Abstract
Introduction
Increasing EFV dose from 600mg to 800mg daily has been suggested with concomitant RFN, as induction of cytochrome p450 isoenzymes may reduce EFV plasma concentrations
Methods
Individuals from the CIPRA-South Africa cohort taking EFV-based ART with concomitant TB were dosed with either increased-(800mg) or standard-(600mg) dose EFV during TB treatment. After TB therapy all took 600mg EFV. Two mid-dosing interval EFV concentrations were determined from each individual: after 4 weeks of concomitant EFV and RFN therapy, and at least 4 weeks after TB therapy completion. Mid-dosing interval EFV concentrations were compared within individuals using the Wilcoxon signed rank test.
Results
Paired-samples were collected from 72 individuals. 45(63%) were women; median weight 59kg (IQR52-67kg). At ART start median CD4 count was114 cells/mm3 (IQR37-165), median viral load 5.5log (IQR5.1–5.9). 38 (53%) took 800mg EFV during TB treatment and 34(47%) took 600mg. EFV concentrations in the 800mg group were higher with RFN [[2.9mg/L (IQR 1.8–5.6)] than without [2.1mg/L (IQR 1.4–3.0)]], p=0.0003. There was no significant difference in EFV concentrations with RFN [2.4mg/L (IQR1.2–5.1)] or without [2.2 mg/L (IQR 1.4 to 3.7)] in the 600mg group. There was no increase in EFV-linked adverse effects in either group. Proportion virologically suppressed at 48 weeks was similar in both groups.
Conclusion
EFV concentrations were significantly increased in the EFV 800mg group on RFN. There was no significant decrease in EFV concentrations when on RFN in the 600mg group. Dose escalation of EFV 600mg to 800mg is not required during concomitant TB therapy in South Africa.
Keywords: antiretroviral therapy, tuberculosis, drug interactions
Introduction and rationale
Antiretroviral treatment options
The South African antiretroviral treatment programme uses a standardised first line regimen that is non-nucleoside reverse transcriptase inhibitor based. [1,2] Efavirenz is the preferred non-nucleoside reverse transcriptase inhibitor, except for women of childbearing potential. The standard dose for efavirenz (EFV) used is 600 mg daily. According to South African guidelines, this dose remains unchanged with concomitant tuberculosis (TB) therapy, but there are limited data during coadministration. [1]
EFV plasma concentrations show marked inter-individual variability.[3] In 2001 Marzolini et al obtained blood samples from130 Swiss people on 600 mg efavirenz. [4] Efavirenz concentrations varied from 125 to 15 230 ug/l at the mid-dose interval (normal range 1–4ug/l). Lower concentrations were linked with virological failure and high concentrations with significant central nervous system toxicity. Other studies have linked higher efavirenz concentrations both with neuropsychiatric side effects and abnormalities of liver function. [5–7] Adding a drug that may potentate EFV metabolism, thus lowering plasma concentrations, could result in failure of antiretroviral therapy. This would be particularly harmful in resource poor setting where therapeutic options are limited.
South Africa has one of the highest incidences of tuberculosis in the developing world, due to a large extent to the HIV epidemic. The numbers of people presenting with TB continue to increase in Africa, in contrast to the decline in TB cases worldwide. The probability of someone with HIV developing TB is 10% per annum in developed countries and likely to be much higher in the developing world. [8] The South African National Tuberculosis guidelines (2000) use rifampicin as a core antimycobacterial in both initial and re-treatment regimens. [9]
In South Africa, many people with symptomatic HIV have tuberculosis and many more develop TB soon after commencing therapy, thus necessitating the concomitant use of TB therapy, including rifampicin, and antiretroviral therapy (ART), including EFV. [10] These two commonly used medications have had previously documented interactions as they are both metabolised by and induce the cytochrome p450 enzyme system (particularly isoenzymes 3A4 and 2B6). While studies revealed that EFV had no impact on rifampicin pharmacokinetics, the reverse did not apply. [11,12]
At least two studies raised concerns for virological breakthrough due to reduced EFV concentrations when dosed at 600mg in the presence of RFN. Data from Lopez-Cortes et al in 2002 in 24 HIV positive individuals revealed a 24% reduction in efavirenz maximum concentration (Cmax) and a 22% reduction in the area under the concentration-time curve (AUC) in a Caucasian population on TB therapy.[12] They noted that an 800mg dose returned serum EFV concentrations to those achieved with 600mg without rifampicin. Information from another 12 healthy normal volunteers, again Caucasian, showed induction of EFV metabolism in the presence of rifampicin.[11] Efavirenz product information states that a 20% reduction in EFV Cmax and a 26% reduction in AUC may be expected with the addition of rifampicin, but that the clinical relevance of this decrease in EFV concentrations had not been established.[13]
