Abstract
Rationale
In humans, exposure to environmental contexts previously associated with heroin intake can provoke relapse to drug use. In rats, exposure to heroin-associated contexts after extinction of drug-reinforced responding in different contexts reinstates heroin seeking. This effect is attenuated by blockade of D1-family receptors in lateral or medial accumbens shell, but not accumbens core.
Objectives
In this study, we further characterized the role of striatal D1-family receptors in context-induced reinstatement by assessing the effect of dorsolateral or dorsomedial injections of the D1-family receptor antagonist SCH 23390 on this reinstatement.
Materials and methods
Rats were trained to self-administer heroin (0.05–0.10 mg/kg per infusion) for 12 days; drug infusions were paired with a discrete tone–light cue. Subsequently, heroin-reinforced lever pressing was extinguished in the presence of the discrete cue in a nondrug context. During reinstatement tests under extinction conditions, the D1-family receptor antagonist SCH 23390 (0.3–1.0µg per side) was injected into the dorsolateral or dorsomedial striatum prior to exposure to heroin self-administration context or the nondrug (extinction) context. We then used a disconnection procedure to examine whether D1-family receptors in the dorsolateral striatum and lateral accumbens shell jointly or independently support context-induced reinstatement.
Results
Dorsolateral but not dorsomedial SCH 23390 injections attenuated context-induced reinstatement of heroin seeking. SCH 23390 injections into the dorsolateral striatum of one hemisphere and lateral accumbens shell of the other hemisphere were ineffective.
Conclusions
Results indicate that dorsolateral striatum D1-family dopamine receptors are critical for context-induced reinstatement of heroin seeking. Results also suggest that D1-receptor-mediated dopamine transmission in the dorsolateral striatum and lateral accumbens shell independently support this reinstatement.
Keywords: Conditioned cues, Dopamine, Dorsal striatum, Drug environment, Extinction, Heroin self-administration, Opiates, Reinstatement, SCH 23390, Ventral striatum
Introduction
Environments previously associated with opiate intake can provoke drug relapse during abstinence (O'Brien et al. 1992; Wikler 1973). Despite this evidence, the role of the drug environment (context) in drug relapse in preclinical models has until recently been largely ignored (Crombag et al. 2008). In recent years, we adapted an ABA renewal procedure (Bouton and Bolles 1979) to study the role of the drug environmental context in the reinstatement of drug seeking (Crombag et al. 2002; Crombag and Shaham 2002). In this procedure, rats are first trained to self-administer drugs in one context (A) where drug infusions are paired with explicit discrete drug cues (e.g., light, tone). Drug-reinforced lever responding is then extinguished in the presence of the discrete drug cue in a different (nondrug) context (B). Subsequently, context-induced reinstatement of drug seeking is assessed by re-exposing rats to the drug-associated context (A). During testing, responding on the previously active lever leads to contingent presentations of discrete drug cues, but not the drug. Using variations of this procedure, we and other authors found reliable context-induced reinstatement of cocaine, heroin, alcohol, and nicotine seeking (Bossert et al. 2004; Diergaarde et al. 2008; Fletcher et al. 2008; Fuchs et al. 2005; Hamlin et al. 2008, 2007; Marinelli et al. 2007; Zironi et al. 2006).
In neuropharmacological studies, we identified a role of dopamine and glutamate transmission in the ventral tegmental area (VTA) and nucleus accumbens shell in context-induced reinstatement of heroin seeking: VTA or medial accumbens shell injections of LY379268 (a mGluR2/3 agonist that decreases evoked glutamate release) or medial or lateral accumbens shell (but not accumbens core) injections of the D1-family receptor antagonist SCH 23390 attenuate this reinstatement (Bossert et al. 2004, 2006, 2007). In the present study, we further characterized the role of striatal D1-family receptors in context-induced reinstatement by assessing the effect of dorsolateral or dorsomedial injections of the D1-family receptor antagonist SCH 23390. Our investigation was inspired by the recent findings of Rogers et al. (2008) who reported that reversible inactivation of dorsolateral striatum attenuates discrete cue-and heroin priming-induced reinstatement of heroin seeking. Additionally, reversible inactivation or dopamine receptor blockade of the dorsolateral striatum attenuates context- and discrete cue-induced reinstatement of cocaine seeking (Fuchs et al. 2006; See et al. 2007) and discrete cue-induced cocaine seeking as assessed in the second-order reinforcement schedule (Belin and Everitt 2008; Di Ciano et al. 2008; Vanderschuren et al. 2005).
We found that SCH 23390 injections into the dorsolateral (but not dorsomedial) striatum attenuated context-induced reinstatement of heroin seeking. We previously reported that SCH 23390 injections into the accumbens shell also attenuated this reinstatement (Bossert et al. 2007). Thus, we used an intrastriatal disconnection procedure (see below) to ask whether D1-receptor mediated dopamine transmission in the accumbens lateral shell and dorsal striatum jointly (i.e., via a serial connection) or independently control context-induced reinstatement. This experiment was inspired by studies of Belin and Everitt (2008), Haber et al. (2000), and Ikemoto (2007) that are discussed below.
