Abstract
Conspecifics are usually considered competitors negatively affecting food intake rates. However, their presence can also inform about resource quality by providing inadvertent social information. Few studies have investigated whether foragers perceive conspecifics as informers or competitors. Here, we experimentally tested whether variation in the density of demonstrators (‘none’, ‘low’ and ‘high’), whose location indicated flower profitability, affected decision-making of bumble-bees Bombus terrestris. Bumble-bees foraged on either ‘simple’ (two colours) or ‘complex’ (four colours) artificial floral communities. We found that conspecifics at low density may be used as sources of information in first flower choices, whereas they appeared as competitors over the whole foraging sequence. Low conspecific densities improved foragers' first-visit success rate in the simple environment, and decreased time to first landing, especially in the complex environment. High conspecific densities did not affect these behavioural parameters, but reduced flower constancy in both floral communities, which may alter the efficiency of pollinating visits. These results suggest that the balance of the costs and benefits of conspecific presence varies with foraging experience, floral community and density. Spatio-temporal scales could thus be an important determinant of social information use. This behavioural flexibility should allow bumble-bees to better exploit their environment.
Keywords: Bombus terrestris, decision-making, competition, foraging behaviour, inadvertent social information, plant–pollinator interactions
1. Introduction
Pollinators forage in complex and changing environments generated by plant communities. They constantly encounter flower types differing in rewards and signals [1]. Choices of pollinators among flowers are crucial for both pollinator and plant populations, because they determine nectar harvest, on one hand, and pollen transfer, on the other. Pollinators are expected to use information about flower profitability to improve their accuracy in appraising the environment [2,3]. Non-social information can be extracted from environmental cues, which are directly or indirectly linked to the presence of reward [4]. Social information, either signals or cues, can be obtained from other foragers. Signals are behavioural traits shaped by selection specifically for communication, whereas social cues are detectable facts that are inadvertently produced by other organisms sharing ecological requirements [3].
The behaviour of conspecifics thus provides foragers with inadvertent social information (ISI) [2,3,5–10]. Because social cues reflect environmental variation, ISI use in decision making could benefit foragers by reducing costs associated with personal trial-and-error sampling. Although initially introduced in vertebrates (reviewed in [6]), the concept of ISI use has recently been extended to insects [2,11,12], and particularly to pollinators (reviewed in [8,13]). For example, ISI leads foraging bumble-bees to visit more rewarding flowers mainly through social attraction to conspecifics that act as demonstrators [13–15]. On the other hand, the exploitation of the same food resource by other individuals reduces food intake [16–18]. Thus, conspecifics also act as competitors, which could penalize foraging pollinators. Previous studies have reported conspecific avoidance to minimize intraspecific competition (i.e. local inhibition [19–21]), leading to broader individual diets in bumble-bees [22].
Despite these contrasting findings, the effect of conspecific density on ISI use by pollinators has never been explicitly tested. We hypothesized that the use of conspecific presence as a cue to find high-quality flowers could be conspecific-density-dependent. Conspecifics should thus be perceived as informers to find high-quality flowers at low density, but as competitors at high density. Since reliance on ISI may be especially useful when foragers need information about novel environments [13,15,20], we also examined whether pollinators adjust their use of ISI with the acquisition of personal experience. We tested these hypotheses in two distinct floral communities, differing in complexity, to investigate their relevance under various environmental conditions. Kawaguchi et al. [23] reported that the response of foragers to conspecifics depends upon the costs of exploiting floral resources. By increasing the number of alternatives, and thus the difficulty of personal learning [24], floral diversity may promote ISI use. Here, we report on a fully crossed factorial experiment including three densities of conspecifics (none, low and high) and two artificial floral communities (simple and complex). We recorded foraging decisions of each bumble-bee (Bombus terrestris L.) during two foraging sequences.
2. Material and methods
(a). Experimental setting
(i). Foraging bumble-bees
Experimental bumble-bees (B. terrestris L., Hymenoptera: Apidae) belonged to two colonies supplied by SARL GTICO (Villeneuve l'Archevêque, France). They were fed with a sucrose solution for 2 h daily and with pollen weekly. As individuals had no foraging experience, we first trained them to handle artificial flowers. Ten worker bees were released each morning in a closed transparent plastic box (17.5 × 11 × 15 cm) containing six white artificial flowers filled with 20 µl of 30 per cent w/w (weight/weight) sucrose solution. Then, one bee was individually allowed to access the foraging area containing 40 white flowers with 5 µl of 30 per cent w/w sucrose solution. A forager was considered trained when it visited three flowers successively, and was then kept in a box until the experiment.
