Abstract
Animal social behaviour is not static with regard to environmental change. Flexibility in cooperative resource use may be an important response to resource decline, mediating the impacts of resource availability on fitness and demography. In forest ecosystems, hollow trees are key den resources for many species, but are declining worldwide owing to forestry. Altered patterns of den sharing may mediate the effects of the decline of this resource. We studied den-sharing interactions among hollow-dependent Australian mountain brushtail possums to investigate how spatial variation in hollow tree availability affects resource sharing and kin selection. Under reduced den availability, individuals used fewer dens and shared them less often. This suggests increased territoriality in the presence of resource competition. Further, there was a switch from kin avoidance to kin preference with decreasing hollow tree availability. This was driven primarily by a change in den sharing among siblings. The inclusive fitness benefits of den sharing with kin are likely to increase under resource-limiting conditions, but are potentially outweighed by the benefits of associating with non-relatives (avoidance of inbreeding or pathogen transmission) where dens are abundant. We discuss how predictions from social evolutionary theory can contribute to understanding animal responses to landscape change.
Keywords: kin selection, population viscosity, resource competition, environmental change, social behaviour, tree hollow
1. Introduction
(a). Environmental change and social behaviour
Habitat degradation and resource decline are key threatening processes for animal populations worldwide [1]. However, in some cases, animals have shown adaptive behavioural responses, such as altered patterns of resource use or social interactions, that have mitigated the negative impacts of environmental changes [2]. Therefore, studying behavioural responses to environmental change may improve our understanding of animal population dynamics in modified environments [3,4]. Further, studies of social interactions occurring in heterogeneous environments (such as spatially or temporally variable resource availability) can provide novel opportunities for testing social evolutionary theories in wild populations [5,6]. In this paper, we used behavioural, genetic and environmental data to test predictions relating to resource use, resource sharing and kin selection by a nocturnal arboreal marsupial in response to the loss of its key diurnal shelter resource, hollow-bearing trees.
(b). The decline of hollow-bearing trees and its effects on forest fauna
Forests contain an important component of the World's biodiversity [7], yet are threatened by human activities such as forest clearing and logging [8]. Many forest animal species depend on hollow-bearing trees for shelter [9]. Large hollows may take hundreds of years to form, and hollow-bearing trees are becoming rare in many forest environments owing to large-scale reductions in forest stand age resulting from short logging intervals [9,10]. The loss of this key resource is a critical factor threatening the persistence of many forest animal species [9–12]. In southeastern Australian tall eucalypt forests, variation in the abundance of arboreal marsupials has been found to be proportional to the local availability of hollow-bearing trees, leading to predictions of declines of these species with the reduced availability of hollow-bearing trees [13]. However, a recent unexpected finding from tall montane forests has been that some species, such as the endangered Leadbeater's possum, Gymnobelidus leadbeateri, and the mountain brushtail possum, Trichosurus cunninghami, have not declined in recent years, despite significant reductions in den availability [14]. The demographic resilience of these species to the decline of this key resource suggests changes in individual resource use and/or resource sharing in response to an overall and per capita decline in resource availability. We studied patterns of den use and den sharing in a mountain brushtail possum population characterized by spatial heterogeneity in hollow tree availability to identify individual-level behavioural responses to resource decline. These responses related to changes in resource use (the number of den trees used per individual), resource sharing and kin selection.
(c). Predictions of social responses to declining den resource availability
Although variation in social behaviour has rarely been addressed specifically in the context of anthropogenic environmental change, extensive laboratory, simulation and theoretical studies of kin selection, cooperation and resource use [15–21] provide a rich background from which to predict social responses to environmental change. We first tested whether the level of individual resource use (number of hollow trees used as dens) varied in response to heterogeneous tree hollow availability. We predicted that as competition increases, resource defence behaviour may develop, favouring the use of a smaller, more defensible resource [22]. Other studies investigating social interactions under increasing resource competition have predicted or demonstrated increased aggression, not cooperation [15,19,23]. Alternatively, a static level of resource use will result in increased resource use overlap as resources decline, and hence the need for increased den sharing and cooperation. We tested whether the number of dens used by an individual, and the rate of den sharing between individuals, varied with den availability.
We predicted kin selection to increase in den-sharing choices in response to resource decline owing to the increased benefit to the recipient of den sharing when these resources are limited. Hamilton's rule predicts that altruism is favoured when rb > c [16], where r is the relatedness of the pair of individuals involved in an interaction, b is the benefit of that interaction to the recipient and c is the cost to the animal performing the action. As den resources decline, individuals should obtain greater inclusive fitness benefits from preferential den sharing with relatives and exclusion of non-relatives. This is because mountain brushtail possums in this region den exclusively in hollow-bearing trees [24]. Thus, under low local availability of hollow-bearing trees, den sharing should have a positive benefit, and non-sharing a negative benefit [15] to the recipient, as there is a substantial risk of going without a den. By contrast, under high local availability of hollow-bearing trees, b is negligible owing to the availability of numerous alternative dens. We predicted no change to c under varying resource availability as dens are not consumed resources. We tested for kin selection via an interacting effect of genetic relatedness and local den resource availability on den-sharing probability among individuals.
Kin selection is unlikely to be the only process driving social interactions with regard to relatedness. We predicted that when the benefits of kin selection in den sharing are negligible (i.e. under high den availability), other influences would predominate over social kin interactions. We predicted that under such conditions, preferential association with unrelated individuals would occur as a form of inbreeding avoidance and as a pathogen-avoidance strategy, because pathogen transmission risk is likely to be greater among relatives with similar genetic susceptibility to pathogens [25,26]. In contrast to kin selection, the strength of selection for kin avoidance associated with these processes should not vary with resource levels, so the pattern of den sharing should switch from kin avoidance to kin selection if the inclusive fitness benefits of kin selection at low resource levels outweigh the negative consequences of kin association.
We tested our predictions by analysing diurnal den use and den-sharing interactions by mountain brushtail possums in relation to genetic relatedness and variation in the local availability of hollow-bearing trees. Our study provides a test of kin selection theory under varying resource levels and investigates animal social responses to environmental variation.