A logical step would be to reduce the risk of virological breakthrough by increasing EFV dose when on concomitant RFN therapy. However, this seems to result in increased drug levels and thus a potential increase in toxicity. A large, as yet unpublished, study (n=65) noted an increase in efavirenz trough concentrations when a dose of 800 or 1000 mg of EFV was given to people on rifampicin, as compared to the 600mg dose without TB treatment. [14] In a further small series, 7 of 9 HIV-positive individuals treated with 800mg of EFV concurrently with rifampicin in 2004 experienced significant central nervous system side effects on this higher dose. Trough EFV concentrations were noted to be high.[15] Others noted central nervous system toxicity with higher EFV concentrations. [7
There remains room for debate to whether the 600mg or 800mg dose of EFV is appropriate when on concurrent TB therapy. The need to achieve adequate EFV concentrations and maintain viral suppression must be weighed against the increased risk of CNS toxicity when a higher dose is used. Differing polymorphisms in the 2B6 cytochrome p450 isoenzyme of an African population compared to the Caucasian populations where the majority of pharmacokinetic work has been completed may explain some of the variation seen. What is true for one population, may not hold true for another.[16] While support for staying with the 600mg dose in developing countries stems from five studies (from South Africa, Brazil, Thailand and India) which have noted good clinical and virological outcomes in people with TB treated with ART containing EFV at the standard 600mg dose, to date there is no information on EFV concentrations at the 800mg dose in an African population, with or without rifampicin.[16–20]
This study compared intra-individual steady state mid-dosing interval plasma concentrations of efavirenz at 800mg or at 600mg during concurrent use of rifampicin, to the standard 600mg EFV dose while on antiretroviral therapy alone in a South African population. It also examined adherence and virological outcomes for both groups as well as any difference in recorded central nervous system or hepatic adverse events.
Methods
This study is a sub-study of the project one of the Comprehensive International Programme for research on AIDS, South Africa (CIPRA-SA project 1): “Safeguard the household” a study of HIV antiviral therapy treatment strategies appropriate for a resource poor country [NCT-0255840].[21] CIPRA-SA project 1 recruited 810 individuals with CD4 counts of <350cells/ul onto antiretroviral therapy between February 2005 and January 2007. Individuals were from two South African sites, one large urban site in Gauteng and one small community in the South Peninsula region of Cape Town.
Antiretroviral therapy was given according to the South African National Antiretroviral Guidelines [1,2] with an initial non-nucleoside reverse transcriptase (NNRTI)-based regimen followed by a protease inhibitor (PI)-based regimen. Efavirenz is the preferred NNRTI for people taking concurrent tuberculosis therapy. First line NNRTIs usually given at the time of this study were stavudine (d4T) and lamivudine (3TC). Scheduled trial visits occurred monthly until week 12 and every 12 weeks thereafter. Viral loads, CD4 counts and adherence assessment by counting of tablet returns were performed at every visit. All adverse event data were recorded. The DAIDS tables for grading the severity of adult and paediatric adverse events (2004) were used to assess each adverse event. [22]
During initial recruitment to the CIPRA-SA project 1 study, from February to November 2005, all participants with concomitant TB were dosed with 800mg of EFV. Later this was amended to allow the investigators to match the South African National ART Guidelines and from December 2005 until January 2007, all people recruited to the same cohort with TB were commenced on the 600mg EFV dose. These doses were specified by the study protocol and amendments and were not left to investigator discretion.
Rifampicin is used for the full duration of tuberculosis (TB) therapy in South Africa. The initial TB treatment regimen comprises 2 two months of four drug therapy (rifampicin, isoniazid, pyrazinamide and ethambutol) followed by four months of rifampicin and isoniazid alone. Rifampicin is dosed according to body weight i.e. 450 mg for patients < 50 kg and 600 mg for patients > 50 kg. Re-treatment of TB comprises 8 months of therapy and rifampicin is again included for the duration of treatment.
