Abstract
Context
The association between low socioeconomic status (SES) and childhood obesity foreshadows life-long inequalities in health. Insight into the causal mechanisms linking childhood adversity to long-term health could be provided by discovering when the negative SES gradient in weight emerges, and what early life experiences are associated with it.
Objective
To examine SES differences in infant weight gain in the first three months of life, and assess contributions of parental BMI, maternal smoking, and feeding method to this association.
Design
Observational study using longitudinal weight data from 2402 families taking part in the Gemini study; a twin birth cohort recruited from all twin births between March and December 2007 in England and Wales.
Outcome Measures
Infant weights at birth and three months converted to standard deviation scores (SDS), change in weight SDS, and rapid growth. SES was indexed by occupation and maternal education.
Results
There were no SES differences in birth weight, but lower SES was associated with higher three-month weight, greater change in weight, and a higher prevalence of rapid growth (all p<0.01); with graded associations across levels of SES. Including parental overweight or smoking in pregnancy in the regression model did not affect the association between SES and weight gain, but including feeding method attenuated the SES effect on weight gain by at least 62% and rendered it non-significant.
Conclusion
The foundations for lifelong socioeconomic inequalities in obesity risk may be laid in early infancy, with infant feeding practices playing a part in the diverging weight trajectories.
Keywords: Socioeconomic status, Obesity, Birth cohort, Weight Gain, Infancy, breastfeeding
Introduction
Rapid growth in infancy has been associated with increased risk of paediatric obesity1-3 and higher cardiovascular and metabolic risk in early adulthood4-9. Many, but not all, studies find that infants from lower socioeconomic status (SES) families have lower birth weights than infants from higher SES families10-12. However, by early-to-middle childhood, the SES gradient is reversed, and children from lower SES families have a higher risk of obesity13-16. To the authors’ knowledge, no studies to date have looked at SES differences in weight gain in early infancy.
SES differences in obesity prevalence are a major contributor to lifelong inequalities in health17, making it crucial to understand the underlying mechanisms. If divergence in weight trajectories begins in the first few months of life, this points to different possible mechanisms than if they emerge when children are mobile and eating a varied diet. One potential mechanism is parental BMI, because it is strongly correlated with the child’s obesity risk18, and an association between higher BMI and lower SES is well-established in women17, although in men, the association is weaker17. SES gradients in weight that emerge in infancy may therefore reflect the unfolding expression of familial risk.
Smoking during pregnancy is a second potentially important factor; it is strongly associated with SES19 and has been implicated in many adverse perinatal outcomes in lower SES groups, including low birth weight20,21; although by six months, infants appear to have overcome the weight effects of exposure to smoking in foetal life22. Higher weight gain in infants from lower SES families could therefore be a consequence of ‘catch-up’ growth following smoking-induced birth weight restriction.
A third potential mechanism for inequalities in growth is maternal choice of feeding method. In many developed countries, higher SES mothers are more likely to breast-feed, and to breastfeed for longer, than lower SES mothers,23 and breastfeeding is associated with slower weight gain in infancy compared with formula feeding24,25. Faster weight gain in lower SES infants could therefore be due to the higher prevalence of formula feeding.
We used data on birth weight and weight at three months collected from a population-based sample of twins, to examine SES differences and investigate the contribution of parental BMI, maternal smoking in pregnancy, and duration of breastfeeding, to explaining associations between SES and weight gain.
Methods
Study population
Participants were families taking part in the Gemini Study, a population-based twin birth cohort study26. In January 2008, the Office for National Statistics (ONS) wrote to all families with twins born in England and Wales between March and December 2007 (n = 6754) to ask for consent to pass their contact details to the Gemini research team. There were 3435 families (51%) that agreed to be contacted and they were sent a baseline questionnaire in early 2008, which was completed by 2402 (70%) families. The geographic distribution of participating families mirrored the UK population density and the sample was representative of national twin statistics on sex, zygosity, gestational age at birth, and birth weight26,27. Ethical approval for Gemini was granted by the University College London Committee for the Ethics of non-National Health Service Human Research, and all aspects of data collection and storage were in accordance with the standards stipulated by this body.
Data were collected in maternally-completed questionnaires and maternally-held health records when the twins were eight months old (mean: 8.2; SD: 2.2 months).
Socioeconomic status
SES was indexed using the National Statistics Socioeconomic Class (NS-SEC) index, which is based on occupation. The NS-SEC was derived using the simplified method described by the ONS28, using the Computer-Assisted Structured Coding Tool29. Using this tool, job descriptions were assigned their corresponding four digit Standard Occupational Classification 2000 code30,31. These codes were linked to an eight category NS-SEC classification, which we reversed so that higher scores represented higher SES. In order to determine household SES, a household reference person was defined by selecting the person with the highest SES. This was the partner in 41% and the mother in 29% of families, and was equal in 18% of families. In the remaining 12%, data were missing or the mother did not have a partner. In this situation the parent with SES data was assigned as household reference person. In order to have adequate group sizes for analysis, NS-SEC scores were grouped into higher (higher and lower managerial and professional occupations), intermediate (intermediate occupations and small employers and own account workers) and lower SES (lower supervisory and technical occupations, (semi-)routine occupations, never worked and long-term unemployed)28.