2. Methods
(a). Study system
Our study focuses on the tall mountain ash (Eucalyptus regnans) forests of Victoria, Australia. Mountain ash trees are the world's tallest flowering plants (the current tallest standing at approx. 100 m), and after 150–200 years, begin to develop hollows that are a critical shelter resource for arboreal mammals and birds [9]. Infrequent, high-severity wildfires are the predominant causes of variation in age between mountain ash forest stands, and therefore in the abundance and type of hollow-bearing trees. More recently, extensive clearfell logging is responsible for the loss of hollow-bearing trees across the landscape [13]. Our study site at Cambarville (37°33.44′ S, 145°53.05′ E, 880–970 m altitude) [27,28] is dominated by an overstorey of mountain ash trees of varying age, resulting from fine-scale variation in past logging and fire patterns.
Our study species, the mountain brushtail possum (T. cunninghami), is a large (adults 2.5–4 kg) and relatively long-lived (approx. 12 years, reproducing after 2–3 years) marsupial of southeast Australian forests [29,30]. It is primarily a nocturnal generalist herbivore and uses hollows in living or dead standing trees for day-time shelter [31]. Because mountain ash trees are the only trees at this site large enough to form hollows suitable for mountain brushtail possums, the varying age of the mountain ash overstorey results in spatial heterogeneity in the availability of den resources (figure 1). Mountain brushtail possums are predominantly solitary, although they occasionally share dens with other individuals and a proportion of individuals form ‘socially monogamous’ pairs, the rate of which varies between populations [31,33]. At our Cambarville study site, the rate of sequential (between years) monogamy was approximately 46 per cent for females and 60 per cent for males, which is higher than expected random mating (M. D. J. Blyton, S. C. Banks, D. B. Lindenmayer & R. Peakall 2010, unpublished data). Females produce a single offspring per year [33].
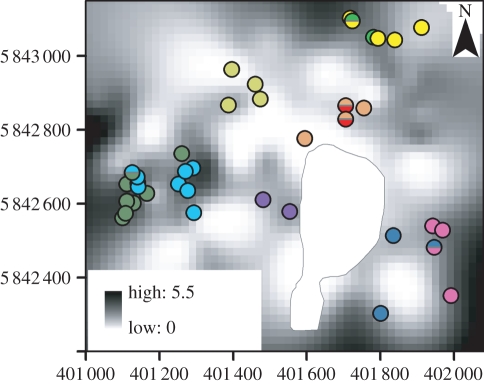
Figure 1.
Map showing spatial variation in the density of hollow-bearing trees across the Cambarville study site. Local density of hollow-bearing trees in this map was interpolated in Anusplin v. 4.37 [32] from 115 regularly spaced survey points as a function of geographical coordinates and forest age (reflecting past fire history). Coloured circles represent the den trees used by a subset of 10 of the 20 individual mountain brushtail possums studied. Each individual is represented by a different coloured circle. Den trees used by more than one individual are represented by circles with more than one colour. The grey polygon in the centre of the map represents a clearing in the study area. The x- and y-axes show eastings and northings coordinates according to the Geodetic Datum of Australia Zone 55S.
(b). Data collection
Our data collection focused on identifying day-time den-sharing interactions among individuals. We recorded interactions with proximity data loggers (Sirtrack Ltd, New Zealand) fitted to mountain brushtail possums at the Cambarville study site. The data loggers were based on a UHF transmitter/receiver and were fitted to animals with a leather collar, along with a VHF transmitter to enable radiotelemetry-based location of individuals. The UHF range coefficient was set such that recorded interactions between den-sharing possums were unlikely to be broken by the movement of individuals and orientation of antennas in shared dens (less than 1 m apart), but that interactions would not be recorded among possums in nearby hollows in the same tree. For free-ranging possums (outside dens), this resulted in the recording of interactions at distances of 2–4 m. Given the low population density of the species in this forest, and our extensive observations, individuals generally did not occur within this distance of one another unless they were interacting.
We fitted proximity data loggers to individuals that had been trapped and sedated [34] as part of a population study ongoing since 1992. In August 2008, we selected individuals known to be adult (greater than 3 years of age) and resident in the population (having a greater than 2 year capture history). Collars were not fitted to juveniles for ethical reasons and because the nature of interactions involving juveniles (e.g. parent–offspring dependence) may differ from those among adults. Following an 8 day pilot study, we recorded interaction data from 18 individuals over 48 days from 26 August to 18 October 2008, after which the collars were removed.
We conducted radio-telemetry to locate the den sites of the collared individuals and to verify that only hollow-bearing trees were used as day-time dens. From a mean of 20 fixes per individual, we found this to be the case, a result consistent with previous radiotracking work at this site [28]. During radiotracking conducted during this study and previously [24], we never found possums outside tree hollows during daylight hours. Thus, we assumed that long-duration (typically all-day) unbroken interactions between sunrise and sunset represented sharing of the denning resource. Based on these day-time den-sharing records, we coded the data as a binary response variable for all possible pairs of possums on each day of data collection, where ‘1’ represented den sharing and ‘0’ represented the inferred use of separate dens by that pair. This allowed us to investigate the probability of den sharing occurring between each pair of resident adult collared possums. Note that this is distinct from the population-level den-sharing rate, which would require the fitting of proximity loggers to every individual in the population. This was not realistic in an open population.
Our set of candidate explanatory variables for analysis included sex, distance, kinship and hollow-bearing tree availability. Distance (log10 distance) was measured between each pair of individuals as the base 10 logarithm of the Euclidean distance between the geographical centroids of each individual's locations as recorded by radiotracking over the duration of the study. The availability of hollow-bearing trees (local den availability) for each individual studied was interpolated at the geographical centroid of that individual's radiotracking-based locations over the duration of the study as a function of geographical location (x- and y-coordinates) and forest overstorey age using the program Anusplin v. 4.37 [32]. The raw data on hollow tree density were collected with a Haglöf factor gauge at 115 survey points spaced at 80 m intervals across the site. Figure 1 shows a map of the interpolated per hectare density of hollow-bearing trees at the site. The effect of kinship was tested as a multilocus relatedness estimator rQG [35], and as a set of specific kinship categories (siblings: a binary variable contrasting full- or half-sibling versus non-siblings). Genetic relationships between individuals were estimated with a panel of seven microsatellites [29] using the program Kingroup v. 2 [36]. A single offspring–mother–father triad was excluded from our analyses as they showed contrasting patterns of interaction when compared with other kin categories (strong maternal association and paternal avoidance). Genetic parentage analyses are described in the electronic supplementary material.