Substudy
All individuals in the CIPRA-SA project 1 cohort who were taking rifampicin-based tuberculosis treatment at the time of commencing efavirenz-based ART were eligible for this sub-study. No extra visits or blood samples were required.
Individuals were divided into two groups: those on 800mg EFV during TB treatment (and 600mg thereafter) and those who remained on 600 mg efavirenz throughout. Demographic information was collected at commencement of ART for each individual, including gender, weight and WHO stage of HIV infection. Dates of commencement and completion of TB treatment were recorded as was date of commencement of ART. The date and time of the efavirenz dose immediately prior to the each sample required was also recorded, as were the date and time of the sample used for mid-dosing interval EFV concentrations. EFV is routinely dosed at night and bloods drawn in the mornings. CD4 counts and viral loads were collected for the first 48 weeks on ART and all adverse events noted were noted during the same time period.
At each scheduled visit during the CIPRA-SA project 1 study plasma was drawn for storage. Samples required for this sub-study were drawn retrospectively from a bank of stored plasma samples after review of each individual’s clinical details to determine which samples were required. Each individual required paired samples for the mid-dosing interval efavirenz assay. The first sample was selected from a visit at least 4 weeks into concurrent TB and ART. The second sample was selected from a visit at least 4 weeks after TB therapy had been discontinued, but while still on ART. As these paired samples were drawn retrospectively, they were not matched with regard to dose to sampling time.
Selected stored samples were pulled from the bank of samples and analysed for efavirenz mid-dosing interval concentrations at the Division of Clinical Pharmacology laboratory at the University of Cape Town which is ISO17025 accredited for this purpose. Efavirenz concentrations were done using validated high-pressure liquid chromatography (HPLC) methods with a mass spectrometer.
Sampling
Due to the large inter-individual variability in EFV concentrations (coefficient of variability (CV) 118%), but less intra-individual variability (CV 30%) (Marzolini 2001), a study using paired observations from one individual is more appropriate for an EFV pharmacokinetic study than comparing between individuals. To detect a more than 30% change in mid-dosing interval EFV concentrations with a power of 90%, at a significance level of 0.05, a sample size of 40 people was required in the EFV 800 mg group. Another sample of 40 was required for the EFV 600 mg group.
Statistical analyses
Statistical analyses were performed using STATA version 10 (STATA Corporation, College Station, TX, USA). Undetectable viral load was defined as a viral load <400 copies/ml. Efavirenz concentrations were classified as subtherapeutic if they were less than 1mg/l.
Continuous variables were summarised using means and standard deviations if normally distributed, and medians and ranges if not normally distributed. Within-group comparisons of continuous variables were made using a paired t test if parametrically distributed and the Wilcoxon signed rank test for paired observations if non-parametrically distributed. Between-group comparisons were made using a t test if parametrically distributed and the Wilcoxon/Mann-Whitney rank sum test if non-parametrically distributed.
Pharmacokinetic analysis
Efavirenz concentrations were determined by validated liquid chromatographytandem mass spectrometry (LC/MS/MS), as previously described [23,24], in the Division of Clinical Pharmacology laboratory, University of Cape Town. The calibration curve was linear over the range of 0.1 to 15 mg/L. Where initial results were above 15mg/ml, specimens were diluted to quantify the concentration.
Ethical considerations
All individuals signed informed consent allowing the use of stored samples for pharmacokinetic work at the commencement of the CIPRA-SA project 1 study. Informed consent documents were approved by both the University of Cape Town and the University of Witwatersrand Ethics Committees. The study was run in accordance with South African Good Clinical Practice Guidelines.
Results
There were 87 participants in the CIPRA-SA project 1 cohort (n=810) who commenced ART while on therapy for tuberculosis and as such were eligible for entry into this sub-study. Seven of these individuals had no second visit for collection of a paired sample either due to death (n=1), transfer to another clinic (n=1), prolonged TB treatment (n=1) or loss to follow-up (n=4) and so were excluded. A further seven individuals with both samples available had no recorded time of efavirenz dosing. These 14 individuals were excluded from the analysis, leaving 72 individuals with paired samples.
Baseline characteristics
The demographics of these groups are described in table 1. There were 38 people who commenced ART with 800mg EFV and 34 people who were on 600mg EFV throughout. The majority of the cohort were women (63%), with a median weight of 59kg (IQR 52–67). Forty-seven percent of the cohort weighed 60kg or more. Baseline CD4 counts were low (122 cells/mm3 (IQR 35–161) for those in the 800mg EFV group and 91 cells/mm3 (IQR 40–165) in the 600mg EFV group) and viral load more than 5 logs at baseline. Baseline characteristics in the two groups were similar.
