Abstract
Adiponectin is an adipokine secreted by differentiated adipocytes. Clinical studies suggest a negative correlation between oxidative stress and adiponectin levels in patients with metabolic syndrome or cardiovascular disease. Natural compounds that can prevent oxidative stress mediated inhibition of adiponectin may be potentially therapeutic. Boldine, an aporphine alkaloid abundant in the medicinal plant Peumus boldus, is a powerful antioxidant. The current study demonstrates the effects of boldine on the expression of adiponectin and its regulators, CCAAT/enhancer binding protein-α (C/EBPα) and peroxisome proliferator-activated receptor (PPAR)-γ, in 3T3-L1 cells. Differentiated 3T3-L1 adipocytes were exposed to either hydrogen peroxide (H2O2) (100 μM) or tumor necrosis factor-α (TNFα) (1 ng/mL) for 24 hours in the presence or absence of increasing concentrations of boldine (5–100 μM). Quantitative polymerase chain reaction showed that both the oxidants decreased the mRNA levels of adiponectin, PPARγ, and C/EBPα to half of the control levels. Boldine, at all concentrations, counteracted the inhibitory effect of H2O2 or TNFα and increased the expression of adiponectin and its regulators. The effect of boldine on adiponectin expression was biphasic, with the lower concentrations (5–25 μM) having a larger inductive effect compared to higher concentrations (50–100 μM). Boldine treatment alone in the absence of H2O2 or TNFα was also able to induce adiponectin at the inductive phase of adipogenesis. Peroxisome proliferator response element-luciferase promoter transactivity analysis showed that boldine interacts with the PPAR response element and could potentially modulate PPAR responsive genes. Our results indicate that boldine is able to modulate the expression of adiponectin and its regulators in 3T3-L1 cells and has the potential to be beneficial in obesity-related cardiovascular disease.
Key Words: adipogenesis, antioxidant, oxidative stress, peroxisome proliferator-activated receptors
Introduction
The adipokine, adiponectin, is up-regulated during the differentiation of preadipocytes into mature adipocytes.1,2 The plasma levels of adiponectin in humans are normally around 30 μg/mL. Even though its exact mechanism of action is not clear, adiponectin plays an important role in modulating lipid metabolism and insulin sensitivity.3,4 Serum adiponectin levels are reduced in both patients with type 2 diabetes and cardiovascular disease.5,6 There is an inverse correlation between obesity and adiponectin.1,7 It is now increasingly recognized that adiponectin is both a potential biomarker for the metabolic syndrome and a possible therapeutic target for the treatment of cardiovascular disease.8–10
Adipocyte differentiation is a highly controlled process. The adipogenic transcription factors, peroxisome proliferator-activated receptor (PPAR)-γ and CCAAT/enhancer binding protein-α (C/EBPα) play a key role in the complex transcriptional cascade that occurs during adipocyte differentiation.11 There is a direct association between the transcriptional activation of genes with the peroxisome proliferator response element (PPRE) and induction of adiponectin gene expression.12,13 The human adiponectin promoter also expresses the putative response elements for C/EBPα.14,15 PPARγ agonists increase adiponectin levels in 3T3-L1 cells, rodents, and humans,16,17 and antidiabetic agents such as thiazolinediones increase the ratio of high-molecular-weight to low-molecular-weight forms of adiponectin.18 PPARγ agonists and adiponectin both increase insulin sensitivity and ameliorate atherosclerosis.6,19
Both inflammation and oxidative stress play an important role during obesity.20,21 Obesity results in increased macrophage infiltration and synthesis of inflammatory markers such as interleukin-6, tumor necrosis factor-α (TNFα), and C-reactive protein by adipose tissue.13,21 Adiponectin has anti-inflammatory activity,22,23 although a recent study indicated that adiponectin might be pro-inflammatory in classic inflammatory diseases.24 Accumulation of lipids in 3T3-L1 cells or adipocytes isolated from mice results in increased oxidative stress and reduced adiponectin expression.25,26 Some of the cardioprotective effects of adiponectin have been attributed to its reduction of oxidative stress.27,28 Any compounds that can increase adiponectin levels and decrease oxidative stress have potential therapeutic properties.29,30
Boldine is an aporphine alkaloid present in the leaves and bark of Boldo (Peumus boldus Mol.), an evergreen shrub native to Chile. It grows in Peru, Brazil, Paraguay, and Argentina and has been introduced to Europe and North America.31,32 Boldo extracts have been used for the treatment of headache, earache, rheumatism, “nervous weakness,” dyspepsia, menstrual pain, and urinary tract inflammation.33 From the pharmacological viewpoint, it is boldine that has attracted the most attention among the many other aporphine-like alkaloids identified from Boldo. In the past 20 years, research has shown that boldine has potent antioxidant properties in biological systems undergoing peroxidative free radical-mediated damage.34–36 In addition, boldine protects enzymes susceptible to peroxidative inactivation such as lysozyme37 and monooxygenases.38 Its mechanism of action has been attributed to the ability of boldine to scavenge free radicals, especially hydroxyl radicals.31,36,39,40 Boldine has anti-inflammatory and antidiabetic properties in animal studies.41–44 Our earlier studies showed that boldine had both antioxidant and anti-atherosclerotic properties in low-density lipoprotein receptor knockout mice.45 The aim of the current study was to compare boldine to other known antioxidants (N-acetyl-l-cysteine [NAC] and α-tocopherol) on their effects on adiponectin and its early regulators (C/EBPα and PPARγ) in 3T3-L1 cells exposed to inflammatory stress (TNFα) or oxidative stress (hydrogen peroxide [H2O2]).
Materials and Methods
Materials
3T3-L1 cells (mouse embryonic fibroblasts–adipose-like cell line) (catalog number CL-173) and Dulbecco's modified Eagle's medium (DMEM) were purchased from American Type Culture Collection (Rockville, MD, USA). 3-Isobutyl-1-methylxanthine, dexamethasone, insulin, α-tocopherol, NAC, Fat Red O, boldine, TNFα, and H2O2 of the highest grade were purchased from Sigma (St. Louis, MO, USA). Fetal bovine serum (FBS) was obtained from HyClone (Logan, UT, USA). Primers for adiponectin, PPARγ, and C/EBPα were obtained from Invitrogen (Carlsbad, CA, USA).
