Abstract
The ability of the foodborne pathogen Listeria monocytogenes to survive antimicrobial treatments is a public health concern; therefore, this study was designed to investigate genetic mechanisms contributing to antimicrobial response in L. monocytogenes. In previous studies, the putative bacteriocin immunity gene lmo2570 was predicted to be regulated by the stress responsive alternative sigma factor, σB. As the alternative sigma factor σL controls expression of genes important for resistance to some antimicrobial peptides, we hypothesized roles for lmo2570, σB, and σL in L. monocytogenes antimicrobial response. Results from phenotypic characterization of a L. monocytogenes lmo2570 null mutant suggested that this gene does not contribute to resistance to nisin or to SdpC, an antimicrobial peptide produced by some strains of Bacillus subtilis. While lmo2570 transcript levels were confirmed to be σB dependent, they were σL independent and were not affected by the presence of nisin under the conditions used in this study. In spot-on-lawn assays with the SdpC-producing B. subtilis EG351, the L. monocytogenes ΔsigB, ΔsigL, and ΔsigB/ΔsigL strains all showed increased sensitivity to SdpC, indicating that both σB and σL regulate genes contributing to SdpC resistance. Nisin survival assays showed that σB and σL both affect L. monocytogenes sensitivity to nisin in broth survival assays; that is, a sigB null mutant is more resistant than the parent strain to nisin, while a sigB null mutation in ΔsigL background leads to reduced nisin resistance. In summary, while the σB-dependent lmo2570 does not contribute to resistance of L. monocytogenes to nisin or SdpC, both σB and σL contribute to the L. monocytogenes antimicrobial response.
Introduction
The Gram-positive, facultative intracellular foodborne pathogen Listeria monocytogenes is the causative agent of listeriosis, which has a human case-fatality rate >20% in the United States (Mead et al., 1999). The vast majority of human listeriosis cases have been reported to occur via consumption of contaminated foods (Mead et al., 1999); therefore, development of more effective methods for controlling the presence of L. monocytogenes in foods is a desirable goal. To that end, various antimicrobial peptides have been investigated as a potential means for inhibiting growth of L. monocytogenes in foods (Muriana, 1996; Cleveland et al., 2001).
Bacteriocins are bacterially produced antimicrobial peptides that are generally most effective against other bacteria that are genetically similar and present in similar ecological niches. To enhance producer strain self-preservation, bacteriocin production is frequently coupled with production of cognate bacteriocin immunity proteins (Venema et al., 1995; Cleveland et al., 2001; Eijsink et al., 2002). For example, NisI, which provides immunity to nisin, is encoded downstream of the nisin biosynthesis genes in Lactococcus lactis (Engelke et al., 1994). Previous studies have reported that bacteriocin production can be influenced by bacterial environmental stress response pathways such as the RecA-dependent SOS response and the ppGpp-dependent stringent response (de los Santos et al., 2005). Bacteriocin production and immunity to antimicrobials are hypothesized to enhance the ability of producer bacteria to vie for limited nutrients in the presence of competitors (Nissen-Meyer and Nes, 1997).
Lactic acid bacteria are recognized as producers of various bacteriocins (Klaenhammer, 1993; Jack et al., 1995). Lactic acid bacteria are commonly present in human foods; therefore, the bacteriocins that they produce, such as pediocin PA-1/AcH, enterocins, and/or sakacins, also may be present in foods (Cleveland et al., 2001). Currently, only nisin, a class I lantibiotic bacteriocin produced by the lactic acid bacterium L. lactis, has generally recognized as safe status for intentional application as an antimicrobial in the U.S. food industry (Jack et al., 1995). Nisin creates membrane-spanning pores in the bacterial cell wall, which enable dissipation of the cell's proton motive force (Bruno et al., 1992; Bonnet et al., 2006). Although, in general, nisin has been demonstrated as an effective antilisterial peptide (Benkerroum and Sandine, 1988), some strains of L. monocytogenes have developed resistance to both nisin and pediocin PA-1 (Gravesen et al., 2002a, 2002b; Gandhi and Chikindas, 2007). L. monocytogenes resistance to nisin is a concern to the segments of the food industry (e.g., dairy and poultry) that currently use this peptide to control pathogen growth (Gandhi and Chikindas, 2007). A better understanding of the molecular mechanisms contributing to antimicrobial resistance in foodborne pathogens could lead to development of improved food safety intervention strategies. One means to that end is to identify and examine putative bacteriocin immunity genes and their physiological roles in protecting the producer strain against either endogenously or exogenously produced antimicrobial peptides.
In a previous study, Kazmierczak et al. (2003) identified lmo2570 as a putative σB-dependent gene with 45% similarity to the Bacillus subtilis bacteriocin immunity gene sdpI (yvaZ) (cmr.jcvi.org), which encodes SdpI. SdpI is a membrane protein conferring resistance to the endogenously produced antimicrobial peptide SdpC (Butcher and Helmann, 2006; Ellermeier et al., 2006). Butcher and Helmann (2006) found that while SdpI has a predominant role in conferring resistance to SdpC, the B. subtilis regulon controlled by σW, an extracytoplasmic function sigma factor, provides secondary immunity to this antimicrobial peptide. Taken together, these data indicate the importance in antimicrobial resistance of both immunity genes and transcriptional level regulatory mechanisms as mediated by alternative sigma factors.
We hypothesized that σB and σL contribute to antimicrobial response in L. monocytogenes. σB has been shown to regulate response to antimicrobial peptides in other Gram-positive bacteria. To illustrate, the B. subtilis σB regulon is upregulated after treatment with either bacitracin or vancomycin (Mascher et al., 2003). In a collection of teicoplanin-resistant Staphylococcus aureus mutants, the majority of the mutations responsible for antimicrobial resistance mapped to rsbW, which encodes the RsbW anti-sigma factor that sequesters σB to prevent it from interacting with RNA polymerase. The teicoplanin-resistant strains with mutations in rsbW showed increased σB activity relative to their parent strain (the MB33 rsbU mutant strain) or to other strains carrying the rsbU wild-type allele (Bischoff and Berger-Bachi, 2001), providing evidence of a link between σB activity and antimicrobial resistance. σB also has been shown to contribute to bacterial stress response regulation in S. aureus (Chan et al., 1998). L. monocytogenes alternative sigma factor σL regulates expression of genes that mediate sensitivity to antimicrobials such as the class IIa bacteriocin, mesentericin Y105 (Robichon et al., 1997); hence, σL also has been associated with antimicrobial response. Therefore, in the studies described below, phenotypic and genotypic assessments were used to determine the contributions of σB, σL, and Lmo2570 to the L. monocytogenes response to SdpC and nisin.