Table 1.
Baseline demographic characteristics of individuals in the 72 paired samples.
| All participants | Participants on 800mg EFV during rifampicin- based antitubercular therapy | Participants on 600mg EFV during rifampicin- based antitubercular therapy | |
|---|---|---|---|
| Number | 72 | 38 | 34 |
| Female | 45 (63%) | 23 (61%) | 22 (65%) |
| Weight (kg), median (IQR) | 58.8 (51.9, 66.9) | 57.9 (51.9, 67.4) | 60.0 (51.4, 65.2) |
| Baseline CD4 count (cells/mm3), median (IQR) | 114 (37, 165) | 122 (32,161) | 91 (40, 165) |
| Baseline viral load (log), median (IQR) | 5.5 (5.1, 5.9) | 5.5 (5.1, 5.9) | 5.7 (5.3, 5.9) |
EFV concentrations
The majority of the participants (92%) had their first sample taken at week 4, 8 or 12 of ART. The majority of the second samples (90%) were taken between week 24 and week 48 on ART.
Table two describes the median EFV concentrations in the 800/600 and 600/600 groups. The median concentration of efavirenz in individuals on 800mg EFV was 2.9 mg/L (IQR 1.8 –5.6mg/L). The median EFV concentration was 2.1 mg/L (IQR 1.4–3.0 mg/L) in these individuals when they had completed TB therapy, after they were changed to the 600mg EFV dose. EFV concentrations were significantly higher on 800mg than that on 600mg (Wilcoxon signed rank p=0.003).
The median concentration of efavirenz in individuals on 600mg EFV during TB therapy was 2.4 mg/L (IQR 1.2 – 5.1mg/L) compared to 2.2 mg/L (IQR 1.4–3.7 mg/L) in the same individuals after tuberculosis therapy was complete. EFV concentrations were similar on and off TB treatment in this group (Wilcoxon signed rank p=0.669). Mean post-dose sampling times were close to 14 hours and there was no significant difference in post-dose sampling times by group (table 2).
Table 2.
EFV median (IQR) plasma concentration in mg/L on and after antitubercular therapy with mean post-dose sampling times.
| On antitubercular therapy | After antitubercular therapy | p value | |
|---|---|---|---|
| EFV 800/600 n=38 | 2.9 (1.8, 5.6) | 2.1 (1.4, 3.0) | 0.0003* |
| Proportion sub- therapeutic n (%) | 1 (3%) | 5 (13%) | 0.089** |
| Mean post-dose sampling time: hours (+/− SD ) | 14.0 (1.5) | 14.3 (1.7) | 0.628# |
| EFV 600/600 n=34 | 2.4 (1.2, 5.1) | 2.2 (1.4, 3.7) | 0.669* |
| Proportion sub- therapeutic n (%) | 4 (12%) | 3 (9%) | 0.690** |
| Mean post-dose sampling time: hours (+/− SD ) | 13.8 (1.7) | 13.8 (1.8) | 0.208# |
Efavirenz therapeutic range: 1.0 –4.0 mg/L
Wilcoxon signrank test
Chi-squared test
t-test.