(c). Data analysis
We tested for variation between individuals in the number of den trees used over the course of the study in relation to spatial variation in the abundance of hollow-bearing trees. The response variable was the total number of unique trees used per individual, as identified by radiotracking. The candidate explanatory variables included the number of radiotracking fixes per individual, sex and local den availability. The modelling of these data was conducted with generalized linear models (GLMs) in R v. 2.11 [37].
We investigated the den-sharing response to den resource (hollow-bearing tree) availability by testing for a statistical interaction between local den availability and relatedness on the per-day den-sharing probability. This enabled us to test whether the den-sharing probability between a pair of individuals varied in response to den availability and to test for kin selection in den sharing in response to a decrease in den resource availability. In the latter case, we would expect a negative statistical interaction between the local density of hollow-bearing trees and genetic relatedness influencing the probability of den sharing.
Because individuals may remain in the same den for a number of days [28], consecutive observations for a pair of individuals may be temporally autocorrelated. Initially, we fitted a logistic regression model using generalized estimating equations (GEEs) [38] using the ‘geepack’ package [39] in R v. 2.11 [37] to include a first-order autoregressive dependence structure to account for correlation in the response variable between days, with pair ID as the subject (grouping variable). Our model-building approach was to add explanatory variables representing distance, sex, relatedness/kinship and spatial variation in hollow-bearing tree availability and drop non-significant (p ≥ 0.05) terms.
Following the GEE-based analysis, we aggregated the data up to the pair level to obtain binomial data (i.e. the number of days a pair shared a den out of the total number of days on which both individuals were collared). We fitted a standard logistic regression model that ignored possible temporal autocorrelation using the glm function in R v. 2.11 [37] to these data and compared the results with the model fitted to the day-level data using GEEs.
(d). Testing alternative interpretations of our data: does variation in population viscosity account for apparent spatial variation in kin selection?
Potentially, variation in philopatry associated with heterogeneous habitat quality [40–42] could lead to spatial variation in relatedness among neighbours (potential den sharers). We used an individual-centred index of local genetic spatial autocorrelation [43] (calculated for each individual with reference to their eight nearest neighbours) as an extra term in our statistical modelling, via an interaction with relatedness (rQG), to test whether den sharing was more likely to occur among relatives when neighbouring groups of individuals were more highly related. If so, did any detected pattern of increased den sharing among relatives with decreasing den availability remain significant after this interaction was fitted? This enabled us to test whether apparent kin selection was simply owing to increased population viscosity or to resource heterogeneity.
3. Results
(a). The number of dens used per individual
Individual possums were found to use between one and six dens over the course of the radiotracking study. There was a significant effect of local den availability on the number of dens that a possum was observed to use over this period (local den availability: estimate = 0.385, s.e. = 0.177, t = 2.18, p = 0.043; intercept: estimate = 1.88, s.e. = 0.56, t = 3.36, p = 0.004). This model predicted a 60 per cent increase in the number of dens used by possums in the highest den availability habitat compared with those in the lowest den availability habitat. No effects of sex, number of radiotracking fixes or period tracked were found (although the latter two variables varied little between the individuals studied).
(b). Den sharing, den availability and relatedness
Of the 7346 potential day-time den-sharing interactions between all pairs of possums fitted with proximity data loggers, we recorded 195 instances of den sharing. These involved 13 of the possible 153 pairs of individuals that were simultaneously fitted with proximity loggers. These 13 pairs shared a den between 1 and 35 days (mean = 16.8) of the 48 days of data recording (figure 2). The 13 pairs involved 15 of the 18 individuals studied (i.e. 15 individuals shared a den with at least one other individual in the collared group). The number of individuals with which these 15 animals shared a den over the course of the field study ranged from one to four (mean = 1.3).
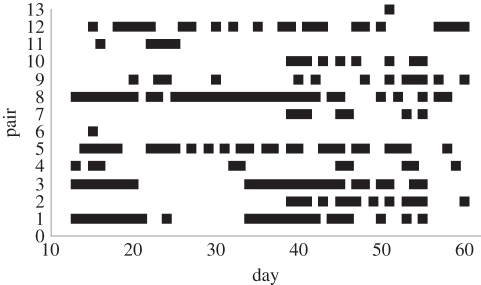
Figure 2.
Den-sharing interactions per day for each of the 13 pairs of individuals recorded interacting over the course of the study. Den sharing (black squares) was identified from interaction records as long-duration interactions typically spanning the entire period from sunrise to sunset for this strictly nocturnal marsupial. Interactions for the first 12 days of the study are not shown, as proximity logger collars were swapped to several new animals between days 10 and 13. The data prior to day 13 were not included in the presented models.
There was a significant negative effect of distance (between the centroids of each individual's recorded locations) on the per-day probability of den sharing between pairs of individuals (table 1). Neighbours were more likely to share dens than individuals separated by greater distances. For instance, model 2 in table 1 predicted that non-sibling pairs in a habitat of average den resource availability had a 27 per cent (s.e. = 2.5%) chance of den sharing when those individuals' range centroids were 30 m apart, but the den-sharing probability dropped to 1.6 per cent (s.e. = 0.3%) for pairs with range centroids 100 m apart. After accounting for the effect of distance, there was a significant negative interaction between genetic relatedness, as estimated by rQG, and local den availability in GEE-based models of the probability of a pair of individuals sharing a den (model 1 in table 1). When we replaced the pairwise relatedness variable rQG with the categorical variable siblings, representing sibling (full- or half-sibling) pairs, we found a similar pattern involving a negative interaction between siblings and local den availability (model 2 in table 1).
Table 1.