Materials and Methods
Bacterial strains and growth conditions
L. monocytogenes parent strain 10403S (serotype 1/2a) and otherwise isogenic sigB and sigL single and double null mutants (ΔsigB, FSL A1-254; ΔsigL, FSL B2-124; ΔsigB/ΔsigL, FSL B2-127) were used in this study. Listeria innocua FSL C2-008 (Woodling and Moraru, 2005), Listeria ivanovii FSL C2-010, Listeria welshimeri FSL N1-064, and Listeria seeligeri FSL N1-067 (Table 1) were used to assess intragenus competition with the L. monocytogenes parent and mutant strains. To examine the susceptibility of the L. monocytogenes parent and mutant strains to a closely related bacterium that produces an antimicrobial peptide, we used strains of B. subtilis that produce SdpC, the bacteriocin whose cognate immunity gene is predicted by sequence similarity to be homologous to L. monocytogenes lmo2570. These strains included B. subtilis prototroph (PY49) (Youngman et al., 1984) and its mutant EG351 (PY79 Pspac-hy-sdpABC) (gift from Dr. J. Helmann, Department of Microbiology, Cornell University), which expresses SdpC under control of an inducible promoter.
Table 1.
Strains Used in This Study
| Strain | Characteristics | Reference or source |
|---|---|---|
| Listeria monocytogenes 10403S | Laboratory parent strain | Bishop and Hinrichs (1987) |
| L. monocytogenes FSL A1-254 | 10403S ΔsigB | Wiedmann et al. (1998) |
| L. monocytogenes FSL P1-002 | 10403S Δlmo2570 | This study |
| L. monocytogenes FSL B2-124 | 10403S ΔsigL | Chaturongakul, unpublished |
| L. monocytogenes FSL B2-127 | 10403S ΔsigB/ΔsigL | Chaturongakul, unpublished |
| Listeria innocua FSL C2-008 | Woodling and Moraru (2005) | |
| Listeria ivanovii FSL C2-010 | Wiedmann, unpublisheda | |
| Listeria welshimeri FSL N1-064 | Fish processing plant environment | |
| Listeria seeligeri FSL N1-067 | Fish processing plant environment | |
| Bacillus subtilis PY79 | Prototroph, parent strain | Youngman et al. (1984) |
| B. subtilis EG351 | PY79 Pspac-hy-sdpABC | Butcher and Helmann (2006) |
Isolate kindly provided (as USDA 2717) by I. Wesley, USDA-ARS.
USDA-ARS, U.S. Department of Agriculture's Agricultural Research Service.
L. monocytogenes strains were grown in brain heart infusion (BHI) broth (Difco, Sparks, MD) at 37°C with shaking (250 rpm) overnight (16–18 h), then were subcultured (1:100) and grown as described below for each experiment. B. subtilis strains were grown in Luria-Bertani broth as described for L. monocytogenes, unless otherwise stated. The ΔsigB/ΔsigL strain grew more slowly than the 10403S, ΔsigB, and ΔsigL strains, requiring an additional incubation time of ∼30 min to reach the same OD600.
Mutant construction
An in-frame 543 base pair deletion within lmo2570 was created in L. monocytogenes 10403S using splicing-by-overlap extension (SOE) polymerase chain reaction (PCR) and allelic exchange mutagenesis (Ho et al., 1989). Primers used were 5′-GGA AGC TTT AAG GCA CTG TGA GCC TGG-3′ (lmo2570 SOEA), 5′-TCA TAC TAG GAA ATA TAC CAA C-3′ (lmo2570 SOEB), 5′-GTT GGT ATA TTT CCT AGT ATG ATT ATT GTT GTT G-3′ (lmo2570 SOEC), and 5′-GGG GTA CCT CAG GTT CAC TGG CAG CTA G-3′ (lmo2570 SOED). Primers were synthesized by IDT Technologies (Coralville, IA). Allelic exchange mutagenesis was confirmed through PCR and subsequent DNA sequencing, the latter of which was performed by the Cornell BioResource Center (Ithaca, NY). The Δlmo2570 mutation did not affect growth rate of the mutant strain relative to the 10403S parent strain when both were grown in BHI at 37°C with shaking at 250 rpm (data not shown).
Spot-on-lawn assays
Spot-on-lawn assays were performed in triplicate as described by Butcher and Helmann (2006). Briefly, to create lawns, 100 μL of a given strain (i.e., 10403S, Δlmo2570, ΔsigB, ΔsigL, or ΔsigB/ΔsigL) that had been grown to an optical density of OD600 = 0.4 was inoculated into 2 mL of 0.7% Luria-Bertani soft agar that had been tempered at 50°C. To induce Pspac-regulated transcription of sdpABC when EG351 was used as the spotting strain, 1 mM isopropyl-β-D-thiogalactopyranoside also was added to the tempered agar that had been inoculated with bacteria. Each mixture was then poured into one well in an eight-well rectangular multidish (26 × 33 mm; Nunc, Rochester, NY). The plates were then dried in a laminar hood for 30 min. Subsequently, 4 μL of the strain being assessed for bacteriocin production (e.g., 10403S, PY79, or EG351), which had been grown to an OD600 = 0.6, was spotted on the agar in the middle of each well. The plates were covered with lids and incubated in a moist container at 37°C for 22–24 h. In addition to the 10403S, PY79, or EG351 test strains, isolates representing five Listeria species also were used as spotting strains to determine if the lawn strains would demonstrate sensitivity to bacteria representing different species within the same genus (Table 1). Sensitivity of lawns to potential bacteriocin producer strains was assessed by measuring the zone of inhibition (zoi) around the growth of the spotted strain. Radii of zoi were determined by measuring the diameters of both the spotted colony and the surrounding zoi in pixels (px). The diameter of the spotted colony was then subtracted from the diameter of the zoi, and the resulting product was divided by 2 (to yield a radius). Measurements were performed using Adobe® Photoshop® CS (Adobe Systems Incorporated, Mountain View, CA).
