Abstract
Inulin, a naturally occurring, functional food ingredient found in various edible plants, has been reported to exert potential health benefits, including decreased risk of colonic diseases, non–insulin-dependent diabetes, obesity, osteoporosis, and cancer. However, the mechanism of the antidiabetic activity of inulin has not yet been elucidated. In this study, we showed that inulin increased the uptake of glucose in C2C12 myotubes, which was associated with both AMP-activated protein kinase (AMPK) and phosphatidylinositol 3-kinase (PI3-K) signaling pathways, but both of these pathways appeared to transmit their signals in an independent manner. Moreover, we found that inulin was able to increase the uptake of glucose in C2C12 myotubes in which insulin resistance was induced by exposing cells to high glucose concentrations. The identical effects of inulin were also observed in HepG2 hepatoma cells. Collectively, we report the antidiabetic activity of inulin and further demonstrate for the first time that such activity is associated with AMPK and PI3-K activation.
Key Words: Akt, AMP-activated protein kinase, glucose uptake, insulin resistance, inulin, phosphatidylinositol 3-kinase
Introduction
Inulin is defined as a polydisperse carbohydrate consisting mainly of β(2 → 1) fructosyl-fructose links ranging from two to 60 units long.1 Classified as a diet fiber, naturally occurring inulin is found in a variety of edible fruits and vegetables, such as wheat, onions, leeks, garlic, asparagus, artichokes, and bananas, and has thus been part of human consumption for centuries. Recent studies have suggested that inulin has many physiological effects such as stimulating the immune system, decreasing the pathogenic bacteria in the intestine, relieving constipation, increasing mineral absorption, lowering the synthesis of triglycerides and fatty acids in the liver, and decreasing their serum levels.2 These effects are associated with the reduction of the risk of some diseases such as intestinal infections, constipation, non–insulin-dependent diabetes, obesity, osteoporosis, colonic cancer, and others.3
It is widely accepted that the addition of dietary fiber to the standardized diabetic diet is beneficial for controlling both insulin- and non–insulin-dependent diabetes. Some clinical studies on the effect of inulin on glycemic control have been reported. Luo et al.4 also reported that chronic ingestion of fructooligosaccharides, an entity that is chemically similar to inulin, decreased basal hepatic glucose production in a process separate from insulin-stimulated glucose metabolism in healthy individuals. In addition, a diet rich in fructooligosaccharides can lower blood triacylglycerols and cholesterol in rats fed high fat diets.5 Despite its common use and beneficial effect on diabetes, its mechanism of glycemic control is poorly understood.
It is well known that insulin plays a central role in glycemic control, and it accelerates glucose transport via activation of phosphatidylinositol 3-kinase (PI3-K), which transmits the signal to the phosphorylation and activation of Akt, leading to glucose transporter 4 (GLUT4) translocation to the plasma membrane in muscle cells.6 Exercise is also known to increase translocation of GLUT4, and AMP-activated protein kinase (AMPK) has been demonstrated to play a critical role in this process.7,8 AMPK is a phylogenetically conserved intracellular energy sensor that plays a central role in the regulation of glucose and lipid metabolism. AMPK, a heterotrimeric complex comprising a catalytic α-subunit and regulatory β- and γ-subunits, is activated when cellular energy is depleted.9 When activated by allosteric binding of AMP or phosphorylation at the Thr172 catalytic subunit by AMPK kinase, AMPK accelerates ATP-generating catabolic pathways, including glucose and fatty acid oxidation,10–12 and simultaneously curtails ATP-consuming anabolic pathways, including cholesterol, fatty acid, and triacylglycerol synthesis.13 Recent investigations suggest that AMPK could potentially be beneficial as a therapeutic target in the treatment of diabetes and obesity.14 The potential role of AMPK in the treatment of type 2 diabetes has been particularly reinforced by the understanding that commonly used antidiabetic drugs, including metformin (dimethylbiguanide) and thiazolidinediones, exert their pharmacological effects via AMPK activation.15,16 In addition, the anti-obesity effects of adiponectin, leptin, and α-lipoic acid are mediated by AMPK.17–19
In the present study, we show that inulin increases the glucose uptake in C2C12 myotubes and HepG2 hepatoma by activating AMPK and PI3-K pathways and that inulin overcomes insulin resistance in both cells, revealing the underlying mechanisms of antidiabetic activity of inulin.