Belin and Everitt (2008) used an intrastriatal disconnection procedure and a second-order reinforcement schedule to study whether a serial anatomical connection between the accumbens and dorsal striatum mediates cue-induced cocaine seeking. In the disconnection procedure, the role a putative serial neuronal pathway plays in a given behavior is inferred from the observation that lesion (permanent or reversible) or receptor blockade of one brain site in one hemisphere together with lesion/receptor blockade of a second brain site in the contralateral (but not ipsilateral) hemisphere disrupts the behavior of interest (Gaffan et al. 1993; Parkinson et al. 2000; Setlow et al. 2002). Belin and Everitt (2008) reported that unilateral blockade of dopamine receptors in the dorsolateral striatum combined with unilateral excitotoxic lesion in the contralateral accumbens core decreases cocaine seeking. They concluded that their findings support the notion of ventral-to-dorsal striatum “spiraling” connection—the anatomical link between the accumbens and dorsal striatum via descending projections to the VTA and substantia nigra and ascending mesoaccumbens and nigrostriatal dopamine projections (Groenewegen and Russchen 1984; Haber et al. 2000; Heimer et al. 1991; Ikemoto 2007; Nauta et al. 1978; Somogyi et al. 1981; Zahm 2000). The spiral anatomical connectivity typically refers to the following: the posteriomedial VTA projects to the medial accumbens shell which projects back to the posteriomedial VTA and lateral VTA, the lateral VTA projects to the lateral shell and accumbens core, which project back to both lateral VTA and substantia nigra compacta, and the substantia nigra compacta projects to the dorsal striatum (Haber et al. 2000; Ikemoto 2007). Regarding the lateral shell, however, results from anatomical studies indicate that it can also connect with the dorsal striatum without the “spiral” connectivity involvement of the core. Thus, some ventrolateral shell neurons project directly to the substantia nigra compacta (Usuda et al. 1998; Zahm and Heimer 1990), which in turn projects to the dorsal striatum. Additionally, ventrolateral shell neurons project to the ventrolateral portion of the ventral pallidum, which in turn projects to the substantia nigra (Usuda et al. 1998; Zahm and Heimer 1990).
Finally, we examined the effect of dorsolateral injections of MK 212 (a 5-HT2C agonist) on context-induced reinstatement of heroin seeking. Although most commonly used as a dopamine D1-family receptor antagonist, SCH 23390 also acts as an agonist at serotonin 5-HT2C (formerly 5-HT1C) receptors (Briggs et al. 1991; Millan et al. 2001; Taylor et al. 1991). Furthermore, investigation of the receptor mechanisms of SCH 23390 in our experiments is of interest because Fletcher et al. (2008) reported that systemic injections of the 5-HT2C receptor agonist Ro 60-0175 decrease context-induced reinstatement of cocaine seeking.
Materials and methods
Subjects
Male Long-Evans rats (total n = 173; Charles River, Raleigh, NC, USA), weighing 350–450 g, were used. After surgery, the rats were housed individually in the animal facility under a reverse 12-h light–dark cycle (lights off at 9 a.m.). Food and water were freely available in the rats' home cages throughout the experiment. Experimental procedures followed the guidelines of the “Principles of Laboratory Animal Care” (NIH publication no. 86–23, 1996). Fifty-two rats were excluded due to catheter problems (n = 15), failure to learn to self-administer heroin (n = 2), poor health (n = 15), misplaced or blocked cannulae (n = 10), technical problems (n = 2), or failure to meet an extinction criterion of less than 25 responses per 3 h over 3 days after 20 extinction days (n = 8).
Intracranial and intravenous surgery
Rats were anesthetized with sodium pentobarbital and chloral hydrate (60 and 25 mg/kg, i.p., respectively), and permanent guide cannulae (23-gauge; Plastics One, Roanoke, VA, USA) were implanted bilaterally 1 mm dorsal to the dorsolateral striatum or dorsomedial striatum or unilaterally 1 mm dorsal to the dorsolateral striatum and 1 mm dorsal to the lateral accumbens shell in the contralateral hemisphere (experiment 3) using stereotaxic coordinates (Paxinos and Watson 2005) that are based on our previous work (Bossert et al. 2007) and previous reports (Belin and Everitt 2008; Fuchs et al. 2006; Vanderschuren et al. 2005). The coordinates for the lateral accumbens shell were AP +2.0 mm, ML ±2.6 mm, and DV −7.2 mm (4° angle), for the dorsolateral striatum were AP +1.2 mm, ML ±3.3 mm, and DV −4.3 mm (2° angle), and for the dorsomedial striatum were AP +1.2 mm, ML ±2.4 mm, and DV −4.3 mm (10° angle). Following cannulae implantation, silastic catheters were inserted into the jugular vein, as described previously (Shaham et al. 1996; Shalev et al. 2001). The catheters were attached to a modified 22-gauge cannula and mounted to the rats' skulls with dental cement. Buprenorphine (0.1 mg/kg, s.c.) was given after surgery to relieve pain and rats were allowed to recover for 7–10 days before heroin self-administration training. During the recovery and training phases, catheters were flushed every 24–48 h with gentamicin (0.08 mg/mL) and sterile saline.
Intracranial injections
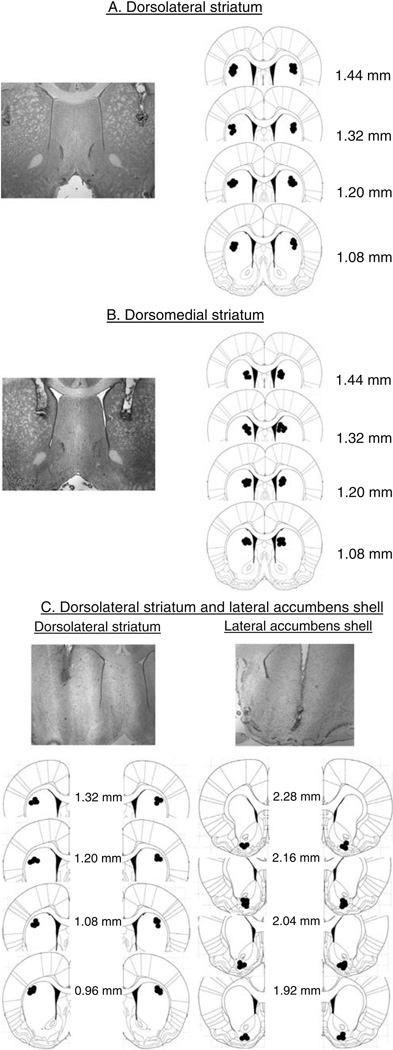
SCH 23390 hydrochloride and MK 212 hydrochloride (Tocris, Ellisville, MO, USA) were dissolved in sterile saline and injected 5–10 min prior to testing. Doses of SCH 23390 hydrochloride (0.3, 0.6, or 1.0µg/0.5µL per side) and MK 212 hydrochloride (0.1µg/0.5µL per side) are based on previous studies (Bossert et al. 2007; Filip and Cunningham 2003; Ramos et al. 2005). Intracranial injections were made using a syringe pump (Harvard Apparatus, Holliston, MA, USA) connected to 10-µL Hamilton syringes that were attached via polyethylene-50 tubing to 30-gauge injectors. SCH 23390, MK 212, and vehicle (saline) injections were made over 1 min and the injectors were left in place for 1 min. After testing, the rats were deeply anesthetized, decapitated, and the brains were removed and stored in formalin. Coronal sections (40–50µm) were sliced on a cryostat and stained with cresyl violet. The brains were then verified for cannulae placement under a microscope. These placements are presented in Fig. 1.
