Abstract
Objective
To examine the association between cardiovascular risk factors and retinal arteriolar tortuosity in a multiethnic child-population.
Methods
Cross sectional study of 986 UK primary-school children of South Asian, black African Caribbean, and white European origin aged 10-11 years. Anthropometric measurements and retinal imaging, were carried out and a fasting blood sample collected. Digital images of retinal arterioles were analysed using a validated semi-automated measure of tortuosity. Associations between tortuosity and cardiometabolic risk factors were analysed using multilevel linear regression, adjusted for gender, age, ethnicity, arteriole branch status, month and school.
Results
Levels of arteriolar tortuosity were similar in boys and girls, and in different ethnic groups. Retinal arteriolar tortuosity was positively associated with levels of triglyceride, total and LDL cholesterol, systolic and diastolic blood pressure. One standard deviation increases in these risk factors were associated with 3.7% (95% CI 1.2, 6.4%), 3.3% (0.9, 5.8%), 3.1% (0.6, 5.6%), 2.0% (−0.3, 4.2%) and 2.3% (0.1, 4.6%) increases in tortuosity respectively. Adiposity, insulin resistance and blood glucose showed no associations with tortuosity.
Conclusion
Established cardiovascular risk factors, strongly linked to coronary heart disease in adulthood, may influence retinal arteriolar tortuosity at the end of the first decade of life.
Keywords: Retina, arteriolar tortuosity, cardiovascular risk
Abnormalities of the retinal micro-circulation in adult life, including micro-aneurysms, arteriolar-venular nicking and arteriolar narrowing, are prospectively and independently related to cardiovascular disease, including both coronary heart disease (CHD) and stroke.1-3 Increased tortuosity of retinal arterioles (assessed subjectively) has been related to risk factors for coronary disease, particularly hypertension, both in adults and children.4;5 Arteriolar narrowing has been related to CHD in later life.6;7 Changes in the retinal microcirculation have also been observed with risk factors for cardiovascular disease; narrower arterioles have been associated with increased blood pressure8 and with BMI.9
Recent studies have suggested that differences in retinal arteriolar morphology associated with cardiovascular risk may emerge early in life. Studies in children have shown strong associations between blood pressure,10 body mass index 11 and retinal arteriolar calibre, which mirror associations previously reported in adults.8;9 As well as measurement of vessel width, tortuosity is another morphological characteristic of the retinal vascular network, relying on the detection of vessel axes as opposed to the exact location of vessel edges.12 Earlier studies of retinal arteriolar tortuosity in adults have suggested that it may be a marker for subsequent CHD risk. However, no studies to date have to our knowledge examined retinal arteriolar tortuosity in children and its association with established cardiovascular risk factors. We set out to use a validated objective measure to assess tortuosity in retinal arterioles (as a novel index of arteriolar function) 13 in a multiethnic population of almost 1000 10-11 year old children, and examine its associations with established cardiometabolic risk markers.
Methods
Study population
This investigation was carried within the Child Heart and Health Study in England (CHASE Study), a school-based survey of the cardiovascular health of British Primary School children living in three UK cities, London, Leicester and Birmingham. Full details of the study design have been reported elsewhere.14 Ethical approval was obtained from the relevant Multicentre Research Ethics Committee and the study was carried out in accordance with the principles expressed in the Declaration of Helsinki. Informed written consent was obtained from each pupil’s parent or guardian. The study was based in a sample of 200 Primary Schools, providing balanced numbers of children of South Asian origin (including Indian, Pakistani and Bangladeshi origin), black African-Caribbean origin (including black African and black Caribbean) and white European origin. The present investigation was based in 46 schools in the final phase of the study, in which children attended for the main survey and returned for measurements of retinal arteriolar tortuosity on a separate occasion. Children were in year 5 (aged 9 to 10 years) for the cardiovascular risk survey, and year 6 (aged 10 to 11 years) for the ocular examination.