Materials and Methods
Materials
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). 2-Deoxy-d-[3H]glucose (6.0 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA, USA). LY294002 was purchased from TOCRIS (Bristol, UK). Compound C was a generous gift from Merck (Darmstadt, Germany). Inulin from chicory and insulin were purchased from Sigma (St. Louis, MO, USA). Antibodies that recognize the phosphorylated acetyl-coenzyme A carboxylase (ACC)α-Ser79, AMPKα-Thr172, and Akt-Ser473 were purchased from Cell Signaling Technology (Danvers, MA, USA). AMPKα antibody was purchased from Upstate Biotechnology (Lake Placid, NY, USA). Antibodies for Akt and α-actinin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture and induction of differentiation
The skeletal muscle cell line C2C12 myoblasts and HepG2 cells were maintained in DMEM supplemented with 10% heat-inactivated FBS at 37°C with 95% air and 5% CO2. To induce differentiation of C2C12 cells, growth medium was replaced with DMEM containing 1% FBS when they were confluent. Experiments were performed in differentiated C2C12 myotubes after 7 days in differentiation medium.
Glucose uptake
Cells were cultured on 12-well cluster dishes, washed with Krebs-Ringer phosphate buffer (25 mM HEPES [pH 7.4], 118 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 1.2 mM KH2PO4, 1.3 mM MgSO4, 5 mM NaHCO3, 0.07% bovine serum albumin, and 5.5 mM glucose), and incubated in serum-free media for 3 hours. After inulin or insulin treatment, cells were incubated in Krebs-Ringer phosphate buffer containing 0.5 μCi of 2-deoxy-d-[3H]glucose for 20 minutes. The reaction was terminated by placing the plates on ice and adding ice-cold phosphate-buffered saline. After three washes with phosphate-buffered saline, the cells were dissolved in 0.1% sodium dodecyl sulfate. Radioactivity was quantified by liquid scintillation counting.
Protein extract and western blot analysis
C2C12 cells grown in a six-well culture plate were washed with cold phosphate-buffered saline, and 150 μL of extraction buffer (50 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.25% sucrose, 0.4 mg/mL digitonin, and 1.5 mM phenylmethylsulfonyl fluoride) was added to the culture dish. The plate was rocked on ice for 3 minutes, and the buffer was collected for western blot analysis. The proteins in the cytosolic fractions were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto a nitrocellulose membrane, and probed using horseradish peroxidase conjugates. Protein levels of α-actinin were used as controls for equal protein loading.
Induction of insulin resistance caused by high glucose concentrations
To develop a model of insulin resistance, C2C12 myoblasts and HepG2 cells were cultured in normal concentrations of glucose (5.5 mM d-glucose) and DMEM supplemented with 10% heat-inactivated FBS for 2 weeks and were then incubated with serum-free DMEM containing high concentrations of glucose (30 mM d-glucose) for 24 hours. Their response to insulin (100 nM for 10 minutes) was then tested as described.20
Statistical analysis
All data are presented as mean ± SE values. Statistical analysis was performed by a two-tailed unpaired Student's t test. A value of P < .05 was accepted as statistically significant.