Fig. 1.
Cannulae placement: representative picture of bilateral cannulae and approximate injector placements of injector tips for all rats (adapted with permission from Paxinos and Watson 2005): a dorsolateral striatum, b dorsomedial striatum, and c unilateral dorsolateral striatum and lateral accumbens shell
Apparatus
The rats were trained and tested in standard Med Associates operant conditioning chambers. Each chamber was equipped with two levers located 9 cm above the grid floor. Lever presses on the active retractable lever activated the infusion pump, whereas lever presses on the inactive nonretractable lever had no programmed consequences. The two contexts differed from each other in their auditory, visual, tactile, and circadian (i.e., morning [session onset at 9 a.m.] vs. afternoon [session onset at 3 p.m.] sessions) cues using procedures identical to those described in Bossert et al. (2004, 2006, 2007). The contexts are referred to as A and B whereby A is the heroin self-administration context and B is the extinction context. The physical environments that provided contexts A and B and circadian cues were counterbalanced.
Procedures
The experiments consisted of three phases: self-administration training (12 days), extinction training (15–20 days), and tests for context-induced reinstatement of heroin seeking (2 days). The experimental sequence was context A (training)–context B (extinction)–contexts A and B (testing).
Heroin self-administration training and extinction
Rats were trained to self-administer heroin for 3 h/day for 12 days. Heroin (diacetylmorphine HCl; NIDA) was dissolved in sterile saline and infused at a volume of 65 µL over 2.3 s at a dose of 0.1 mg/kg per infusion (first six sessions) and 0.05 mg/kg per infusion (last six sessions). During training, heroin infusions were earned under a fixed ratio 1 (FR-1), 2.3-s timeout reinforcement schedule and were paired with a compound tone–light cue for 2.3 s. During the extinction phase that occurred in context B, responses on the previously active lever led to the presentation of the tone–light cue, but not heroin. Tests for reinstatement were conducted under extinction conditions (lever presses led to the presentation of the tone–light cue but not heroin) and started after a minimum of 15 daily extinction sessions when the rats met the extinction criterion. The different groups of rats that were subsequently injected intracranially with SCH 23390 or MK 212 were matched for their heroin intake and number of active lever presses during training and for the number of active lever presses during the extinction phase.
Experiment 1: Effect of dorsal striatum SCH 23390 injections on context-induced reinstatement
Eight groups of rats were used. Rats were injected with vehicle or one dose of SCH 23390 (0.3, 0.6, or 1.0µg per side/0.5 µL) into the dorsolateral (four groups; n = 8–12 per group) and dorsomedial (four groups; n = 8–12 per group) striatum. Each rat was tested twice with their assigned dose: once prior to exposure to context A (heroin context) and once prior to exposure to context B (extinction context). The order of testing in contexts A and B was counterbalanced and tests were separated by 48 h (rats remained in an animal housing room between tests).
Experiment 2: Effect of dorsal striatum injections of SCH 23390 on sucrose self-administration
We assessed the effect of dorsal striatum SCH 23390 injections on sucrose self-administration in heroin-experienced rats in order to rule out motor deficits or other nonspecific effects of these injections on reinstatement. Two days after the completion of experiment 1, 14 rats (six dorsolateral and eight dorsomedial) were food restricted to maintain 85–90% of their free-feeding weight (~20 g of food per day). Three days later, sucrose self-administration began and the rats were trained to lever press for 5% oral sucrose solution (FR-1 reinforcement schedule, 8 s timeout, 0.2 ml per reward delivery over 4 s) for 1 h/day as previously described (Bossert et al. 2006, 2007). After 5 days of training, food was available ad libitum and tests with SCH 23390 injections began when rats displayed stable lever presses (<10% variability over 2 days). Using a counterbalanced within-subjects design, we tested the effect of vehicle and SCH 23390 (1.0µg per side/0.5µL) into the dorsolateral and dorsomedial striatum on sucrose self-administration. Injections were given every 48 h and a self-administration session (with no injections) was given between the two test sessions. Vehicle and SCH 23390 were injected bilaterally into the dorsolateral (n = 6) and dorsomedial (n = 8) striatum.
Experiment 3: Effect of SCH 23390 injections into the dorsolateral striatum of one hemisphere and lateral accumbens shell of the other hemisphere
Two groups of rats were used. The vehicle group (n = 12) was injected with saline into the dorsolateral striatum of one hemisphere and lateral accumbens shell of the other hemisphere. The SCH 23390 group (n = 11) was injected with the drug into the dorsolateral striatum of one hemisphere (1.0µg/0.5µL) and lateral accumbens shell (0.6µg/0.5µL) of the other hemisphere. Each rat was tested twice with their assigned dose: once prior to exposure to context A (heroin context) and once prior to exposure to context B (extinction context). The tests were separated by 48 h (rats remained in an animal housing room between tests). The order of testing in contexts A and B was counterbalanced and implantation of cannulae into either left or right hemisphere in the dorsolateral striatum and lateral accumbens shell was counterbalanced.