Cardiovascular risk factor assessment
A single survey team including three trained research nurses and a support fieldworker carried out all survey measurements between June 2006 and February 2007. Survey methods have been described in detail elsewhere.14 Participating children provided a blood sample after an overnight fast and had measurements of height, weight and waist circumference. Right-sided skinfold thicknesses were measured in four sites (biceps, triceps, subscapular, suprailiac); analyses are based on the sum of the four measurements. Leg to arm bioimpedance was measured using the Bodystat 1500 bioimpedance monitor (Bodystat Ltd, Isle of Man, UK); fat mass was derived using equations derived specifically for children using dual energy X-ray absorptiometry (DXA) validation 15 and presented as a fat mass index (fat mass/height5) which was independent of height. Seated blood pressure was measured twice in the right arm after 5 minutes rest using an Omron 907 blood pressure recorder, with an appropriately sized cuff; the average of the two measures was used. Pubertal status was measured in the girls using Tanner scales.16 Participating children provided questionnaire information on parental and grandparental country of birth and reported any current health problems. The parent or guardian was asked to provide information on the ethnicity of both parents and that of the child (coded using a classification similar to the 2001 UK Census), and on their occupation, coded using the National Statistics Socioeconomic Classification (NS-SEC). Ethnicity of the children was defined using the ethnicity of both parents or (if not available) the ethnicity of the child; in a small proportion of cases in which parental information was not available (1%), child information on the place of birth of parents and grandparents was used to define ethnic origin, described in more detail elsewhere.14 Children were defined as ‘white European’ (including white British, white Irish, white European, or a combination of these), ‘black African-Caribbean’ (black African, black Caribbean, black British, black other, or a combination of these), ‘South Asian’ (Indian, Pakistani, Bangladeshi or a combination of these), or ‘other Asian’. The latter group included those with a specified Asian place of origin (mainly Afghanistan, China, and Turkey) other than ‘South Asian’, so that subjects of Indian, Pakistani, Bangladeshi, and Sri Lankan origin are excluded from ‘other Asian’. All laboratory analyses were carried out blind to participant ethnicity. Analyses of HbA1c, glucose and blood lipids were carried out in the Department of Clinical Biochemistry, Newcastle Hospitals NHS Trust, which received blood samples within 48 hours of collection. Glucose was measured in plasma using the hexokinase method. HbA1c was measured in whole blood by ion exchange high performance liquid chromatography; HbA1c values were recalculated to adjust for abnormal haemoglobin variants or for increased amounts of normal variant fetal haemoglobin (HbF) where present. Triglyceride and HDL-cholesterol were measured in serum using an Olympus auto-analyser. Serum, separated and frozen on dry ice after collection, were used for measurement of insulin (Department of Medicine, University of Newcastle, UK) using an ELISA method which does not cross-react with proinsulin 17 and C-reactive protein, which was assayed by ultra sensitive nephelometry (Dade Behring, Milton Keynes, UK). The homeostasis model assessment (HOMA) model equations were used to provide an estimate of insulin resistance.18
Ocular examination
Ocular assessment included the measurement of vision, visual acuity, and open-field autorefraction (SRW-5000, Shin-Nippon Commerce Inc., Tokyo, Japan) without cycloplegia, and noncontact ocular biometry (Zeis IOL Master, Carl Zeiss Meditec, UK).13;19 Fundus imaging included 2 digital images (30°, 1280×960 pixels) centred on the optic disc of each child’s eye recorded in subdued lighting using the Nidek NM-200D handheld fundus camera (Nidek Co., Ltd., Hiroishi, Japan).13 Image processing was carried out using the Computer Assisted Image Analysis of the Retina (CAIAR) program.13;20;21 CAIAR identifies vessel segments (typically 10 to 16 per eye) and returns measures of tortuosity for each segment beyond a circle 120 pixels in diameter (equivalent to 1.8mm in the objective plane of an emmetropic eye) centred on the optic disc (to exclude most overlapping vessels emerging from the disc) to a diameter of 400 pixels (equivalent to a measurement area of 100mm2). A simple tortuosity measure based on the mean change in subdivided chord lengths was used.13 The units of tortuosity, which are dimensionless as they represent a ratio measure, have been validated against subjective measures of tortuosity in this age group, and show good agreement.13 Images from children of different ethnic origin are shown in Figure 1 with low, medium and high levels of tortuosity (1st, median and 99th percentile of measure). Measures of vessel tortuosity were obtained for arterioles and different levels of bifurcation (primary, secondary, tertiary, 4 or more branches) assessed subjectively. Time taken to process 400 vessels is approximately 3 hours.13
Figure 1.
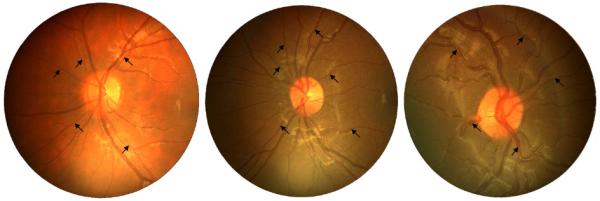
Images of children with low (1st percentile, 3.7×10−3, white European), median (7.0×10−3, black African Caribbean) and high (99th percentile, 16.5×10−3, black African Caribbean) levels of tortuosity index (averaged over all arterioles in the image). Major arterioles are labelled with black arrows.