Radii of zoi produced on the various lawn strains were initially compared to zoi produced on the 10403S reference lawn using one-way analysis of variance (ANOVA) with Dunnett's t-test, using SAS® 9.0 (SAS Institute, Cary, NC). To determine if there were statistically significant interaction effects between the sigB and the sigL deletions, a two-way ANOVA (with Tukey's adjustment for multiple comparisons) was performed. In this model, the dependent variable was zoi radius, and the independent variables included sigB + sigL + sigB*sigL + replicate. The factors sigB and sigL in the model indicate the presence or absence of that gene in the strains tested. An adjusted p < 0.05 was considered significant in this and all other statistical analyses.
Nisin minimum inhibitory concentration determination
The objective of this experiment was to determine a minimal inhibitory concentration of nisin for log-phase L. monocytogenes to enable selection of an appropriate sublethal concentration for subsequent quantitative reverse-transcriptase (qRT)–PCR experiments. Nisin's solubility and activity are optimal at pH 3 and 3.5, respectively (Abee and Delves-Broughton, 2003); therefore, nisin is typically dissolved in an acidified solution before use (Liu and Hansen, 1990; Huot et al., 1996). As σB expression and activity are induced at low pH (Becker et al., 1998; Sue et al., 2004), we predicted that addition of nisin in an acidified solution to the various cultures would upregulate expression of the σB regulon, thus conferring a survival advantage to the wild type over the ΔsigB strain (Wiedmann et al., 1998; Ferreira et al., 2001, 2003) and, hence, confounding interpretation of our experimental results. Therefore, to avoid induction of σB activity, nisin was dissolved in sterile distilled water (1000 AU/mL), and the pH of the final solution was adjusted to 7.0 using 0.01 N sodium hydroxide. Nisin solutions at pH 7.0 were used throughout these experiments. The nisin solutions were filter sterilized with a 0.2 μm, 25 mm syringe filter (NALGENE®; Thermo Fisher Scientific, Waltham, MA) and diluted to the test concentrations. The minimum inhibitory concentration of nisin (Sigma, St. Louis, MO) was determined for all strains of L. monocytogenes by measuring absorbance at OD600 using a Fusion™ Universal Microplate Analyzer (PerkinElmer, Shelton, CT). Strains were grown overnight, subcultured 1:100, and grown to OD600 = 0.4. Strains were inoculated to a final concentration of 1 × 104 CFU/well into 96-well round bottom microplates (Costar, Corning, NY), and the edges were sealed with Parafilm® (Alcan Packaging, Neenah, WI) to prevent evaporation. OD600 measurements were taken after 24 h incubation at 37°C with shaking. The lowest concentration that inhibited growth for all strains after a 24 h incubation in a 96-well plate format was 100 AU nisin/mL, as determined in three replicate trials. Therefore, a sublethal concentration of 75 AU nisin/mL was used for TaqMan qRT-PCR assays.
Total RNA isolation
L. monocytogenes 10403S, ΔsigL, and ΔsigB were grown to logarithmic phase (OD600 = 0.4) and collected after (i) incubation for 10 min after addition of nisin in sterile distilled water to yield a final concentration of 75 AU/mL nisin, (ii) incubation for 10 min after addition of an equivalent volume of sterile distilled water without nisin, and (iii) incubation for 10 min without any addition. RNA isolation and purification was performed as previously described (Sue et al., 2004; Raengpradub et al., 2008), except that DNase treatments were performed using TURBO™ DNase (Ambion, Austin, TX) following the manufacturer's instructions. Total nucleic acid concentrations and purity were estimated using absorbance readings (260 nm/280 nm) on a NanoDrop™ ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE).
qRT-PCR
Transcript levels of lmo2570, as well as of two housekeeping genes, rpoB and gap, were quantified using TaqMan primers and probes and the ABI Prism® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) as previously described (Kazmierczak et al., 2006). Data were analyzed using the ABI Prism 7000 Sequence Detection System software (Applied Biosystems) as previously described by Sue et al. (2004). Primer Express® 1.0 software (Applied Biosystems) was used to design oligonucleotide primers and TaqMan probes for lmo2570: forward primer (5′-AAG TGG CGG TGC ATT TCG-3′), reverse primer (5′-TAA GCC AAG CCA CTT TTG CAT-3′), and probe (6FAM 5′-ACG GAC TTC TCC CCA GAT-3′ MGB-NFQ). Primers and probes for gap and rpoB were previously described (Sue et al., 2004; Schwab et al., 2005), respectively.
Transcript levels of lmo2570, as determined by qRT-PCR, were log10 transformed and then normalized to the geometric mean of transcript levels from the housekeeping genes rpoB and gap as previously described (Kazmierczak et al., 2006). Statistical analyses of normalized lmo2570 transcript levels were performed using one-way ANOVA and Tukey's studentized range (honestly significant difference or HSD) test, performed in SAS 9.0 (SAS Institute).
Nisin survival assay
The objective of this assay was to measure relative survival characteristics of stationary phase L. monocytogenes 10403S, ΔsigB, ΔsigL, ΔsigB/ΔsigL, and Δlmo2570 strains in the presence of an initially lethal concentration of nisin (150 AU/mL nisin). Strains were grown in BHI at 37°C with shaking overnight, followed by a 1% subculture and growth to logarithmic phase (OD600 = 0.4), followed by a second subculture and growth to stationary phase (OD600 = 1.0 + 3 h), followed by a third 1% subculture (0.5 into 50 mL, final concentration of ∼2 × 107 CFU/mL) in a 300 mL flask (Bellco, Vineland, NJ). Nisin (150 AU/mL, pH 7.0) was added to the BHI, and cultures were incubated at 37°C with shaking for an additional 9 h. Bacterial numbers were determined before and after the addition of nisin. Specifically, samples were taken at 0, 0.5, 1, 2, 3, 4, 5, 6, and 9 h postaddition and spiral plated on BHI agar using a Spiral Biotech Autoplate® 4000 (Spiral Biotech, Norwood, MA). Colonies were enumerated with a QCount™ (Spiral Biotech) after 24 h incubation at 37°C. Colony counts were transformed to log10 CFU/mL.