Cell culture
3T3-L1 cells were grown in T75 cell culture flasks. The following cell culture media were used: Basal Medium (BM) (DMEM + 10% FBS), Induction Medium (IM) (DMEM + 10% FBS + 0.5 mM 3-isobutyl-1-methylxanthine +10 μg/mL insulin + 1 μM dexamethasone), and Maintenance Medium (MM) (DMEM + 10% FBS + 10 μg/mL insulin). Cells were grown to 100% confluence in BM. Two days after full confluence, the cells were transferred to IM for 2 days followed by MM for 2 days. The cells were transferred back to BM and grown for 10 days with regular medium change every 2 days. At the end of 10 days in BM, the 3T3-L1 cells become fully differentiated as confirmed by the change in morphology (increased lipid-loaded cells compared to fibroblast-like morphology of preadipocytes) and Fat Red O staining of the lipid droplets.46
Cell treatment
Effect of antioxidants on adiponectin gene expression exposed to an oxidative stress (H2O2) or inflammatory cytokine (TNFα). In order to investigate if known antioxidants (α-tocopherol or NAC) and boldine prevent the decrease in adiponectin gene expression by oxidative or inflammatory stress, fully differentiated 3T3-L1 cells after 10 days in BM were washed and treated with 0–25 μM antioxidants (α-tocopherol, NAC, or boldine) in the presence or absence of 100 μM H2O2 or 1 ng/mL TNFα for 24 hours. All the antioxidants were suspended in 100% ethanol. The final concentration of the ethanol was kept to a minimum to avoid any potential cytotoxicity. Vehicle controls were run simultaneously. At the end of the treatment, the cell supernatant was collected for Western blotting of secreted adiponectin, and the cells were collected in TRI Reagent® (Sigma, St. Louis, MO, USA) for mRNA isolation. Three independent experiments were performed with separate batches of 3T3-L1 cells.
Concentration effect of boldine on adiponectin gene expression
Fully differentiated 3T3-L1 cells were treated with either H2O2 (100 μM) or TNFα (1 ng/mL) in the presence or absence of increasing concentrations of boldine (5–100 μM) or increasing concentrations of boldine (5–100 μM) alone without the oxidants for 24 hours. At the end of incubation, the cell supernatant was collected for Western blotting of the secreted adiponectin, and the cells were transferred to TRI Reagent for mRNA isolation. The gene expression of adiponectin and its transcriptional regulators, C/EBPα and PPARγ, were determined using real-time quantitative polymerase chain reaction (RT-qPCR).
Effect of boldine on different time points during the adipocyte differentiation
Control experiments were also performed to investigate when boldine exerts its beneficial effect during the adipocyte differentiation process. To demonstrate this, 3T3-L1 preadipocytes were treated with 10 μM boldine at different phases of the adipogenesis cascade, i.e., 0 day, 2 days (induction phase in IM), 4 days (maintenance phase in MM), and 6 days (post-differentiation phase in BM). The mRNA was isolated from the cells that were collected at the end of the various time points. The gene expression of adiponectin was determined using RT-qPCR. Three independent experiments were performed with separate batches of 3T3-L1 cells.
RT-qPCR
RNA was isolated using TRI Reagent according to the manufacturer's protocol (Sigma). RNA concentrations were determined using the NanoDrop (Nanodrop Technologies Inc., Thermo Scientific, Wilmington, DE, USA). Total RNA (1 μg) was reverse-transcribed using an iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Aliquots of cDNA were applied to RT-qPCR. RT-qPCR was performed by using iQ SYBR™ (Molecular Probes, Eugene, OR, USA) Green Supermix (Bio-Rad). The primers for adiponectin were as follows: sense 5′-GCA GAG ATG GCA CTC CTG GA-3′ and antisense 5′-CCC TTC AGC TCC TGT CAT TCC-3′. The other primers used in this study were as follows: PPARγ, sense 5′-GTC TCA CAA TGC CAT CAG GTT-3′ and antisense 5′-TGA TTT GTC CGT TGT CTT TCC-3′; C/EBPα, sense 5′-GGG TGA GTT CAT GGA GAA TGG-3′ and antisense 5′-CAG TTT GGC AAG AAT CAG AGC A-3′. β-Actin was used as a housekeeping gene: sense 5′-CTA CCT CAT GAA GAT CCT CAG CGA-3′ and antisense 5′-TTC TCC TTA ATG TCA CGC ACG ATT-3′. RT-qPCR was performed in the Bio-Rad iQ™5 instrument. All samples were run in triplicates. Results were calculated using the method of Pfaffl (2−ΔΔCt)47 and expressed as differences in fold change ± SEM in the experimental gene in antioxidant-treated cells compared to untreated controls.
Western blotting of adiponectin protein
The cell supernatant at the end of each experiment was collected and lyophilized (Labconco, Kansas City, MO, USA) for Western blotting of secreted adiponectin. The lyophilized samples were suspended in RIPA buffer (50 mM Tris, 150 mM sodium chloride, 1% Triton, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 5 mM EDTA) containing protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). Lyophilized protein (50 μg) from each sample was electrophoresed on 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After Western blotting the adiponectin protein was detected using anti-mouse adiponectin antibody (R&D Systems, Minneapolis, MN, USA) (0.1 μg/mL) and anti-goat immunoglobulin G secondary antibody (diluted 1:8,000). A 30-kDa adiponectin protein band was detected using the chemiluminescence kit from Amersham (Piscataway, NJ, USA) in an EC3 Bio-Imaging System (UVP, Upland, CA, USA). The intensity of the bands was determined using densitometry and expressed as a percentage of control ± SEM.
PPRE-luciferase reporter assay
In order to investigate if the effect of boldine on adiponectin expression is due to its interaction with PPAR or its response element, a PPRE-luciferase reporter activation assay was performed in 3T3-L1 preadipocytes. 3T3-L1 cells were cultured in DMEM with 10% FBS and subcultured into six-well plates for 24 hours prior to transfection. Cells were transfected with PPRE-luciferase reporter constructs ([PPRE]3-TK-Luc) using the Fugene® 6 method (Roche) according to the manufacturer's protocols. Twenty-four hours after transfection, the transfected 3T3-L1 cells were exposed to treatment with either boldine (10 μM) alone or boldine (10 μM) along with 1 μM 15-PGJ2 (a PPARγ agonist). Control experiments were also run with only 1 μM 15-PGJ2 treatment. Luciferase activity was determined 24 hours after treatment using the Promega (Madison, WI, USA) single luciferase assay kit following the manufacturer's protocol. The changes in relative chemiluminesence were measured using a Berthold luminometer (Berthold Technologies, Bad Wildbad, Germany). The results were expressed as relative luminescence units ± SEM. Five independent experiments were performed with separate batches of 3T3-L1 cells.