Maternal educational qualifications were reported in the baseline questionnaire in seven levels: 0 – no qualifications; 1 – Basic high school education ((General) Certificate of Secondary Education, Ordinary level); 2 – Vocational qualification (General National Vocational Qualification, Business and Technology Education Council National Diploma); 3 – Advanced high school education (Advanced or Advanced Supplementary Level); 4 – Higher National Certificate or Diploma (HNC or HND); 5 – Undergraduate degree; 6 – Postgraduate degree. These were grouped into higher (scores 4, 5 and 6), intermediate (scores 2 and 3) and lower (scores 0 and 1) educational level. These groups were used to confirm that results obtained using NS-SEC could be replicated using another SES indicator.
Infant weights
Parents were asked to report their children’s recorded weights from birth onwards using the measurements made by health professionals and recorded in each child’s personal health record. Parents were asked to photocopy the relevant pages of their child’s health record or copy all available measurements for each twin into the questionnaire. Parents reported a mean of 9.6 (SD 5.4, range 1-45) weight measurements per child between birth and on average 6.5 (SD 2.5, range 1.5-22) months of age. Data were checked and cleaned for implausible values. Weight at ‘three months’ was derived for each twin. In order to maximise the size of the sample with available data the measurement occasion closest to three months that occurred between two and four months was selected to represent ‘three-month’ weight. Age at the ‘three months’ measurement occasion was also noted.
Weight standard deviation scores (SDS) at birth and three months were calculated adjusting for age, sex and gestational age based on British 1990 growth reference data using the LMS growth macro for excel32,33. Change in weight SDS from birth to three months was calculated by subtracting weight SDS at birth from weight SDS at three months so that positive values represented faster growth than expected, negative values represented slower growth than expected, and a value of zero represented tracking of growth along the same centile. Rapid growth was defined as a change in weight SDS from birth to three months of >0.67 as described by Ong and Loos3.
Potential explanatory variables
Smoking status during pregnancy was self-reported using the question: ‘Did you smoke any cigarettes while pregnant’, with the response option, yes/no.
Maternal and paternal current heights and weights were self-reported in the baseline questionnaire and used to compute BMI (weight / height2). Missing values (3% of mothers and 11% of partners) were imputed by taking the mean BMI for each sex. Sensitivity analyses using the non-imputed scores did not alter the conclusions. BMI was categorised as ‘desirable’ (BMI ≤ 25), ‘overweight’ (BMI 25 – 29.9) or ‘obese’ (BMI ≥ 30) for descriptive purposes but was used as a continuous variable in the analyses. Because the group of parents that could be classified as ‘underweight’ (BMI < 18.5) was small (57 mothers and 9 fathers), they were included in the ‘desirable’ BMI group.
Infant feeding methods were assessed by asking the questions: “Are you currently breastfeeding your twins” (response options: yes both, yes 1st born; yes 2nd born; neither); ‘If you are no longer breastfeeding, when did you stop’ (response options: number of weeks after birth); and for mothers that reported ever bottle feeding “How soon after birth did you start bottle feeding your twins” (response options: number of minutes/hours/days after birth). Duration of any breastfeeding was categorised as: ‘none’, ‘<1 month’, ‘1–2 months’, ‘2–3 months’ and ‘3 months or longer’; Introduction of bottle feeding was categorised as: ‘never’, ‘<1 month’, ‘1–2 months’, ‘2–3 months’ and ‘3 months or longer’. An exclusive breastfeeding (between 0-3 months) variable was created by combining the breastfeeding and bottle feeding categories so exclusive breastfeeders were defined as those who breastfed for 3 months or more and didn’t introduce a bottle until after 3 months. Information on exclusive breastfeeding was missing for 11% of the sample, therefore for the main analysis, duration of any breastfeeding was used to indicate infant feeding practices in order to maximise the sample size. The exclusive breastfeeding variable was used to confirm the results.
Covariates
Parental age at birth, and infant sex and gestational age were assessed through maternally completed questionnaires. Gestational age was dichotomised to preterm (before 37 weeks) and term (≥ 37 weeks) for descriptive purposes, but was used as a continuous variable in the analyses.