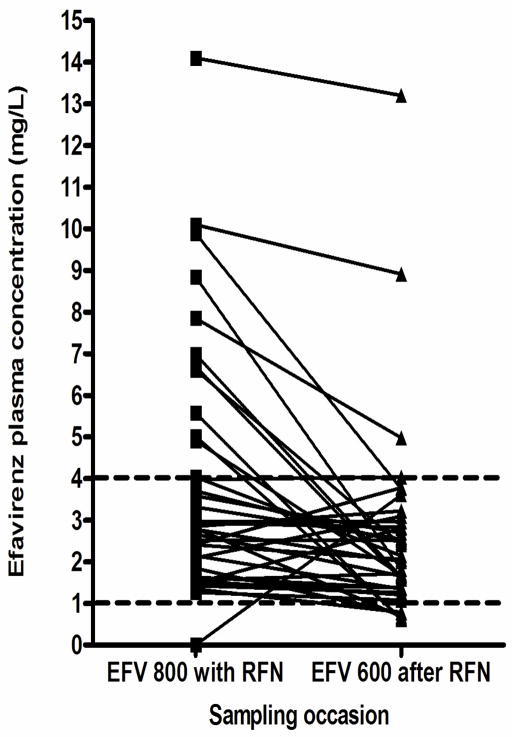
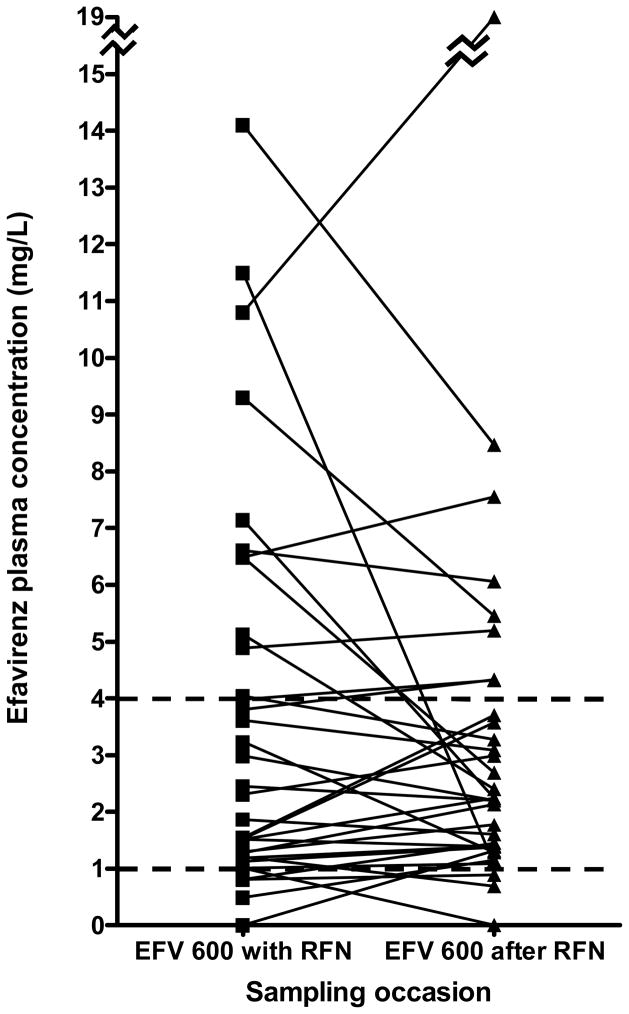
Figure 1 shows the paired values for all individuals, both in the 800/600 group (figure 1a) and in the 600/600 group (figure 1b). The great majority of individuals had stable or decreasing concentrations of efavirenz once rifampicin treatment was completed. In the 800/600 group there was less variability in the EFV concentrations and only one individual had a sub-therapeutic EFV concentration while on RFN. In the 600/600 group the variability of EFV concentrations was greater on RFN and four individuals had sub-therapeutic concentrations, however there was no significant difference in the proportion of those sub-therapeutic either within or across the groups (table 2).
Figure 1.
Figure 1a: Paired graphs showing individual efavirenz concentrations in the 800/600 EFV group. Dotted lines represent normal range (1–4 mg/L), n=38.
Figure 1b: Paired graphs showing individual efavirenz concentrations in the 600/600 EFV group. Dotted lines represent normal range (1–4 mg/L), n=34.
Using linear regression, there was no correlation found between weight as a continuous variable and efavirenz concentration at either time point, in either group. Participants were also categorised into those weighing <60kg and those weighing>=60kg within each dosing group while on rifampicin therapy and although EFV levels in the >=60kg group were slightly lower, there was no significant difference in median efavirenz concentrations (600mg group: <60 kg median EFV level 2.5 mg/L (IQR 1.3, 4.0), >=60 kg median EFV level 1.5 mg/L (IQR 1.2, 5.1); 800mg group: <60 kg median EFV level 3.6 mg/L (IQR 2.7, 7.0), p=0.593, >=60 kg median EFV level 2.4 mg/L (IQR 1.5, 3.7), p=0.103).
Virological and adherence outcomes
Ninety-two percent of the group were suppressed to <50 copies/ml at 48 weeks of ART. There was no significant difference by group. There was no association between EFV concentrations of <1 mg/land a detectable viral load at the time of EFV sampling (χ2 p=0.979 for first sample, p=0.276 for second sample).