Data from the nisin survival assay were used to calculate two parameters: (i) bacterial reduction after 0.5 h of nisin exposure and (ii) growth rate during recovery and re-growth (between 1 and 9 h after nisin exposure). Linear regression was used to determine the slope representing the change in bacterial numbers for each strain from 1 to 9 h (i.e., the period when viable cell numbers were increasing [re-growth]); this value represents the bacterial growth rate in log10 growth/h. Statistical analyses were then performed on both parameters. First, a one-way ANOVA (with Dunett's t-test or Tukey's studentized range [HSD] test) was performed to determine if (i) bacterial reduction or (ii) growth rate differed between the mutant strains and the parent strain. To determine if there were statistically significant interaction effects between the sigB and the sigL deletions, a two-way ANOVA (with Tukey's adjustment for multiple comparisons) was performed. In this model, the dependent variable was either (i) bacterial reduction after nisin exposure or (ii) growth rate during re-growth; the independent variables included sigB + sigL + sigB*sigL + replicate. The factors sigB and sigL in the model indicate the presence or absence of that gene in the strains tested.
Results
No intragenus competition was evident between L. monocytogenes and the other Listeria strains tested (Table 1). Specifically, no zoi occurred between any of the listerial species that were used as spotting strains (L. innocua, L. ivanovii, L. welshimeri, or L. seeligeri) and any of the L. monocytogenes lawn strains (10403S, ΔsigB, ΔsigL, or ΔsigB/ΔsigL; data not shown).
lmo2570 is σB, but not σL dependent and does not contribute to resistance to nisin or SdpC
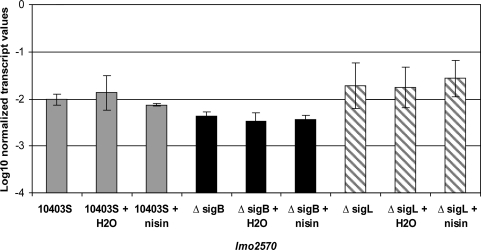
qRT-PCR was initially used to determine whether either σB or σL contributes to transcription of lmo2570, a putative bacteriocin immunity gene (Fig. 1). lmo2570 transcript levels were consistently and significantly lower in the ΔsigB strain as compared to the 10403S parent strain (p < 0.05; Fig. 1), indicating σB-dependent transcription of lmo2570. While lmo2570 transcript levels were consistently higher in the ΔsigL strain as compared to the parent strain, this difference was not significant (p > 0.05; Fig. 1). The presence of nisin at a sublethal concentration (75 AU/mL) did not affect lmo2570 transcript levels (Fig. 1). Normalized lmo2570 transcript levels in 10403S were low, ranging from 0.007 to 0.034. To put these low lmo2570 transcript levels into biological context, in 10403S, ∼0.02 transcripts of lmo2570 were present relative to the mean transcript levels of the highly expressed housekeeping genes, rpoB and gap, as indicated in Fig. 1. The lmo2570 transcript levels observed in the present study are on the same order of magnitude as those reported previously for other σB-dependent genes [e.g., opuCA and bsh (Chan et al., 2007)].
FIG. 1.
Normalized log-transformed lmo2570 transcript levels for Listeria monocytogenes 10403S (gray bars), ΔsigB (black bars), and ΔsigL (hatched bars). Transcript levels were determined by quantitative reverse-transcriptase–polymerase chain reaction using RNA isolated from logarithmic phase (OD600 = 0.4) L. monocytogenes that had been (i) incubated for 10 min after addition of nisin in sterile distilled water to yield a final concentration of 75 AU/mL nisin; (ii) incubated for 10 min after addition of an equivalent volume of sterile distilled water without nisin; or (iii) incubated for 10 min without any addition. Transcript levels were log transformed and normalized to the geometric mean of the transcript levels for the housekeeping genes rpoB and gap. Values represent mean transcript levels from three independent RNA collections; error bars indicate one standard deviation from each mean. Overall ANOVA showed a significant effect of the factor strain, but no effect of the factor condition (i.e., no addition, addition of water, or addition of nisin). Tukey's test showed significantly lower transcript levels for lmo2570 in the ΔsigB strain as compared to the parent strain; transcript levels did not differ significantly between the ΔsigL strain and the parent strain.
Spot-on-lawn assays were used to compare the sensitivities of L. monocytogenes 10403S and Δlmo2570 to SdpC, the antimicrobial peptide whose cognate immunity gene shares amino acid similarity with Lmo2570. Specifically, B. subtilis PY79 (which naturally produces SdpC) and B. subtilis EG351 (which overexpresses SdpC in the presence of isopropyl-β-D-thiogalactopyranoside) were spotted on lawns of either 10403S or Δlmo2570. Zoi for 10403S and Δlmo2570 (Table 2) did not differ significantly (p > 0.05), indicating that lmo2570 does not contribute to SdpC resistance. Neither 10403S nor Δlmo2570 showed inhibition by 10403S (Table 2) or by any other Listeria species (data not shown), indicating absence of intragenus inhibition, at least among the strains tested.
Table 2.
Spot-on-Lawn Assay Results
| |
Mean zoi radii (SD) for L. monocytogenes strainsb |
||||
|---|---|---|---|---|---|
| Strain spotteda | 10403S | Δlmo2570 | ΔsigB | ΔsigL | ΔsigB/ΔsigL |
| L. monocytogenes 10403S | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| B. subtilis PY79 | 11.8 (1.6) | 10.0 (2.2) | 11.5 (4.9) | 16.7 (0.6) | 20.5c (2.3) |
| B. subtilis EG351 (IPTG) | 13.5 (3.9) | 8.3 (2.9) | 28.2c (1.6) | 20.7c (5.3) | 32.0c (2.6) |
Strains spotted on the lawns are listed in left column; the average zoi radius around each spot is shown for each lawn, with SD in parentheses.