Results
Inulin increases glucose uptake and activates AMPK and Akt in C2C12 myotubes
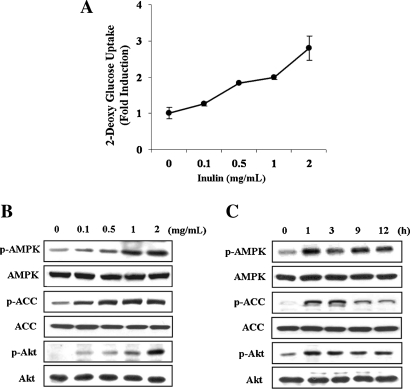
We first examined the effect of inulin on glucose uptake in C2C12 myotubes. Glucose uptake was induced in a dose-dependent manner, showing a maximum induction of approximately threefold at 2 mg/mL for 1 hour (Fig. 1A). Inulin did not influence the viability of C2C12 cells within a dose range of 0–1 mg/mL for 48 hours, but higher concentrations (2 mg/mL) revealed a slight toxicity (data not shown). Thus, concentrations of inulin up to 1 mg/mL were used in subsequent experiments. We then investigated the kinetics of AMPK and Akt activation in C2C12 myotubes that were exposed to inulin at the indicated concentrations for 1 hour (Fig. 1B) or at 1 mg/mL for the indicated time periods (Fig. 1C). Akt is a serine/threonine protein kinase that plays a critical role in the translocation of GLUT4 to the plasma membrane via a signal transduction cascade involving insulin, the insulin receptor substrate family, and PI3-K.21 The phosphorylation level of Thr172 in the active site of the AMPKα catalytic subunit, which is essential for the enzyme activity,22 was increased in a dose- and time-dependent manner (Fig. 1B and C). Furthermore, phosphorylation of Ser79 in ACC, which is the best-characterized phosphorylation site by AMPK,9 was also increased with similar kinetics. The phosphorylation of ACC-Ser79 has been shown to tightly correlate with endogenous AMPK activity.23 Under these conditions, the phosphorylation level of Akt-Ser473, which indicates activation of upstream PI3-K, was induced in a dose- and time-dependent manner. The total amount of AMPK, ACC, and Akt was the same. Therefore, these results indicate that AMPK and PI3-K/Akt pathways were activated by inulin treatment in C2C12 myotubes.
FIG. 1.
Inulin stimulates glucose uptake and activates both PI3-K/Akt and AMPK in C2C12 myotubes. C2C12 myotubes were incubated with the indicated concentrations of inulin for 1 hour or with 1 mg/mL inulin for the indicated time periods. (A) Glucose uptake was measured as described in Materials and Methods. Data are mean ± SD values for two independent assays performed in triplicate. (B and C) Protein extracts were prepared and subjected to western blot assay using anti-phosphospecific ACC-Ser79 (p-ACC), total ACC (ACC), anti-phosphospecific AMPK-Thr172 (p-AMPK), total AMPK (AMPK-α), anti-phosphospecific Akt-Ser473 (p-Akt), and total Akt (AKT) antibodies, varying (B) inulin concentration or (C) incubation time. Experiments were repeated three times with similar results, and a representative result is shown.
Inulin-induced increase of glucose uptake is associated with both AMPK and PI3-K/Akt activities
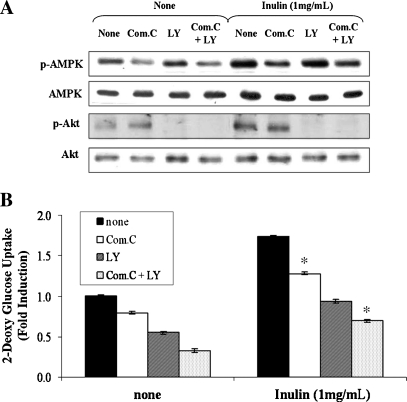
To determine whether activated AMPK or Akt was involved in the effect of inulin on glucose uptake, we attempted to inhibit the AMPK or Akt activity using a pharmacological approach. C2C12 myotubes were pretreated with compound C, an AMPK inhibitor,24 or LY294002, a PI3-K inhibitor, for 30 minutes and then exposed to 1 mg/mL inulin for 1 hour. The phosphorylations of AMPKα-Thr172 and Akt-Ser473 induced by inulin were attenuated by compound C and LY294002, respectively (Fig. 2A). Compound C and LY294002 blocked the inulin-induced glucose uptake, indicating a critical role of AMPK and PI3-K/Akt in the process (Fig. 2B). However, inhibition of PI3-K/Akt pathway by LY294002 led to an almost complete block of inulin-induced glucose uptake, whereas treatment with the AMPK inhibitor partially attenuated the inulin effect. To clarify the role of AMPK on inulin-induced glucose uptake, we evaluated the synergistic effect of both inhibitors on glucose uptake. The combined treatment (Compound C + LY294002) resulted in remarkably decreased glucose uptake. Furthermore, AMPK inhibition by Compound C exerted no effect on the inulin-induced phosphorylation of Akt-Ser473. Conversely, LY294002 did not alter inulin-induced phosphorylation of AMPK-Thr172. Thus, both AMPK and PI3-K/Akt signal pathways are required for inulin-induced glucose uptake, but both of these pathways seemed to transmit their signals in an independent manner.