Adherence by tablet count of efavirenz returns was uniformly excellent, with a median adherence of 100% (IQR 96–100%) at 12 weeks as well as at 48 weeks (IQR 96.5–100%) and no significant difference was noted by group. There was no evidence of a difference in adherence at week 12 and week 48 between those with sub-therapeutic EFV levels and those with levels above 1mg/L (data not shown).
Adverse events
During the first 60 weeks on antiretroviral therapy, 105 adverse events were reported in this cohort. Thirty-three (32%) adverse events in 21 individuals were thought to be possibly or probably related to the ART: two (2%) due to efavirenz and 31 (30%) due to the use of stavudine. Both EFV-related events were elevations in transaminase concentrations, one to ACTG grade 2 (2.5 – 5 times the upper limit of normal) and the other to ACTG grade 3 (5 –10 ten times the upper limit of normal). [http://rcc.tech-res.com/safetyandpharmacovigilance/] Both occurred in individuals who were only exposed to 600mg doses of EFV and both occurred during concomitant tuberculosis therapy. Both individuals had EFV levels outside of the 75% interquartile range at the time of the toxicity, namely 9.3 mg/L and 10.8 mg/L respectively. In general, adverse events were evenly distributed across the two groups with 11 individuals (28%) in the 600/600 group and 10 (22%) individuals in the 800/600 group experiencing an adverse event.
Discussion
Although current recommendations suggest that EFV dosage should be increased during concomitant antitubercular therapy, particularly in individuals weighing more than 60kg [25, 26], this remains a point of debate [27]. Current evidence suggests a dose increase may not be necessary in the South African context.[17,19] A comparison of intra-individual mid-dose EFV concentrations in our population revealed that EFV concentrations did not decrease when using standard 600 mg dosing during concomitant rifampicin containing antitubercular therapy across a range of weights. This data complements the recently published data from Cohen et al, which similarly describes no significant difference in EFV concentrations in individuals dosed with 600mg EFV throughout TB treatment in a very similar population, as well as data from Boulle et al which showed no impact on virological outcomes in individuals treated for TB while on a standard 600mg dose of EFV. [17,19]
In addition, data from this study show that EFV concentrations were significantly increased when individuals were exposed to the recommended increased dose of EFV (800mg) during therapy with rifampicin as compared to standard 600mg EFV on completion of TB treatment. Despite these increased concentrations, no particular increase in EFV-related toxicity was noted, although the EFV concentrations in the two individuals experiencing an EFV-related adverse event during concomitant RFN therapy were higher than expected. Although adverse event data was collected throughout the CIPRA study, only ACTG grade 3 and 4 events were captured in the database. It is possible that an increased frequency of minor (ACTG Grade 1 and 2) adverse effects was missed through the data capture process, but it is clear that no serious toxicity EFV-related toxicity was reported. The clinicians were not required to enquire specifically for EFV toxicities. Raised EFV concentrations do however still cause anxiety about potential EFV toxicity, especially if the higher EFV dose is used. [7,18]
Virologic suppression was maintained throughout rifampicin treatment in both the EFV 800mg and EFV 600mg groups, despite a minority of individuals having sub-therapeutic concentrations noted. This is consistent with other recent studies in the same population, where no difference was noted in virological outcome in a large group in individuals commencing EFV-based ART with or without concomitant TB therapy. [17] Adherence to EFV in these sub-therapeutic individuals did not differ from those with therapeutic levels, but adherence as assessed by tablet returns is not a sensitive enough measure to note a missed dose immediately prior to a study visit. One of the strengths of this study is the tight control of visit schedule and monitoring due to being a sub-study of a larger randomised controlled study, reflected in the consistency in post-dose sampling times. [21] Most individuals with TB were retained on the study for the duration of their treatment so the majority who entered the study had a paired sample available for intra-individual analysis as preferred for EFV pharmacokinetic sampling due to large inter-individual variability. [3]
This study has several limitations. Recent data on efavirenz suggests that the pharmacogenetic profile of the cytochrome p450 enzyme system, in particular the 2B6 G526T polymorphism may have a greater impact on the metabolism of EFV than rifampicin. [19, 27,28]. This study could not determine relationships between the 2B6 polymorphisms and EFV concentrations as genetic samples were not available for the majority of individuals. Individuals were not randomised to the study arms, but enrolled sequentially into the 800mg and then the 600mg EFV groups. This may have led to a bias, such as the more ill individuals enrolling early, however we have shown that the baseline clinical data of the groups did not differ (table 1). The use of samples collected at routine clinical visits did not allow us to examine the impact of rifampicin on EFV at time points other than mid-dosing, although mid-dosing concentrations are accepted as the standard pharmacokinetic measure for EFV therapeutic drug monitoring. Our recorded time for EFV dosing was based on subjective patient reporting and was not objectively observed or standardised. We only collected one sample from each individual while taking rifampicin and another sample after rifampicin treatment, thus we could not control for intra-individual variability which may have been a source of error when assessing a single sample without objective dosing times. Furthermore we did not collect data on rifampicin adherence as it was assumed to be uniformly adequate due to daily tuberculosis therapy being directly observed either in the clinic or by community-supporters in South Africa.
