regimen (table 3). The median time to development of atropine toxicity was 7 hours (interquartile range [IQR] 3-15) following atropinisation in all hospitals. The most frequent manifestation was agitated delirium, requiring either physical restraint (tied to bed) or sedation (with diazepam 5 to 10mg IV or haloperidol 5mg IM prn). In the titrated dose group only 3/126 (2%) patient’s required any form of restraint. By contrast, 48/100 (48%) of the ad hoc dose group were restrained. The rates of delirium (17% vs 1%) and hallucinations (35% vs 1%) or either was significantly higher in the ad hoc dose regime. Moreover anti-cholinergic delirium with hallucinations was noted in 36 patients but only one (1/36; 3%) of these was in the titrated atropine dose group. These patients were noted to have dilated pupils, dry mucosal membranes, flushed skin, and decreased bowel sounds and to have needed urinary catheterization, further strengthening the diagnosis of atropine toxicity. The median duration of hospital stay was significantly shorter in the titrated atropine dose group (table 3).
Table 2.
Summary of atropine dosing regime
|
Ad hoc dose N=100 |
Titrated dose N=126 |
P Value | |
|---|---|---|---|
| Atropine Bolus (mg) | 15 (10-20) | 3.9 (1.2-19.2) | 0.0013* |
| Atropine Infusion (mg/hour) | 2.1 (1.18-3.39) | 1.39 (0.46-2.32) | <0.0001* |
| Total atropine dose (mg.) over | 65.4 | 37.3 | <0.0001* |
| initial 24 hours |
Data are Median (IQR)
Results that are significantly different and corresponding P value
Table 3.
Summary of atropine toxicity in patients with prospective observations
|
Ad hoc dose N=100 |
Titrated dose N=126 |
P Value | |
|---|---|---|---|
| Restrained | |||
| Physical | 42 (42%) | 2 (2%) | <0.0001* |
| Diazepam | 5 (5%) | 1 (1%) | N/S |
| Haloperidol | 1 (1%) | 0 (0%) | N/S |
| Delirium€ | 17 (17%) | 1 (1%) | <0.0001* |
| Hallucination | 35 (35%) | 1 (1%) | <0.0001* |
| Ventilated | 3(3%) | 23 (18%) | <0.0004* |
| Hospital stay (days)¥ | 4.7 (3-6) | 3.1 (2-3) | <0.0001* |
| Deaths from atropine subgroup | 7 (7%) | 14(11%) | N/S |
| Deaths from whole cohort | 8 (7%) | 17 (13%) | N/S |
Delirium was evaluated by using CAM scoring system
Data are number (%) unless otherwise indicated
Median (IQR).
Results that are significantly different and corresponding P value
There was no statistical difference in case fatality between groups for either the entire cohort or the subgroup monitored for atropine effect (table 3).
Ventilation rates were higher in the titrated atropine group [23% vs 1%, relative risk 10.62 (95% CI 7.1, 15.3), P=<0.0001]. This difference was due to an excess of dimethoate poisonings in the titrated group and was not seen for other pesticides (table 4). Oxime therapy did not significantly alter the relative risk for ventilation in either group: 0.37 (95% CI 0.043 to 3.21, p value= 0.398) and 1.58 (95% CI 0.598 to 4.15, p value= 0.378) for the ad hoc and titrated group respectively.
Table 4.
Summary of major outcomes of pesticide type
| Ad hoc dose | Titrated dose | P Value | |
|---|---|---|---|
| Chlorpyrifos | N=93 | N=56 | |
|
| |||
| Ventilated | 2 (2%) | 5 (9%) | N/S |
| Death | 3 (3%) | 2 (4%) | N/S |
|
| |||
| Dimethoate | N=3 | N=28 | |
|
| |||
| Ventilated | 0 (0%) | 14 (50%) | N/S |
| Death | 2 (67%) | 8 (29%) | N/S |
|
| |||
| Carbamates | N=5 | N=13 | |
|
| |||
| Ventilated | 0 (0%) | 1 (8%) | N/S |
|
| |||
| Death | 1 (20%) | 2 (15%) | N/S |
|
| |||
| Others and unknown OPs£ | N=18 | N=56 | |
|
| |||
| Ventilated | 3 (17%) | 4 (7%) | N/S |
| Death | 2 (11%) | 5 (9%) | N/S |
Data are number (%)
Diazinon, Phethoate, Acephate, Profenopos, Fenthion
Discussion
Atropine is the only universally accepted specific treatment in the management of anticholinesterase poisoning. Despite this universal acceptance, there are no data to guide administration with a consequent wide variation in recommendations for dosing [3].