Statistical analysis
Statistical analyses were carried out using STATA/SE software (Stata/SE 10.1 for Windows, StataCorp LP, College Station, TX, USA). The tortuosity index (the outcome / dependent variable) exhibited a positive skew and was log transformed to normalize the distribution prior to analysis. Histograms of the tortuosity index before and after transformation are shown in Figure 2. Other variables requiring log transformation, included ponderal index, fat mass index, waist circumference, sum of skinfolds, insulin, triglyceride, and C reactive protein. Gender and ethnic differences in these variables were examined as fixed effects using multilevel linear regression models with school as a random effect to allow for the clustering of children within school. Analyses of tortuosity index additionally included a random effect for child to allow for the correlation of multiple measures of tortuosity within the same child; this avoids loss of data by summarising vessel measures within the same individual as a single mean. The ‘xtmixed’ command in STATA was used, which allows a distinct variance for each random effect within a random-effects equation and assumes that all covariances are zero. The percentage difference in arteriolar tortuosity for a SD increase in cardiometabolic risk factor (log transformed where appropriate) was examined; cardiometabolic risk factors were chosen a priori. All analyses were adjusted for age group, gender, ethnicity, month and arteriole branch status (all as fixed effects); adjustment for the latter was made as levels of tortuosity increased with branch status. Effect of additionally adjusting tortuosity differences for axial length or spherical equivalent refraction was examined. Tests for interaction were used to examine whether associations of arteriolar tortuosity to cardiometabolic risk differ by gender or ethnic group; interactions were not considered in the absence of main effects.
Figure 2.
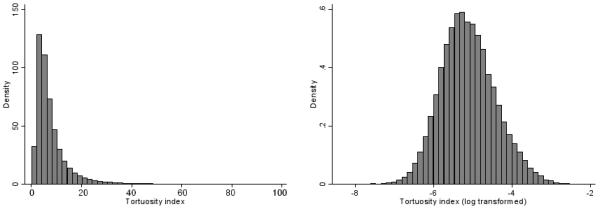
Histograms showing untransformed and log transformed tortuosity index (units of tortuosity are dimensionless but are multiplied by 103)
Results
Of 1642 children invited to participate in this phase of CHASE, 1176 (72%) took part (mean age 9.8 years, 48% male). Fundus imaging and refractive assessment were carried out in 986 children, blood sample data were available for 872 of these children. Participation rates were similar among children of white Europeans (77%), South Asians (77%), Asian others (76%), and lower amongst those of black African Caribbean origin (63%). Measures of tortuosity were obtained for a total of 16,670 retinal arterioles from 1963 eyes (8.5 arterioles per eye). The difference in the number of arterioles measured per eye between ethnic groups was not statistically significant (Likelihood Ratio Test, P=0.2); South Asians 9.2 arterioles per eye, Asian other 7.0, white Europeans 8.6, and black African Caribbeans 8.4 arterioles.
There was appreciable variation in tortuosity within this population (overall mean index 6.8×10−3, SD 2×10−3; Figure 2). Levels of arteriolar tortuosity by sex and ethnic group are summarised in Table 1. Arteriolar tortuosity did not differ appreciably between boys and girls. Compared to white Europeans, South Asian and black African Caribbean children had similar levels of tortuosity; Asian other and other miscellaneous ethnic groups had lower levels of tortuosity. Adjusted associations of age, body size and cardiovascular risk factors with retinal tortuosity are shown in Table 2. Diastolic and to a lesser extent systolic blood pressure showed positive associations with arteriolar tortuosity, while levels of triglyceride and LDL cholesterol showed stronger positive associations. The associations of retinal arteriolar tortuosity to blood pressure (systolic and diastolic), levels of triglycerides and LDL cholesterol (by quintiles) were generally graded (Figure 3). Adiposity markers (ponderal index, waist circumference, sum of skinfolds, fat mass index) and diabetic risk factors (HbA1c, glucose, insulin, insulin resistance) all showed weak positive associations with arteriolar tortuosity, but none of these were statistically significant. The effect of adjustment for axial length (as well as height) made little difference to the associations (Table 2); percentage differences were similar after adjustment for spherical equivalent refractions instead of axial length (data not presented). The mutual independence of these risk factor associations was examined. The positive associations between triglycerides, cholesterol and arteriolar tortuosity were unaffected by adjustment for SBP. Associations with blood pressure were marginally weakened (by approximately a quarter) after adjustment for LDL cholesterol (Table 2). The associations between cardiometabolic risk factors and retinal arteriolar tortuosity were generally similar for boys and girls and in different ethnic groups (all tests for interaction P>0.1).
Table 1.