Statistical Analysis
As social class is nested within families and is therefore the same for both twins, we selected one twin per pair for these analyses to avoid problems with clustering, using SPSS 16.0 for Windows. Means and 95% confidence intervals (CIs) of the different weight measures were calculated by levels of SES, maternal smoking, parental weight status and breastfeeding duration, and compared using ANOVA for birth weight SDS, three-month weight SDS and change in weight SDS, and using χ2 for rapid growth. Weighted tests for trend were performed and significant deviation from linearity was checked using ANOVA. Linear regression (for birth weight SDS, three-month weight SDS and change in weight SDS) and logistic regression models (for rapid growth) were used to examine the association between SES and infant weight controlling successively for the potential explanatory variables. The basic model (model 1) predicted weight SDS at birth or three months by SES adjusting for gender, gestational age (and three-month age when three-month weight SDS was the outcome). Change in weight SDS was modelled by using three-month weight SDS as the dependent variable and birth weight SDS as a predictor. Model 2 was model 1 with inclusion of maternal smoking during pregnancy; model 3 was model 1 with inclusion of maternal and paternal BMI; model 4 was model 1 with inclusion of infant feeding practices; and model 5 included all potential explanatory variables and covariates.
Using Stata version 10.1, the role of each of the potential explanatory variables was determined by the percentage attenuation in the coefficient for SES in each successive model, using the formula: 100 × (βmodel 1 – βmodel 1+variable )/ (βmodel 1) for linear regression models, and 100 × ((1-ORmodel 1) – (1-ORmodel 1+variable))/ (1-ORmodel 1) for logistic regression models. The 95% CI for the percentage attenuation was calculated using a bias-corrected accelerated bootstrap method with 10 000 resamplings.
Results
Baseline data were available from 2402 families. Data on birth weight SDS, three-month SDS, change in weight SDS and rapid growth were available on 2313, 2099, 2081 and 2081 families, respectively and SES was missing for eight families. Table 1 shows the characteristics of the study population at baseline by SES category; 63% of families were classified as higher SES, 17% as intermediate and 20% as lower SES. In each SES group numbers of male and female infants were equal. Over half the infants were born at term.
Table 1.
Baseline characteristics by household socio-economic status
| SES1 | |||
|---|---|---|---|
| Higher (n = 1515) |
Intermediate (n = 407) |
Lower (n = 472) |
|
| Infant characteristics | % (n) | ||
| Girls | 50.4 (763) | 51.8 (211) | 49.4 (233) |
| Gestation ≥ 37 weeks | 56.4 (855) | 54.8 (223) | 56.1 (265) |
| Mean (SE) | |||
| Child age at 3 month measurement (weeks) | 12.9 (0.04) | 12.8 (0.08) | 12.8 (0.08) |
| Parent characteristics | Mean (SE) | ||
| Age of mother at birth (years) | 34.0 (0.11) | 32.3 (0.27) | 30.1 (0.28)* |
| Age of partner at birth (years) | 36.4 (0.15) | 35.1 (0.34) | 33.8 (0.37)* |
| % (n) | |||
| Mother with university degree | 58.8 (891) | 16.5 (67) | 10.0 (47)* |
| Smoked during pregnancy | 6.5 (99) | 11.5 (47) | 25.8 (122)* |
| Mother overweight or obese2 | 39.6 (600) | 47.2 (192) | 51.9 (245)* |
| Partner overweight or obese2 | 61.5 (931) | 63.6 (259) | 70.8 (334)* |
| Breastfeeding duration ≥ 3 months | 36.4 (551) | 21.6 (88) | 17.1 (81)* |
Data are for one randomly selected twin from each pair.
Higher SES: managerial and professional occupations; Intermediate SES: intermediate occupation; lower SES: routine and manual occupations
BMI > 25
p for trend <0.01
SES and weight
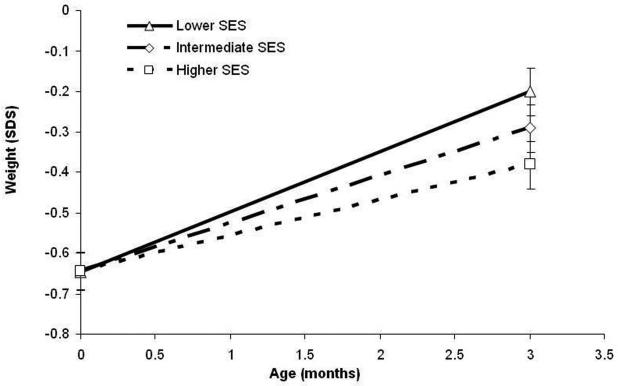
Weights at birth and three months are shown in Table 2. The mean birth-weight was 2.46 kg (SD=0.54), and the mean weight at three months was 5.16 kg (SD=0.90). There were no SES differences in birth weight (p-value for trend: 0.93). However, at three months, infants from lower SES families had a higher weight SDS than infants from higher SES families, and also a greater change in weight SDS (p for trend across SES categories < 0.01 for both). Infants from lower SES families also had a 36% (95% CI: 7% - 72%) higher chance of rapid growth. The association between SES and weight change is illustrated in Figure 1.
Table 2.