The suggestion to increase EFV dose during concomitant TB and HIV therapy has been based on relatively scant data, largely from non-African populations, and data as to the correct dosing remains limited. [11–14, 29] Data from this CIPRA-SA sub-study, together with recent published data from two other South African studies, demonstrate that the 600mg dose of EFV maintains adequate drug-concentrations during concomitant TB therapy, even in those weighing more than 60kg, and that the dose escalation to 800mg is not required in a South African population.
Acknowledgments
The authors would like to acknowledge the valuable support from the staff of the Division of Clinical Pharmacology laboratory at the University of Cape Town where the EFV concentrations were analysed as well as the NHLS laboratory in Johannesburg where study samples were stored.
Thanks go to the Soweto and Masiphumelele clinical teams who managed the patients on the CIPRA-SA project 1 study.
Footnotes
Disclosure statement
Catherine Orrell, Francesca Conradie, Jennifer Pitt, Prudence Ive, Ian Sanne and Robin Wood were all partially funded by CIPRA-SA. Funding for the CIPRA-SA trial was by the Division of AIDS (DAIDS) of the National Institutes of Allergy and Infectious Diseases, the National Institutes of Health, through grant No 1U19AI53217-01 and registered with ClinicalTrials.gov, NCT00255840.
References
- 1.National antiretroviral treatment guidelines. National Department of Health; South Africa: 2004. (Updated April 2004, accessed 27 June 2010) Available from http://hst.org.za/uploads/files/sa_ART_Guidelines1.pdf. [Google Scholar]
- 2.National Department of Health SA. Clinical Guidelines For The Management Of HIV & AIDS In Adults And Adolescents. 2010 (updated April 2010, accessed June 6, 2010). Available from http://www.doh.gov.za/docs/
- 3.Ståhle L, Moberg L, Svensson JO, Sönnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004 Jun;26(3):267–70. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez F, Navarro A, Padilla S, et al. Prediction of neuro-psychiatric adverse events associated with long term efavirenz therapy, using plasma level monitoring. Clin Infect Dis. 2005 Dec 1;41(11):1648–53. doi: 10.1086/497835. [DOI] [PubMed] [Google Scholar]
- 6.Kappelhoff BS, van Leth F, Robinson PA, et al. for the 2NN study group. Are adverse events of nevirapine and efavirenz related to plasma levels? Antivir Ther. 2005;10(4):489–98. [PubMed] [Google Scholar]
- 7.Núñez M, González de Requena D, Gallego L, Jiménez-Nácher I, González-Lahoz J, Soriano V. Higher efavirenz plasma levels correlate with development of insomnia. J Acquir Immune Defic Syndr. 2001 Dec 1;28(4):399–400. doi: 10.1097/00126334-200112010-00015. [DOI] [PubMed] [Google Scholar]
- 8.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 9.National Department of Health SA. South African Tuberculosis Control Programme Practical Guidelines. 2000 (updated 2000, accessed 27 June 2010). Available from http://www.capegateway.gov.za/Text/2003/tb_guidelines2000.pdf.
- 10.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and “unmasking” of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedek I, Wea JAF. Pharmacokinetic interaction between rifampicin and efavirenz in healthy volunteers. [Abstract]. 12th World AIDS Conference; Geneva. 1998. [Google Scholar]
- 12.Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, Alarcon-Gonzalez A, Gomez-Mateos J, Leon-Jimenez E, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–690. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 13.Sustiva Prescribing Information. [accessed 27 June 2010];Bristol-Meyers Squibb Company. 2008 Available from http://packageinserts.bms.com/pi/pi_sustiva.pdf.