Geometric mean retinal arteriolar tortuosity (95% CI) by gender and ethnic group
| N | Geometric Mean Arteriolar Tortuosity (95% CI) ×103 |
p (difference)† | ||
|---|---|---|---|---|
| Boys | 462 | 6.7 | (6.4, 7.0) | |
| Girls | 524 | 6.6 | (6.3, 6.9) | 0.42 |
| White European | 222 | 6.9 | (6.6, 7.3) | |
| Black African-Caribbean | 243 | 6.8 | (6.4, 7.2) | 0.52 |
| South Asian | 276 | 6.7 | (6.4, 7.1) | 0.40 |
| Asian other | 65 | 5.8 | (5.3, 6.4) | <0.001 |
| Other | 180 | 6.4 | (6.1, 6.8) | 0.03 |
All means are adjusted for age groups, gender (except by sex), ethnicity (except by ethnic group), month and branch status and a random effect for child and school. Log transformed tortuosity values have been exponentiated to give geometric means and 95% confidence limits. Note, units of tortuosity are dimensionless.
P-values for difference compare boy with girls, white Europeans with children of other ethnic group
Table 2.
Percentage differences in arteriolar tortuosity for a one SD/log SD increase in a range of cardiometabolic risk factors
| % Difference in Arteriolar Tortuosity (95% CI) p(Difference) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean† | SD/GSD† | Adjustment 1 | Adjustment 2 | Adjustment 3 | |||||||
| Age (years) | 9.8 | 0.4 | −1.9 | (−4.7, 0.8) | 0.17 | −2.0 | (−4.7, 0.8) | 0.16 | |||
| Height (cm) | 139.2 | 7.0 | 0.2 | (−2.1, 2.5) | 0.88 | 0.5 | (−1.8, 2.9) | 0.67 | |||
| Ponderal index (kg/m3)* | 13.2 | 1.2 | 0.8 | (−1.4, 3.0) | 0.48 | 1.0 | (−1.2, 3.2) | 0.40 | |||
| Waist circumference (cm)* | 63.7 | 1.1 | 0.2 | (−2.0, 2.5) | 0.84 | 0.5 | (−1.7, 2.7) | 0.68 | |||
| Sum of skinfolds (mm)* | 42.6 | 1.6 | 1.4 | (−0.9, 3.7) | 0.24 | 1.5 | (−0.7, 3.9) | 0.18 | |||
| Fat mass index (kg/m5)* | 1.9 | 1.5 | 0.6 | (−1.6, 2.9) | 0.59 | 0.7 | (−1.5, 3.0) | 0.52 | |||
| Glucose (mmol/L) | 4.5 | 0.3 | 0.9 | (−1.6, 3.4) | 0.48 | 0.9 | (−1.6, 3.4) | 0.48 | |||
| HbA1c (%) | 5.3 | 0.3 | 0.7 | (−1.6, 3.2) | 0.55 | 0.7 | (−1.7, 3.1) | 0.56 | |||
| Insulin (mU/L)* | 7.2 | 1.8 | 0.6 | (−1.9, 3.2) | 0.65 | 0.7 | (−1.8, 3.3) | 0.57 | |||
| Insulin resistance* | 0.9 | 1.8 | 0.6 | (−1.9, 3.2) | 0.65 | 0.7 | (−1.8, 3.3) | 0.57 | |||
| Triglyceride (mmol/L)* | 0.9 | 1.5 | 3.7 | (1.2, 6.4) | 0.004 | 3.7 | (1.1, 6.3) | 0.005 | 3.8 | (1.0, 6.6) | 0.01 |
| Total cholesterol (mmol/L) | 4.5 | 0.8 | 3.3 | (0.9, 5.8) | 0.01 | 3.3 | (0.9, 5.7) | 0.01 | 3.2 | (0.8, 5.7) | 0.01 |
| LDL cholesterol (mmol/L) | 2.7 | 0.7 | 3.1 | (0.6, 5.6) | 0.01 | 3.1 | (0.6, 5.6) | 0.01 | 2.9 | (0.4, 5.5) | 0.02 |
| HDL cholesterol (mmol/L) | 1.5 | 0.3 | −0.2 | (−2.5, 2.2) | 0.89 | −0.3 | (−2.7, 2.1) | 0.79 | |||
| Systolic BP (mmHg) | 104.4 | 10.7 | 1.9 | (−0.3, 4.2) | 0.09 | 2.0 | (−0.3, 4.2) | 0.09 | 1.5 | (−1.0, 4.0) | 0.24 |
| Diastolic BP (mmHg) | 63.2 | 9.1 | 2.3 | (0.1, 4.6) | 0.04 | 2.3 | (0.1, 4.6) | 0.04 | 1.5 | (−0.9, 3.9) | 0.23 |
| C reactive protein (mg/L)* | 0.6 | 3.7 | −0.2 | (−2.5, 2.2) | 0.86 | −0.1 | (−2.4, 2.2) | 0.92 | |||
Analyses of age and anthropometric measurements are based on 986 subjects; analyses of blood measurements are based on 872 subjects
Log transformed variable
Arithmetic mean and SD or geometric mean and geometric SD for log transformed variables (95% central range is geometric mean × GSD2, is geometric mean × GSD2)
All percentage differences in tortuosity are for a one SD increase in risk factor
Adjustment 1: includes gender, age groups (except for age), ethnicity, observer (physical measurements only), month, retinal branch status and a random effect for child within school
Adjustment 2: includes all variables in adjustment 1 plus axial length
Adjustment 3: includes all variables in adjustment 2, plus adjustment for height, sum of skinfolds, fasting insulin and (i) SBP for Triglyceride, LDL and total cholesterol associations, (ii) LDL cholesterol for BP associations.