Infant growth by household socioeconomic status and covariates
| Birth weight | Three-month weight | Change in weight1 | Rapid growth | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | SDS (95% CI) | n | SDS (95% CI) | n | SDS (95% CI) | % | OR (95% CI) | ||
| Overall | 2313 | −0.56 (−0.59, −0.52) | 2099 | −0.27 (−0.32, −0.23) | 2081 | 0.28 (0.23, 0.32) | 34 | - | |
| SES2 | Higher | 1470 | −0.56 (−0.61, −0.51) | 1367 | −0.33 (−0.39, −0.27) | 1351 | 0.23 (0.18, 0.28) | 32 | Ref |
| Intermediate | 394 | −0.52 (−0.61, −0.42) | 348 | −0.16 (−0.27, −0.05) | 348 | 0.33 (0.22, 0.43) | 34 | 1.08 (0.85, 1.40) | |
| Lower | 449 | −0.57 (−0.65, −0.49) | 384 | −0.18 (−0.28, −0.07) | 3832 | 0.40 (0.30, 0.50) | 39 | 1.36 (1.07, 1.72) | |
| p for trend (weighted) |
0.93 | 0.003 | 0.001 | 0.0133 | |||||
| Potential Explaining variables | |||||||||
| Smoking | No | 2062 | −0.54 (−0.58, −0.50) | 1888 | −0.27 (−0.32, −0.22) | 1873 | 0.27 (0.22, 0.31) | 33 | Ref |
| Yes | 256 | −0.69 (−0.80, −0.57) | 217 | −0.30 (−0.44, −0.16) | 214 | 0.36 (0.23, 0.50) | 40 | 1.34 (1.00, 1.78) | |
| p for difference | 0.02 | 0.72 | 0.17 | 0.06 | |||||
| Mother BMI4 | Desirable | 1319 | −0.61 (−0.66, −0.56) | 1197 | −0.30 (−0.36, −0.24) | 1185 | 0.31 (0.25, 0.36) | 35 | Ref |
| Overweight | 692 | −0.49 (−0.57, −0.42) | 625 | −0.32 (−0.40, −0.23) | 622 | 0.18 (0.10, 0.26) | 32 | 0.90 (0.73, 1.11) | |
| Obese | 309 | −0.45 (−0.56, −0.35) | 284 | −0.08 (−0.20, 0.04) | 281 | 0.37 (0.26, 0.48) | 34 | 0.97 (0.74, 1.28) | |
| p for trend (weighted) |
0.001 | Deviation: 0.03 | Deviation: 0.002 | 0.553 | |||||
| Partner BMI4 | Desirable | 846 | −0.57 (−0.64, −0.50) | 770 | −0.33 (−0.41, −0.26) | 763 | 0.23 (0.16, 0.30) | 32 | Ref |
| Overweight | 1168 | −0.56 (−0.62, −0.51) | 1054 | −0.26 (−0.33, −0.20) | 1045 | 0.30 (0.25, 0.36) | 35 | 1.12 (0.92, 1.36) | |
| Obese | 306 | −0.49 (−0.58, −0.39) | 282 | −0.16 (−0.28, −0.03) | 280 | 0.30 (0.19, 0.41) | 34 | 1.08 (0.81, 1.44) | |
| p for trend (weighted) |
0.28 | 0.02 | 0.17 | 0.413 | |||||
| Breast feeding duration |
No breastfeeding | 529 | −0.53 (−0.61,−0.45) | 449 | −0.08 (−0.18, 0.02) | 445 | 0.44 (0.35, 0.52) | 40 | Ref |
| 0 – 1 month | 480 | −0.53 (−0.62, −0.45) | 453 | −0.08 (−0.18, 0.01) | 450 | 0.45 (0.37, 0.54) | 39 | 0.95 (0.72, 1.24) | |
| 1 – 2 months | 323 | −0.63 (−0.74, −0.53) | 292 | −0.28 (−0.41, −0.15) | 289 | 0.36 (0.26, 0.45) | 34 | 0.77 (0.57, 1.05) | |
| 2 – 3 months | 173 | −0.54 (−0.68, −0.39) | 158 | −0.28 (−0.45, −0.11) | 156 | 0.21 (0.06, 0.37) | 31 | 0.68 (0.46, 1.00) | |
| ≥ 3 months | 705 | −0.56 (−0.63, −0.49) | 661 | −0.53 (−0.61, −0.45) | 655 | 0.03 (−0.04, 0.11) | 26 | 0.53 (0.41, 0.68) | |
| p for trend (weighted) |
0.56 | <0.001 | <0.001 | <0.001 | |||||
Abbreviation: BMI, Body Mass Index; CI, confidence interval; SDS, standard deviation score; OR, odds ratio
Change in weight SDS: weight at three months SDS minus birth weight SDS
Higher SES: managerial and professional occupations; Intermediate SES: intermediate occupation; lower SES: routine and manual occupations
p-value for overall SES effect
Mother and partner BMI: desirable BMI ≤ 25; overweight: BMI 25 – 30; obese: BMI > 30
Figure 1.