FIG. 2.
Effect of inulin on glucose uptake is dependent upon both AMPK and Akt in C2C12 myotubes. C2C12 cells were pretreated with or without 20 μM compound C (Com.C) or 25 μM LY24002 for 30 minutes and then treated with 1 mg/mL inulin for 1 hour. (A) Protein extracts were prepared and subjected to western blot analysis using anti-phosphospecific AMPK-Thr172 (p-AMPK), total AMPK (AMPK-α), anti-phosphospecific Akt-Ser473 (p-Akt), and total Akt (AKT) antibodies. Experiments were repeated three times with similar results, and a representative result is shown. (B) Glucose uptake was measured as described in Materials and Methods. Data are mean ± SD values for two independent assays performed in triplicate. *P < .05 versus inulin alone.
Inulin overcomes insulin resistance in a PI3-K/Akt- and AMPK-dependent manner
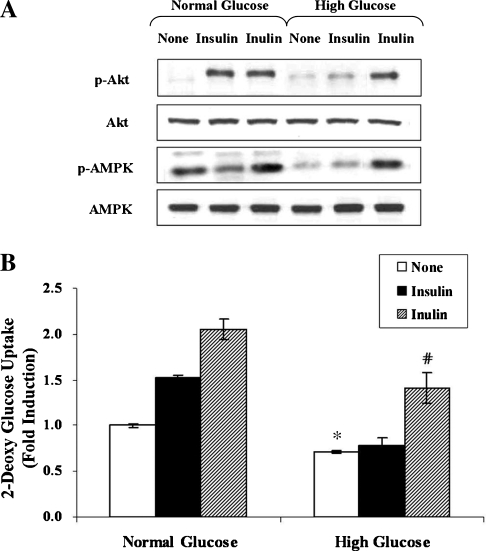
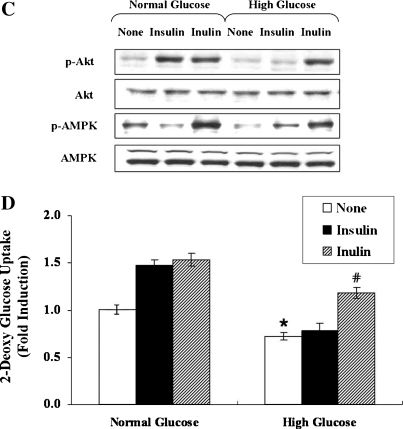
To examine the effect of inulin on glycemic control under insulin resistance conditions, we first attempted to create insulin resistance by exposing C2C12 cells to high concentrations of glucose (30 mM for 24 hours). Indeed, such treatment almost abolished phosphorylation of Akt in response to insulin (100 nM for 10 minutes) (Fig. 3A, lanes 2 and 5). Simultaneously, the basal phosphorylation level of AMPK was also decreased without a change in total AMPK protein levels (Fig. 3A, lanes 1 and 4). Next, to determine whether inulin is able to overcome insulin resistance, the cells were incubated in the absence or presence of inulin (1 mg/mL) for 1 hour. The phosphorylation of Akt and AMPK was markedly up-regulated after treatment of insulin-resistant C2C12 cells with inulin, compared to the basal level (Fig. 3A, lanes 4 and 6). In concert with these changes in phosphorylation of Akt and AMPK, glucose uptake that was decreased by high glucose concentrations was dramatically increased by almost 1.8-fold after treatment of the cells with inulin (Fig. 3B). These results demonstrate that exposure to high concentrations of glucose induces an insulin resistance-like condition, including an inhibition of the Akt and AMPK pathways, but that inulin is able to overcome the insulin resistance, resulting in increased glucose uptake via activation of Akt and AMPK pathways.
