Abstract
The antimicrobial activity of essential oils of oregano, thyme, basil, marjoram, lemongrass, ginger, and clove was investigated in vitro by agar dilution method and minimal inhibitory concentration (MIC) determination against Gram-positive (Staphylococcus aureus and Listeria monocytogenes) and Gram-negative strains (Escherichia coli and Salmonella Enteritidis). MIC90% values were tested against bacterial strains inoculated experimentally in irradiated minced meat and against natural microbiota (aerobic or facultative, mesophilic, and psychrotrophic bacteria) found in minced meat samples. MIC90% values ranged from 0.05%v/v (lemongrass oil) to 0.46%v/v (marjoram oil) to Gram-positive bacteria and from 0.10%v/v (clove oil) to 0.56%v/v (ginger oil) to Gram-negative strains. However, the MIC90% assessed on minced meat inoculated experimentally with foodborne pathogen strains and against natural microbiota of meat did not show the same effectiveness, and 1.3 and 1.0 were the highest log CFU/g reduction values obtained against tested microorganisms.
Introduction
The new technologies of food preservation include nonthermal inactivation, such as ionization radiation, high hydrostatic pressure, and pulsed electric fields; modified atmosphere and active packaging; biopreservation; and natural antimicrobial compounds (Devlieghere et al., 2004). Plants are a source of bioactive molecules and have been widely used both traditionally and commercially to increase the shelf-life and safety of foods (Sasidharan et al., 2008).
Biological properties of essential oils and their antimicrobial activity have been attributed to phenolic compounds, such as the carvacrol, eugenol (2-methoxy-4-(2-propenyl) phenol), and thymol (Seydim and Sarikus, 2006). These compounds have hydrophobic characteristics and interact with different sites of microbial cell (e.g., cell wall and cytoplasmic membrane), causing loss of cellular constituents, collapse of membrane structure, and cell death (Burt, 2004). Bactericidal or bacteriostatic activity of essential oils, in vitro and in food assays, against Salmonella enterica, Escherichia coli O157:H7, Staphylococcus aureus, Listeria monocytogenes, Lactobacillus plantarum, Saccharomyces cerevisiae, and Candida albicans strains has been reported (Lambert et al., 2001; Chorianopoulos et al., 2004; Friedman et al., 2004; Kim et al., 2004). Studies in vitro have used spices as antimicrobials in laboratory media although the levels of spices and their essential oils to inhibit microorganisms in food have been found to be higher than those assays performed using culture media (Burt and Reinders, 2003; Uhart et al., 2006).
Thus, we aimed to determine in vitro the minimal inhibitory concentration (MIC) of essential oils from Thymus vulgaris (thyme), Origanum majorana (marjoram), Origanum vulgare (oregano), Ocimum basilicum (basil), Zingiber officinale (ginger), Cymbopogon citratus (lemon grass), and Caryophyllus aromaticus (clove) against E. coli, S. aureus, L. monocytogenes, and Salmonella Enteritidis strains. MIC90% values were evaluated in minced meat irradiated and experimentally inoculated with these pathogenic bacteria and against natural microbiota of minced meat (mesophiles and psycrotrophs).
Materials and Methods
Plants and essential oils
Fresh leaves of oregano, thyme, basil, marjoram, and lemon grass; rhizome of ginger; and dried inflorescence clove were purchased from the local market. Steam distillation using the Clevenger system was used to obtain the essential oils, which were stored at 5°C in sealed glass vials before use.
Bacterial strains
Five strains of L. monocytogenes, S. aureus, E. coli, and Salmonella Enteritidis isolated from food samples were used. ATTC strains of each bacterial species (Lm ATTC 7644, Sa ATCC 25923, SE ATCC 13076, and Ec ATCC 25922) were also tested.
In vitro antibacterial assay: MIC test
Mueller–Hinton agar (Difco, Sparks, NV) plates containing essential oils (0.025%v/v, 0.05%v/v, 0.1%v/v, 0.3%v/v, 0.5%v/v, 0.7%v/v, 1.0%v/v, 1.5%v/v, 2.0%v/v, and 3.0%v/v) and Tween 80 (0.2%) were inoculated by Sterr's inoculator with bacterial strains incubated overnight at 36°C in brain heart infusion (Difco) and standardized in sterile saline (104–105 CFU/mL). The bacterial growth was indicated as the presence and/or absence of colonies on media after at 37°C/24 h, and the MIC was the lowest concentration showing no growth at Mueller–Hinton agar (NCCLS, 2004). Afterward, MIC90% values were calculated.