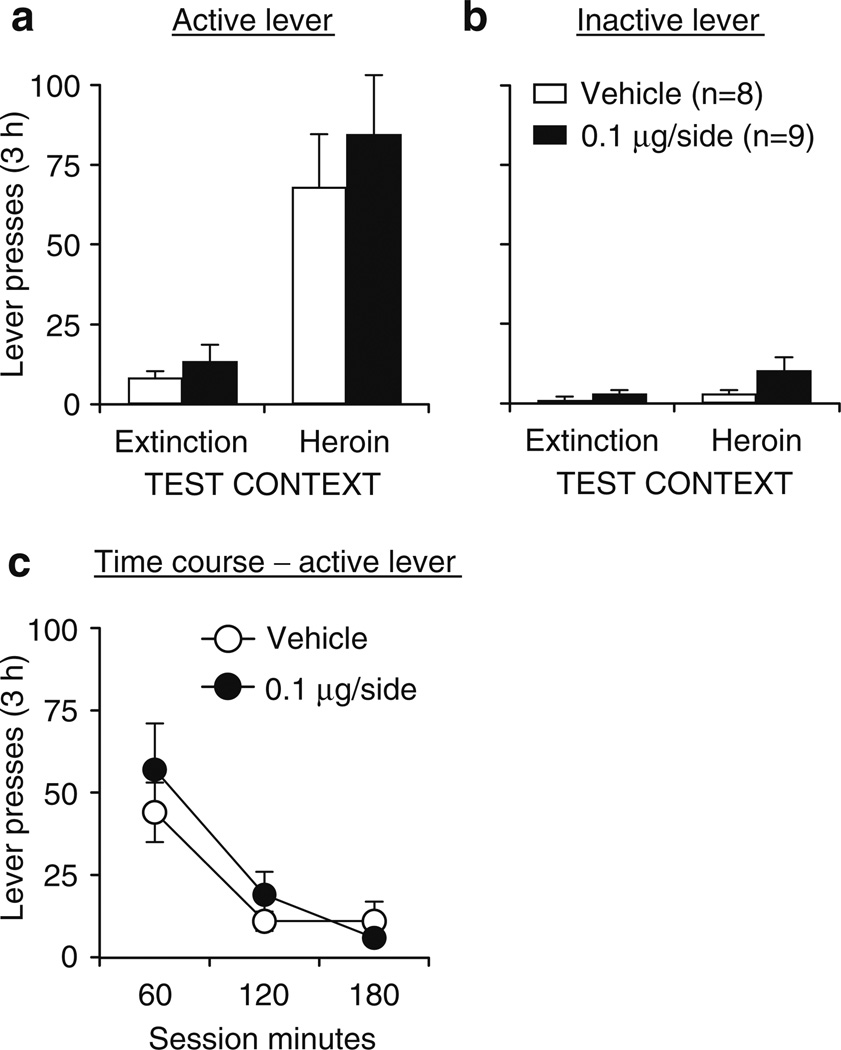
The unilateral dorsolateral striatum and lateral accumbens shell doses of SCH 23390 were 1.0 and 0.6µg, respectively, because these were the most effective doses in these sites after bilateral injections (Fig. 3; Bossert et al. 2007).
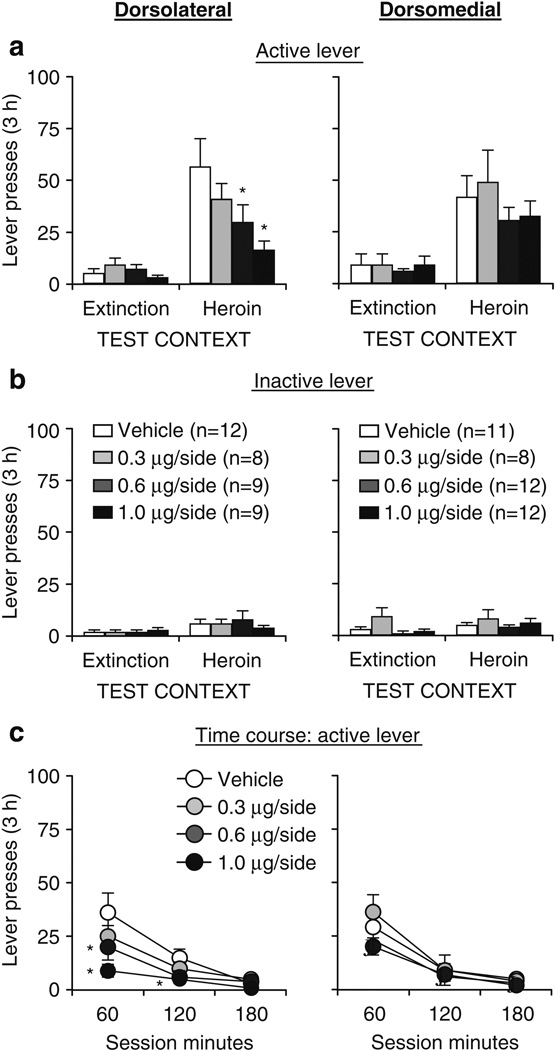
Fig. 3.
Effects of dorsolateral (left panel) and dorsomedial (right panel) striatum SCH 23390 injections on context-induced reinstatement of heroin seeking. a Active lever: mean ± SEM number of presses on the active lever after bilateral injections of vehicle or SCH 23390 prior to exposure to the heroin or the extinction context. b Inactive lever: mean ± SEM number of presses on the inactive lever during testing. c Time course—active lever: mean ± SEM number of presses on the active lever at each hour over the 3-h tests after vehicle or SCH 23390 injections prior to exposure to the heroin context. *p<0.05, different from the vehicle condition
Experiment 4: Effect of dorsal striatum MK 212 injections on context-induced reinstatement
Two groups of rats were used. Rats were injected with vehicle or MK 212 (0.1µg per side/0.5 µL) into the dorsolateral striatum (n = 8–9 per group). Each rat was tested twice with their assigned dose: once prior to exposure to context A (heroin context) and once prior to exposure to context B (extinction context). The order of testing in contexts A and B was counterbalanced and tests were separated by 48 h (rats remained in an animal housing room between tests).