Figure 3.
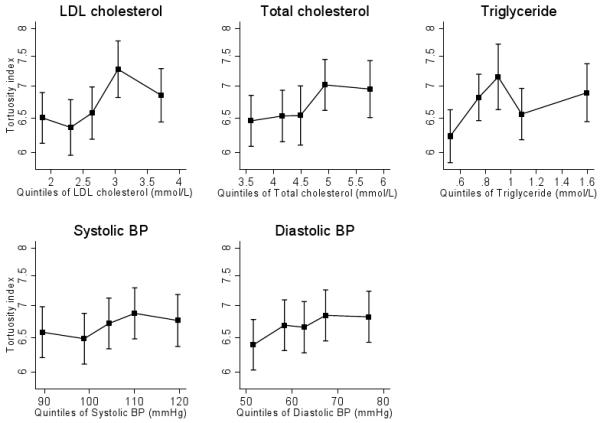
Level of tortuosity index (log scale) by quintiles of LDL and total cholesterol (mmol/L), triglyceride (mmol/L), systolic and diastolic blood pressure (mm Hg).
Discussion
This study provides evidence that retinal arteriolar tortuosity shows appreciable variation between individuals in childhood and is positively associated with established cardiovascular risk factors (including triglyceride, total and LDL cholesterol, systolic and diastolic blood pressure). These findings raise the possibility that early markers of cardiovascular disease, such as higher blood lipids (in particular triglyceride levels) and blood pressure, influence retinal arteriolar tortuosity during the first decade of life.
Cardiovascular disease has long been viewed as a disease originating in middle age. However, there is now substantial evidence from pathological studies,22-24 epidemiological studies25-31 and combined pathological-epidemiological studies32;33 that CHD risk originates earlier in life and that abnormalities in arterial structure and function are apparent before adult life. Abnormalities of retinal microvasculature, particularly affecting the arterioles, are known to be related to cardiovascular disease and CHD in adult life.1-3;34;35 However, less is known about changes in the morphology of retinal vessels amongst populations without overt cardiometabolic disease. Studies that do exist have focused on the measurement of vessel width, both in adults 8;9 and children.10;11 Measures of width are more difficult in children with prominent vessel reflexes (especially on arterioles), and in different ethnic groups with varying levels of refractive error (with higher levels of myopia amongst Asians)19;36;37 and background levels of retinal pigmentation (with higher levels amongst black African-Caribbeans – see Figure 1). Measuring vessel tortuosity offers another morphological characteristic of the vascular network, which we found was less sensitive to these difficulties.12 and is effectively independent of vessel width. The findings from this study are consistent with earlier studies that have shown a positive association between retinal vessel tortuosity (assessed subjectively) and hypertension, both in child and adult populations,4;5;38 and amongst those with severe coronary disease.39 However, not all studies have been consistent in their findings.40;41 This may reflect the unreliability of subjective assessment of tortuosity in many of these earlier studies. Objective measures of tortuosity will avoid measurement error inherent with subjective assessment (especially at lower levels of tortuosity).13
Strengths and limitations
We have used a novel measure of tortuosity based on a subdivided chord length method (chosen a priori) that has been validated against subjective assessment in this age group, which shows good agreement and repeatability across a broad range of vessel tortuosity,13 from smoothly curved to highly tortuous retinal vessels in infants with retinopathy of prematurity.20 Moreover, unlike dimensional measurements (such as width), we have also shown that the tortuosity measure is relatively unaffected by refractive error 13 and is therefore likely to be particularly valid in this multi-ethnic population with large differences in ametropia and ocular biometry.19 Other strengths of this study include the appreciable sample size and multi-ethnic population, designed to detect modest differences in risk markers between major ethnic groups (white European, South Asian, black African Caribbean).14 Overall response rates were high with little difference between ethnic groups. The slightly lower response rate in black African-Caribbeans is unlikely to invalidate the results as there was no strong evidence of ethnic difference in the pattern of association between tortuosity and cardiovascular risk factors (except perhaps for blood cholesterol). Response rates for blood sampling were slightly lower (as expected), but this is unlikely to have affected the associations observed, especially as those with extremes in arteriolar tortuosity are unlikely to know and choose not to participate. The cross sectional nature of the present study means that it cannot be assumed that cardiovascular risk factors caused increased retinal arteriolar tortuosity. Indeed, it is plausible that more tortuous microcirculation may lead to higher blood pressure.