(ii). Artificial flowers
Experiments were carried out during July 2006 in a 220 × 110 cm and 100 cm high foraging arena, which was illuminated with six incandescent lamps (40 W each) protected by mesh and directed towards the reflective white roof from 08.00 to 21.00 h. Eight patches of five artificial flowers were randomly set on a green platform. Four patches were composed of high-quality flowers (30% w/w sucrose solution) and four of low-quality ones (10% w/w sucrose solution). Artificial flowers consisted of a 40 × 40 × 6 mm Plexiglas support covered with a coloured plastic sheet and mounted on a 38 mm pedicel. A nectary (4 mm deep and 5 mm in diameter) was drilled on the upper face of each Plexiglas support to hold the reward. Flowers were washed with 30 per cent ethanol solution and replenished with nectar between test sessions. The visual distances among the four flower colours (dark blue, light blue, orange and yellow) were computed in the hexagonal colour space of bumble-bees [25]. Flower colours were all distinguishable from each other by bumble-bees as the separating distances—from 0.182 (yellow/orange) to 0.768 (dark blue/orange) hexagon units—were higher than the discrimination threshold of 0.062 hexagon units [26].
(b). Experimental design
(i). Density of conspecifics
Bumble-bees perceive either inorganic models [27,28] or dead individuals [15,23] as demonstrators in experimental ISI studies. The density of conspecifics was manipulated using dead bees from another nest conserved at −20°C. This allowed us to control the spatial distribution of conspecifics and avoid resource depletion. Demonstrators were always set on high-quality flowers (30% w/w), so that their presence provided valuable information about the distribution of high-quality resources. The ‘low’ treatment involved one demonstrator per high-quality patch, so that 10 per cent of flowers were occupied (four demonstrators in total), whereas the ‘high’ treatment involved three demonstrators per high-quality patch, so that 30 per cent of flowers were occupied (12 demonstrators in total).
(ii). Complexity of floral communities
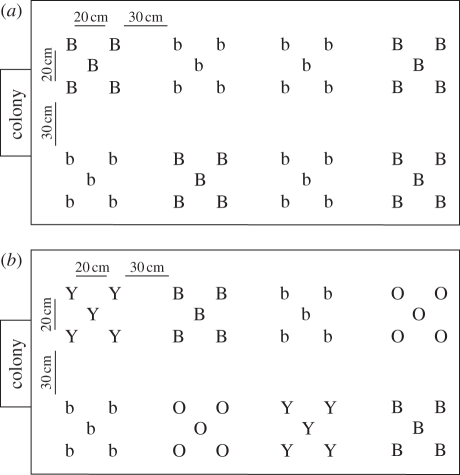
We created two types of community, offering the same nectar volumes but differing in the number of flower types (e.g. figure 1). The ‘simple’ community contained 20 dark blue high-quality flowers filled with 5 µl of 30 per cent sucrose solution and 20 light blue low-quality flowers filled with 5 µl of 10 per cent sucrose solution (i.e. four patches of each colour). The ‘complex’ community encompassed 10 dark blue plus 10 orange high-quality flowers (5 µl 30% sucrose solution) and 10 light blue plus 10 yellow low-quality flowers (5 µl 10% sucrose solution; i.e. two patches of each colour). No significant innate colour preferences were detected for bumble-bees foraging alone (simple community: 30% of first visits on dark-blue flowers versus 70% on light-blue ones, χ2 = 1.6, d.f. = 1, p = 0.21, n = 10; complex community: 30% of first visits on yellow, 30% on dark blue, 10% on orange and 30% on light blue, χ2 = 1.2, d.f. = 3, p = 0.75, n = 10).
Figure 1.
Examples of the spatial arrangements of flower patches for the (a) simple and (b) complex floral communities. Each community contained 40 flowers organized in eight patches of five similar flowers. ‘colony’ indicates the location of a beehive. The ‘simple’ floral community was composed of 20 high-quality (30% w/w sucrose solution) dark-blue (B) and 20 low-quality (10% w/w sucrose solution) light-blue (b) flowers. The ‘complex’ floral community was composed of 10 high-quality dark blue (B), 10 high-quality orange (O), 10 low-quality light blue (b) and 10 low-quality yellow (Y) flowers.