Summaries of models of the per-day probability of a den-sharing interaction occurring between pairs of mountain brushtail possums. (The models featured a binomial response (logit link function) and were fitted by generalized estimating equations (GEEs) with pair ID as the subject (grouping factor) and a first-order autoregressive time structure. Models 2 and 3 were also fitted as logistic generalized linear models (GLMs) with observations aggregated over the 48 days for each pair.)
| logistic model fitted by GEE |
binomial GLM |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| model | variable | estimate | s.e. | Wald | p | estimate | s.e. | Wald | p |
| 1 | intercept | 4.61 | 1.00 | 21.34 | <0.001 | 5.29 | 0.51 | 10.34 | <0.001 |
| log10 dist | −5.12 | 0.72 | 51.02 | <0.001 | −5.37 | 0.31 | −17.58 | <0.001 | |
| local den availability | 0.60 | 0.25 | 5.65 | 0.018 | 0.54 | 0.07 | 7.73 | <0.001 | |
| rQG | 5.32 | 3.13 | 2.88 | 0.090 | 5.12 | 0.80 | 6.43 | <0.001 | |
| local den availability × rQG | −1.53 | 0.74 | 4.21 | 0.040 | −1.59 | 0.24 | −6.54 | <0.001 | |
| 2 | intercept | 5.02 | 0.95 | 27.65 | <0.001 | 5.58 | 0.51 | 10.87 | <0.001 |
| log10 dist | −5.73 | 0.75 | 57.85 | <0.001 | −5.90 | 0.35 | −17.02 | <0.001 | |
| local den availability | 0.79 | 0.23 | 12.21 | <0.001 | 0.73 | 0.08 | 9.69 | <0.001 | |
| siblings | 5.38 | 2.29 | 5.50 | 0.019 | 5.22 | 0.58 | 9.03 | <0.001 | |
| local den availability × siblings | −1.44 | 0.48 | 8.95 | 0.003 | −1.44 | 0.17 | −8.69 | <0.001 | |
Given the large standard errors for the correlation parameters in the GEE-fitted models (table 1, model 1: estimate = 0.46, s.e. = 1.56; table 1, model 2: estimate = 0.33, s.e. = 2.39), we considered it unnecessary to fit the temporal correlation structure. Therefore, we fitted binomial GLMs to the data representing the number of interactions per pair aggregated over the 48 day period, and obtained very similar parameter estimates for both models (table 1). We drew our inference from the GLMs as they were the more parsimonious models and the inference was more exact.
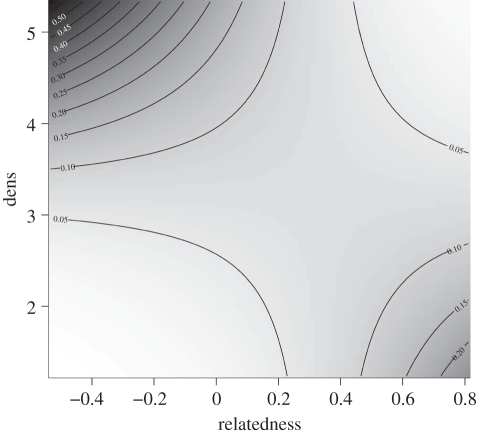
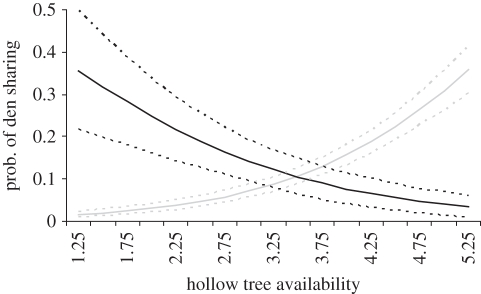
The interpretation of both of these models with regard to the den-sharing rate is that the per-day probability of a pair of individuals sharing a den was positively related to local den availability (den sharing decreases as den availability decreases). With regard to kin selection, model 1 (table 1) predicts that under high den resource availability, unrelated individuals were preferred in den-sharing interactions (figure 3). However, as den availability decreases, kin are increasingly preferred over non-kin in den-sharing interactions. Model 2 (table 1) was similar: siblings were less likely than non-siblings to share a den under high den availability. However, as den availability decreased, siblings became more likely than non-sibling pairs to share a den (figure 4). We believe that the apparent shift to kin selection in den sharing under low den resource availability is primarily driven by sibling interactions, as the significant statistical interaction between rQG and local hollow tree density became non-significant (Wald statistic = 0.325, p = 0.568) at the expense of siblings (Wald statistic = 6.65, p = 0.009) when the latter variable was added to model 1.
Figure 3.
Contour plot of the per-day probability of a pair of mountain brushtail possums sharing a den during daylight hours in relation to genetic relatedness (relatedness) and local density of dens (dens). Predictions were from GLM model 1 in table 1 with the distance variable set to 60 m, as pairs at this distance were realistically likely to interact.
Figure 4.
The per-day probability of sibling (black lines) and non-sibling pairs (grey lines) of mountain brushtail possums sharing a den during daylight hours in relation to the availability of denning resources (hollow trees). The predictions were derived from GLM model 2 (table 1), with distance set to 60 m, as pairs at this distance were realistically likely to interact. The error bars represent 95% confidence intervals around the predictions.
When we added the individual-centred index of local spatial autocorrelation [43] to the analysis, we found a significant interaction between this variable and rQG, indicating that den sharing is more likely to occur between relatives when relatedness among neighbours is high. However, the negative interaction between local den availability and relatedness remained significant in this model. Therefore, spatial variation in population viscosity is unlikely to account for the apparent pattern of kin selection under low den resource availability. Indeed, an exploratory graphical analysis of local spatial autocorrelation suggested that there was no increase in genetic similarity among neighbours (indicating greater philopatry, or population viscosity) in association with spatial variation in den availability (electronic supplementary material).