Statistical analyses
Data were analyzed with the statistical program SPSS (GLM procedure) and significant effects (p<0.05) were followed by Fisher's PLSD post hoc tests. Data from experiments 1, 3, and 4 were analyzed for total (non-reinforced) active and inactive lever presses. For the statistical analysis of experiments 1, 3, and 4, the between-subjects factor was SCH 23390 or MK 212 dose and the within-subjects factors were lever (active or inactive) and test context (heroin [A] or extinction [B]). Data for the dorsolateral and dorsomedial striatum were analyzed separately. For the statistical analysis of experiment 2, the within-subjects factors were SCH 23390 dose and lever.
Results
Training and extinction
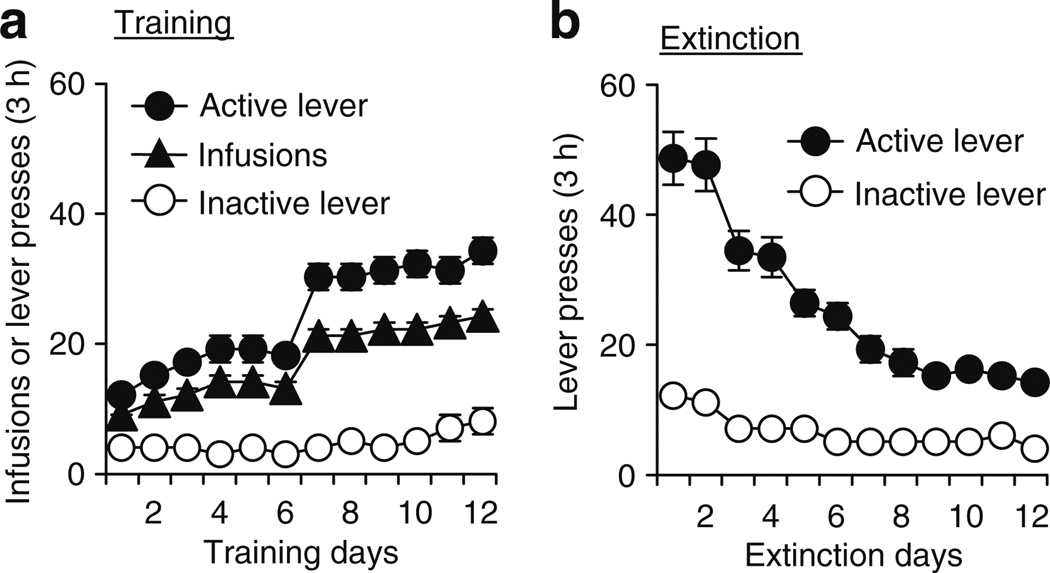
Figure 2a shows the mean ± SEM number of heroin infusions and presses on the active and inactive levers for all rats (dorsolateral and dorsomedial striatum) tested in the reinstatement experiments (experiments 1, 3, and 4). The rats demonstrated reliable heroin self-administration, as indicated by the increase in lever presses when the heroin dose was decreased from 0.1 to 0.05 mg/kg per infusion on training day7 (p<0.01). Figure 2b shows the mean ± SEM number of lever presses on the previously active and inactive levers during the first 12 extinction sessions for these rats. As expected, during the extinction phase, response rates decreased over time (p<0.01).
Fig. 2.
Heroin self-administration training and extinction of the drug-reinforced responding (experiments 1, 3, and 4). a Training: mean ± SEM number of infusions and active and inactive lever responses during the 12 days of heroin self-administration training. b Extinction: mean ± SEM number of presses on the previously active lever and on the inactive lever during the first 12 extinction sessions conducted in the absence of heroin and in a context different from the training context. Data are from all rats in experiments 1, 3, and 4 (n = 121)
Experiment 1: Effect of dorsal striatum injections of SCH 23390 on context-induced reinstatement
SCH 23390 injections into the dorsolateral but not dorsomedial striatum attenuated the reinstatement of active lever presses when rats were exposed to the heroin (training) context after extinction in a different (nondrug) context (Fig. 3). SCH 23390 injections into either brain area had no effect on active lever presses in the extinction context.
Dorsolateral striatum
The statistical analysis (n = 8–12 per dose) revealed significant effects of test context (F1,34 = 64.5, p<0.01), lever (F1,34 = 35.9, p<0.01), SCH 23390 dose by test context (F3,34 = 5.0, p<0.01), lever by test context (F1,34 = 38.9, p<0.01), and SCH 23390 dose by lever by test context (F3,34 = 4.4, p<0.01; Fig. 3a left panel). For inactive lever presses, the statistical analyses revealed a significant effect only for test context (F1,34 = 10.9, p<0.01); no other effects were significant (Fig. 3b). Analysis of the time course of active lever presses in the heroin context revealed significant effects of session time (F2,68 = 31.4, p<0.01), SCH 23390 dose (F3,34 = 3.4, p<0.05), and session time by SCH 23390 dose (F6,68 = 2.5, p<0.05; Fig. 3c).
Dorsomedial striatum
The statistical analysis (n = 8–12 per dose) revealed significant effects of test context (F1,39 = 59.4, p<0.01), lever (F1,39 = 41.5, p<0.01), and lever by test context (F1,39 = 43.8, p<0.01), but not of SCH 23390 dose or interaction between SCH 23390 dose and the other factors (p>0.05; Fig. 3a right panel). For inactive lever presses, the statistical analyses revealed a significant effect only for test context (F1,39 = 5.6, p<0.01); no other effects were significant (Fig. 3b). Analysis of the time course of active lever presses in the heroin context revealed significant effects of session time (F2,78 = 74.0, p<0.01), but not of SCH 23390 dose or session time by SCH 23390 dose (p>0.05; Fig. 3c).