Adherence to some protocols will lead to lengthy delays in time to optimum atropinisation which can result in death from central respiratory depression, hypoxia (due to bronchospasm and bronchorrhoea) and hypotension (due to bradycardia and myocardial depression)[3]. Conversely, some protocols may lead to excessive anti-cholinergic toxicity, which in severe cases may even be fatal[3].
The titrated atropine dose group received significantly lower total doses of atropine. The rates of atropine toxicity, restraint, and the duration of hospital stay were all significantly lower in this group despite having indirect evidence of higher toxicity on admission, as shown by more patients with lung signs of poisoning, higher rates of ventilation and estimated doses of ingestion, and having ingested more dangerous pesticides.
The bolus doses and subsequent infusion rates were higher in the ad hoc atropine dose group. The median time to initiate atropine treatment is 4 hours. The fact that atropine toxicity developed a median of 7 hours after starting the infusion in both groups suggests that toxicity was due to higher infusion rates. Since the infusions rates in the titrated group are guided by the initial atropinisation loading dose (typically 10-20% of the cumulative bolus doses per hour), theoretically this might lead to less toxicity.
There were no significant differences in death rates between the two groups despite baseline characteristics suggesting that the titrated dose group had a higher risk of death. The ad hoc dose group had predominately ingested chlorpyrifos which has a lower case-fatality ratio (8.0) than most organophosphates [6]. Patients in the titrated group had taken a higher proportion of highly lethal OPs and are more likely to have significant aging at the time of admission [6]. This is particularly the case for dimethoate poisoning with a high case-fatality ratio of 23% [6]. Dimethoate poisoning patients were more ill, unconscious, hemodynamically unstable on admission requiring inotropes and rapidly deteriorated into early respiratory failure and largely belonged to the titrated group.
The lower use of pralidoxime in the titrated dose group was mainly due to local clinical practice based on lack of documented evidence of its efficacy at these doses of oxime in rural Asia[7]. In addition the doses of 1 gm qid is likely to be inadequate for dimethoate ingestions. This study however is underpowered to detect a therapeutic effect of pralidoxime although theoretically higher use of oxime should result in a lower atropine requirement.
Although rates of ventilation were significantly higher in the titrated group, there was no difference in ventilatory rates for dimethoate, chlorpyrifos, carbamates, unknown OP’s and other types of organophosphorous ingestions.
Despite the high proportion of patients who were admitted after being transferred in the titrated group the median time to admission was similar in both groups. This may be attributed due to a primary delay in transport in the ad hoc dose group as this population come from a mountainous area where transportation is slower.
The impairment in consciousness on admission patients (GCS 9-14) was more frequently found in the ad hoc dose group, although the proportion of patients with severe impairment (GCS<9) was more in the titrated group. The reasons remain unclear but may relate to less pre-transfer and inter-transfer atropine treatment in the ad hoc dose group and ingestion of OP’s with higher case fatality ratio in the titrated group.
The incidence of delirium may be a significant clinical issue in situations where resources are scarce as such patients require higher nursing time. Delirium may contribute to increased morbidity[8, 9]. Hyperthermia and physical restraining may further contribute to poor outcomes. Prolonged periods of untreated delirium has been shown to result in longer hospital stays, increased higher short and long-term mortality [10, 11]
Rates of alcohol use were comparable in both groups and seem unlikely to have contributed to such a large difference in delirium.