The association between SES and weight SDS at birth and three-months, unadjusted
Potential explanatory variables
Data were missing on 0.1% and 5% of the sample for smoking and feeding method respectively. As shown in Table 1, lower SES mothers were more likely to have smoked during pregnancy, were more likely to be overweight or obese, and were less likely to have breastfed for at least three months (all p’s for trends across categories < 0.01).
Infants of mothers who reported smoking in pregnancy had significantly lower birth weight SDS (p = 0.02; Table 2), although differences in weight SDS had disappeared at three months (p = 0.72). Differences in weight change by smoking status were not significant (p = 0.17), although rapid growth approached significance (p = 0.06).
Infants of overweight or obese mothers had higher birth weight SDS than infants of normal-weight mothers (p for trend across weight categories < 0.01), but there was no linear trend across maternal weight groups for three-month weight SDS, change in weight SDS (trend test violated for both), or rapid growth (p = 0.55). Partner’s BMI was not associated with birth weight SDS (p = 0.28), change in weight SDS (p = 0.17) or rapid growth (p = 0.41), but was linked to higher three-month weight SDS (p for trend = 0.02).
Duration of breastfeeding was not associated with birth weight. However, longer breastfeeding duration was associated with lower weight SDS at three months, a smaller change in weight SDS from birth to three months, and being less likely to show rapid growth (p for trend < 0.01 for all).
Explaining the association between SES and infant weight change
SES was significantly associated with weight at three months, change in weight from birth to three months and rapid growth, so these associations were examined further with a set of models which progressively included the potential explanatory variables. The SES effect for weight at three months remained significant after including smoking during pregnancy or parental BMI in the models. After including breastfeeding, the association between SES and three-month weight SDS was attenuated by 68% and no longer statistically significant (Table 3).
Table 3.
The association of household socioeconomic status with weight measures, corrected for covariates
| Birth weight SDS | Three-month weight SDS |
Change in weight SDS1 |
Rapid Growth | ||
|---|---|---|---|---|---|
| Model 1 | Estimate (SE)2 | 0.002 (0.02) | −0.090 (0.03) | −0.089 (0.02) | 0.849 (0.78, 0.92) |
| P-value | 0.94 | 0.003 | <0.001 | <0.001 | |
| Model 2 | Estimate (SE)2 | −0.015 (0.02) | −0.096 (0.03) | −0.085 (0.03) | 0.876 (0.81, 0.95) |
| Plus smoking | P-value | 0.53 | 0.002 | 0.001 | 0.002 |
| % Attenuation | N/A3 | −8.8 (−44.9, 6.4) | 3.1 (−12.5, 19.3) | 16.1 (−1.9, 86.4) | |
| Model 3 | Estimate (SE)2 | 0.014 (0.02) | −0.080 (0.03) | −0.088 (0.02) | 0.840 (0.77, 0.91) |
| Plus parental BMI | P-value | 0.56 | 0.008 | <0.001 | <0.001 |
| % Attenuation | N/A3 | 10.8 (4.2 40.5) | 1.1 (−7.5, 10.8) | −9.7 (−50.4, 0.1) | |
| Model 4 | Estimate (SE)2 | 0.015 (0.03) | −0.026 (0.03) | −0.032 (0.03) | 0.940 (0.83, 1.06) |
| Plus breastfeeding | P-value | 0.56 | 0.4 | 0.21 | 0.32 |
| % Attenuation | N/A3 | 68.4 (35.4, 230.0) | 62.2 (35.0, 152.4) | 52.6 (18.7, 307.1) | |
| Model 5 | Estimate (SE)2 | 0.011 (0.03) | −0.031 (0.03) | −0.036 (0.03) | 0.940 (0.83, 1.07) |
| Plus all variables | P-value | 0.67 | 0.34 | 0.17 | 0.34 |
| % Attenuation | N/A3 | 61.1 (27.8, 237.7) | 57.2 (29.2, 154.5) | 53.4 (14.1, 377.6) |
Model 1: SES effect corrected for gender, gestational age, 3 month age.
Model 2: SES effect corrected for gender, gestational age, 3 month age and smoking.
Model 3: SES effect corrected for gender, gestational age, 3 month age and parental BMI.
Model 4: SES effect corrected for gender, gestational age, 3 month age and breastfeeding.
Model 5: SES effect corrected for gender, gestational age, 3 month age and smoking, parental BMI, breastfeeding.