Oils' antimicrobial activity in minced meat assay
Two antimicrobial tests were carried out on minced meat: (1) antimicrobial activity of essential oils against pathogenic bacterial strains (L. monocytogenes, S. aureus, E. coli, and Salmonella Enteritidis) experimentally inoculated on irradiated meat and (2) antimicrobial activity against the natural microbiota (mesophilic and psychrotrophic bacteria) of meat. MIC90% values achieved in vitro tests according to the Gram-positive bacteria (L. monocytogenes and S. aureus) and Gram-negative bacteria (E. coli and Salmonella Enteritidis) were tested in meat because the susceptibility profile was similar among the essential oils.
Minced meat samples (25 g) were packaged in individual portions, frozen (−20°C), and irradiated with 10.2 kGy, aiming to eliminate natural microbial populations. The irradiated meat samples were experimentally inoculated (104–105 CFU/g) with bacterial strains, and essential oils (%v/g) were added to meat and maintained at 5°C/3 h. Serial dilutions and pour plate method were performed with plate count agar (PCA) (Difco), and after 35°C/24–48 h the CFU/g was recorded. Tests were performed in duplicate.
The mesophilic and psychrotrophic enumeration were performed on minced meat, and individual samples (25 g) were tested with or without essential oils according to the MIC90% values from in vitro sensibility tests. The meat samples with oils and the controls were kept at 5°C, and serial dilutions were performed at 0 h (sample without oil) and 6 and 24 h (samples with and without oil) counting CFU/g in PCA. The psychrotrophics were recorded at 0 and 24 h after contact with essential oils using serial dilution and inoculation on PCA and incubation at 5°C/7 days.
Statistical analysis
The nonparametric test of Kruskal–Wallis was employed to compare independent treatments. Student–Newman–Keuls test was applied to multiple comparisons. To meat assays, nonparametric test of Mann–Whitney was used for groups (with or without oil), while the nonparametric variance analysis and Kruskal–Wallis was performed to bacteria.
Results and Discussion
All strains were susceptible to essential oils, and clove showed the highest antimicrobial activity (MIC90% = 0.09%v/v), followed by lemongrass (0.25%v/v) and thyme (0.26%v/v). Lemon grass was the most effective against Gram-positive (0.05%v/v), followed by clove, ginger (0.09%v/v), and thyme (0.10%v/v) oils and clove oil for Gram-negative strains (0.10%v/v). In vitro studies have demonstrated the antibacterial activity of essential oils against L. monocytogenes, Salmonella Typhimurium, E. coli O157:H7, Shigella dysenteria, Bacillus cereus, and S. aureus, and Gram-negative bacteria were less susceptible than Gram-positive bacteria (Burt, 2004).
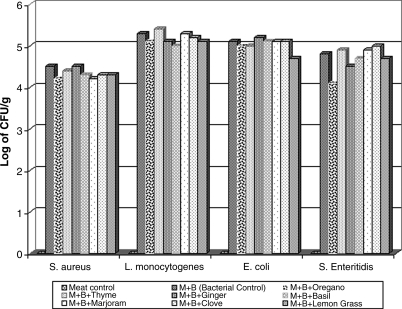
The CFU/g (log) values of S. aureus, L. monocytogenes, E. coli, and Salmonella Enteritidis assays with or without essential oil zacontact in meat experimentally inoculated are shown in Fig. 1. The reduction between tests and control treatments was not significant, and the oils were able to reduce 1 log compared with the control. Although no significant differences were found, the bacteriostatic effect of oils was verified, and no bacterial developments were recorded at 5°C/3 h for all bacterial strains.
FIG. 1.
Log of CFU/g values from Staphylococcus aures, Escherichia coli, Listeria Monocytogenes, and Salmonella Enteritidis experimentally inoculated in irradiated minced meat added with essential oils and maintained at 5°C/3 h.