(c). Data records and analyses
(i). Behavioural observations
The behaviour of focal foragers was recorded using Observer software (v. 2.01; Noldus Information Technology). For each treatment, 10 focal bees were independently followed during two test sessions of 20 visits (variable ‘sequence rank’). Between the two test sessions, which were performed during the same day, bees were kept alone in boxes. Treatments were randomized over the course of the experiment. Bumble-bees from the two colonies were equally allocated to experimental treatments (5 ± 1 individuals of each colony per experimental treatment). Because speed and accuracy of choice have potential fitness consequences, we focused on the time bees took to land on their first flower (time to first landing), as well as the proportion of visits to high-quality flowers (success rate) for the first visit and during the entire foraging sequence.
(ii). Statistical analyses
Statistical analyses were carried out using R v. 2.10.1 [29]. To examine differences in behavioural parameters according to the experimental treatments, we carried out statistical models including ‘floral community’, ‘demonstrator density’, ‘sequence rank’ and their interactions as fixed factors (class variables). To account for the non-independence of foraging sequences or visits made by the same bee, individual identity was included as a random effect (cluster option in survival models and grouping factor in binomial models). Models were selected by Akaike's information criterion and the effects were tested by comparing nested models using log-likelihood ratio tests (χ2-tests). Multiple comparisons among demonstrator density levels were then performed using orthogonal contrasts.
Time to first landing was analysed using failure-time models, also known as survival models. This approach, adapted to time data [30,31], involves modelling the time to failure of a component (death in survival models, or landing on a flower here). We used non-parametric Cox proportional hazard regression models, based on the ranks of the landing times (Coxph function from survival package), to test the effect of experimental factors on the instantaneous risk of first landing across time (hazard function). We analysed first-visit success rate and mean success rate over the entire sequence with generalized linear mixed models (lmer function from lme4 package). The first-visit success rate was estimated for a binomial model fitted to the binary response variable: 1 for a visit to a high-quality flower and 0 for a visit to a low-quality flower. The mean success rate was estimated for a binomial model fitted to the succession of visits to high- and low-quality flowers within foraging sequences. To investigate the potential implications of bumble-bee behaviour on pollination, we measured flower constancy of individual bees (i.e. the tendency to move to a similar flower while taking colour preference into account [32]). We computed the constancy index = (c − e)/(c + e − 2ce), adapted from Jacobs [33], where c is the observed proportion of moves between the same colour and e is the expected proportion of moves between the same colour based on the overall frequency of each colour selected in a given foraging sequence. Values range from −1 (complete inconstancy) through 0 (random foraging) to +1 (complete constancy). Linear mixed models were fitted to constancy index data (lme function from nlme package) and analyses of variance were performed.
To investigate whether the presence of conspecifics induced repulsion or attraction at the flower scale, we compared theoretical with observed proportions of visits to occupied flowers among visits to high-quality flowers with two-sided proportion tests and Wilcoxon signed-rank tests for analyses of first visits and the sequence, respectively. Theoretical proportions depended only on the proportion of occupied flowers among high-quality flowers: 0.2 at low and 0.6 at high density of conspecifics. Observed proportions corresponded to the proportion of visits to occupied flowers among high-quality flowers. Observed and theoretical proportions were computed for the first visit (proportion of bumble-bees) and over the entire foraging sequence (mean proportion of visits by each bumble-bee).