Radii were determined from three independent experiments by measuring diameters of the zoi in pixels using Adobe® Photoshop® CS.
Values that are significantly different (p < 0.05; one-way ANOVA with Dunnett's t-test) from the zoi produced on the L. monocytogenes 10403S lawn.
IPTG, isopropyl-β-D-thiogalactopyranoside; zoi, zone of inhibition; SD, standard deviations; ANOVA, analysis of variance.
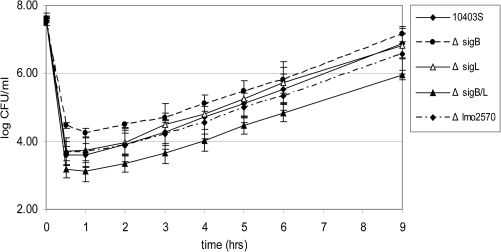
To characterize the responses of stationary phase L. monocytogenes 10403S and Δlmo2570 to nisin, we evaluated survival of ∼2 × 107 CFU/mL 10403S or Δlmo2570 in BHI in the presence of 150 AU nisin/mL (Fig. 2). Exposure to nisin for 30 min led to 4.0 and 3.9 log reductions in bacterial numbers for 10403S and Δlmo2570, respectively (Fig. 2), indicating no difference in nisin susceptibility between these strains. After the initial killing by nisin, bacterial numbers increased between 1 and 9 h post-nisin exposure, reflecting growth of cells that had survived nisin exposure. Specifically, after 9 h, bacterial numbers were 6.9 and 6.6 log for the parent strain and Δlmo2570, respectively, further supporting that Δlmo2570 and 10403S susceptibilities to nisin do not differ.
FIG. 2.
Viable numbers of stationary-phase L. monocytogenes 10403S, Δlmo2570, ΔsigB, ΔsigL, and ΔsigB/ΔsigL at various time points after exposure to 150 AU/mL nisin. Values are reported as log CFU/mL. Data shown represent the average of four independent experiments; error bars represent one standard deviation from each mean.
σB and σL both contribute to resistance to the SdpC antimicrobial peptide produced by B. subtilis
Spot-on-lawn assays were used to compare the sensitivities of L. monocytogenes 10403S, ΔsigB, ΔsigL, and ΔsigB/ΔsigL to the antimicrobial peptide SdpC. The zoi produced by B. subtilis PY79 did not differ between L. monocytogenes 10403S and ΔsigB or ΔsigL; the zoi produced by PY79 on the ΔsigB/ΔsigL lawn was significantly larger than the zoi produced on 10403S (p < 0.05; Table 2), suggesting the possibility that σB and σL contribute to SdpC resistance in an additive fashion. B. subtilis EG351 produced significantly larger zoi on ΔsigB (28.2 ±1.6 px), ΔsigL (20.7 ± 5.3 px), and ΔsigB/ΔsigL lawns (32.0 ±2.6 px), as compared to the zoi produced on 10403S (13.5 ± 3.9; Table 2). Two-way ANOVA analyses of zoi data showed no significant “sigB*sigL” interaction effect on SdpC sensitivity (p > 0.05), further supporting the notion of additive (as compared to multiplicative) contributions of σB and σL to SdpC resistance. Overall, results from this assay indicate that alternative sigma factors σB and σL both contribute to resistance to SdpC.
σB and σL both contribute to response to the bacteriocin nisin
A 30 min exposure to nisin (150 AU/mL) resulted in a 4.0 log reduction in bacterial numbers for stationary phase 10403S. By comparison, reduction of ΔsigB bacterial numbers was significantly less (3.0 log reduction; p < 0.05; Fig. 2), indicating increased nisin resistance of this strain relative to that of 10403S. Bacterial numbers for the ΔsigL and ΔsigB/ΔsigL strains were reduced by 3.9 and 4.5 log; these reductions were not significantly different from that of 10403S (p > 0.05; Dunett's t-test). Interestingly, log reduction for the ΔsigB/ΔsigL strain was significantly (p > 0.05; Tukey's HSD) greater (4.5 log) as compared to the ΔsigB strain (3.0 log reduction), indicating a significant effect of the sigL deletion on nisin killing in a ΔsigB background. Based on the reductions in bacterial numbers between 0 and 0.5 h, two-way ANOVA analyses found a significant sigL*sigB interaction effect on survival after nisin exposure, indicating that the effect of one sigma factor on survival differs depending on the presence or absence of the other sigma factor.
After initial killing by nisin, all strains showed re-growth between 1 and 9 h post-nisin exposure. Rates of re-growth, as represented by the slopes of the graphs between 1 and 9 h postexposure, were compared among the strains. While the growth rate for the ΔsigL strain (0.39 log CFU/h) was not significantly different from that of the parent strain (0.41 log CFU/h), both the ΔsigB and the ΔsigB/ΔsigL strains showed significantly slower growth rates (0.36 log CFU/h for both; p < 0.05, Dunett's t-test) as compared to the parent strain. Although no significant sigB*sigL interaction effect on nisin survival was identified by two-way ANOVA analysis, the sigB deletion had a significant effect on growth rate, with sigB deletion strains (i.e., ΔsigB and ΔsigBΔsigL) showing slower growth rates as compared to the corresponding strains with intact sigB genes.
Discussion
We hypothesized that alternative sigma factors σB and σL and the hypothetical bacteriocin immunity gene, lmo2570, contribute to L. monocytogenes antimicrobial response. This hypothesis was based on previous observations, including (i) σB and its homolog σF contribute to antimicrobial response in other Gram-positive organisms (Bischoff and Berger-Bachi, 2001; Mascher et al., 2003; Michele et al., 1999), (ii) L. monocytogenes σL controls sensitivity to class IIa bacteriocins, mesentericin Y105, pediocin PA-1, and enterocin A, and (iii) the putative σB-dependent lmo2570 has sequence homology to the B. subtilis bacteriocin immunity gene, sdpI. To test our hypothesis, we assessed the sensitivities of strains with null mutations in sigB, sigL, sigB/sigL, or lmo2570 to the antimicrobial peptides SdpC and nisin. We also characterized transcription of lmo2570 in 10403S, ΔsigB, and ΔsigL strains exposed to a subminimal inhibitory concentration of nisin. Our results show that (i) while lmo2570 is σB dependent, it does not contribute to resistance to SdpC or nisin and (ii) both σB and σL contribute to resistance to the antimicrobial peptide SdpC, as shown by results from spot-on-lawn assays. In addition, both σB and σL affect L. monocytogenes sensitivity to nisin in broth survival assays. Specifically, while loss of only sigB renders the resulting strain more resistant to nisin than the parent strain, loss of sigB in a ΔsigL background leads to reduced nisin resistance relative to the original parent strain.