Abbreviations: BMI, Body Mass Index; CI, confidence interval; N/A, not applicable; OR, odds ratio; SE, standard error
Weight change: three-month weight SDS as the dependent variable and birth weight SDS as a predictor
For Rapid growth: estimate OR (95% CI)
Not applicable as SES did not have a significant effect on weight in model 1
Controlling for smoking during pregnancy or parental BMI did not affect the association between SES and growth rate, but including breastfeeding in the model attenuated the association by 62% for change in weight SDS from birth to three months and 53% for the odds of rapid growth. When the analyses were repeated using exclusive breastfeeding duration, the pattern of results was the same (results not shown).
Replicating the analyses using education as the indicator of SES
The analyses were repeated using maternal education as the SES indicator and the same pattern was found. There were no education differences in birth weight SDS, but mothers with lower levels of education had infants with higher three-month weight SDS and weight gain, and a higher prevalence of rapid weight gain. As with the analyses based on occupation, only inclusion of infant feeding practices in the model attenuated the education effect: by 88% for three-month weight SDS, 82% for change in weight SDS, and 64% for rapid growth (results not shown).
Discussion
We found clear evidence of an SES gradient in early infant growth in this British sample drawn from a twin cohort. There were no SES differences in birth weight, but compared with infants from higher SES families, infants from lower SES families were heavier at three months, had a higher change in weight from birth to three months, and more of them met criteria for rapid growth; all of which are related to a higher risk of later chronic disease2,5,7. We are not aware of any other studies that have investigated SES differences in weight in this early period of infancy, although the relationship between SES and weight in childhood is well established 13-16.
We tested three possible mechanisms for the SES difference in infant weight gain: maternal smoking in pregnancy, parental BMI, and infant feeding method. Lower SES mothers were more likely to have smoked in pregnancy, but SES differences in weight gain remained significant after controlling for maternal smoking during pregnancy, indicating that weight differences by SES were unlikely to be due to catch-up growth as a result of a smoking-induced growth-restriction in-utero34. Nor did the higher parental BMI observed in the lower SES families explain the SES difference in infant weight at three months. However, SES differences in infant weight gain largely disappeared after controlling for SES differences in infant feeding method; giving strong support for a feeding-mediated influence on infant growth.
Many studies in developed countries have observed that lower SES mothers are less likely to breast-feed, or if they do, to breast-feed for a shorter time24,25,35. Breastfeeding is thought to produce slower weight gain through its relatively low protein content than infant formula, and by providing hormones, enzymes and growth factors that regulate energy intake, energy expenditure, and cellular chemistry36. It also reduces the scope for maternal control (for example formula feeders can give extra bottles of formula),37,38 and encourages the infant’s emerging capabilities of self-regulation of energy intake36. It is also possible that the physical closeness and tenderness involved in breastfeeding has its own effects on development, given the growing evidence from animal studies that maternal-infant interactions can affect the epigenome39.
Because the Gemini study is a twin sample, birth weights were lower than the 1990-born singletons used for reference data to calculate weight SDS32. Higher weight gain in the first three months in twins is likely to be the result of catch-up growth, which has been shown in twins to be most dramatic in the first three months40. However, the gradient in the rate and amount of weight gain in twins from different SES backgrounds indicate that some twins catch-up more than others. The reasons for SES differences in growth are likely to be driven by similar factors in twins and singletons.
Other studies have reported SES differences in catch-up growth in length41,42; although in these studies higher SES infants and children tended to catch-up more. Combined with the results of the present study, differential gain in weight and length might help explain SES differences in obesity rates in later childhood. Future studies should investigate the growth in length and weight by SES in the same study to confirm if this explains later social class variation in BMI.
The strengths of this study include its large, population-based sample, the range of potential explaining variables, and the use of weight data based on child health records measured by health professionals. However, there were several limitations. The data were from twins and the weight trajectories of twins are known to differ from singletons, although there is no obvious reason to expect the SES patterning to be different. All information was reported by parents, which could result in inaccuracy and underreporting of smoking and BMI. The NS-SEC used to index SES was derived using the simplified method which does not take into account employment status or organisation size, and although it correctly allocates 83% of cases compared with the full method, it slightly overestimates SES28. Because of this overestimation of the SES, the effect on early infant growth will be underestimated. Compared with the general UK population, Gemini families had higher SES43, but there were sufficient numbers in each SES group to allow comparisons. In Gemini, 76% of mothers initiated breastfeeding, which is comparable to UK singleton data44, although the average duration of breastfeeding was shorter than with singletons (26% of Gemini mothers breastfeeding for at least four months compared with 40% in the population) 44. As this study found a significant effect for breastfeeding with this shorter than average breastfeeding duration, the effect in the population is likely to be even larger.
This study does not allow us to determine whether the breast-feeding effect is causal. Many studies support the idea that breast-feeding is protective against excess weight gain45 although one randomised trial had no effect46. However, genetically determined differences in appetite are associated with weight47,48 and it is possible that mothers switch from breast to formula feeding because their baby appears less satisfied; i.e. weight gain susceptibility in the infant could influence parental feeding behaviour. However, there is unanimous agreement that breastfeeding is best for most babies and that it may well afford some protection from excessive weight gain. Effective strategies are needed to ensure that infants from all SES groups get the best nutritional start in life; but efforts could be targeted towards lower SES groups, and especially those families with parental obesity.