3. Results
(a). Time to first landing
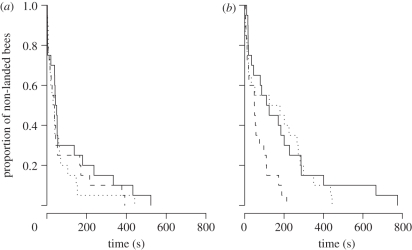
No significant interactions between explanatory variables were detected on time to first landing, but analyses revealed significant main effects. The complexity of floral community increased the time to first landing (χ2 = 4.09, d.f. = 1, p = 0.04), which was twice as long in the complex community (median time to first landing = 75 s; 95% confidence interval, CI = 49–170) as in the simple community (39 s; 95% CI = 26–52; figure 2). Demonstrator density also affected time to first landing (χ2 = 6.80, d.f. = 2, p = 0.03), which was reduced by 20 per cent in the presence of demonstrators (‘none’ versus ‘low–high’: p = 0.02) mainly because of the low-density treatment (none versus low: p = 0.01; none versus high: p = 0.10). The median time to first landing at low density was reduced by 21 per cent in the simple floral community (from 44 s; 95% CI = 39–180 at none to 36 s; 95% CI = 16–165 at low; figure 2a) and by 58 per cent in the complex one (from 111 s; 95% CI = 45–286 at none to 49 s; 95% CI = 20–110 at low; figure 2b). Foraging sequence rank affected time to first landing (χ2 = 7.56, d.f. = 1, p < 0.01). Median time to first landing was reduced by 63 per cent between the first (70 s; 95% CI = 52–165) and second (26 s; 95% CI = 17–45) foraging sequence, suggesting that bumble-bees acquired experience.
Figure 2.
Kaplan–Meier survival curves of the time to first landing for the (a) simple and (b) complex floral communities, depending on the density of demonstrators: ‘none’, ‘low’ and ‘high’. These discrete, stepped survivorship curves represent the cumulative proportion of flying bees before the first landing (‘non-landed bees’) over time. Solid lines indicate the distributions of bumble-bees foraging at none, dashed lines at low and dotted lines at high density of demonstrators. The two successive sequences were not individually represented (n = 10 individuals per experimental treatment).
(b). Success rates
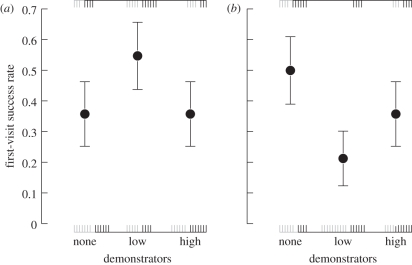
The effect of demonstrator density on the first-visit success rate depended upon floral community complexity (interaction between demonstrator density and floral community: χ2 = 6.24, d.f. = 2, p = 0.04). The first-visit success rate at low demonstrator density was the highest in the simple community and the lowest in the complex one (figure 3). However, the first-visit success rate was not influenced by the sequence rank of a given bumble-bee (χ2 = 1.98, d.f. = 1, p = 0.16).
Figure 3.
First-visit success rate of focal bumble-bees for the (a) simple and (b) complex floral communities, depending on the density of demonstrators: none, low and high. Values are the estimated mean ± s.e. predicted by the selected statistical model (generalized linear mixed effects model with repeated measures, lmer function, link = logit). The statistical model fitted empirical binary data corresponding to the vertical dashed lines: first visit to a high-quality flower at the top and first visit to a low-quality flower at the bottom of the plot. Grey vertical dashed lines represent the numbers of individual first visits for the first sequence and black ones for the second sequence (n = 10 individuals per experimental treatment).
Contrary to first visits, mean success rates over the entire sequence remained unchanged in the presence of demonstrators (χ2 = 3.26, d.f. = 2, p = 0.20), but varied with floral community complexity (χ2 = 4.75, d.f. = 1, p = 0.03) and sequence rank (χ2 = 9.49, d.f. = 1, p < 0.01). Mean success rates increased from 0.45 ± 0.05 to 0.52 ± 0.05 between the first and second sequence in the simple community, and from 0.30 ± 0.04 to 0.47 ± 0.05 in the complex one.
(c). Attraction or repulsion to occupied flowers
For first visits, observed and theoretical proportions of visits to occupied flowers did not differ statistically, regardless of the experimental treatment (χ2 = 5.38, d.f. = 4, p = 0.25), even though observed proportions seemed slightly higher than theoretical ones (table 1). Over the entire sequence, observed proportions were significantly lower than theoretical ones at low density (W = 187, d.f. = 1, p = 0.02), but not significantly so at high density (W = 284, d.f. = 1, p = 0.46). Bumble-bees tended to visit occupied flowers less than expected by chance, which reflects an average slight repulsion from demonstrators at the flower level along a foraging sequence, especially for the second sequence (table 1).
Table 1.