The effects of antimicrobial peptides on L. monocytogenes appear to differ depending on the class of peptide, the strain, initial number of bacteria, growth phase, and the assay used for evaluation. To illustrate, σB was reported previously to contribute to L. monocytogenes tolerance to nisin or lacticin 3147 in broth assays (Begley et al., 2006), but not to nisin, lacticin 3147, or sakacin A resistance in agar overlay assays (Moorhead and Dykes, 2003; Begley et al., 2006). Moorhead and Dykes (2003) showed that an L. monocytogenes serotype 1/2a wild-type strain was less resistant to nisin than a serotype 4c wild-type strain, suggesting differences in antimicrobial sensitivities among strains. It is also likely that other environmental stresses (in addition to the presence of the antimicrobial peptide) imposed upon the cells also evoke differential phenotypic responses from the cells (e.g., exposure to low pH induces σB activity in L. monocytogenes), which may provide cross-resistance to multiple stresses (Ferreira et al., 2003).
lmo2570 is σB dependent, but does not contribute to antimicrobial resistance
L. monocytogenes Lmo2570 is 45% similar at the amino acid level to the B. subtilis immunity protein SdpI, which confers immunity against SdpC (Butcher and Helmann, 2006; Ellermeier et al., 2006); therefore, we hypothesized that lmo2570 may play a role in antimicrobial immunity in L. monocytogenes. lmo2570 was predicted as σB dependent in previous microarray experiments (Kazmierczak et al., 2003). As previous reports have shown that bacteriocin immunity genes can contribute to resistance to multiple antimicrobials (Matsumoto-Nakano and Kuramitsu, 2006), in addition to examining its role in SdpC resistance, we also tested the contributions of lmo2570 to resistance to the commercially available bacteriocin, nisin. The Δlmo2570 strain did not show reduced sensitivity to either SdpC or nisin. Exposure to nisin did not induce transcription of lmo2570 in either the wild-type or any of the mutant strains. lmo2570 thus does not appear to be important for SdpC or nisin resistance in L. monocytogenes. A role for this gene in resistance to other bacteriocins or in contributing to nisin and SdpC resistance under environmental conditions not tested here cannot be excluded by our data, however. Our confirmation of lmo2570 as σB dependent suggests a role for lmo2570 in L. monocytogenes survival or growth under conditions that remain to be defined.
σB and σL both contribute to L. monocytogenes response to SdpC and nisin
We found clear evidence that alternative sigma factors σL and σB both contribute to SdpC resistance. Specifically, as determined in a spot-on-lawn assay, we showed that both the ΔsigB and the ΔsigL strains were significantly more susceptible to the bactericidal effect of the antimicrobial peptide SdpC produced by B. subtilis EG351 than the otherwise isogenic 10403S parent strain. Characterization of a ΔsigB/ΔsigL double-mutant strain suggested that deletion of both genes had an additive, but not an interactive, effect on SdpC resistance. However, deletions of both ΔsigB and ΔsigL had an interactive effect on L. monocytogenes resistance to nisin. Specifically, while the ΔsigB/ΔsigL strain showed decreased resistance to nisin as compared to the ΔsigB strain, the ΔsigB strain showed increased resistance to nisin as compared to the parent strain, which has both sigB and sigL intact. The interactive effect observed after the loss of both sigB and sigL may indicate that at least some genes important for recovery and re-growth after nisin exposure are coregulated, either directly or indirectly, by these alternative sigma factors. We also found that re-growth of both the ΔsigB and the ΔsigB/ΔsigL strains after nisin exposure was slower than that of the parent strain, consistent with previous observation that σB is important for B. subtilis recovery after rifampin treatment (Bandow et al., 2002). The overall observation that a deletion of the gene encoding σL (i.e., a single deletion) does not affect L. monocytogenes resistance to nisin is consistent with observations by Dalet et al. (2000), who reported that σL (which has also been designated as RpoN) is not involved in L. monocytogenes nisin resistance. Relative to its otherwise isogenic parent, a ΔrpoN L. monocytogenes strain (i.e., a strain lacking σL) has previously shown increased resistance to the class IIa nonlantibiotic bacteriocins mesentericin Y105, pediocin PA-1, and enterocin A (Robichon et al., 1997; Dalet et al., 2000), consistent with our finding that σL contributes to resistance to some bacteriocins (i.e., SdpC). Our findings, as well as previous findings by others (e.g., Dalet et al., 2000), thus support that different regulatory elements are critical for the ability of L. monocytogenes, and other bacteria, to respond to different bacteriocins, a notion consistent with the diverse nature of this group of antibacterial compounds.
Overall, our data indicate that σB and σL both contribute to the ability of L. monocytogenes to respond to antimicrobials. Regulatory interactions among multiple alternative sigma factors also have been shown to contribute to antibiotic resistance in B. subtilis. Specifically, three (σM, σW, and σX) of the seven B. subtilis extracytoplasmic function alternative sigma factors have overlapping regulons that contribute to antibiotic resistance, as demonstrated by the greatly enhanced sensitivity of a triple MWX mutant to various antimicrobials, including nisin (Mascher et al., 2007). Strains bearing single or double mutations in the genes encoding these alternative sigma factors displayed considerably less antimicrobial sensitivity than the strain with the triple mutation (Mascher et al., 2007). Thus, in combination with previous studies, our data support a model in which multiple alternative sigma factors contribute to regulatory networks important for fine-tuning transcriptional regulation of gene expression to help optimize bacterial cell resistance to antimicrobial peptides.