Statistics
For the RT-qPCR analysis, statistics were performed at the level of ΔCt, in order to exclude potential bias due to averaging of data transformed through the Pfaffl equation 2−ΔΔCt. All data are presented as fold change, percentage of control, or relative luminescence units ± SEM obtained from three independent sets of experiments. The statistical significance was determined using Student's t test. A probability value of P < .05 was considered to be statistically significant.
Results
Effect of oxidative stress (100 μM H2O2) and antioxidants on expression of adiponectin and its transcription regulators PPARγ and C/EBPα in 3T3-L1 cells
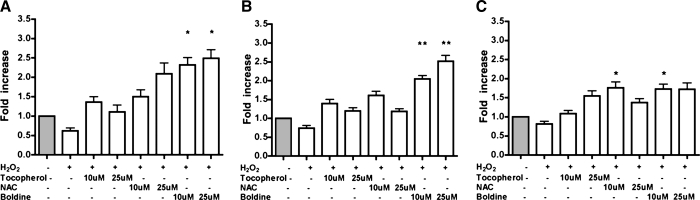
Fully differentiated 3T3-L1 cells were treated with 100 μM H2O2 alone or with H2O2 with α-tocopherol (10–25 μM), NAC (10–25 μM), or boldine (10–25 μM) for 24 hours. As shown in Figure 1A, H2O2 treatment suppressed adiponectin gene expression to half of the control levels. All the tested antioxidants protected adiponectin expression from oxidative stress. At similar concentrations, boldine had the highest potency in increasing adiponectin expression (P ≤ .019 at 10 μM, P ≤ .022 at 25 μM).
FIG. 1.
Effect of H2O2 and antioxidants on mRNA levels of (A) adiponectin and its transcription regulators, (B) PPARγ and (C) C/EBPα. Differentiated 3T3-L1 adipocytes were treated with H2O2 (100 μM) in the absence or presence of various antioxidants (α-tocopherol, NAC, and boldine) at 5–25 μM for 24 hours. After treatments, RT-qPCR was performed on isolated mRNA. The results are expressed as differences in fold change in antioxidant-treated cells compared to vehicle controls. Data are mean ± SEM values for three independent experiments performed in triplicate. *P ≤ .05, **P ≤ .01 versus control.
H2O2 treatment also suppressed expression of the regulators of adipogenesis and adiponectin gene expression, PPARγ and C/EBPα (Fig. 1B and C, respectively). All the antioxidant treatments increased both PPARγ and C/EBPα expression. Compared to the other antioxidants, boldine increased PPARγ mRNA expression significantly (P ≤ .007 at 10 μM, P ≤ .009 at 25 μM).
Concentration effect of boldine on expression of adiponectin and its regulators, PPARγ and C/EBPα, in the presence of H2O2 in 3T3-L1 adipocytes
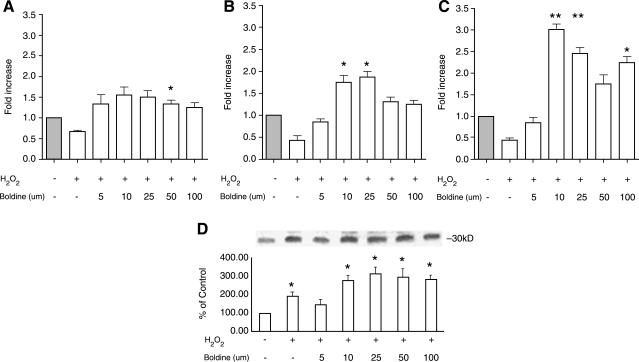
Fully differentiated 3T3-L1 cells were treated with 100 μM H2O2 in the absence or presence of increasing concentrations of boldine (5–100 μM) for 24 hours. As shown in Figure 2A, H2O2 treatment decreased adiponectin gene expression to half of the control levels. Boldine treatment prevented the oxidative stress-mediated decrease in adiponectin gene expression (P ≤ .049). Boldine treatment had a biphasic effect on adiponectin expression. At lower concentrations (5–25 μM), boldine increased adiponectin expression, which reached a plateau around 50 μM, after which the induction was lower. This biphasic effect of boldine was also observed on its effect on the levels of the transcription factors PPARγ and C/EBPα (Fig. 2B and C, respectively). The inductive effect of boldine on PPARγ mRNA expression (P ≤ .041 at 10 μM, P ≤ .023 at 25 μM), and C/EBPα expression (P ≤ .004 at 10 μM, P ≤ .009 at 25 μM) reached its peak around 10–25 μM.
FIG. 2.
Effect of increasing concentrations of boldine on mRNA levels of (A) adiponectin and its transcription regulators, (B) PPARγ and (C) C/EBPα, in the presence of H2O2. Differentiated 3T3-L1 adipocytes were treated with H2O2 (100 μM) in the absence or presence of 5–100 μM boldine for 24 hours. After treatments, RT-qPCR was performed on isolated mRNA. The mRNA results are expressed as differences in fold change in antioxidant treated cells compared to vehicle controls. (D) The levels of secreted adiponectin protein were measured using Western blot analysis of the lyophilized cell supernatant from antioxidant-treated cells and are expressed as the percentage of their immunoblot intensity relative to vehicle controls. Data are mean ± SEM values for three independent experiments performed in triplicate. *P ≤ .05, **P ≤ .01 versus control.
Western blotting of the lyophilized cell supernatant from this experiment for secreted adiponectin showed a dose-dependent increase in adiponectin protein by boldine (Fig. 2D). The adiponectin protein levels were increased significantly at 10–25 μM (P < .02) concentrations of boldine.