The Confusion Assessment Method (CAM) has become the most widely used instrument for detection of delirium world-wide, because of both its strong validation results as well as its ease of use, it has a sensitivity of 94-100%, specificity of 90-95%, and high inter-observer reliability [12]. There were 18 patients (9%) in the entire cohort which fulfilled the criterion for delirium according to CAM. However we had very high rates of hallucinations, suggesting that CAM is not sensitive for anti-cholinergic delirium or were poorly administered or did not culturally translate.
Poor resources may restrict the ability to titrate doses and may contribute to the evolution of clinical practices of over treatment as a therapeutic goal. Arguably over atropinisation may be preferable to under atropinisation in some situations such as transportation, high patient load or limited monitoring. In this cohort the protocol associated with high rates of atropine toxicity was not associated with improved mortality despite the fact they received a higher level of monitoring than they would receive in most secondary hospitals.
A potential limitation of our study was that a large number of patients (129/153; 84%) in the titrated groups had been transfers from a primary hospital. In general these patients would receive bolus atropine doses prior to transfer although these details on pre-transfer treatment were poorly documented or not available. If this is the case it would minimize the difference between the initial bolus doses and suggest toxicity being related to higher infusion rates.
Conclusions
This study suggests that there is no therapeutic advantage in over atropinisation of patients in situations where the patient can be monitored. Titrated doses of atropine are less likely to cause toxicity. As the infusion rate appears to be associated with anti-cholinergic toxicity this study suggests that the maintenance infusion dose should be calculated based on the cumulative dose of atropine used to optimally atropinise the patient on admission. Use of a structured monitoring sheet may aid in tailoring doses to clinical response.
Figure.
Flow chart of major outcomes following anticholinesterase pesticide poisoning for all patients in the cohort.
Acknowledgements
We would like to thank Dr Michael Eddelston, Professors Nick Buckley and Kent Oslen for their comments. The clinical research assistants and respective trial coordinators for their help with data collection and entry. The cooperation of the treating physicians, nurses and staff in all hospitals is gratefully acknowledged. SACTRC is funded by the Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant GR071669MA.
Reference
- 1.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Qjm. 2000;93(11):715–731. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 2.Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Advances in clinical chemistry. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 3.Eddleston M, Buckley NA, Checketts H, Senarathna L, Mohamed F, Sheriff MH, Dawson A. Speed of initial atropinisation in significant organophosphorus pesticide poisoning--a systematic comparison of recommended regimens. Journal of toxicology. 2004;42(6):865–875. doi: 10.1081/clt-200035223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bird SB, Gaspari RJ, Dickson EW. Early death due to severe organophosphate poisoning is a centrally mediated process. Acad Emerg Med. 2003;10(4):295–298. doi: 10.1111/j.1553-2712.2003.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 5.Eddleston M, Dawson A, Karalliedde L, Dissanayake W, Hittarage A, Azher S, Buckley NA. Early management after self-poisoning with an organophosphorus or carbamate pesticide - a treatment protocol for junior doctors. Critical care (London, England) 2004;8(6):R391–397. doi: 10.1186/cc2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, et al. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366(9495):1452–1459. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 7.Buckley NA, Eddleston M, Szinicz L. Oximes for acute organophosphate pesticide poisoning. Cochrane database of systematic reviews (Online) 2005;(1):CD005085. doi: 10.1002/14651858.CD005085. [DOI] [PubMed] [Google Scholar]
- 8.Association AP. Practice guidelines for the treatment of patients with delirium. Am J Psychiatry. 1999;vol. 156:1–20. [PubMed] [Google Scholar]
- 9.Meanger D. Delirium: optimizing management. BMJ. (Clinical research ed) 2001;322(7279):144–149. doi: 10.1136/bmj.322.7279.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Seminars in respiratory and critical care medicine. 2001;22(2):115–126. doi: 10.1055/s-2001-13826. [DOI] [PubMed] [Google Scholar]
- 11.Ely EW, Gautam S, Margolin R, Francis J, May L, Speroff T, Truman B, Dittus R, Bernard R, Inouye SK. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of internal medicine. 1990;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]