In conclusion, these findings suggest that the foundations for socioeconomic inequalities in obesity may be laid down in early infancy, with differences in infant feeding practices playing an important role.
Acknowledgements
Funding/Support: Gemini is funded by a grant from Cancer Research UK to JW (C1418/A7974). The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; and preparation, review or approval of the manuscript.
Additional Contributions: We thank the Gemini families who are participating in the study and the Office of National Statistics for their help in recruiting them.
Footnotes
Conflict of interest statement: The authors report no conflict of interest
References
- (1).Simmons R. Perinatal programming of obesity. Semin Perinatol. 2008;32:371–374. doi: 10.1053/j.semperi.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring) 2006;14:491–499. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- (3).Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95:904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- (4).Herman KM, Craig CL, Gauvin L, Katzmarzyk PT. Tracking of obesity and physical activity from childhood to adulthood: the Physical Activity Longitudinal Study. Int J Pediatr Obes. 2009;4:281–288. doi: 10.3109/17477160802596171. [DOI] [PubMed] [Google Scholar]
- (5).Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113:475–482. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- (6).Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116:473–480. doi: 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- (7).Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey SG. Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am J Clin Nutr. 1998;67:1111–1118. doi: 10.1093/ajcn/67.6.1111. [DOI] [PubMed] [Google Scholar]
- (8).Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91(Suppl):1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301:2234–2242. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- (10).Dubois L, Girard M. Determinants of birthweight inequalities: population-based study. Pediatr Int. 2006;48:470–478. doi: 10.1111/j.1442-200X.2006.02256.x. [DOI] [PubMed] [Google Scholar]
- (11).Jansen PW, Tiemeier H, Looman CW, et al. Explaining educational inequalities in birthweight: the Generation R Study. Paediatr Perinat Epidemiol. 2009;23:216–228. doi: 10.1111/j.1365-3016.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- (12).Kramer MS, Seguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14:194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- (13).Semmler C, Ashcroft J, van Jaarsveld CH, Carnell S, Wardle J. Development of overweight in children in relation to parental weight and socioeconomic status. Obesity (Silver Spring) 2009;17:814–820. doi: 10.1038/oby.2008.621. [DOI] [PubMed] [Google Scholar]
- (14).Langnase K, Mast M, Muller MJ. Social class differences in overweight of prepubertal children in northwest Germany. Int J Obes Relat Metab Disord. 2002;26:566–572. doi: 10.1038/sj.ijo.0801956. [DOI] [PubMed] [Google Scholar]
- (15).Stamatakis E, Wardle J, Cole TJ. Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes (Lond) 2010;34:41–47. doi: 10.1038/ijo.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes (Lond) 2006;30:610–617. doi: 10.1038/sj.ijo.0803141. [DOI] [PubMed] [Google Scholar]
- (17).McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- (18).Whitaker KL, Jarvis MJ, Beeken RJ, Boniface D, Wardle J. Comparing maternal and paternal intergenerational transmission of obesity risk in a large population-based sample. Am J Clin Nutr. 2010;91:1560–1567. doi: 10.3945/ajcn.2009.28838. [DOI] [PubMed] [Google Scholar]
- (19).Laaksonen M, Rahkonen O, Karvonen S, Lahelma E. Socioeconomic status and smoking: analysing inequalities with multiple indicators. Eur J Public Health. 2005;15:262–269. doi: 10.1093/eurpub/cki115. [DOI] [PubMed] [Google Scholar]
- (20).Morgen CS, Bjork C, Andersen PK, Mortensen LH, Nybo Andersen AM. Socioeconomic position and the risk of preterm birth--a study within the Danish National Birth Cohort. Int J Epidemiol. 2008;37:1109–1120. doi: 10.1093/ije/dyn112. [DOI] [PubMed] [Google Scholar]
- (21).Lanting CI, Buitendijk SE, Crone MR, Segaar D, Bennebroek Gravenhorst J, Wouwe JP. Clustering of Socioeconomic, Behavioural, and Neonatal Risk Factors for Infant Health in Pregnant Smoker. Plos One. 2009:4. doi: 10.1371/journal.pone.0008363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Conter V, Cortinovis I, Rogari P, Riva L. Weight growth in infants born to mothers who smoked during pregnancy. BMJ. 1995;310:768–771. doi: 10.1136/bmj.310.6982.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).van Rossem L, Oenema A, Steegers EA, et al. Are starting and continuing breastfeeding related to educational background? The generation R study. Pediatrics. 2009;123:e1017–e1027. doi: 10.1542/peds.2008-2663. [DOI] [PubMed] [Google Scholar]