Effect of increasing concentrations of boldine on adiponectin and PPARγ and C/EBPα expression in the presence of TNFα in differentiated 3T3-L1 adipocytes
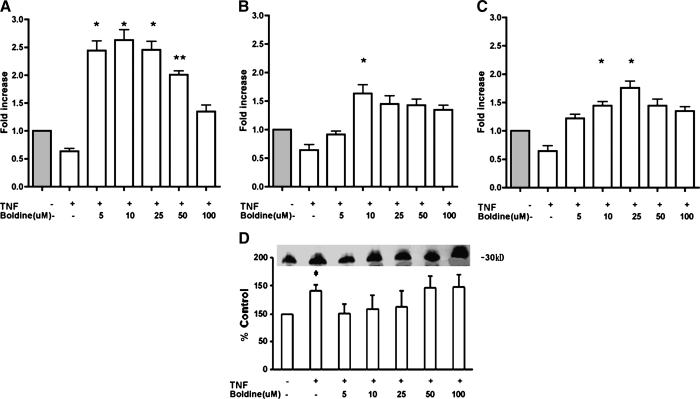
Differentiated 3T3-L1 cells were treated with 1 ng/mL TNFα alone or in the presence of increasing concentrations of boldine (5–100 μM) for 24 hours. As shown in Figure 3A, TNFα treatment decreased adiponectin gene expression to half of the control levels. Boldine treatment attenuated the TNFα-induced decrease in adiponectin gene expression (P ≤ .014 at 5 μM, P ≤ .013 at 10 μM, P ≤ .011 at 25 μM, P ≤ .005 at 50 μM). Boldine again demonstrated a biphasic effect on adiponectin gene expression in TNFα-treated 3T3-L1 adipocytes, with lower concentrations (5–25 μM) having a higher inductive effect compared to higher concentrations (50–100 μM).
FIG. 3.
Effect of increasing concentrations of boldine on mRNA levels of (A) adiponectin and its transcription regulators, (B) PPARγ and (C) C/EBPα, in the presence of TNFα. Differentiated 3T3-L1 adipocytes were treated with 1 ng/mL TNFα in the absence or presence of 5–100 μM boldine for 24 hours. After treatments, RT-qPCR was performed on isolated mRNA. The mRNA results are expressed as differences in fold change in antioxidant treated cells compared to vehicle controls. (D) The levels of secreted adiponectin protein were measured using Western blot analysis of the lyophilized cell supernatant from antioxidant-treated cells and are expressed as the percentage of their immunoblot intensity relative to vehicle controls. Data are mean ± SEM values for three independent experiments performed in triplicate. *P ≤ .05, **P ≤ .01 versus control.
TNFα treatment decreased both PPARγ and C/EBPα gene expression. Boldine attenuated the TNFα-induced decrease in both PPARγ (Fig. 3B) and C/EBPα (Fig. 3C) expression. The inductive effect of boldine on PPARγ mRNA expression (P ≤ .049 at 10 μM) and C/EBPα expression (P ≤ .029 at 10 μM, P ≤ .026 at 25 μM) reached saturation at around 10–25 μM.
Western blotting of the lyophilized cell supernatant for secreted protein from this experiment also showed that boldine increased adiponectin protein levels (Fig. 3D). Because of large variations in the mean percentage between replicate experiments, statistical significance was not achieved.
Concentration effect of boldine alone on expression of adiponectin and its transcription regulators, PPARγ and C/EBPα
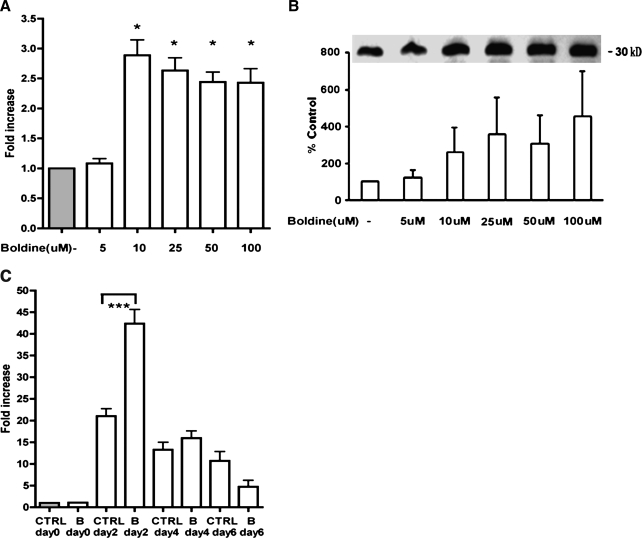
Boldine treatment by itself without any oxidants could also increase adiponectin expression. When fully differentiated 3T3-L1 cells were treated with increasing concentrations of boldine without any oxidants (Fig. 4A), adiponectin gene expression was significantly increased (P ≤ .018 at 10 μM, P ≤ .016 at 25 μM, P ≤ .012 at 50 μM, P ≤ .025 at 100 μM).
FIG. 4.
Effect of treatment with increasing concentrations of boldine on adiponectin levels in the absence of H2O2: (A) mRNA levels for adiponectin and (B) Western blotting of secreted adiponectin protein. Differentiated 3T3-L1 adipocytes were exposed to increasing concentrations of boldine (5–100 μM) in the absence of any oxidants for 24 hours. After treatments, RT-qPCR was performed on isolated mRNA, and Western blotting was performed on the lyophilized cell supernatant. (C) Effect of boldine on adiponectin mRNA levels at various phases during the adipogenesis cascade. 3T3-L1 preadipocytes were exposed to 10 μM boldine at different phases during the adipogenesis process: day 0, day 2, day 4, and day 6. After treatments, RT-qPCR was performed on isolated mRNA. The mRNA results are expressed as differences in fold change in treated cells compared to untreated controls. Western blot results are expressed as percentage change in level of secreted adiponectin protein in treated cells compared to untreated controls. Data are mean ± SEM values for three independent experiments performed in triplicate. *P ≤ .05, ***P ≤ .005 versus control (CTRL).
Western blotting of the lyophilized cell supernatant for secreted protein from this experiment also showed that boldine caused a dose-dependent increase in adiponectin protein (Fig. 4B). Because of variations in the mean percentages between replicate experiments, a statistical significance was not achieved.
To investigate when boldine exerts a beneficial effect during the adipocyte differentiation cascade, 3T3-L1 preadipocytes were treated either at the initial phase (day 0), during the induction phase (day 2), during the differentiation phase (day 4), or after differentiation (day 6) with 10 μM boldine. As observed in Figure 4C, boldine at 10 μM had its maximum effect on adiponectin at a much earlier phase in the adipocyte differentiation process, the induction phase (day 2) (10–15-fold induction, P ≤ .004), after which time its inductive effect decreased to only two- to fourfold at later phases (day 4 and day 6).