Conclusions
Alternative sigma factors have been shown to regulate genes and operons critical for resistance to antimicrobials in various bacteria, including B. subtilis, L. monocytogenes, Salmonella enterica serovar Typhimurium, S. aureus, and Vibrio cholerae (Robichon et al., 1997; Crouch et al., 2005; Zhang et al., 2005; Butcher and Helmann, 2006; Mathur et al., 2007). Our data indicate that σB and σL, as well as the simultaneous presence of both σB and σL, contribute to antimicrobial response in L. monocytogenes in a manner that is dependent on the antimicrobial that is present. The results reported in this study provide further evidence of the importance of regulatory networks for fine-tuning L. monocytogenes responses to changing environmental conditions (Chaturongakul et al., 2008).
Acknowledgments
The project described was supported by National Institutes of Health Award No. 5R01AI052151-07 (to K.J.B.). The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors thank Dr. J. Helmann for the gift of B. subtilis strains used in this study, and Dr. S. Chaturongakul and B. Bowen for the creation of the ΔsigL and ΔsigB/ΔsigL strains. We also thank Dr. T. Bergholz for helpful discussions concerning statistical analyses.
Disclosure Statement
No competing financial interests exist.
References
- Abee T. Delves-Broughton J. Bacteriocins—nisin. In: Russell NJ, editor; Gould GW, editor. Food Preservatives. New York City: Springer; 2003. pp. 146–169. [Google Scholar]
- Bandow JE. Brotz H. Hecker M. Bacillus subtilis tolerance of moderate concentrations of rifampin involves the σB-dependent general and multiple stress response. J Bacteriol. 2002;184:459–467. doi: 10.1128/JB.184.2.459-467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LA. Cetin MS. Hutkins RW. Benson AK. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J Bacteriol. 1998;180:4547–4554. doi: 10.1128/jb.180.17.4547-4554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley M. Hill C. Ross RP. Tolerance of Listeria monocytogenes to cell envelope-acting antimicrobial agents is dependent on SigB. Appl Environ Microbiol. 2006;72:2231–2234. doi: 10.1128/AEM.72.3.2231-2234.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkerroum N. Sandine WE. Inhibitory action of nisin against Listeria monocytogenes. J Dairy Sci. 1988;71:3237–3245. doi: 10.3168/jds.S0022-0302(88)79929-4. [DOI] [PubMed] [Google Scholar]
- Bischoff M. Berger-Bachi B. Teicoplanin stress-selected mutations increasing σB activity in Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1714–1720. doi: 10.1128/AAC.45.6.1714-1720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DK. Hinrichs DJ. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- Bonnet M. Rafi MM. Chikindas ML. Montville TJ. Bioenergetic mechanism for nisin resistance, induced by the acid tolerance response of Listeria monocytogenes. Appl Environ Microbiol. 2006;72:2556–2563. doi: 10.1128/AEM.72.4.2556-2563.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno ME. Kaiser A. Montville TJ. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992;58:2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher BG. Helmann JD. Identification of Bacillus subtilis σW-dependent genes that provide intrinsic resistance to antimicrobial compounds produced by Bacilli. Mol Microbiol. 2006;60:765–782. doi: 10.1111/j.1365-2958.2006.05131.x. [DOI] [PubMed] [Google Scholar]
- Chan YC. Boor KJ. Wiedmann M. σB-dependent and σB-independent mechanisms contribute to transcription of Listeria monocytogenes cold stress genes during cold shock and cold growth. Appl Environ Microbiol. 2007;73:6019–6029. doi: 10.1128/AEM.00714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PF. Foster SJ. Ingham E. Clements MO. The Staphylococcus aureus alternative sigma factor σB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol. 1998;180:6082–6089. doi: 10.1128/jb.180.23.6082-6089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturongakul S. Raengpradub S. Wiedmann M. Boor KJ. Modulation of stress and virulence in Listeria monocytogenes. Trends Microbiol. 2008;16:388–396. doi: 10.1016/j.tim.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland J. Montville TJ. Nes IF. Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1–20. doi: 10.1016/s0168-1605(01)00560-8. [DOI] [PubMed] [Google Scholar]
- Crouch M-L. Becker LA. Bang I-S. Tanabe H. Ouellette AJ. Fang FC. The alternative sigma factor σE is required for resistance of Salmonella enterica serovar Typhimurium to anti-microbial peptides. Mol Microbiol. 2005;56:789–799. doi: 10.1111/j.1365-2958.2005.04578.x. [DOI] [PubMed] [Google Scholar]
- Dalet K. Briand C. Cenatiempo Y. Hechard Y. The rpoN gene of Enterococcus faecalis directs sensitivity to subclass IIa bacteriocins. Curr Microbiol. 2000;41:441–443. doi: 10.1007/s002840010164. [DOI] [PubMed] [Google Scholar]
- de los Santos PE. Parret AHA. de Mot R. Stress-related Pseudomonas genes involved in production of bacteriocin LlpA. FEMS Microbiol Lett. 2005;244:243–250. doi: 10.1016/j.femsle.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Eijsink VG. Axelsson L. Diep DB. Havarstein LS. Holo H. Nes IF. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie Leeuwenhoek. 2002;81:639–654. doi: 10.1023/a:1020582211262. [DOI] [PubMed] [Google Scholar]
- Ellermeier CD. Hobbs EC. Gonzalez-Pastor JE. Losick R. A three-protein signaling pathway governing immunity to a bacterial cannibalism toxin. Cell. 2006;124:549–559. doi: 10.1016/j.cell.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Engelke G. Gutowski-Eckel Z. Kiesau P. Siegers K. Hammelmann M. Entian KD. Regulation of nisin biosynthesis and immunity in Lactococcus lactis 6F3. Appl Environ Microbiol. 1994;60:814–825. doi: 10.1128/aem.60.3.814-825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A. O'Byrne CP. Boor KJ. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl Environ Microbiol. 2001;67:4454–4457. doi: 10.1128/AEM.67.10.4454-4457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A. Sue D. O'Byrne CP. Boor KJ. Role of Listeria monocytogenes σB in survival of lethal acidic conditions and in the acquired acid tolerance response. Appl Environ Microbiol. 2003;69:2692–2698. doi: 10.1128/AEM.69.5.2692-2698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M. Chikindas ML. Listeria: a foodborne pathogen that knows how to survive. Int J Food Microbiol. 2007;113:1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Gravesen A. Jydegaard Axelsen AM. Mendes da Silva J. Hansen TB. Knochel S. Frequency of bacteriocin resistance development and associated fitness costs in Listeria monocytogenes. Appl Environ Microbiol. 2002a;68:756–764. doi: 10.1128/AEM.68.2.756-764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravesen A. Ramnath M. Rechinger KB. Andersen N. Jansch L. Hechard Y. Hastings JW. Knochel S. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiology. 2002b;148:2361–2369. doi: 10.1099/00221287-148-8-2361. [DOI] [PubMed] [Google Scholar]