- (24).Kramer MS, Guo T, Platt RW, et al. Feeding effects on growth during infancy. J Pediatr. 2004;145:600–605. doi: 10.1016/j.jpeds.2004.06.069. [DOI] [PubMed] [Google Scholar]
- (25).Rzehak P, Sausenthaler S, Koletzko S, et al. Period-specific growth, overweight and modification by breastfeeding in the GINI and LISA birth cohorts up to age 6 years. Eur J Epidemiol. 2009;24:449–467. doi: 10.1007/s10654-009-9356-5. [DOI] [PubMed] [Google Scholar]
- (26).van Jaarsveld CH, Johnson L, Llewellyn C, Wardle J. Gemini: A UK Twin Birth Cohort With a Focus on Early Childhood Weight Trajectories, Appetite and the Family Environment. Twin Res Hum Genet. 2010;13:72–78. doi: 10.1375/twin.13.1.72. [DOI] [PubMed] [Google Scholar]
- (27).Office for National Statistics . Review of the Registrar General on births and patterns of family building in England and Wales. National Statistics; Newport: United Kingdom: 2006. [Google Scholar]
- (28).Office for National Statistics . National Statistics Socio-economic Classification: User Manual. Office for National Statistics; 2005. [Google Scholar]
- (29).Jones R, Elias P. CASCOT: Computer Assisted Coding Tool. http://www2.warwick.ac.uk/fac/soc/ier/publications/software/cascot/
- (30).Office for National Statistics . Standard Occupational Classification 2000 (SOC 2000) Vol. 1. Office for National Statistics; 2000. [Google Scholar]
- (31).Office for National Statistics . Standard Occupational Classification 2000 (SOC 2000) Vol. 2. Office for National Statistics; 2000. [Google Scholar]
- (32).Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Cole TJ. Software for LMS method: LMSGrowth PC. http://homepage.mac.com/tjcole/FileSharing1.html.
- (34).Pollack H, Lantz PM, Frohna JG. Maternal smoking and adverse birth outcomes among singletons and twins. Am J Public Health. 2000;90:395–400. doi: 10.2105/ajph.90.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80:1579–1588. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- (36).Bartok CJ, Ventura AK. Mechanisms underlying the association between breastfeeding and obesity. Int J Pediatr Obes. 2009;4:196–204. doi: 10.3109/17477160902763309. [DOI] [PubMed] [Google Scholar]
- (37).Taveras EM, Scanlon KS, Birch L, Rifas-Shiman SL, Rich-Edwards JW, Gillman MW. Association of breastfeeding with maternal control of infant feeding at age 1 year. Pediatrics. 2004;114:e577–e583. doi: 10.1542/peds.2004-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Taveras EM, Rifas-Shiman SL, Scanlon KS, Grummer-Strawn LM, Sherry B, Gillman MW. To what extent is the protective effect of breastfeeding on future overweight explained by decreased maternal feeding restriction? Pediatrics. 2006;118:2341–2348. doi: 10.1542/peds.2006-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Szyf M. The early life environment and the epigenome. Biochim Biophys Acta. 2009;1790:878–885. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- (40).Wilson RS. Twin growth: initial deficit, recovery, and trends in concordance from birth to nine years. Ann Hum Biol. 1979;6:205–220. doi: 10.1080/03014467900007212. [DOI] [PubMed] [Google Scholar]
- (41).Teranishi H, Nakagawa H, Marmot M. Social class difference in catch up growth in a national British cohort. Arch Dis Child. 2001;84:218–221. doi: 10.1136/adc.84.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Li L, Manor O, Power C. Early environment and child-to-adult growth trajectories in the 1958 British birth cohort. Am J Clin Nutr. 2004;80:185–192. doi: 10.1093/ajcn/80.1.185. [DOI] [PubMed] [Google Scholar]
- (43).Office for National Statistics Census 2001. http://www.statistics.gov.uk/census2001/census2001.asp.
- (44).Griffiths LJ, Smeeth L, Hawkins SS, Cole TJ, Dezateux C. Effects of infant feeding practice on weight gain from birth to 3 years. Arch Dis Child. 2009;94:577–582. doi: 10.1136/adc.2008.137554. [DOI] [PubMed] [Google Scholar]
- (45).Arenz S, Ruckerl R, Koletzko B, von KR. Breast-feeding and childhood obesity-- a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247–1256. doi: 10.1038/sj.ijo.0802758. [DOI] [PubMed] [Google Scholar]
- (46).Kramer MS, Matush L, Vanilovich I, et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86:1717–1721. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- (47).Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr. 2008;88:22–29. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- (48).Carnell S, Haworth CM, Plomin R, Wardle J. Genetic influence on appetite in children. Int J Obes (Lond) 2008;32:1468–1473. doi: 10.1038/ijo.2008.127. [DOI] [PubMed] [Google Scholar]