Effect of boldine on PPAR promoter activation
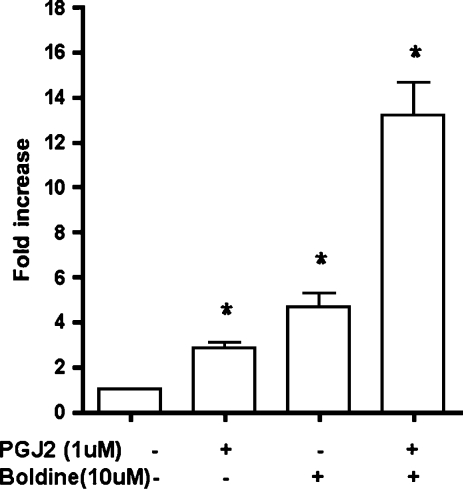
To investigate if the effect of boldine on adiponectin expression and its transcriptional regulators is due to its direct interaction with the PPARγ promoter or a PPRE, 3T3-L1 preadipocytes were transfected with PPRE-luciferase reporter construct, [PPRE]3-TK-Luc, and were treated with boldine (10 μM) treatment alone or boldine (10 μM) along with 1 μM 15-PGJ2 (a PPARγ agonist). Promoter activity after 24 hours showed that boldine at 10 μM (P ≤ .04) had a much stronger activation of PPRE-luciferase compared to 1 μM PPARγ agonist, 15-PGJ2 (P ≤ .015) (Fig. 5). Boldine in combination with 15-PGJ2 had an additive induction of PPAR promoter activity. This suggests that boldine may either directly activate the PPAR promoter or interact with genes containing a PPRE and may alter the expression of PPAR-regulated downstream genes, like adiponectin.
FIG. 5.
Effect of boldine on PPRE-promoter activity. 3T3-L1 preadipocytes transfected with a PPRE-luciferase reporter construct was exposed to boldine (10 μM), the PPARγ agonist 15-PGJ2 (1 μM), or boldine (10 μM) + 15-PGJ2 (1 μM). PPRE-luciferase activity was measured as a change in chemiluminesence in a luminometer after a 24-hour treatment. The results are expressed as change in relative luminescence units (RLU) in treated cells compared to untreated controls. Data are mean ± SEM vales for five independent experiments performed in triplicate. *P ≤ .05 versus control.
Discussion
Obesity is a leading worldwide public health concern.48 The association between obesity and chronic diseases such as cardiovascular disease, type 2 diabetes, metabolic syndrome, and cancer is becoming highly apparent.48–50 Increases in adipose mass and alterations in adipokines are tightly linked to obesity. Several studies have been recently initiated to investigate the beneficial effect of natural and synthetic compounds on adipogenesis and adipokines.51–55 Transcription factors such as PPARγ and C/EBPα play an important role in the adipogenic pathway and in the synthesis and secretion of several adipokines, including adiponectin.56 Adiponectin is inversely related to adiposity, and low concentrations of this protein are a predictive marker for cardiovascular disease and type 2 diabetes.4,6,9,57 Adiponectin can be regulated by both oxidative stress and inflammation.22–26,57 There are recent studies showing that H2O2 can decrease adiponectin gene expression in 3T3-L1 adipocytes within 10 minutes of exposure.20,58–62 Several natural and synthetic antioxidant compounds have been investigated for their role to protect adiponectin expression.30,63
Boldine, the aporphine alkaloid abundant in the leaves and bark of the medicinal plant Boldo, is a powerful antioxidant with anti-inflammatory and anti-atherosclerotic properties as shown in several in vitro and in vivo studies.33 In the current study, the ability of boldine to protect adiponectin expression from the effects of the known inhibitor H2O2 or inflammatory cytokine (TNFα) was demonstrated in 3T3-L1 adipocytes. The results show that boldine at all concentrations tested can protect adiponectin from both oxidative stress and inflammatory cytokines. Western blotting for secreted adiponectin showed similar trends between mRNA and protein expression, suggesting a role for boldine at both the transcriptional and translational level of adiponectin. At similar (or equivalent) concentrations, boldine exhibited the most potent beneficial effect on adiponectin expression compared to the other antioxidants (α-tocopherol and NAC) tested, suggesting that boldine might be a much more powerful antioxidant. Because boldine is known to have antioxidant and anti-inflammatory properties, the mechanism by which boldine protects adiponectin expression from the action of H2O2 and TNFα can be due to both of these properties. Many of the in vitro studies have shown a beneficial effect of boldine at <50 μM concentration and in vivo studies at <50 mg/kg doses.33,64 Studies from our laboratory have shown that boldine can inhibit oxidation of low-density lipoprotein even at concentrations of <5 μM. We have also shown that oral feeding of boldine at doses of 1 or 5 mg/kg decreased the development of atherosclerotic lesions in low-density lipoprotein receptor knockout mice.45 In this study, we used boldine at physiologically relevant concentrations and found beneficial effects on adiponectin expression.
At increasing concentrations, boldine had a biphasic effect on adiponectin levels, with lower concentrations (10–25 μM) of boldine giving better protection than higher concentrations (≥50 μM). This biphasic effect of boldine has been observed by other investigators. Milian et al.36 observed that boldine along with several other similar analogs inhibited generation of reactive oxygen species from activated neutrophils; this effect of boldine was more prominent at lower concentrations (10–25 μM) than at higher concentrations (100 μM). Recently, Konrath et al.65 observed that boldine can have both antioxidant and pro-oxidant properties depending on its concentrations. They observed that boldine at lower concentrations (10 μM) protected cellular damage to rat hippocampal slices exposed to oxygen-glucose deprivation in vitro; however, at higher concentrations (100 μM) boldine increased the cellular damage. These authors found that at lower concentrations boldine had three times higher total reactive antioxidant potential levels compared to Trolox (a peroxyl radical scavenger); however, at higher concentrations boldine increased lipid peroxidation parameters in these tissue. This biphasic effect of boldine might be therapeutically relevant and needs further investigation.