- Ho SN. Hunt HD. Horton RM. Pullen JK. Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Huot E. Barrena-Gonzalez C. Petitdemange H. Comparative effectiveness of nisin and bacteriocin J46 at different pH values. Lett Appl Microbiol. 1996;22:76–79. doi: 10.1111/j.1472-765x.1996.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Jack RW. Tagg JR. Ray B. Bacteriocins of Gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak MJ. Mithoe SC. Boor KJ. Wiedmann M. Listeria monocytogenes σB regulates stress response and virulence functions. J Bacteriol. 2003;185:5722–5734. doi: 10.1128/JB.185.19.5722-5734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak MJ. Wiedmann M. Boor KJ. Contributions of Listeria monocytogenes σB and PrfA to expression of virulence and stress response genes during extra- and intracellular growth. Microbiology. 2006;152:1827–1838. doi: 10.1099/mic.0.28758-0. [DOI] [PubMed] [Google Scholar]
- Klaenhammer TR. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–85. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Liu W. Hansen JN. Some chemical and physical properties of nisin, a small-protein antibiotic produced by Lactococcus lactis. Appl Environ Microbiol. 1990;56:2551–2558. doi: 10.1128/aem.56.8.2551-2558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T. Hachmann A-B. Helmann JD. Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function σ factors. J Bacteriol. 2007;189:6919–6927. doi: 10.1128/JB.00904-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T. Margulis NG. Wang T. Ye RW. Helmann JD. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol Microbiol. 2003;50:1591–1604. doi: 10.1046/j.1365-2958.2003.03786.x. [DOI] [PubMed] [Google Scholar]
- Mathur J. Davis BM. Waldor MK. Antimicrobial peptides activate the Vibrio cholerae σE regulon through an OmpU-dependent signalling pathway. Mol Microbiol. 2007;63:848–858. doi: 10.1111/j.1365-2958.2006.05544.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto-Nakano M. Kuramitsu HK. Role of bacteriocin immunity proteins in the antimicrobial sensitivity of Streptococcus mutans. J Bacteriol. 2006;188:8095–8102. doi: 10.1128/JB.00908-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS. Slutsker L. Dietz V. McCaig LF. Bresee JS. Shapiro C. Griffin PM. Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michele TM. Ko C. Bishai WR. Exposure to antibiotics induces expression of the Mycobacterium tuberculosis sigF gene: implications for chemotherapy against Mycobacterial persistors. Antimicrob Agents Chemother. 1999;43:218–225. doi: 10.1128/aac.43.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead SM. Dykes GA. The role of the sigB gene in the general stress response of Listeria monocytogenes varies between a strain of serotype 1/2a and a strain of serotype 4c. Curr Microbiol. 2003;46:461–466. doi: 10.1007/s00284-002-3867-6. [DOI] [PubMed] [Google Scholar]
- Muriana PM. Bacteriocins for control of Listeria spp. in food. J Food Prot. 1996;59(Supplement):54–63. doi: 10.4315/0362-028X-59.13.54. [DOI] [PubMed] [Google Scholar]
- Nissen-Meyer J. Nes IF. Ribosomally synthesized antimicrobial peptides: their function, structure, biogenesis, and mechanism of action. Arch Microbiol. 1997;167:67–77. [PubMed] [Google Scholar]
- Raengpradub S. Wiedmann M. Boor KJ. Comparative analysis of the σB-dependent stress responses in Listeria monocytogenes and Listeria innocua strains exposed to selected stress conditions. Appl Environ Microbiol. 2008;74:158–171. doi: 10.1128/AEM.00951-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichon D. Gouin E. Debarbouille M. Cossart P. Cenatiempo Y. Hechard Y. The rpoN (sigma54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J Bacteriol. 1997;179:7591–7594. doi: 10.1128/jb.179.23.7591-7594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab U. Bowen B. Nadon C. Wiedmann M. Boor KJ. The Listeria monocytogenes prfAP2 promoter is regulated by sigma B in a growth phase dependent manner. FEMS Microbiol Lett. 2005;245:329–336. doi: 10.1016/j.femsle.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Sue D. Fink D. Wiedmann M. Boor KJ. σB-dependent gene induction and expression in Listeria monocytogenes during osmotic and acid stress conditions simulating the intestinal environment. Microbiology. 2004;150:3843–3855. doi: 10.1099/mic.0.27257-0. [DOI] [PubMed] [Google Scholar]
- Venema K. Venema G. Kok J. Lactococcal bacteriocins: mode of action and immunity. Trends Microbiol. 1995;3:299–304. doi: 10.1016/s0966-842x(00)88958-1. [DOI] [PubMed] [Google Scholar]
- Wiedmann M. Arvik TJ. Hurley RJ. Boor KJ. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J Bacteriol. 1998;180:3650–3656. doi: 10.1128/jb.180.14.3650-3656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodling SE. Moraru CI. Influence of surface topography on the effectiveness of pulsed light treatment for the inactivation of Listeria innocua on stainless-steel surfaces. J Food Sci. 2005;70:m345–m351. [Google Scholar]
- Youngman P. Perkins JB. Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]
- Zhang H. Morikawa K. Ohta T. Kato Y. In vitro resistance to the CSαβ-type antimicrobial peptide ASABF-α is conferred by overexpression of sigma factor sigB in Staphylococcus aureus. J Antimicrob Chemother. 2005;55:686–691. doi: 10.1093/jac/dki070. [DOI] [PubMed] [Google Scholar]