Experiment 2: Effect of dorsolateral and dorsomedial striatum SCH 23390 injections on sucrose self-administration
SCH 23390 injections (1.0µg per side/0.5 µL) into the dorsolateral or dorsomedial striatum had no effect on sucrose self-administration (p>0.05). The mean ± SEM number of responses on the active lever for rats injected with vehicle or SCH 23390 into the dorsolateral striatum was 103±12 and 114±9, respectively. The mean ± SEM number of responses on the active lever for rats injected with vehicle or SCH 23390 into the dorsomedial striatum was 140±14 and 133±19, respectively.
Experiment 3: Effect of SCH 23390 injections into dorsolateral striatum of one hemisphere and lateral accumbens shell of the other hemisphere
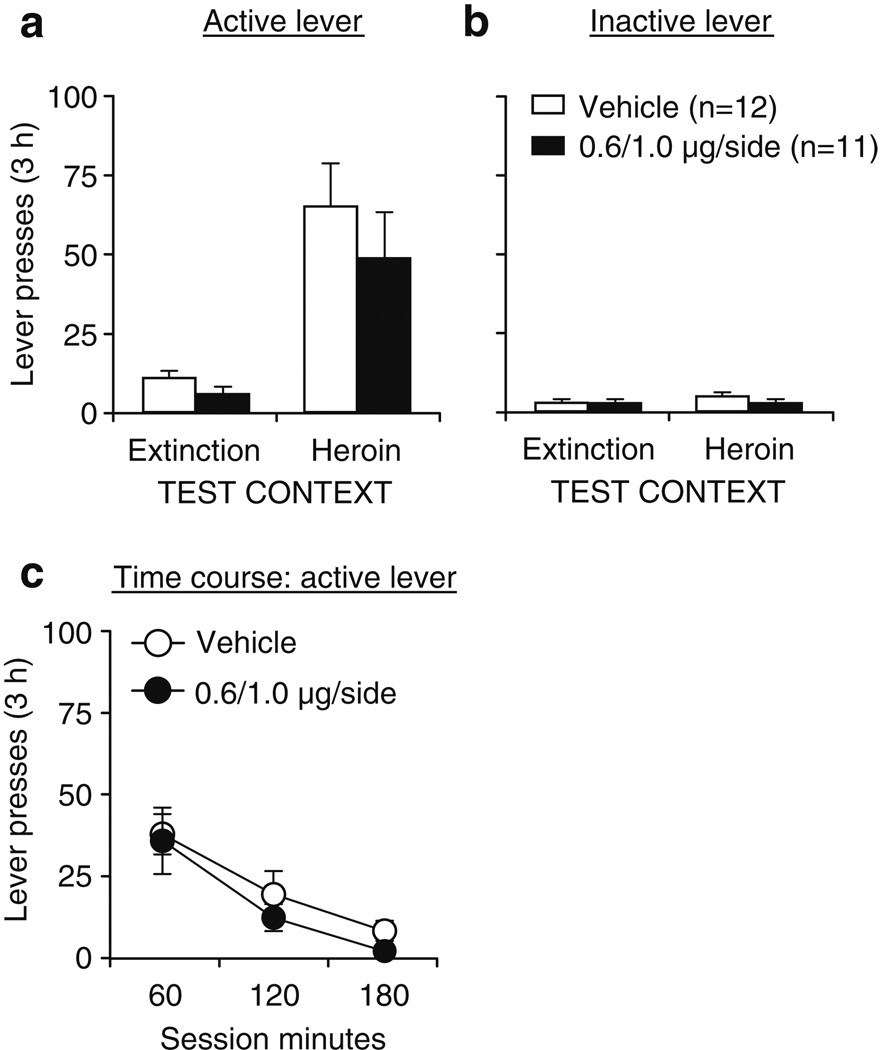
Unilateral SCH 23390 injections into the dorsolateral striatum (1.0µg/0.5µL) contralateral to unilateral SCH 23390 injections into the lateral accumbens shell (0.6µg/0.5µL) had no effect on reinstatement of active lever presses when rats were exposed to the heroin (training) context after extinction in a different (nondrug) context (Fig. 4a). The statistical analysis (n = 11–12 per dose) revealed significant effects of test context (F1,21 = 40.0, p<0.01), lever (F1,21 = 28.5, p<0.01), and lever by test context (F1,21 = 28.1, p<0.01), but not of SCH 23390 dose or interactions between SCH 23390 dose and the other factors (p>0.05). There were no significant effects for inactive lever presses (Fig. 4b). Analysis of the time course of active lever presses in the heroin context revealed significant effects of session time (F2,42 = 30.0, p<0.01), but not of SCH 23390 dose or session time by SCH 23390 dose (p>0.05; Fig. 4c).
Fig. 4.
Effect of SCH 23390 injections into the dorsolateral striatum of one hemisphere and lateral accumbens shell of the other hemisphere on context-induced reinstatement of heroin seeking. a Active lever: mean ± SEM number of presses on the active lever after vehicle or SCH 23390 injections into the dorsolateral striatum of one hemisphere and lateral accumbens shell of the other hemisphere prior to exposure to the heroin or the extinction context. b Inactive lever: mean ± SEM number of presses on the inactive lever during testing. c Time course—active lever: mean ± SEM number of presses on the active lever at each hour over the 3-h tests after vehicle or SCH 23390 injections prior to exposure to the heroin context
Experiment 4: Effect of dorsolateral striatum injections of MK 212 on context-induced reinstatement
MK 212 injections into the dorsolateral striatum (0.1µg/0.5µL) had no effect on reinstatement of active lever presses when rats were exposed to the heroin (training) context after extinction in a different (nondrug) context (Fig. 5a). The statistical analysis (n = 8–9 per dose) revealed significant effects of test context (F1,15 = 40.6, p<0.01), lever (F1,15 = 28.8, p<0.01), and lever by test context (F1,15 = 27.0, p<0.01), but not of MK 212 dose or interactions between MK 212 dose and the other factors(p>0.05). There were no significant group effects for inactive lever presses (Fig. 5b). Analysis of the time course of active lever presses in the heroin context revealed significant effects of session time (F2,30 = 25.7, p<0.01), but not of MK 212 dose or session time by MK 212 dose (p>0.05; Fig. 5c).