The most interesting finding of this study was the observation that boldine treatment by itself in the absence of any oxidants could also induce adiponectin expression (Fig. 4A and B). It was also observed that this effect of boldine on adiponectin was at a much earlier phase in the adipogenesis cascade (Fig. 4C), i.e., at the inductive phase (day 2). This suggests that boldine's effect might be upstream of adiponectin synthesis and might actually be interacting with the early regulators of the adipogenesis pathway and adiponectin regulatory genes, i.e., PPARγ and C/EBPα. This was supported by our studies where boldine was able to protect these transcription factors from oxidants and its ability to increase PPRE-luciferase promoter activity. Boldine was able to induce PPAR promoter activity by approximately twofold higher than the induction by a known PPARγ agonist, 15-PGJ2. In combination with 15-PGJ2, boldine was able to increase the activity several-fold higher (15-fold). These results suggest that boldine might be able to interact with the PPAR promoter directly and/or modulate PPRE responsive genes, including adiponectin. It also suggests that the inductive effect of boldine on adiponectin in the absence of any oxidants might be independent of its antioxidant property. Whether this effect is due to its anti-inflammatory property or some other unknown mechanism is yet to be investigated.
Oxidative stress is an important player in both atherosclerosis and obesity-related diseases. Controversy exists over the beneficial effects of popular antioxidants such as vitamin E,66,67 but an appropriate natural compound like boldine that has both antioxidant and anti-inflammatory properties might be beneficial in the prevention or treatment of these diseases. Although this is yet to be established in vivo, our study, for the first time, shows that, apart from its other beneficial properties, the aporphine alkaloid boldine, because of its effect on regulators of adipogenesis and adiponectin levels may also have strong beneficial effects on obesity-related diseases.
Acknowledgments
The authors acknowledge support from grants HL074239 (to N.S.) and 5P20RR016477 from the National Institutes of Health. The authors thank Dr. Gary Rankin, Ms. Amy Deborde, and Dr. Elsa Mangiarua for their help with proofreading our manuscript.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Hu E. Liang P. Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 2.Scherer PE. Williams S. Fogliano M. Baldini G. Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 3.Swarbrick MM. Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lara-Castro C. Fu Y. Chung BH. Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 5.Sheng T. Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008;35:321–326. doi: 10.1016/S1673-8527(08)60047-8. [DOI] [PubMed] [Google Scholar]
- 6.Tarquini R. Lazzeri C. Laffi G. Gensini GF. Adiponectin and the cardiovascular system: from risk to disease. Intern Emerg Med. 2007;2:165–176. doi: 10.1007/s11739-007-0027-9. [DOI] [PubMed] [Google Scholar]
- 7.Stern N. Osher E. Greenman Y. Hypoadiponectinemia as a marker of adipocyte dysfunction—Part I: the biology of adiponectin. J Cardiometab Syndr. 2007;2:174–182. doi: 10.1111/j.1559-4564.2007.06597.x. [DOI] [PubMed] [Google Scholar]
- 8.Lu JY. Huang KC. Chang LC. Huang YS. Chi YC. Su TC. Chen CL. Yang WS. Adiponectin: a biomarker of obesity-induced insulin resistance in adipose tissue and beyond. J Biomed Sci. 2008;15:565–576. doi: 10.1007/s11373-008-9261-z. [DOI] [PubMed] [Google Scholar]
- 9.Giannessi D. Maltinti M. Del Ry S. Adiponectin circulating levels: a new emerging biomarker of cardiovascular risk. Pharmacol Res. 2007;56:459–467. doi: 10.1016/j.phrs.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Mao X. Hong JY. Dong LQ. The adiponectin signaling pathway as a novel pharmacological target. Mini Rev Med Chem. 2006;6:1331–1340. doi: 10.2174/138955706778992978. [DOI] [PubMed] [Google Scholar]
- 11.Brun RP. Kim JB. Hu E. Altiok S. Spiegelman BM. Adipocyte differentiation: a transcriptional regulatory cascade. Curr Opin Cell Biol. 1996;8:826–832. doi: 10.1016/s0955-0674(96)80084-6. [DOI] [PubMed] [Google Scholar]
- 12.Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond) 2005;29(Suppl 1):S13–S16. doi: 10.1038/sj.ijo.0802907. [DOI] [PubMed] [Google Scholar]
- 13.Sharma AM. Staels B. Peroxisome proliferator-activated receptor gamma and adipose tissue—understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 14.Qiao L. Maclean PS. Schaack J. Orlicky DJ. Darimont C. Pagliassotti M. Friedman JE. Shao J. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes. 2005;54:1744–1754. doi: 10.2337/diabetes.54.6.1744. [DOI] [PubMed] [Google Scholar]
- 15.Kita A. Yamasaki H. Kuwahara H. Moriuchi A. Fukushima K. Kobayashi M. Fukushima T. Takahashi R. Abiru N. Uotani S. Kawasaki E. Eguchi K. Identification of the promoter region required for human adiponectin gene transcription: association with CCAAT/enhancer binding protein-beta and tumor necrosis factor-alpha. Biochem Biophys Res Commun. 2005;331:484–490. doi: 10.1016/j.bbrc.2005.03.205. [DOI] [PubMed] [Google Scholar]
- 16.Hiuge A. Tenenbaum A. Maeda N. Benderly M. Kumada M. Fisman EZ. Tanne D. Matas Z. Hibuse T. Fujita K. Nishizawa H. Adler Y. Motro M. Kihara S. Shimomura I. Behar S. Funahashi T. Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin level. Arterioscler Thromb Vasc Biol. 2007;27:635–641. doi: 10.1161/01.ATV.0000256469.06782.d5. [DOI] [PubMed] [Google Scholar]