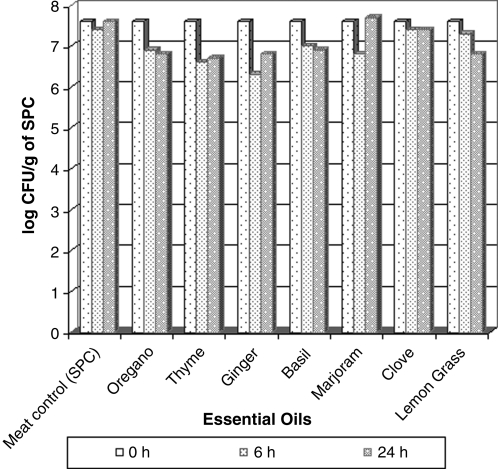
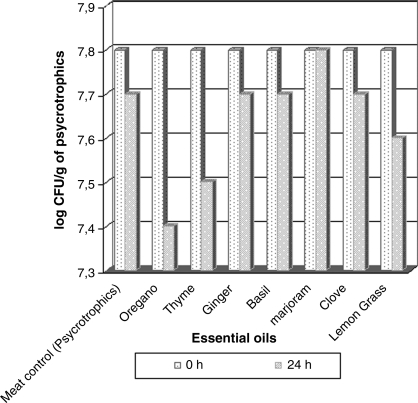
The log CFU/g values for mesophilic aerobic bacteria from minced meat recorded at 0 h (positive control) and 6 and 24 h after adding oil to meat are presented in Fig. 2. No significant differences were verified, and 1.3 and 1.0 were log CFU/g reduction values to ginger and thyme oils, respectively. The psychrotrophic reduction tests (Fig. 3) after 24 h of oils and meat contact produced the highest log reduction with oregano oil (0.4).
FIG. 2.
Log of CFU/g of standard plate count (SPC) values recorded on minced meat samples after 5°C/6 and 24 h of essential oil addition.
FIG. 3.
Log of CFU/g for psychrotrophic microorganism values recorded on minced meat samples after 5°C/24 h of essential oil addition.
Although the antimicrobial activity in vitro of oils has been moderately effective on meat model, the potential use of these oils in food preservation technologies should be found in optimal concentrations to ensure the safety of the food, appropriated organoleptical characteristics, and accepted by consumers. Studies aiming to elucidate the interaction between essential oils and components of food matrices or additives, stability of oils during food processing, and the standardization of antibacterial methods are still needed.
Acknowledgments
This research was supported by Fundação de Amparo a Pesquisa de São Paulo (FAPESP 05/56110-2 and 05/55039-2). We thank the Companhia Brasileira de Esterilização (CBE) for meat sample irradiation, Dr. Luciano Barbosa for statistical analysis, and Dr. José Maurício Sforcin for critical review of the article.
References
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:233–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Burt SA. Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O 157:H7. Lett Appl Microbiol. 2003;36:162–167. doi: 10.1046/j.1472-765x.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- Chorianopoulos N. Kalpoutzakis E. Aligiannis N. Mitaku S. Nychas G. Haroutounian SA. Essential oils of Sartureja, Origanum, and Thymus species: chemical composition an antibacterial activities against foodborne pathogens. J Agric Food Chem. 2004;52:8261–8267. doi: 10.1021/jf049113i. [DOI] [PubMed] [Google Scholar]
- Devlieghere F. Vermeiren L. Debevere J. New preservation technologies: possibilities and limitations. Int Dairy J. 2004;14:273–285. [Google Scholar]
- Friedman M. Henika PR. Levin CE. Mandrell RE. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J Agric Food Chem. 2004;52:6042–6048. doi: 10.1021/jf0495340. [DOI] [PubMed] [Google Scholar]
- Kim JW. Kim YS. Kyung KH. Inhibitory activity of essential oils garlic an onion against bacteria and yeasts. J Food Prot. 2004;67:499–504. doi: 10.4315/0362-028x-67.3.499. [DOI] [PubMed] [Google Scholar]
- Lambert RJW. Skandamis PN. Coote PJ. Nychas GJE. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 2001;91:453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- [NCCLS] National Committee for Clinical Laboratory Standards. Method for Dilution Antimicrobial Susceptibility Tests for Bacterial that Grow Aerobically. 7th. NCCLS; Wayne, PA: 2004. Approved Standard M7.A6. [Google Scholar]
- Sasidharan S. Zuraini Z. Yoga Latha L. Sngetha S. Suryani S. Antimicrobial activities of Psophocarpus tetragonolobus (L.) DC extracts. Food Pathog Dis. 2008;5:303–309. doi: 10.1089/fpd.2007.0078. [DOI] [PubMed] [Google Scholar]
- Seydim AC. Sarikus G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res Int. 2006;39:639–644. [Google Scholar]
- Uhart M. Maks N. Ravishankar S. Effect of spices on growth and survival of Salmonella Typhimurium DT 104 in ground beef stored at 4 and 8°C. J Food Saf. 2006;26:115–125. [Google Scholar]