4. Discussion
(a). Resource use and resource sharing in heterogeneous habitat
We found reduced overall den sharing but increased kin selection in den-sharing interactions among mountain brushtail possums as the local availability of hollow-bearing (den) trees declined. This is consistent with other studies which have demonstrated that increased cooperation is not a favoured response as resource competition increases [19,23]. Reduced tolerance and increased aggression appear to be more common [19,23]. Our findings suggest increased territoriality and resource defence in response to resource decline, as the reduction in the number of dens used per individual with den resource decline may be a behavioural response enabling more efficient resource defence [22,23].
(b). Kin selection and cooperation in heterogeneous habitat
In addition to using fewer den trees and sharing them less often, individuals switched from preference of non-kin to preference of kin in den-sharing interactions as den resources declined. This is consistent with both the ‘light’ and ‘dark’ sides of Hamilton's rule under increasing resource competition [15,44]. As the rule (rb > c; where r is relatedness, b is the benefit to the recipient and c is the cost to the giver [16]) relates to resource use, sharing may convey greater benefits to the recipient under increasing resource competition: b increases, so the inclusive fitness benefit to the giver increases and kin selection is favoured. Here, this was observed in the form of a significant increase in the per-night probability of den sharing among kin as local den availability declined. On the ‘dark side’ of Hamilton's rule, the negative consequences to the recipient of non-sharing are increased under resource decline—in this system, there is an increased risk of going without a den. Thus, ‘not sharing’ becomes an action with a negative b value. Under local resource competition, such actions should be favoured towards individuals with negative relatedness [15,16,44]. Here, this occurred in the form of a significant decline in the per-day probability of den sharing among non-kin as local den availability declined (figures 3 and 4).
Our study is a demonstration of variation in kin selection owing to environmentally mediated variation in b. A similar kin selection response was observed in Siberian jays (Perisoreus infaustus) where the benefit of antipredator vigilance to nearby individuals was greater in relatively exposed, human-managed habitat compared with pristine habitat with greater visual cover from predators [45]. Antipredator vigilance by breeders was greater in managed than in pristine territories when offspring were retained in the group, but there was no antipredator vigilance response to landscape change by breeders without kin in the group. We predict that a common consequence of landscape change for socio-biology will relate to changes in b (benefit to the recipient) of social actions.
(c). Resource availability mediates alternative processes driving kin interactions
The shift to kin selection that we documented under low den resource availability is particularly striking given the strong den-sharing preference for unrelated individuals in habitat with high den resource availability (figures 3 and 4). When b is low under high local den availability resource levels, the inclusive fitness outcomes of kin selection (or intolerance of non-kin) are negligible and other influences on social kin interactions may predominate. We hypothesized that the avoidance of inbreeding or pathogen transmission associated with den sharing with kin would favour kin avoidance in such interactions. In the presence of fine-scale genetic structure (which exists within the scale of this study area for both sexes; [27]), random mating with regard to local levels of genetic relatedness may confer an inbreeding risk [46]. Thus, inbreeding avoidance may be represented by the avoidance of related individuals in social interactions. A proportion of individuals at this site form pair bonds involving regular den sharing [47,48], and inbreeding avoidance is a component of mate choice in this population (M. D. J. Blyton, S. C. Banks, R. Peakall & D. B. Lindenmayer 2010, unpublished data). Pathogen transmission risk may be greater among relatives [25,26], as they may share genetic susceptibility to common pathogens and long-duration interactions at close proximity, such as den sharing, may facilitate pathogen transmission. The strong preference for den sharing among unrelated individuals that we observed under conditions of high local den availability supports the overall predictions of these hypotheses. However, in contrast to kin selection and den sharing, these hypotheses do not predict any variation in the cost of den sharing with kin under heterogeneous resource availability. Hence, our results suggest that the environmentally variable fitness benefits of sharing with kin outweigh the ‘environmentally static’ benefit of kin avoidance when den resource availability is low. An important set of questions for future research relates to the consequences of compromising on kin avoidance in low den resource habitat for inbreeding avoidance and pathogen transmission.
(d). Population viscosity, spatial genetic structure and kin selection
Hamilton [16,17] suggested that one of the ways in which kin selection could operate was population viscosity, whereby limited dispersal results in elevated relatedness among neighbours, and kin selection operates by a general altruism towards neighbours. Recent theory suggests the former prediction does not hold, as kin competition also increases with population viscosity [18,42,49]. In our study system, there is the possibility that spatial variation in population viscosity may lead to spatial variation in the relatedness of den sharers. Increased population viscosity might evolve in resource-rich regions of the study area where the incentive to remain philopatric is greater [40]. There is fine-scale genetic structure in this population: individuals separated by less than approximately 200 m were found to be significantly more related than ‘random’ across the population [27]. Indeed (and intuitively), this study showed that den sharers were more likely to be kin when the individuals involved in the interaction were surrounded by related neighbours (i.e. the local index of spatial autocorrelation was positive). However, spatial variation in fine-scale genetic structure was not associated with the local availability of hollow-bearing trees (electronic supplementary material), and the pattern of increased preference for kin in den-sharing interactions under den resource decline remained significant after accounting for variable fine-scale genetic structure. Hence, our findings do not support an effect of variable population viscosity underlying the spatially heterogeneous pattern of preference for den sharing with kin. Other studies of natural systems have yielded similar findings, involving kin selection via the discrimination of kin (not general altruism towards related neighbours resulting from population viscosity) under scenarios of spatial genetic structure [50,51].
(e). Social and demographic responses to forest resource decline
In many ecosystems, variation in the availability of key resources results from natural or anthropogenic factors. In Australian tall montane forests, logging and fire are major drivers in spatial variation of a key den resource for arboreal marsupials [14]. This study shows that the social response to the decline of this resource is not one of ‘generosity of spirit’ but rather reduced resource sharing, with intolerance of non-kin and selection for kin in den-sharing interactions. Potentially, the demographic ‘buffer’ against resource decline involves the shift by individuals to using a smaller and more defensible set of den trees in an increasingly intolerant local neighbourhood, with reduced den-use overlap leading to a greater proportion of the trees in a forest stand being occupied, as observed in recent field surveys [14].