FIG. 3.
Inulin overcomes insulin resistance in a PI3-K/Akt- and AMPK-dependent manner. C2C12 cells were incubated in serum-free medium overnight and incubated in serum-free medium containing either normal (5.5 mM) or high (30 mM) concentrations of d-glucose for an additional 24 hours. Before harvesting, the cells were stimulated with 1 mg/mL inulin or 100 nM insulin for 1 hour. (A) The phosphorylation of AMPK and Akt was analyzed with anti-phospho-Ser473 Akt and anti-phospho-Thr172 AMPK antibodies, respectively. Experiments were repeated three times with similar results, and a representative result is shown. (B) Glucose uptake was measured as described in Materials and Methods. Data are mean ± SD values for two independent assays performed in triplicate. *P < .05 versus normal glucose; #P < .05 versus high glucose alone.
Inulin increases glucose uptake and overcomes insulin resistance in HepG2 cells
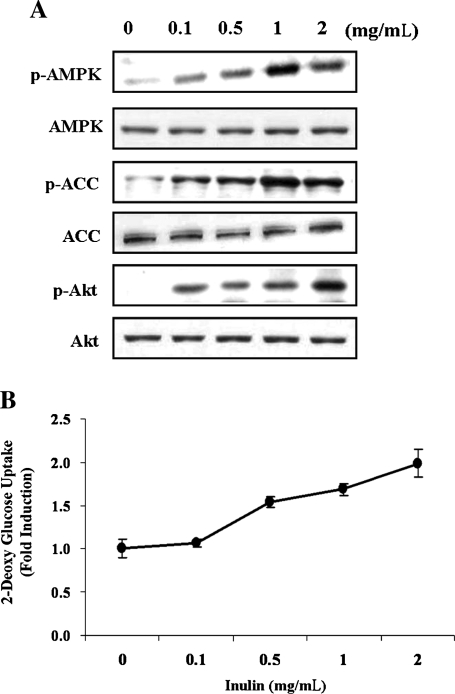
To determine the effect of inulin on glycemic control and its mechanism of action, we carried out identical experiments in HepG2 hepatoma. Inulin induced activation of AMPK and Akt (Fig. 4A) and increased glucose uptake in a dose-dependent manner (Fig. 4B). The effect of inulin on AMPK in hepatoma cells was less than that in muscle cells (Figs. 1B and 4A), which might be due to the various expression patterns of AMPK isoforms between tissues.25
FIG. 4.