- 17.Mineo H. Oda C. Chiji H. Kawada T. Shimizu K. Taira T. Thiazolidinediones exhibit different effects on preadipocytes isolated from rat mesenteric fat tissue and cell line 3T3-L1 cells derived from mice. Cell Biol Int. 2007;31:703–710. doi: 10.1016/j.cellbi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Pajvani UB. Hawkins M. Combs TP. Rajala MW. Doebber T. Berger JP. Wagner JA. Wu M. Knopps A. Xiang AH. Utzschneider KM. Kahn SE. Olefsky JM. Buchanan TA. Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 19.Moller DE. Berger JP. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S17–S21. doi: 10.1038/sj.ijo.0802494. [DOI] [PubMed] [Google Scholar]
- 20.Furukawa S. Fujita T. Shimabukuro M. Iwaki M. Yamada Y. Nakajima Y. Nakayama O. Makishima M. Matsuda M. Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferroni P. Basili S. Falco A. Davi G. Inflammation, insulin resistance, and obesity. Curr Atheroscler Rep. 2004;6:424–431. doi: 10.1007/s11883-004-0082-x. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi N. Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24–30. doi: 10.1016/j.cca.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takemura Y. Walsh K. Ouchi N. Adiponectin and cardiovascular inflammatory responses. Curr Atheroscler Rep. 2007;9:238–243. doi: 10.1007/s11883-007-0025-4. [DOI] [PubMed] [Google Scholar]
- 24.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–330. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Hattori Y. Akimoto K. Gross SS. Hattori S. Kasai K. Angiotensin-II-induced oxidative stress elicits hypoadiponectinaemia in rats. Diabetologia. 2005;48:1066–1074. doi: 10.1007/s00125-005-1766-7. [DOI] [PubMed] [Google Scholar]
- 26.Soares AF. Guichardant M. Cozzone D. Bernoud-Hubac N. Bouzaidi-Tiali N. Lagarde M. Geloen A. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med. 2005;38:882–889. doi: 10.1016/j.freeradbiomed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Li R. Wang WQ. Zhang H. Yang X. Fan Q. Christopher TA. Lopez BL. Tao L. Goldstein BJ. Gao F. Ma XL. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E1708. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 28.Tao L. Gao E. Jiao X. Yuan Y. Li S. Christopher TA. Lopez BL. Koch W. Chan L. Goldstein BJ. Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 29.Shimada M. Mochizuki K. Sakurai N. Goda T. Dietary supplementation with epigallocatechin gallate elevates levels of circulating adiponectin in non-obese type-2 diabetic Goto-Kakizaki rats. Biosci Biotechnol Biochem. 2007;71:2079–2082. doi: 10.1271/bbb.70174. [DOI] [PubMed] [Google Scholar]
- 30.Cho SY. Park PJ. Shin HJ. Kim YK. Shin DW. Shin ES. Lee HH. Lee BG. Baik JH. Lee TR. (-)-Catechin suppresses expression of Kruppel-like factor 7 and increases expression and secretion of adiponectin protein in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2007;292:E1166–E1172. doi: 10.1152/ajpendo.00436.2006. [DOI] [PubMed] [Google Scholar]
- 31.Schmeda-Hirschmann G. Rodriguez JA. Theoduloz C. Astudillo SL. Feresin GE. Tapia A. Free-radical scavengers and antioxidants from Peumus boldus Mol. (“Boldo”) Free Radic Res. 2003;37:447–452. doi: 10.1080/1071576031000090000. [DOI] [PubMed] [Google Scholar]
- 32.Speisky H. Cassels BK. Boldo and boldine: an emerging case of natural drug development. Pharmacol Res. 1994;29:1–12. doi: 10.1016/1043-6618(94)80093-6. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien P. Carrasco-Pozo C. Speisky H. Boldine and its antioxidant or health-promoting properties. Chem Biol Interact. 2006;159:1–17. doi: 10.1016/j.cbi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Speisky H. Cassels BK. Lissi EA. Videla LA. Antioxidant properties of the alkaloid boldine in systems undergoing lipid peroxidation and enzyme inactivation. Biochem Pharmacol. 1991;41:1575–1581. doi: 10.1016/0006-2952(91)90156-y. [DOI] [PubMed] [Google Scholar]
- 35.Kringstein P. Cederbaum AI. Boldine prevents human liver microsomal lipid peroxidation and inactivation of cytochrome P4502E1. Free Radic Biol Med. 1995;18:559–563. doi: 10.1016/0891-5849(94)e0138-9. [DOI] [PubMed] [Google Scholar]
- 36.Milian L. Estelles R. Abarca B. Ballesteros R. Sanz MJ. Blazquez MA. Reactive oxygen species (ROS) generation inhibited by aporphine and phenanthrene alkaloids semi-synthesized from natural boldine. Chem Pharm Bull (Tokyo) 2004;52:696–699. doi: 10.1248/cpb.52.696. [DOI] [PubMed] [Google Scholar]
- 37.Jimenez I. Lissi EA. Speisky H. Free-radical-induced inactivation of lysozyme and carbonyl residue generation in protein are not necessarily associated. Arch Biochem Biophys. 2000;381:247–252. doi: 10.1006/abbi.2000.1963. [DOI] [PubMed] [Google Scholar]
- 38.Kubinova R. Machala M. Minksova K. Neca J. Suchy V. Chemoprotective activity of boldine: modulation of drug-metabolizing enzymes. Pharmazie. 2001;56:242–243. [PubMed] [Google Scholar]
- 39.Jimenez I. Garrido A. Bannach R. Gotteland M. Speisky H. Protective effects of boldine against free radical-induced erythrocyte lysis. Phytother Res. 2000;14:339–343. doi: 10.1002/1099-1573(200008)14:5<339::aid-ptr585>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 40.Ubeda A. Montesinos C. Paya M. Alcaraz MJ. Iron-reducing and free-radical-scavenging properties of apomorphine and some related benzylisoquinolines. Free Radic Biol Med. 1993;15:159–167. doi: 10.1016/0891-5849(93)90055-y. [DOI] [PubMed] [Google Scholar]
- 41.Backhouse N. Delporte C. Givernau M. Cassels BK. Valenzuela A. Speisky H. Anti-inflammatory and antipyretic effects of boldine. Agents Actions. 1994;42:114–117. doi: 10.1007/BF01983475. [DOI] [PubMed] [Google Scholar]
- 42.Lanhers MC. Joyeux M. Soulimani R. Fleurentin J. Sayag M. Mortier F. Younos C. Pelt JM. Hepatoprotective and anti-inflammatory effects of a traditional medicinal plant of Chile, Peumus boldus. Planta Med. 1991;57:110–115. doi: 10.1055/s-2006-960043. [DOI] [PubMed] [Google Scholar]
- 43.Jang YY. Song JH. Shin YK. Han ES. Lee CS. Protective effect of boldine on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Pharmacol Res. 2000;42:361–371. doi: 10.1006/phrs.2000.0705. [DOI] [PubMed] [Google Scholar]