(f). Kin selection and environmental change
Anthropogenic and natural environmental variation has wide-ranging effects on social behaviour [2,52]. Despite strong links between cooperation, kin selection and fitness, few studies have investigated how these aspects of behaviour respond to environmental change [45]. There is, however, a strong literature based on theoretical, simulation and laboratory research from which we can predict how patterns of kin selection and cooperation will vary in response to environmental changes. Many forms of environmental change are likely to affect the key selective drivers of kin selection, r, b and c. For instance, r may be affected by habitat fragmentation, which can reduce dispersal and increase the spatial clustering of relatives [53]. This presents greater opportunity for kin selection through altruism towards neighbours [44], but potentially also greater resource competition among kin [15,18,21,41,49]. The benefit to the recipient of cooperative actions, b, may be influenced by environmental changes where those interactions relate to resource-use overlap and sharing. In the case of cooperative antipredator behaviour (e.g. vigilance), the benefits of such actions are likely to be increased in riskier habitats such as cleared landscapes or habitat edges [45]. Variation in resource distribution and competition has been a strong focus of theoretical, simulation and laboratory studies of kin selection and cooperation [15,21,23,49,54]. Potentially, the cost, c, of altruistic actions could be increased under scenarios of environmental change, such as in cases of sharing of consumable resources that are declining in availability. Undoubtedly, controlled settings such as computer simulations or laboratory experimental environments enable more precise quantification and prediction of behavioural responses to changes in these key drivers of cooperative behaviour. However, studies of natural systems can be important, not only in testing such predictions in ‘real-world’ systems, but also in identifying how kin selection interacts with other aspects of behaviour such as individual resource-use patterns and inbreeding avoidance.
5. Conclusions
Many animal populations will need to respond rapidly to the ecological and evolutionary challenges presented by anthropogenic environmental change [3,5]. Here, we identified significant changes in social behaviour and resource use in response to environmental heterogeneity. Landscape ecology and conservation-oriented demographic modelling rarely consider adaptive social or behavioural responses when predicting the demographic consequences of resource variation. However, social behaviour has implications for individual fitness and population viability [2,52], and we argue that a stronger focus on evolutionary aspects of social and behavioural processes may improve our ability to understand and predict animal responses to environmental change [3].
Acknowledgements
The field research was conducted under ANU Animal Experimentation Ethics Committee permit C.RE. 58.09.
Mr John Stein provided advice and assistance with GIS data and analyses. Comments from two anonymous reviewers and the subject editor Dr Chris Faulkes greatly improved the manuscript. This research was funded by the Australian Research Council (DP0984876), the Hermon Slade Foundation (HSF 08-4) and a private donation from Jim Atkinson and Di Stockbridge.
References
- 1.Fischer J., Lindenmayer D. B. 2007. Landscape modification and habitat fragmentation: a synthesis. Glob. Ecol. Biogeogr. 16, 265–280 10.1111/j.1466-8238.2007.00287.x (doi:10.1111/j.1466-8238.2007.00287.x) [DOI] [Google Scholar]
- 2.Banks S. C., Piggott M. P., Stow A. J., Taylor A. C. 2007. Sex and sociality in a disconnected world: a review of the impacts of habitat fragmentation on animal social interactions. Can. J. Zool. 85, 1065–1079 10.1139/Z07-094 (doi:10.1139/Z07-094) [DOI] [Google Scholar]
- 3.Hendry A. P., et al. 2010. Evolutionary biology in biodiversity science, conservation, and policy: a call to action. Evolution 64, 1517–1528 [DOI] [PubMed] [Google Scholar]
- 4.Nocera J. J., Forbes G. J. 2010. Incorporating social information to improve the precision of models of avian habitat use. Condor 112, 235–244 10.1525/cond.2010.090237 (doi:10.1525/cond.2010.090237) [DOI] [Google Scholar]
- 5.Kinnison M. T., Hairston N. G. 2007. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 21, 444–454 10.1111/j.1365-2435.2007.01278.x (doi:10.1111/j.1365-2435.2007.01278.x) [DOI] [Google Scholar]
- 6.Zhang F., Tao Y., Li Z. Z., Hui C. 2010. The evolution of cooperation on fragmented landscapes: the spatial Hamilton rule. Evol. Ecol. Res. 12, 23–33 [Google Scholar]
- 7.Vié J.-C., Hilton-Taylor C., Stuart S. N. 2009. Wildlife in a changing world. An analysis of the 2008 IUCN Red List of Threatened Species. Gland, Switzerland: International Union for Conservation of Nature and Natural Resources [Google Scholar]
- 8.Perry D. A., Oren R., Hart S. 2008. Forest ecosystems. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 9.Gibbons P., Lindenmayer D. B. 2002. Tree hollows and wildlife conservation in Australia. Melbourne, Australia: CSIRO Publishing [Google Scholar]