Theoretical and observed proportions of visits to occupied flowers among visits to high-quality flowers in each experimental combination with conspecifics (the asterisk indicates that the observed proportion of visits to occupied flowers could not be computed because bees did not visit high-quality flowers).
| demonstrator density | floral community | sequence | theoretical proportion | observed proportion first visit | observed proportion sequence (mean ± s.e.) |
|---|---|---|---|---|---|
| low | simple | 1 | 0.2 | 0.20 | 0.18 ± 0.05 |
| simple | 2 | 0.2 | 0 | 0.09 ± 0.03 | |
| complex | 1 | 0.2 | * | 0.18 ± 0.06 | |
| complex | 2 | 0.2 | 0.50 | 0.14 ± 0.04 | |
| high | simple | 1 | 0.6 | 0.75 | 0.56 ± 0.09 |
| simple | 2 | 0.6 | 0.67 | 0.49 ± 0.05 | |
| complex | 1 | 0.6 | 0.67 | 0.62 ± 0.09 | |
| complex | 2 | 0.6 | 1 | 0.53 ± 0.10 |
(d). Flower constancy
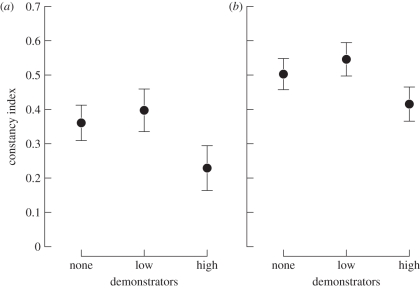
No significant interactions between explanatory variables were detected on flower constancy. Overall, bumble-bees were more constant when foraging in complex than in simple communities, as indicated by the significant floral community effect (F1,56 = 10.87, p < 0.01). The constancy index increased from 0.33 ± 0.03 in the simple community to 0.49 ± 0.03 in the complex one (figure 4). Demonstrator density also affected foragers' flower constancy (F2,56 = 3.52, p = 0.04). Bumble-bees were less constant at high density (contrast high versus ‘none–low’, p = 0.01). Constancy indexes decreased by 40 per cent between none-plus-low and high demonstrator densities in the simple floral community (figure 4a) and by 22 per cent in the complex one (figure 4b). The sequence rank did not significantly impact a forager's flower constancy (F1,59 = 2.10, p = 0.16).
Figure 4.
Flower constancy (mean ± s.e.) of focal bumble-bees on the (a) simple and (b) complex floral communities, depending on the density of demonstrators: none, low and high. The two successive sequences were not individually represented (n = 10 individuals per experimental treatment).
4. Discussion
We investigated whether bumble-bees adjust their behaviour to conspecific density when foraging on two contrasting floral communities. A summary of results (table 2) underlines the high flexibility in their responses to conspecifics depending on floral community and foraging experience. Although low densities of conspecifics could be used as a source of information in first decision-making, bees treated them as competitors with the acquisition of experience over the whole foraging sequence. This supports the hypothesis that relying on social information may be useful when foragers need information about their environment [13,15,20].
Table 2.
Summary of the impact of conspecific density treatments in each floral community on the studied behavioural variables. The reference situation is the ‘none’ demonstrator treatment in each floral community (Ø, no impact; +, positive impact; −, negative impact).
| demonstrator density | floral community | time to first landing | success rates |
flower constancy | |
|---|---|---|---|---|---|
| first visit | first visit | sequence | sequence | ||
| low | simple | + | + | Ø | Ø |
| complex | + | − | Ø | Ø | |
| high | simple | Ø | Ø | Ø | − |
| complex | Ø | Ø | Ø | − | |
(a). Conspecifics as a source of information
In the simple floral community, low densities of conspecifics tended to enhance the proportions of first visits to high-quality flowers (table 2). The accuracy of first choices was enhanced by the presence of demonstrators, reflecting the profitability of floral food sources, a form of ISI [2,3,6]. This confirms that bumble-bees can extract cues from conspecifics to better assess their environment, as previously described for experiments involving either only one [13–15] or more than one demonstrator [27,28]. Local enhancement, whereby the presence of a conspecific attracts individuals to a particular location [34,35], was not detected at flower level, contrary to Leadbeater & Chittka [27] and Kawaguchi et al. [15]. Such differences might result from the spatial arrangement of same flower types in patches, but a low statistical power for the analysis of first visits to occupied flowers could not be excluded. The attractiveness of the whole patch, including non-occupied flowers, was thus enhanced by demonstrators, as in studies in which bees preferentially visited occupied inflorescences [13,23] or larger patches [14]. Social attraction at the patch level might enable bumble-bees to use ISI while simultaneously avoiding intraspecific competition for occupied flowers. This strategy might be especially efficient for bumble-bees to exploit patchily distributed resources, such as aggregated flowers, inflorescences or plants [14].