- 14.Lewthwaite P, Gibbons S, Vilar F, Khoo S. Efavirenz, rifampicin and rifabutin - a case for therapeutic drug monitoring. [Abstract]. IAS Conference on Pathogenesis and Treatment; Paris. 2003. [Google Scholar]
- 15.Brennan-Benson P, Lyus R, Harrison T, Pakianathan M, Macallan D. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS. 2005;19:1541–1543. doi: 10.1097/01.aids.0000183519.45137.a6. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Kumar P, Ramesh K, Anitha S, et al. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob Agents Chemother. 2009;53:863–868. doi: 10.1128/AAC.00899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boulle A, Van Cutsem G, Cohen K, Hilderbrand K, Mathee S, Abrahams M, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–539. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 18.Pedral-Sampaio DB, Alves CR, Netto EM, Brites C, Oliveira AS, Badaro R. Efficacy and safety of Efavirenz in HIV patients on Rifampin for tuberculosis. Braz J Infect Dis. 2004;8:211–216. doi: 10.1590/s1413-86702004000300004. [DOI] [PubMed] [Google Scholar]
- 19.Cohen K, Grant A, Dandara C, McIlleron H, Pemba L, Fielding K, Charalombous S, Churchyard G, Smith P, Maartens G. Effect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516G>T polymorphism on efavirenz concentrations in adults in South Africa. Antivir Ther. 2009;14(5):687–95. [PMC free article] [PubMed] [Google Scholar]
- 20.Manosuthi W, Kiertiburanakul S, Sungkanuparph S, Ruxrungtham K, Vibhagool A, Rattanasiri S, Thakkinstian A. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS. 2006 Jan 2;20(1):131–2. doi: 10.1097/01.aids.0000196181.18916.9b. [DOI] [PubMed] [Google Scholar]
- 21.Sanne I, Orrell C, Fox MP, et al. for the CIPRA-SA Study Team. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010 Jun 15; doi: 10.1016/S0140-6736(10)60894-X. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Division of AIDS table for grading the severity of adult and pediatric adverse events. Version 1.0. 2004 December; (Clarification August 2009, accessed 25 June 2010). Available from http://rcc.tech-res.com/safetyandpharmacovigilance/
- 23.Ren Y, Nuttall JJ, Egbers C, Eley BS, Meyers TM, Smith PJ, Maartens G, McIlleron HM. High prevalence of subtherapeutic plasma concentrations of efavirenz in children. J Acquir Immune Defic Syndr. 2007;45:133–136. doi: 10.1097/QAI.0b013e31805c9d52. [DOI] [PubMed] [Google Scholar]
- 24.Chi J, Jayewardene AL, Stone JA, Motoya T, Aweeka FT. Simultaneous determination of five HIV protease inhibitors nelfinavir, indinavir, ritonavir, saquinavir and amprenavir in human plasma by LC/MS/MS. J Pharm Biomed Anal. 2002;30:675–684. doi: 10.1016/s0731-7085(02)00357-6. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Managing drug interactions in the treatment of HIV-related tuberculosis. Updated 18 May 2008, accessed 04 January 2010. Available from http://www.cdc.gov/tb/publications/guidelines/HIV_AIDS.htm.
- 26.Robertson SM, Oenzak SR, Pau A. Drug interactions in the management of HIV infection: an update. Expert Opin Pharmacother. 2007;8(17):2947–2963. doi: 10.1517/14656566.8.17.2947. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Kumar P, Ramesh K, Anitha S, Narendran G, Menon P, Gomathi C, Swaminathan S. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob Agents Chemother. 2009 Mar;53(3):863–8. doi: 10.1128/AAC.00899-08. Epub 2009 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uttayamakul S, Likanonsakul S, Manosuthi W, Wichukchinda N, Kalambaheti T, Nakayama EE, Shioda T, Khusmith S. Effects of CYP2B6 G516T polymorphisms on plasma efavirenz and nevirapine levels when co-administered with rifampicin in HIV/TB co-infected Thai adults. AIDS Res Ther. 2010 Mar 26;7:8. doi: 10.1186/1742-6405-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DiGiacinto JL, Chan-Tack KM, Robertson SM, Reynolds KS, Struble KA. Are literature references sufficient for dose recommendations? An FDA case study of efavirenz and rifampin. J Clin Pharmacol. 2008 Apr;48(4):518–23. doi: 10.1177/0091270008315308. Epub 2008 Feb 26. [DOI] [PubMed] [Google Scholar]