Implications
We have observed appreciable variation in retinal arteriolar tortuosity and associations with established cardiovascular risk factors that were consistent in boys and girls and across ethnic groups. Our results suggest that unfavourable cardiovascular risk profiles in childhood may be having adverse effects on arteriolar structure and function in the first decade of life. The strength, consistency and graded associations observed between several established cardiovascular risk factors and retinal arteriolar tortuosity suggest that the associations, very unlikely to have occurred by chance, may be causal. However, biological mechanisms for the findings remain uncertain, especially the strong positive association with triglycerides. The mechanisms by which blood lipids influence the wall of larger arteries are not likely to apply to arterioles; blood pressure could be having a mechanical influence on arteriolar structure and function.42 Further research in population based studies is needed to clarify the associations between cardiovascular risk factors and retinal arteriolar tortuosity, and the relations between retinal arteriolar tortuosity and other markers of vascular structure and function. Prospective studies will also be useful in establishing the time sequence of these associations. In addition, more evidence on other potential determinants of retinal arteriolar tortuosity, including early life factors (such as birthweight and/or gestation),43;44 childhood lifestyle (including physical activity) and genetic predisposition41 may also be important in this context. We examined objective measures of physical activity in our study,45 but found no evidence of an association with arteriolar tortuosity (data not presented). The associations observed suggest that retinal arteriolar tortuosity may be influenced by cardiovascular risk factors in early life.
Acknowledgements
We are grateful to the members of the CHASE study team (in particular Alicja Kiedzik, Devina Jo-Anne Joysurry and Melanie Prescott who made the ocular measurements) and to participating schools, pupils and parents. We also wish to acknowledge Sindy Lee, Ruth Whincup, Fahad Zaman, Rahil Hosseini, Mahdi Mazinani and Yousef Ebrahim Doost Kanafi for their work on processing the images. We are grateful to the Birmingham Optical Group (Birmingham, UK) who provided a Nidek NM-200D handheld fundus camera and to Dr Nicola Logan (Aston University, Birmingham, UK) who provided an auto-refractor (Shin-Nippon Commerce Inc., Tokyo, Japan) for the duration of the study.
Sources of support
This work was supported by a grant from the BUPA Foundation (755/G25), and carried out during a cardiovascular survey funded by the British Heart Foundation (PG/07/033/22770). The Child Heart And Health Study in England (CHASE) was supported by a grant from the Wellcome Trust (068362/Z/02/Z).
Footnotes
Conflicts of interest
None to declare
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, et al. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287(9):1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- (2).Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001;358(9288):1134–1140. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- (3).Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, et al. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110(5):933–940. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- (4).Daniels SR, Lipman MJ, Burke MJ, Loggie JM. Determinants of retinal vascular abnormalities in children and adolescents with essential hypertension. J Hum Hypertens. 1993;7(3):223–228. [PubMed] [Google Scholar]
- (5).de Margerie J, Boyd TA. A statistical investigation of the correlation of retinal arteriolar tortuosity with blood pressure and age. Trans Can Ophthalmol Soc. 1961;24:6–17. [PubMed] [Google Scholar]
- (6).Wang JJ, Liew G, Wong TY, Smith W, Klein R, Leeder SR, et al. Retinal vascular calibre and the risk of coronary heart disease-related death. Heart. 2006;92(11):1583–1587. doi: 10.1136/hrt.2006.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Wong TY, Kamineni A, Klein R, Sharrett AR, Klein BE, Siscovick DS, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the Cardiovascular Health Study. Arch Intern Med. 2006;166(21):2388–2394. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- (8).Leung H, Wang JJ, Rochtchina E, Tan AG, Wong TY, Klein R, et al. Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci. 2003;44(7):2900–2904. doi: 10.1167/iovs.02-1114. [DOI] [PubMed] [Google Scholar]