Inulin increases glucose uptake and overcomes insulin resistance in HepG2 hepatoma cells. HepG2 cells were incubated with the indicated concentrations of inulin for 1 hour. (A) Protein extracts were prepared and subjected to western blot assay using anti-phosphospecific ACC-Ser79 (p-ACC), total ACC (ACC), anti-phosphospecific AMPK-Thr172 (p-AMPK), total AMPK (AMPK-α), anti-phosphospecific Akt-Ser473 (p-Akt), and total Akt (AKT) antibodies. Experiments were repeated three times with similar results, and a representative result is shown. (B) Glucose uptake was measured as described in Materials and Methods. Data are mean ± SD values for two independent assays performed in triplicate. (C) HepG2 cells were incubated in serum-free medium overnight and incubated in serum-free medium containing either normal (5.5 mM) or high (30 mM) concentrations of d-glucose for an additional 24 hours. Before harvesting, the cells were stimulated with 1 mg/mL inulin or 100 nM insulin for 1 hour. The phosphorylation of AKT, and AMPK was analyzed with anti-phospho-Ser473 Akt and anti-phospho-Thr172 AMPK antibodies, respectively. Experiments were repeated three times with similar results, and a representative result is shown. (D) Glucose uptake was measured as described in Materials and Methods. Data are mean ± SD values for two independent assays performed in triplicate. *P < .05 versus normal glucose; #P < .05 versus high glucose alone.
In a model of insulin resistance induced by high glucose concentration, inulin treatment led to activation of Akt and AMPK pathways (Fig. 4C) as well as a 1.5-fold increase in glucose uptake (Fig. 4D). The results in HepG2 cells were consistent with those in C2C12 cells.
Discussion
The present study demonstrated that inulin stimulated glucose uptake and overcame insulin resistance by activating the AMPK and PI3-K/Akt pathways in C2C12 skeletal muscle cells and HepG2 hepatoma cells. Although many beneficial properties of inulin have been reported, especially effects on diabetes, the mechanisms are poorly understood. Thus, to our knowledge, this is the first report demonstrating the antidiabetic effects of inulin and its mechanisms of action.
At this point, we do not understand the mechanism underlying AMPK activation by inulin. AMPK is activated by metabolic stresses that deplete cellular ATP, such as glucose deprivation, hypoxia, oxidative stress, hyperosmolarity, and exercise in muscle.26 Sorbitol induced AMPK activation by hyperosmolar stress (120 mM),26 but AMPK activation of inulin is not likely to be linked to hyperosmolarity considering the low concentration of inulin (0.2 mM) used in our study. On the other hand, upstream kinases like LKB1 can activate AMPK regardless of the AMP:ATP ratio.27 Further studies are required to elucidate the signaling pathways through which inulin activates AMPK.
The mechanism by which insulin enhances glucose uptake in muscle has been well established. Insulin stimulation leads to activation of PI3-K and Akt, which promotes GLUT4 translocation to plasma membrane. In contrast, accumulating evidence suggests that AMPK is likely to mediate the effect of insulin-independent stimuli for glucose uptake. Indeed, AMPK activation in response to muscle contraction, hypoxia, and hyperosmolarity correlates closely with an increase in glucose uptake in muscle.15,28 More recently, it was demonstrated that expression of a constitutively active form of AMPK stimulated glucose uptake into the cells, in association with enhanced GLUT4 translocation to the plasma membrane.29 Consistent with these reports, AMPK activation by inulin increased glucose uptake. This effect of AMPK did not involve any part of the insulin-dependent pathway such as PI3-K/Akt signaling (Fig. 3).
In summary, the current study established that inulin exerts beneficial therapeutic effects against diabetes, at least, partially via the insulin-dependent PI3-K/Akt pathway and the insulin-independent AMPK pathway. Although the mechanism by which inulin activated AMPK remains somewhat elusive, inulin could be considered a potential agent in the treatment of many metabolic diseases due to its activation of PI3-K/Akt and AMPK. Our results also suggest that the salutary effects of inulin on the lowered glucose uptake associated with insulin-resistant states depend on Akt. This is the first report to suggest a possible mechanism by which inulin affects glycemic control. Additionally, recent studies demonstrated that the AMPK cascade plays a central role in the regulation of lipid metabolism in response to the adipocyte-derived hormones, leptin and adiponectin. Thus, further research into the possible effects of inulin on lipid metabolism via AMPK activation will contribute to our understanding of the pathogenesis of obesity and diabetes.