- 10.Michel A. K., Winter S. 2009. Tree microhabitat structures as indicators of biodiversity in Douglas-fir forests of different stand ages and management histories in the Pacific Northwest, USA. For. Ecol. Manage. 257, 1453–1464 10.1016/j.foreco.2008.11.027 (doi:10.1016/j.foreco.2008.11.027) [DOI] [Google Scholar]
- 11.Meijaard E., Sheil D., Nasi R., Stanley S. A. 2006. Wildlife conservation in Bornean timber concessions—art. no. 47. Ecol. Soc. 11, 47–47 [Google Scholar]
- 12.Ochoa J. 2000. Effects of logging on small-mammal diversity in the lowland forests of the Venezuelan Guyana region. Biotropica 32, 146–164 10.1646/0006-3606(2000)032[0146:EDLEND]2.0.CO;2 (doi:10.1646/0006-3606(2000)032[0146:EDLEND]2.0.CO;2) [DOI] [Google Scholar]
- 13.Lindenmayer D. B., Cunningham R. B., Donnelly C. F. 1997. Decay and collapse of trees with hollows in eastern Australian forests: impacts on arboreal marsupials. Ecol. Appl. 7, 625–641 10.1890/1051-0761(1997)007[0625:DACOTW]2.0.CO;2 (doi:10.1890/1051-0761(1997)007[0625:DACOTW]2.0.CO;2) [DOI] [Google Scholar]
- 14.Lindenmayer D. B., Wood J., McBurney L., Michael D., Crane M., Macgregor C., Montague-Drake R., Banks S. C., Gibbons P. In press Cross-sectional versus longitudinal research: a case study linking large trees with hollows to populations of cavity-dependent arboreal marsupials in Australian forests. Ecol. Monogr. [Google Scholar]
- 15.Gardner A., West S. A. 2004. Spite and the scale of competition. J. Evol. Biol. 17, 1195–1203 10.1111/j.1420-9101.2004.00775.x (doi:10.1111/j.1420-9101.2004.00775.x) [DOI] [PubMed] [Google Scholar]
- 16.Hamilton W. D. 1964. Genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16 10.1016/0022-5193(64)90038-4 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 17.Hamilton W. D. 1964. Genetical evolution of social behaviour. 2. J. Theor. Biol. 7, 17–52 10.1016/0022-5193(64)90039-6 (doi:10.1016/0022-5193(64)90039-6) [DOI] [PubMed] [Google Scholar]
- 18.Queller D. C. 1992. Does population viscosity promote kin selection. Trends Ecol. Evol. 7, 322–324 10.1016/0169-5347(92)90120-Z (doi:10.1016/0169-5347(92)90120-Z) [DOI] [PubMed] [Google Scholar]
- 19.Ross-Gillespie A., Gardner A., Buckling A., West S. A., Griffin A. S. 2009. Density dependence and cooperation: theory and a test with bacteria. Evolution 63, 2315–2325 10.1111/j.1558-5646.2009.00723.x (doi:10.1111/j.1558-5646.2009.00723.x) [DOI] [PubMed] [Google Scholar]
- 20.West S. A., Griffin A. S., Gardner A. 2007. Evolutionary explanations for cooperation. Curr. Biol. 17, R661–R672 10.1016/j.cub.2007.06.004 (doi:10.1016/j.cub.2007.06.004) [DOI] [PubMed] [Google Scholar]
- 21.West S. A., Pen I., Griffin A. S. 2002. Conflict and cooperation—cooperation and competition between relatives. Science 296, 72–75 10.1126/science.1065507 (doi:10.1126/science.1065507) [DOI] [PubMed] [Google Scholar]
- 22.Brown J. H. 1964. The evolution of diversity of avian territorial systems. Wilson Bull. 76, 160–169 [Google Scholar]
- 23.Dubois F. D., Giraldeau L. A. 2005. Fighting for resources: the economics of defense and appropriation. Ecology 86, 3–11 10.1890/04-0566 (doi:10.1890/04-0566) [DOI] [Google Scholar]
- 24.Lindenmayer D. B., Welsh A., Donnelly C. F. 1998. The use of nest trees by the mountain brushtail possum (Trichosurus caninus) (Phalangeridae: Marsupialia). V. Synthesis of studies. Wildl. Res. 25, 627–634 10.1071/WR97081 (doi:10.1071/WR97081) [DOI] [Google Scholar]
- 25.Deter J., et al. 2008. Kinship, dispersal and hantavirus transmission in bank and common voles. Arch. Virol. 153, 435–444 10.1007/s00705-007-0005-6 (doi:10.1007/s00705-007-0005-6) [DOI] [PubMed] [Google Scholar]
- 26.Grear D. A., Samuel M. D., Scribner K. T., Weckworth B. V., Langenberg J. A. 2010. Influence of genetic relatedness and spatial proximity on chronic wasting disease infection among female white-tailed deer. J. Appl. Ecol. 47, 532–540 10.1111/j.1365-2664.2010.01813.x (doi:10.1111/j.1365-2664.2010.01813.x) [DOI] [Google Scholar]
- 27.Banks S. C., Knight E. J., Dubach J. E., Lindenmayer D. B. 2008. Microhabitat heterogeneity influences offspring sex allocation and spatial kin structure in possums. J. Anim. Ecol. 77, 1250–1256 10.1111/j.1365-2656.2008.01448.x (doi:10.1111/j.1365-2656.2008.01448.x) [DOI] [PubMed] [Google Scholar]
- 28.Lindenmayer D. B., Welsh A., Donnelly C. F., Meggs R. A. 1996. Use of nest trees by the mountain brushtail possum (Trichosurus Caninus) (Phalangeridae, Marsupialia). 1. Number of occupied trees and frequency of tree use. Wildl. Res. 23, 343–361 10.1071/WR9960343 (doi:10.1071/WR9960343) [DOI] [Google Scholar]
- 29.Banks S. C., Dubach J., Viggers K. L., Lindenmayer D. B. 2010. Adult survival and microsatellite diversity in possums: effects of major histocompatibility complex-linked microsatellite diversity but not multilocus inbreeding estimators. Oecologia 162, 359–370 10.1007/s00442-009-1464-0 (doi:10.1007/s00442-009-1464-0) [DOI] [PubMed] [Google Scholar]
- 30.Lindenmayer D. B., Dubach J., Viggers K. L. 2002. Geographic dimorphism in the mountain brushtail possum (Trichosurus caninus): the case for a new species. Aust. J. Zool. 50, 369–393 10.1071/ZO01047 (doi:10.1071/ZO01047) [DOI] [Google Scholar]
- 31.Lindenmayer D. B., Welsh A., Donnelly C. F. 1997. Use of nest trees by the mountain brushtail possum (Trichosurus caninus) (Phalangeridae, Marsupialia). III. Spatial configuration and co-occupancy of nest trees. Wildl. Res. 24, 661–677 10.1071/WR96112 (doi:10.1071/WR96112) [DOI] [Google Scholar]