Fig. 5.
Effect of MK 212 injections into the dorsolateral striatum on context-induced reinstatement of heroin seeking. a Active lever: mean ± SEM number of presses on the active lever after vehicle or MK 212 injections into the dorsolateral striatum prior to exposure to the heroin or the extinction context. b Inactive lever: mean ± SEM number of presses on the inactive lever during testing. c Time course—active lever: mean ± SEM number of presses on the active lever at each hour over the 3-h tests after vehicle or MK 212 injections prior to exposure to the heroin context
Discussion
We found that blockade of dorsolateral but not dorsomedial striatum D1-family receptors with SCH 23390 attenuated context-induced reinstatement of heroin seeking. This finding indicates a critical role of D1-receptor-mediated dopamine transmission in the dorsolateral striatum in this reinstatement. Additionally, using an intrastriatal disconnection procedure (Belin and Everitt 2008), we found that SCH 23390 injections into the dorsolateral striatum of one hemisphere and lateral accumbens shell of the other hemisphere had no effect on context-induced reinstatement. This finding suggests that D1-receptor-mediated dopamine transmission in the dorsolateral striatum and lateral accumbens shell independently control this reinstatement. Finally, we found that dorsolateral striatum injections of the 5-HT2C receptor agonist MK 212 had no effect on context-induced reinstatement of heroin seeking. This finding suggests that, under our experimental conditions, SCH 23390 behavioral effects are not mediated by 5-HT2C receptors.
Methodological considerations
There are several methodological considerations that should be taken into account in the interpretation of the present data. First, the dopamine projection from the substantia nigra to the dorsal striatum plays an important role in motor performance (Ungerstedt 1968). Thus, the effects of dorsolateral striatum SCH 23390 injections on context-induced reinstatement may be due to motor deficits. However, this possibility is unlikely because, in heroin-experienced rats, the highest SCH 23390 dose (1.0µg per side) had no effect on high rates of lever presses for oral sucrose.
Second, results from studies in which SCH 23390 is injected intracranially to identify a role of a specific brain site in behavior should be interpreted with caution because SCH 23390 is a lipophilic drug that rapidly diffuses away from the injection site (Caine et al. 1995). However, the fact that dorsomedial striatum SCH 23390 injections (~0.9–1.0 mm medial to dorsolateral striatum) were ineffective suggests that the drug effect on context-induced reinstatement is mediated by D1-family receptors in dorsolateral striatum.
Third, a potential methodological issue in experiment 4 is the use of a single dose of MK 212. However, the dose we used (0.1µg per side) is similar or 20 times higher than the MK 212 doses found effective in reversing cocaine- or MDMA-induced locomotion after prefrontal cortex injections, respectively (Filip and Cunningham 2003; Ramos et al. 2005). Thus, while the results of our single-dose experiment do not rule out a potential role of 5-HT2C receptors in the dorsal striatum in context-induced reinstatement, these results indicates that it is unlikely that the effect of SCH 23390 on this reinstatement is due to its effect on 5-HT2C receptors.
Finally, the negative results in experiment 3 (intrastriatal disconnection) should be interpreted with caution because accumbens shell SCH 23390 injections were localized to the lateral shell, leaving the medial shell in both hemispheres intact and thus able to support behavior. As mentioned before, either medial or lateral accumbens shell SCH 23390 injections block context-induced reinstatement (Bossert et al. 2007). Thus, while our data suggest that D1-receptor-mediated dopamine transmission in the dorsolateral striatum and accumbens shell independently supports context-induced reinstatement, our negative findings could reflect incomplete D1-family receptor blockade in the accumbens shell.
Does D1-receptor-mediated dopamine transmission in the dorsolateral striatum and lateral accumbens shell independently or jointly support context-induced reinstatement?
We found that blockade of dopamine D1-family receptors in either dorsolateral striatum (Fig. 3) or medial or lateral accumbens shell (Bossert et al. 2007) attenuates context-induced reinstatement of heroin seeking. Based on these data, we used an intrastriatal disconnection procedure (Belin and Everitt 2008) to study whether D1-receptor-mediated dopamine transmission in dorsolateral striatum and accumbens shell is part of a serial connection that supports context-induced reinstatement. As mentioned in the introduction, this was inspired by previous work on the ventral-to-dorsal spiraling connection—the anatomical link between the accumbens and dorsal striatum via descending projections to midbrain dopamine neurons of the VTA and substantia nigra and ascending mesoaccumbens and nigrostriatal dopamine projections (Haber et al. 2000; Ikemoto 2007; Nauta et al. 1978; Somogyi et al. 1981). Based on this anatomical connectivity, we assessed the role of D1-receptor-mediated dopamine transmission in the lateral accumbens shell because, from a spiral connectivity perspective, it is more proximal to the dorsal striatum. We found that unilateral injections of SCH 23390 into the dorsolateral striatum combined with unilateral SCH 23390 injections into the contralateral accumbens lateral shell were ineffective (Fig. 4). Based on these negative findings, we did not assess the effect of ipsilateral SCH 23390 injections into these two brain areas. As most neuronal pathways are ipsilateral, lesioning or blocking receptors in ipsilateral brain areas should not affect behavior because the contralateral side is intact and compensates for the ipsilateral loss. Thus, ipsilateral manipulations are a critical control condition only when the contralateral disconnection manipulation is effective (Setlow et al. 2002).