Acknowledgments
This work was supported by the Seoul R&BD program.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Flamm G. Glinsmann W. Kritchevsky D. Prosky L. Roberfroid M. Inulin and oligofructose as dietary fiber: a review of the evidence. Crit Rev Food Sci Nutr. 2001;41:353–362. doi: 10.1080/20014091091841. [DOI] [PubMed] [Google Scholar]
- 2.Kaur N. Gupta AK. Applications of inulin and oligofructose in health and nutrition. J Biosci. 2002;27:703–714. doi: 10.1007/BF02708379. [DOI] [PubMed] [Google Scholar]
- 3.Roberfroid MB. Functional foods: concepts and application to inulin and oligofructose. Br J Nutr. 2002;87(Suppl 2):S139–S143. doi: 10.1079/BJNBJN/2002529. [DOI] [PubMed] [Google Scholar]
- 4.Luo J. Rizkalla SW. Alamowitch C, et al. Chronic consumption of short-chain fructooligosaccharides by healthy subjects decreased basal hepatic glucose production but had no effect on insulin-stimulated glucose metabolism. Am J Clin Nutr. 1996;63:939–945. doi: 10.1093/ajcn/63.6.939. [DOI] [PubMed] [Google Scholar]
- 5.Kok NN. Taper HS. Delzenne NM. Oligofructose modulates lipid metabolism alterations induced by a fat-rich diet in rats. J Appl Toxicol. 1998;18:47–53. doi: 10.1002/(sici)1099-1263(199801/02)18:1<47::aid-jat474>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q. Somwar R. Bilan PJ, et al. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurth-Kraczek EJ. Hirshman MF. Goodyear LJ. Winder WW. 5′AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes. 1999;48:1667–1671. doi: 10.2337/diabetes.48.8.1667. [DOI] [PubMed] [Google Scholar]
- 8.Musi N. Fujii N. Hirshman MF, et al. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50:921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- 9.Hardie DG. Carling D. The AMP-activated protein kinase—fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 10.Makinde AO. Gamble J. Lopaschuk GD. Upregulation of 5′-AMP-activated protein kinase is responsible for the increase in myocardial fatty acid oxidation rates following birth in the newborn rabbit. Circ Res. 1997;80:482–489. doi: 10.1161/01.res.80.4.482. [DOI] [PubMed] [Google Scholar]
- 11.Ai H. Ihlemann J. Hellsten Y, et al. Effect of fiber type and nutritional state on AICAR- and contraction-stimulated glucose transport in rat muscle. Am J Physiol Endocrinol Metab. 2002;282:E1291–E1300. doi: 10.1152/ajpendo.00167.2001. [DOI] [PubMed] [Google Scholar]
- 12.Zong H. Ren JM. Young LH, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henin N. Vincent MF. Gruber HE. Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995;9:541–546. doi: 10.1096/fasebj.9.7.7737463. [DOI] [PubMed] [Google Scholar]
- 14.Kahn BB. Alquier T. Carling D. Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Musi N. Hirshman MF. Nygren J, et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 16.LeBrasseur NK. Kelly M. Tsao TS, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291:E175–E181. doi: 10.1152/ajpendo.00453.2005. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T. Kamon J. Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 18.Minokoshi Y. Kim YB. Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 19.Kim MS. Park JY. Namkoong C, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 20.Zang M. Zuccollo A. Hou X, et al. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 21.Alessi DR. Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 22.Hawley SA. Davison M. Woods A, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 23.Lee M. Hwang JT. Lee HJ, et al. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 24.Zhou G. Myers R. Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung PC. Salt IP. Davies SP. Hardie DG. Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J. 2000;346:659–669. [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi T. Hirshman MF. Fujii N. Habinowski SA. Witters LA. Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- 27.Hardie DG. The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi T. Hirshman MF. Kurth EJ. Winder WW. Goodyear LJ. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- 29.Fryer LG. Foufelle F. Barnes K. Baldwin SA. Woods A. Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem J. 2002;363:167–174. doi: 10.1042/0264-6021:3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]