- 44.Chi TC. Lee SS. Su MJ. Antihyperglycemic effect of aporphines and their derivatives in normal and diabetic rats. Planta Med. 2006;72:1175–1180. doi: 10.1055/s-2006-947199. [DOI] [PubMed] [Google Scholar]
- 45.Santanam N. Penumetcha M. Speisky H. Parthasarathy S. A novel alkaloid antioxidant, Boldine and synthetic antioxidant, reduced form of RU486, inhibit the oxidation of LDL in-vitro and atherosclerosis in vivo in LDLR-/- mice. Atherosclerosis. 2004;173:203–210. doi: 10.1016/j.atherosclerosis.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 46.Fox KE. Fankell DM. Erickson PF. Majka SM. Crossno JT., Jr Klemm DJ. Depletion of cAMP-response element-binding protein/ATF1 inhibits adipogenic conversion of 3T3-L1 cells ectopically expressing CCAAT/enhancer-binding protein (C/EBP) alpha, C/EBP beta, or PPAR gamma 2. J Biol Chem. 2006;281:40341–40353. doi: 10.1074/jbc.M605077200. [DOI] [PubMed] [Google Scholar]
- 47.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336–352. doi: 10.1111/j.1365-2796.2008.01922.x. [DOI] [PubMed] [Google Scholar]
- 49.Pradhan A. Obesity, metabolic syndrome, and type 2 diabetes: inflammatory basis of glucose metabolic disorders. Nutr Rev. 2007;65:S152–S156. doi: 10.1111/j.1753-4887.2007.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 50.Lane G. Obesity and gynaecological cancer. Menopause Int. 2008;14:33–37. doi: 10.1258/mi.2007.007036. [DOI] [PubMed] [Google Scholar]
- 51.Hsu CL. Yen GC. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J Agric Food Chem. 2007;55:8404–8410. doi: 10.1021/jf071695r. [DOI] [PubMed] [Google Scholar]
- 52.Cho KJ. Moon HE. Moini H. Packer L. Yoon DY. Chung AS. Alpha-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J Biol Chem. 2003;278:34823–34833. doi: 10.1074/jbc.M210747200. [DOI] [PubMed] [Google Scholar]
- 53.Pinent M. Blade MC. Salvado MJ. Arola L. Hackl H. Quackenbush J. Trajanoski Z. Ardevol A. Grape-seed derived procyanidins interfere with adipogenesis of 3T3-L1 cells at the onset of differentiation. Int J Obes (Lond) 2005;29:934–941. doi: 10.1038/sj.ijo.0802988. [DOI] [PubMed] [Google Scholar]
- 54.Lin J. Della-Fera MA. Baile CA. Green tea polyphenol epigallocatechin gallate inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Obes Res. 2005;13:982–990. doi: 10.1038/oby.2005.115. [DOI] [PubMed] [Google Scholar]
- 55.Rayalam S. Della-Fera MA. Yang JY. Park HJ. Ambati S. Baile CA. Resveratrol potentiates genistein's antiadipogenic and proapoptotic effects in 3T3-L1 adipocytes. J Nutr. 2007;137:2668–2673. doi: 10.1093/jn/137.12.2668. [DOI] [PubMed] [Google Scholar]
- 56.Salma N. Xiao H. Imbalzano AN. Temporal recruitment of CCAAT/enhancer-binding proteins to early and late adipogenic promoters in vivo. J Mol Endocrinol. 2006;36:139–151. doi: 10.1677/jme.1.01918. [DOI] [PubMed] [Google Scholar]
- 57.Steffes MW. Gross MD. Lee DH. Schreiner PJ. Jacobs DR., Jr Adiponectin, visceral fat, oxidative stress, and early macrovascular disease: the Coronary Artery Risk Development in Young Adults Study. Obesity (Silver Spring) 2006;14:319–326. doi: 10.1038/oby.2006.41. [DOI] [PubMed] [Google Scholar]
- 58.Iwata C. Wang X. Uchida K. Nakanishi N. Hattori Y. Buthionine sulfoximine causes endothelium dependent hyper-relaxation and hypoadiponectinemia. Life Sci. 2007;80:873–878. doi: 10.1016/j.lfs.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Kamigaki M. Sakaue S. Tsujino I. Ohira H. Ikeda D. Itoh N. Ishimaru S. Ohtsuka Y. Nishimura M. Oxidative stress provokes atherogenic changes in adipokine gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2006;339:624–632. doi: 10.1016/j.bbrc.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 60.Magalang UJ. Rajappan R. Hunter MG. Kutala VK. Kuppusamy P. Wewers MD. Marsh CB. Parinandi NL. Adiponectin inhibits superoxide generation by human neutrophils. Antioxid Redox Signal. 2006;8:2179–2186. doi: 10.1089/ars.2006.8.2179. [DOI] [PubMed] [Google Scholar]
- 61.Ouedraogo R. Wu X. Xu SQ. Fuchsel L. Motoshima H. Mahadev K. Hough K. Scalia R. Goldstein BJ. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes. 2006;55:1840–1846. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 62.Chen B. Lam KS. Wang Y. Wu D. Lam MC. Shen J. Wong L. Hoo RL. Zhang J. Xu A. Hypoxia dysregulates the production of adiponectin and plasminogen activator inhibitor-1 independent of reactive oxygen species in adipocytes. Biochem Biophys Res Commun. 2006;341:549–556. doi: 10.1016/j.bbrc.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Gong H. Ni Y. Guo X. Fei L. Pan X. Guo M. Chen R. Resistin promotes 3T3-L1 preadipocyte differentiation. Eur J Endocrinol. 2004;150:885–892. doi: 10.1530/eje.0.1500885. [DOI] [PubMed] [Google Scholar]
- 64.Stevigny C. Bailly C. Quetin-Leclercq J. Cytotoxic and antitumor potentialities of aporphinoid alkaloids. Curr Med Chem Anticancer Agents. 2005;5:173–182. doi: 10.2174/1568011053174864. [DOI] [PubMed] [Google Scholar]
- 65.Konrath EL. Santin K. Nassif M. Latini A. Henriques A. Salbego C. Antioxidant and pro-oxidant properties of boldine on hippocampal slices exposed to oxygen-glucose deprivation in vitro. Neurotoxicology. 2008;29:1136–1140. doi: 10.1016/j.neuro.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Parthasarathy S. Khan-Merchant N. Penumetcha M. Khan BV. Santanam N. Did the antioxidant trials fail to validate the oxidation hypothesis? Curr Atheroscler Rep. 2001;3:392–398. doi: 10.1007/s11883-001-0077-9. [DOI] [PubMed] [Google Scholar]
- 67.Traber MG. Frei B. Beckman JS. Vitamin E revisited: do new data validate benefits for chronic disease prevention? Curr Opin Lipidol. 2008;19:30–38. doi: 10.1097/MOL.0b013e3282f2dab6. [DOI] [PubMed] [Google Scholar]