Our results do not support the hypothesis that ventral-to-dorsal striatum neuronal connectivity is critical for the normal expression of context-induced reinstatement heroin seeking. However, this conclusion should only be tentatively accepted for several reasons. First, as mentioned above, in our intrastriatal disconnection procedure, D1-receptor-mediated dopamine function remains intact in the medial accumbens shell, which is also critical for context-induced reinstatement (Bossert et al. 2007). However, from a spiral connectivity perspective, it is unlikely that different results would have been obtained had we injected SCH 23390 into the medial accumbens shell because the spiral connections from the medial shell to the dorsal striatum pass through the accumbens lateral shell and core (Haber et al. 2000; Ikemoto 2007). Second, it is possible that the ventral-to-dorsal striatum neuronal connectivity in context-induced reinstatement occurs via activation of D2-family receptors, a contribution to dopamine neurotransmission that we did not assess in either accumbens shell or dorsal striatum. This is an empirical question for future research because we previously found that systemic injections of the D2-family receptor raclopride is as effective as SCH 23990 in attenuating context-induced reinstatement of cocaine seeking (Crombag et al. 2002).
Conclusions
We found that blockade of D1-family receptors in the dorsolateral striatum attenuated context-induced reinstatement of heroin seeking. This finding extends previous reports on the important role of the dorsolateral striatum in discrete cue- and heroin priming-induced reinstatement (Rogers et al. 2008), context- and discrete cue-induced reinstatement of cocaine seeking (Fuchs et al. 2006; See et al. 2007), and discrete cue-induced cocaine seeking as assessed in the second-order reinforcement schedule (Belin and Everitt 2008; Di Ciano et al. 2008; Vanderschuren et al. 2005). Our findings and these previous findings parallel those from studies in humans demonstrating that cue-induced heroin or cocaine craving is associated with increased dorsal striatum dopamine release (Volkow et al. 2006; Zijlstra et al. 2008) and neuronal activity in this brain region (Garavan et al. 2000). This concordance across drug classes in humans and rats supports the view that dopamine transmission in the dorsal striatum is critically involved in drug craving and relapse (Belin et al. 2009; Everitt and Robbins 2005; White 1996). We also found that unilateral injections of SCH 23390 into the dorsolateral striatum combined with unilateral SCH 23390 injections into the contralateral accumbens shell had no effect on context-induced reinstatement. As discussed above, despite several potential issues in interpreting these negative findings, these findings suggest that D1-receptor-mediated dopamine transmission in the accumbens shell and dorsolateral striatum independently control this reinstatement.
Acknowledgments
The work was supported by the Intramural Research Program of the National Institute on Drug Abuse. We thank Makeda Carroll for the technical assistance and Drs. Geoff Schoenbaum, Satoshi Ikemoto, and Stefanie Geisler for the excellent comments regarding data interpretation and neuroanatomy.
References
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979;10:445–466. [Google Scholar]
- Briggs CA, Pollock NJ, Frail DE, Paxson CL, Rakowski RF, Kang CH, Kebabian JW. Activation of the 5-HT1C receptor expressed in Xenopus oocytes by the benzazepines SCH 23390 and SKF 38393. Br J Pharmacol. 1991;104:1038–1044. doi: 10.1111/j.1476-5381.1991.tb12546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Crombag H, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1007–1016. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Crombag H, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, de Vries W, Raaso H, Schoffelmeer AN, De Vries TJ. Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist rimonabant (SR141716A) Neuropharmacology. 2008;55:712–716. doi: 10.1016/j.neuropharm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Filip M, Cunningham KA. Hyperlocomotive and discriminative stimulus effects of cocaine are under the control of serotonin(2C) (5-HT(2C)) receptors in rat prefrontal cortex. J Pharmacol Exp Ther. 2003;306:734–743. doi: 10.1124/jpet.102.045716. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT(2C) receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate–putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffan D, Murray EA, Fabre-Thorpe M. Interaction of the amygdala with the frontal lobe in reward memory. Eur J Neurosci. 1993;5:968–975. doi: 10.1111/j.1460-9568.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Li Z, Le AD. Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci. 2007;26:2815–2823. doi: 10.1111/j.1460-9568.2007.05898.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Newman-Tancredi A, Quentric Y, Cussac D. The “selective” dopamine D1 receptor antagonist, SCH23390, is a potent and high efficacy agonist at cloned human serotonin2C receptors. Psychopharmacology (Berl) 2001;156:58–62. doi: 10.1007/s002130100742. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, Mclellan TA, Ehrman R. Classical conditioning in drug dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical–ventral striatopallidal systems. Behav Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th edn. San Diego: Elsevier Academic; 2005. [Google Scholar]
- Ramos M, Goni-Allo B, Aguirre N. Administration of SCH 23390 into the medial prefrontal cortex blocks the expression of MDMA-induced behavioral sensitization in rats: an effect mediated by 5-HT2C receptor stimulation and not by D1 receptor blockade. Neuropsychopharmacology. 2005;30:2180–2191. doi: 10.1038/sj.npp.1300735. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned responses. Behav Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking under opioid maintenance: the effects of opioid withdrawal, heroin priming and stress. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Bolam JP, Totterdell S, Smith AD. Monosynaptic input from the nucleus accumbens–ventral striatum region to retrogradely labelled nigrostriatal neurones. Brain Res. 1981;217:245–263. doi: 10.1016/0006-8993(81)90002-0. [DOI] [PubMed] [Google Scholar]
- Taylor LA, Tedford CE, McQuade RD. The binding of SCH 39166 and SCH23390 to 5-HT1C receptors in porcine choroid plexus. Life Sci. 1991;49:1505–1511. doi: 10.1016/0024-3205(91)90051-c. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8870. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: multiple partial actions on memory systems. Addiction. 1996;91:921–949. discussion 951–965. [PubMed] [Google Scholar]
- Wikler A. Dynamics of drug dependence, implication of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–446. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Booij J, van den Brink W, Franken IH. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 2008;18:262–270. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]