- (9).Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47(6):2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Mitchell P, Cheung N, de HK, Taylor B, Rochtchina E, Islam FM, et al. Blood pressure and retinal arteriolar narrowing in children. Hypertension. 2007;49(5):1156–1162. doi: 10.1161/HYPERTENSIONAHA.106.085910. [DOI] [PubMed] [Google Scholar]
- (11).Cheung N, Saw SM, Islam FM, Rogers SL, Shankar A, de HK, et al. BMI and retinal vascular caliber in children. Obesity (Silver Spring) 2007;15(1):209–215. doi: 10.1038/oby.2007.576. [DOI] [PubMed] [Google Scholar]
- (12).Lotmar W, Freiburghaus A, Bracher D. Measurement of vessel tortuosity on fundus photographs. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1979;211(1):49–57. doi: 10.1007/BF00414653. [DOI] [PubMed] [Google Scholar]
- (13).Owen CG, Rudnicka AR, Mullen R, Barman SA, Monekosso D, Whincup PH, et al. Measuring retinal vessel tortuosity in 10-year-old children: validation of the Computer-Assisted Image Analysis of the Retina (CAIAR) program. Invest Ophthalmol Vis Sci. 2009;50(5):2004–2010. doi: 10.1167/iovs.08-3018. [DOI] [PubMed] [Google Scholar]
- (14).Whincup PH, Nightingale CM, Owen CG, Rudnicka AR, Gibb I, McKay C, et al. Early emergence of ethnic differences in type 2 diabetes precursors in the UK: the Child Heart And health Study in England (CHASE Study) PLoS Med. 2010;7(4):e1000263. doi: 10.1371/journal.pmed.1000263. doi:10.1371/journal.pmed.1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Clasey J, Bradley K, Bradley J, Long DA. A new BIA equation estimating the body composition of young children. Obesity. 2007;15:A127. doi: 10.1038/oby.2011.158. [DOI] [PubMed] [Google Scholar]
- (16).Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatric and Perinatal Epidemiology. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- (17).Andersen L, Dinesen B, Jorgensen PN, Poulsen F, Roder ME. Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem. 1993;39(4):578–582. [PubMed] [Google Scholar]
- (18).Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- (19).Rudnicka AR, Owen CG, Nightingale CM, Cook D, Whincup P. Ethnic differences in the prevalence of myopia and ocular biometry in 10-11 year old children: the Child Heart And Health Study in England (CHASE) Invest Ophthalmol Vis Sci. 2010;51(12):6270–6276. doi: 10.1167/iovs.10-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wilson CM, Cocker KD, Moseley MJ, Paterson C, Clay ST, Schulenburg WE, et al. Computerized analysis of retinal vessel width and tortuosity in premature infants. Invest Ophthalmol Vis Sci. 2008;49(8):3577–3585. doi: 10.1167/iovs.07-1353. [DOI] [PubMed] [Google Scholar]
- (21).Ng J, Clay ST, Barman SA, Fielder AR, Moseley MJ, Parker KH, et al. Maximum likelihood estimation of vessel parameters from scale space analysis. Image and Vision Computing. 2010;28(1):55–63. [Google Scholar]
- (22).Matturri L, Ottaviani G, Corti G, Lavezzi AM. Pathogenesis of early atherosclerotic lesions in infants. Pathol Res Pract. 2004;200(5):403–410. doi: 10.1016/j.prp.2004.03.004. [DOI] [PubMed] [Google Scholar]
- (23).Enos WF, Holmes RH, Beyer JC. Coronary heart disease among United States soldiers killed in action in Korea. JAMA. 1953;152:1090–1093. doi: 10.1001/jama.1953.03690120006002. [DOI] [PubMed] [Google Scholar]
- (24).Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman WP, III, Herderick EE, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999;281(8):727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- (25).Nelson MJ, Ragland DR, Syme SL. Longitudinal prediction of adult blood pressure from juvenile blood pressure levels. Am J Epidemiol. 1992;136(6):633–645. doi: 10.1093/oxfordjournals.aje.a116543. [DOI] [PubMed] [Google Scholar]
- (26).Webber LS, Srinivasan SR, Wattigney WA, Berenson GS. Tracking of serum lipids and lipoproteins from childhood to adulthood. The Bogalusa Heart Study. Am J Epidemiol. 1991;133(9):884–899. doi: 10.1093/oxfordjournals.aje.a115968. [DOI] [PubMed] [Google Scholar]
- (27).Kemper HC, Snel J, Verschuur R, Storm-van Essen L. Tracking of health and risk indicators of cardiovascular diseases from teenager to adult: Amsterdam Growth and Health Study. Prev Med. 1990;19(6):642–655. doi: 10.1016/0091-7435(90)90061-n. [DOI] [PubMed] [Google Scholar]
- (28).Leeson CP, Whincup PH, Cook DG, Mullen MJ, Donald AE, Seymour CA, et al. Cholesterol and arterial distensibility in the first decade of life: a population-based study. Circulation. 2000;101(13):1533–1538. doi: 10.1161/01.cir.101.13.1533. [DOI] [PubMed] [Google Scholar]