Contrary to our expectations, the presence of demonstrators did not improve the proportion of first visits to high-quality flowers in the complex floral community (table 2). As indicated by the lowest first-visit success rate, low densities of demonstrator even decreased the attractiveness of the whole of the high-quality patches, without modifying the attractiveness of the specific occupied flowers. That foraging bumble-bees avoided occupied patches in the complex community but not in the simple one is difficult to explain. First, the complexity of the cues resulting from demonstrators scattered on two distinct colours might have affected the bees' ability to process the information accurately [5]. This seems unlikely, because decision-making was accurate, as indicated by the observed strong avoidance of occupied patches. Second, because foragers can exhibit contrasting responses to conspecifics depending on the flower type on which they were landed [23], our results might have been explained if bumble-bees were attracted to blue flower patches, whatever the floral community, and repulsed by orange ones, which could occur only in the complex one. However, analyses of first-visit success rate for each colour suggest that this was not the case, because both blue and orange high-quality flowers were visited less during low than none demonstrator treatments in the complex community (blue flowers: three versus five visits at low and none; orange flowers: one versus five at low and none, respectively).
Furthermore, the presence of conspecifics, especially at low density, reduced the time until the first landing in both floral communities (table 2), which is consistent with results on food-finding times in bumble-bees by Kawaguchi et al. [15]. Conspecific presence might have improved flower detection, facilitated choice and/or increased flight speed towards flowers. The shortening of decision time may constitute another advantage of the presence of conspecifics. This underlines the importance of considering not only the success but also the rapidity of food finding, especially in complex situations [36], to predict fitness consequences. Overall, our results highlight the flexibility in the way ISI benefits foragers, depending on the complexity of the environment. During first visits, ISI use might benefit foragers by improving the accuracy of flower choice (success rate) in simple floral communities, whereas only the rapidity of flower choice (time to first landing) may be improved in complex communities.
(b). Conspecifics as a source of competition
The success rate for first visits was not improved at high densities of demonstrators in the simple floral community. Similarly, the time to first landing was not reduced at high densities of demonstrators in the complex floral community (table 2). This contrast suggests that variation in demonstrator density may affect two intertwined ecological factors with contrasting implications: the availability of ISI and the intensity of competition. For selection of breeding habitat, recent theoretical studies predict that conditions are optimal at intermediate densities of competitors [37–39]. Very low densities of conspecifics may provide insufficient information for habitat selection, whereas high densities may indicate too much competition, thus outweighing any benefits of finding a richer habitat [40,41]. Our results support a similar balance between information gain and competition costs in foraging pollinators [42].
At the whole-sequence scale, the lower success rates in the complex community indicate the difficulty of exploiting more diverse resources for bumble-bees. The proportions of visits to high-quality flowers were also increased with the acquisition of experience in both floral communities. Nevertheless, the presence of demonstrators did not affect the success rates over the foraging sequence, although the fact that bumble-bees visited occupied flowers less than expected by chance suggests repulsion to conspecifics (table 1). This differs from Leadbeater & Chittka [27], who observed attraction to occupied flowers over the foraging sequence, probably because of continuous reward replenishment. Here, associative learning between conspecific presence and reward depletion could have occurred during training, hence the perception of occupied flowers as potentially empty. The use of inanimate demonstrators rather than live ones did not allow response to the nectar-feeding behaviour that could be used as a cue to find reward, but it was not responsible for these differences, because the same experimental artifice was used in previous experiments [15,23,27]. Even though demonstrators could not deplete resources, their presence was sufficient to prevent foraging bumble-bees from exploiting occupied flowers. This suggests that interference between individuals might play an important role in foraging bumble-bees. Conspecific presence could therefore be used as a source of information to estimate both patch quality and competition intensity.