- 32.Hutchinson M. F. 2004. Anusplin, v. 4.3. Canberra, Australia: Fenner School of Environment and Society, Australian National University [Google Scholar]
- 33.Martin J. K., Martin A. A. 2007. Resource distribution influences mating system in the bobuck (Trichosurus cunninghami: Marsupialia). Oecologia 154, 227–236 10.1007/S00442-007-0823-y (doi:10.1007/S00442-007-0823-y) [DOI] [PubMed] [Google Scholar]
- 34.Viggers K. L., Lindenmayer D. B. 1995. The use of tiletamine hydrochloride and zolazepam hydrochloride for sedation of the mountain brushtail possum, Trichosurus caninus Ogilby (Phalangeridae, Marsupialia). Aust. Vet. J. 72, 215–216 10.1111/j.1751-0813.1995.tb03523.x (doi:10.1111/j.1751-0813.1995.tb03523.x) [DOI] [PubMed] [Google Scholar]
- 35.Queller D. C., Goodnight K. F. 1989. Estimating relatedness using genetic-markers. Evolution 43, 258–275 10.2307/2409206 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 36.Konovalov D., Manning C., Henshaw M. T. 2004. Kingroup: a program for pedigree relationship reconstruction and kin group assignments using genetic markers. Mol. Ecol. Not. 4, 779–782 10.1111/j.1471-8286.2004.00796.x (doi:10.1111/j.1471-8286.2004.00796.x) [DOI] [Google Scholar]
- 37.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 38.Hardin J., Hilbe J. 2003. Generalized estimating equations. London, UK: Chapman and Hall [Google Scholar]
- 39.Højsgaard S., Halekoh U. J. Y. 2005. The R package geepack for generalized estimating equations. J. Stat. Soft. 15, 1–11 [Google Scholar]
- 40.Julliard R. 2000. Sex-specific dispersal in spatially varying environments leads to habitat-dependent evolutionary stable offspring sex ratios. Behav. Ecol. 11, 421–428 10.1093/beheco/11.4.421 (doi:10.1093/beheco/11.4.421) [DOI] [Google Scholar]
- 41.Lion S., Van Baalen M. 2008. Self-structuring in spatial evolutionary ecology. Ecol. Lett. 11, 277–295 10.1111/j.1461-0248.2007.01132.x (doi:10.1111/j.1461-0248.2007.01132.x) [DOI] [PubMed] [Google Scholar]
- 42.Queller D. C. 1994. Genetic relatedness in viscous populations. Evol. Ecol. 8, 70–73 10.1007/BF01237667 (doi:10.1007/BF01237667) [DOI] [Google Scholar]
- 43.Double M. C., Peakall R., Beck N. R., Cockburn A. 2005. Dispersal, philopatry, and infidelity: dissecting local genetic structure in superb fairy-wrens (Malurus cyaneus). Evolution 59, 625–635 [PubMed] [Google Scholar]
- 44.Hamilton W. D. 1970. Selfish and spiteful behaviour in an evolutionary model. Nature 228, 1218–1220 10.1038/2281218a0 (doi:10.1038/2281218a0) [DOI] [PubMed] [Google Scholar]
- 45.Griesser M., Nystrand M. 2009. Vigilance and predation of a forest-living bird species depend on large-scale habitat structure. Behav. Ecol. 20, 709–715 10.1093/beheco/arp045 (doi:10.1093/beheco/arp045) [DOI] [Google Scholar]
- 46.Stow A. J., Sunnucks P. 2004. Inbreeding avoidance in Cunningham's skinks (Egernia cunninghami) in natural and fragmented habitat. Mol. Ecol. 13, 443–447 10.1046/j.1365-294X.2003.02060.x (doi:10.1046/j.1365-294X.2003.02060.x) [DOI] [PubMed] [Google Scholar]
- 47.Banks S. C., Ward S. J., Lindenmayer D. B., Finlayson G. R., Lawson S. J., Taylor A. C. 2005. The effects of habitat fragmentation on the social kin structure and mating system of the agile antechinus Antechinus agilis. Mol. Ecol. 14, 1789–1801 10.1111/j.1365-294X.2005.02535.x (doi:10.1111/j.1365-294X.2005.02535.x) [DOI] [PubMed] [Google Scholar]
- 48.Freeman-Gallant C. R., Meguerdichian M., Wheelwright N. T., Sollecito S. V. 2003. Social pairing and female mating fidelity predicted by restriction fragment length polymorphism similarity at the major histocompatibility complex in a songbird. Mol. Ecol. 12, 3077–3083 10.1046/j.1365-294X.2003.01968.x (doi:10.1046/j.1365-294X.2003.01968.x) [DOI] [PubMed] [Google Scholar]
- 49.West S. A., Murray M. G., Machado C. A., Griffin A. S., Herre E. A. 2001. Testing Hamilton's rule with competition between relatives. Nature 409, 510–513 10.1038/35054057 (doi:10.1038/35054057) [DOI] [PubMed] [Google Scholar]
- 50.Maher C. R. 2009. Effects of relatedness on social interaction rates in a solitary marmot. Anim. Behav. 78, 925–933 10.1016/j.anbehav.2009.06.027 (doi:10.1016/j.anbehav.2009.06.027) [DOI] [Google Scholar]
- 51.Reynolds S. M., Christman M. C., Uy J. A. C., Patricelli G. L., Braun M. J., Borgia G. 2009. Lekking satin bowerbird males aggregate with relatives to mitigate aggression. Behav. Ecol. 20, 410–415 10.1093/beheco/arn146 (doi:10.1093/beheco/arn146) [DOI] [Google Scholar]
- 52.Foster S. A. 1999. The geography of behaviour: an evolutionary perspective. Trends Ecol. Evol. 14, 190–195 10.1016/S0169-5347(98)01577-8 (doi:10.1016/S0169-5347(98)01577-8) [DOI] [PubMed] [Google Scholar]
- 53.Banks S. C., Lindenmayer D. B., Ward S. J., Taylor A. C. 2005. The effects of habitat fragmentation via forestry plantation establishment on spatial genotypic structure in the small marsupial carnivore, Antechinus agilis. Mol. Ecol. 14, 1667–1680 10.1111/j.1365-294X.2005.02525.x (doi:10.1111/j.1365-294X.2005.02525.x) [DOI] [PubMed] [Google Scholar]
- 54.Brockhurst M. A., Buckling A., Gardner A. 2007. Cooperation peaks at intermediate disturbance. Curr. Biol. 17, 761–765 10.1016/j.cub.2007.02.057 (doi:10.1016/j.cub.2007.02.057) [DOI] [PubMed] [Google Scholar]