- (29).Paffenbarger RS, Wing AL. Chronic disease in former college students. X. The effects of single and multiple characteristics on risk of fatal coronary heart disease. Am J Epidemiol. 1969;90(6):527–535. doi: 10.1093/oxfordjournals.aje.a121099. [DOI] [PubMed] [Google Scholar]
- (30).Klag MJ, Ford DE, Mead LA, He J, Whelton PK, Liang KY, et al. Serum cholesterol in young men and subsequent cardiovascular disease. N Engl J Med. 1993;328(5):313–318. doi: 10.1056/NEJM199302043280504. [DOI] [PubMed] [Google Scholar]
- (31).McCarron P, Davey Smith G, Okasha M, McEwen J. Blood pressure in young adulthood and mortality from cardiovascular disease. Lancet. 2000;355(9213):1430–1431. doi: 10.1016/S0140-6736(00)02146-2. [DOI] [PubMed] [Google Scholar]
- (32).Newman WP, III, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, et al. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med. 1986;314(3):138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- (33).Malcom GT, Oalmann MC, Strong JP. Risk factors for atherosclerosis in young subjects: the PDAY Study. Pathobiological Determinants of Atherosclerosis in Youth. Ann N Y Acad Sci. 1997;817:179–188. doi: 10.1111/j.1749-6632.1997.tb48205.x. [DOI] [PubMed] [Google Scholar]
- (34).Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47(5):975–981. doi: 10.1161/01.HYP.0000216717.72048.6c. [DOI] [PubMed] [Google Scholar]
- (35).Ikram MK, Janssen JA, Roos AM, Rietveld I, Witteman JC, Breteler MM, et al. Retinal vessel diameters and risk of impaired fasting glucose or diabetes: the Rotterdam study. Diabetes. 2006;55(2):506–510. doi: 10.2337/diabetes.55.02.06.db05-0546. [DOI] [PubMed] [Google Scholar]
- (36).Ip JM, Huynh SC, Robaei D, Kifley A, Rose KA, Morgan IG, et al. Ethnic differences in refraction and ocular biometry in a population-based sample of 11-15-year-old Australian children. Eye (Lond) 2008;22(5):649–656. doi: 10.1038/sj.eye.6702701. [DOI] [PubMed] [Google Scholar]
- (37).Kleinstein RN, Jones LA, Hullett S, Kwon S, Lee RJ, Friedman NE, et al. Refractive error and ethnicity in children. Arch Ophthalmol. 2003;121(8):1141–1147. doi: 10.1001/archopht.121.8.1141. [DOI] [PubMed] [Google Scholar]
- (38).Wolffsohn JS, Napper GA, Ho SM, Jaworski A, Pollard TL. Improving the description of the retinal vasculature and patient history taking for monitoring systemic hypertension. Ophthalmic Physiol Opt. 2001;21(6):441–449. doi: 10.1046/j.1475-1313.2001.00616.x. [DOI] [PubMed] [Google Scholar]
- (39).Michelson EL, Morganroth J, Nichols CW, MacVaugh H., III Retinal arteriolar changes as an indicator of coronary artery disease. Arch Intern Med. 1979;139(10):1139–1141. [PubMed] [Google Scholar]
- (40).Kwa VI, van der Sande JJ, Stam J, Tijmes N, Vrooland JL. Retinal arterial changes correlate with cerebral small-vessel disease. Neurology. 2002;59(10):1536–1540. doi: 10.1212/01.wnl.0000033093.16450.5c. [DOI] [PubMed] [Google Scholar]
- (41).Taarnhoj NC, Munch IC, Sander B, Kessel L, Hougaard JL, Kyvik K, et al. Straight versus tortuous retinal arteries in relation to blood pressure and genetics. Br J Ophthalmol. 2008;92(8):1055–1060. doi: 10.1136/bjo.2007.134593. [DOI] [PubMed] [Google Scholar]
- (42).Kylstra JA, Wierzbicki T, Wolbarsht ML, Landers MB, III, Stefansson E. The relationship between retinal vessel tortuosity, diameter, and transmural pressure. Graefes Arch Clin Exp Ophthalmol. 1986;224(5):477–480. doi: 10.1007/BF02173368. [DOI] [PubMed] [Google Scholar]
- (43).Tapp RJ, Williams C, Witt N, Chaturvedi N, Evans R, Thom SA, et al. Impact of size at birth on the microvasculature: the Avon Longitudinal Study of Parents and Children. Pediatrics. 2007;120(5):e1225–e1228. doi: 10.1542/peds.2006-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Mitchell P, Liew G, Rochtchina E, Wang JJ, Robaei D, Cheung N, et al. Evidence of arteriolar narrowing in low-birth-weight children. Circulation. 2008;118(5):518–524. doi: 10.1161/CIRCULATIONAHA.107.747329. [DOI] [PubMed] [Google Scholar]
- (45).Owen CG, Nightingale CM, Rudnicka AR, Cook DG, Ekelund U, Whincup PH. Ethnic and gender differences in physical activity levels among 9-10-year-old children of white European, South Asian and African-Caribbean origin: the Child Heart Health Study in England (CHASE Study) Int J Epidemiol. 2009;38:1082–1093. doi: 10.1093/ije/dyp176. [DOI] [PMC free article] [PubMed] [Google Scholar]