Such behavioural responses by bumble-bees to conspecifics could have important implications for plant pollination. Flower constancy was higher in the complex floral community, probably owing to the greater variety of floral types: this promoted constancy either because of cognitive constraints or, more likely, because of increased flower dissimilarity [1,32,43,44]. Most importantly, flower constancy of focal bumble-bees was strongly reduced at a high density of demonstrators in both floral communities (table 2). This probably resulted from avoidance of many occupied flowers, which could increase the probability of switching among flower types. In natural systems, reduction of flower constancy can be translated in a lower efficiency of pollinating visits, by increasing the amount of heterospecific pollen deposition [45]. Variation in conspecific density could therefore alter the quantity and quality of pollinating visits.
To conclude, our experiments underline the high behavioural flexibility of foraging bumble-bees to conspecifics (table 2). Bumble-bees might adjust their choices according to the balance between information gain and competition costs defined by conspecific density. Moreover, spatio-temporal scales of decision-making appear to determine their responses to conspecifics. Temporally, conspecifics could be used as a source of information only in first decision-making, whereas they consistently appeared as competitors with the acquisition of experience during the foraging sequence. Spatially, the presence of conspecifics could provide information about patch quality in a simple floral community, a strategy that should allow bumble-bees to better exploit their patchy environment. By studying the reciprocal links between floral communities and decision-making of a pollinating insect, our work underlines the importance of integrating behavioural ecology with community ecology.
Acknowledgements
We are grateful to Colin Fontaine and Carine L. Collin for their help and comments on the experiment and the article. Facilities and bumble-bee platform were designed and built thanks to Jacques Mériguet and Colin Fontaine. We thank Céline Hauzy, Cathy Neill, Gérard Lacroix and Thomas Tully for advice on the analyses, and Martin Giurfa (CRCA Toulouse) and Emmanuelle Tastard (EDB Toulouse) for providing the spectral sensitivity functions of bumble-bees. We also thank the Associate Editor and two anonymous referees for constructive and useful comments.
References
- 1.Chittka L., Thomson J. D., Waser N. M. 1999. Flower constancy, insect psychology, and plant evolution. Naturwissenschaften 86, 361–377 10.1007/s001140050636 (doi:10.1007/s001140050636) [DOI] [Google Scholar]
- 2.Danchin E., Giraldeau L. A., Cézilly F. 2008. Behavioural ecology. New York, NY: Oxford University Press [Google Scholar]
- 3.Wagner R. H., Danchin E. 2010. A taxonomy of biological information. Oikos 119, 203–209 10.1111/j.1600-0706.2009.17315.x (doi:10.1111/j.1600-0706.2009.17315.x) [DOI] [Google Scholar]
- 4.Goulson D. 2003. Bumbleebees: behaviour and ecology. Oxford, UK: Oxford University Press [Google Scholar]
- 5.Bonnie K. E., Earley R. L. 2007. Expanding the scope for social information use. Anim. Behav. 74, 171–181 10.1016/j.anbehav.2006.12.009 (doi:10.1016/j.anbehav.2006.12.009) [DOI] [Google Scholar]
- 6.Danchin E., Giraldeau L. A., Valone T. J., Wagner R. H. 2004. Public information: from nosy neighbors to cultural evolution. Science 305, 487–491 10.1126/science.1098254 (doi:10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- 7.Kavaliers M., Choleris E., Agmo A., Braun W. J., Colwell D. D., Muglia L. J., Ogawa S., Pfaff D. W. 2006. Inadvertent social information and the avoidance of parasitized male mice: a role for oxytocin. Proc. Natl Acad. Sci. USA 103, 4293–4298 10.1073/pnas.0600410103 (doi:10.1073/pnas.0600410103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leadbeater E., Chittka L. 2007. Social learning in insects—from miniature brains to consensus building. Curr. Biol. 17, R703–R713 10.1016/j.cub.2007.06.012 (doi:10.1016/j.cub.2007.06.012) [DOI] [PubMed] [Google Scholar]
- 9.Nocera J. J., Forbes G. J., Giraldeau L. A. 2006. Inadvertent social information in breeding site selection of natal dispersing birds. Proc. R. Soc. B 273, 349–355 10.1098/rspb.2005.3318 (doi:10.1098/rspb.2005.3318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parejo D., Danchin E., Silva N., White J. F., Dreiss A. N., Aviles J. M. 2008. Do great tits rely on inadvertent social information from blue tits? A habitat selection experiment. Behav. Ecol. Sociobiol. 62, 1569–1579 10.1007/s00265-008-0586-4 (doi:10.1007/s00265-008-0586-4) [DOI] [Google Scholar]
- 11.Coolen I., Dangles O., Casas J. 2005. Social learning in noncolonial insects? Curr. Biol. 15, 1931–1935 10.1016/j.cub.2005.09.015 (doi:10.1016/j.cub.2005.09.015) [DOI] [PubMed] [Google Scholar]
- 12.Mery F., Varela S. A. M., Danchin É., Blanchet S., Parejo D., Coolen I., Wagner R. H. 2009. Mate copying and stimulus generalization in an invertebrate: personal versus public information use. Curr. Biol. 19, 730–734 10.1016/j.cub.2009.02.064 (doi:10.1016/j.cub.2009.02.064) [DOI] [PubMed] [Google Scholar]
- 13.Leadbeater E., Chittka L. 2005. A new mode of information transfer in foraging bumblebees? Curr. Biol. 15, R447–R448 10.1016/j.cub.2005.06.011 (doi:10.1016/j.cub.2005.06.011) [DOI] [PubMed] [Google Scholar]
- 14.Baude M., Dajoz I., Danchin E. 2008. Inadvertent social information in foraging bumblebees: effects of flower distribution and implications for pollination. Anim. Behav. 76, 1863–1873 10.1016/j.anbehav.2008.08.010 (doi:10.1016/j.anbehav.2008.08.010) [DOI] [Google Scholar]
- 15.Kawaguchi L. G., Ohashi K., Toquenaga Y. 2006. Do bumble bees save time when choosing novel flowers by following conspecifics? Funct. Ecol. 20, 239–244 10.1111/j.1365-2435.2006.01086.x (doi:10.1111/j.1365-2435.2006.01086.x) [DOI] [Google Scholar]
- 16.Heinrich B. 1979. Resource heterogeneity and patterns of movement in foraging bumblebees. Oecologia 40, 235–245 10.1007/BF00345321 (doi:10.1007/BF00345321) [DOI] [PubMed] [Google Scholar]
- 17.Makino T. T., Sakai S. 2005. Does interaction between bumblebees (Bombus ignitus) reduce their foraging area?: bee-removal experiments in a net cage. Behav. Ecol. Sociobiol. 57, 617–622 10.1007/s00265-004-0877-3 (doi:10.1007/s00265-004-0877-3) [DOI] [Google Scholar]
- 18.Thomson J. D., Peterson S. C., Harder L. D. 1987. Response of traplining bumble bees to competition experiments—shifts in feeding location and efficiency. Oecologia 71, 295–300 10.1007/BF00377298 (doi:10.1007/BF00377298) [DOI] [PubMed] [Google Scholar]
- 19.Goulson D., Hawson S. A., Stout J. C. 1998. Foraging bumblebees avoid flowers already visited by conspecifics or by other bumblebee species. Anim. Behav. 55, 199–206 10.1006/anbe.1997.0570 (doi:10.1006/anbe.1997.0570) [DOI] [PubMed] [Google Scholar]
- 20.Slaa E. J., Wassenberg J., Biesmeijer J. C. 2003. The use of field-based social information in eusocial foragers: local enhancement among nestmates and heterospecific in stingless bees. Ecol. Entomol. 28, 369–379 10.1046/j.1365-2311.2003.00512.x (doi:10.1046/j.1365-2311.2003.00512.x) [DOI] [Google Scholar]
- 21.Stout J. C., Goulson D. 2001. The use of conspecific and interspecific scent marks by foraging bumblebees and honeybees. Anim. Behav. 62, 183–189 10.1006/anbe.2001.1729 (doi:10.1006/anbe.2001.1729) [DOI] [Google Scholar]
- 22.Fontaine C., Collin C. L., Dajoz I. 2008. Generalist foraging of pollinators: diet expansion at high density. J. Ecol. 96, 1002–1010 10.1111/j.1365-2745.2008.01405.x (doi:10.1111/j.1365-2745.2008.01405.x) [DOI] [Google Scholar]
- 23.Kawaguchi L. G., Ohashi K., Toquenaga Y. 2007. Contrasting responses of bumble bees to feeding conspecifics on their familiar and unfamiliar flowers. Proc. R. Soc. B 274, 2661–2667 10.1098/rspb.2007.0860 (doi:10.1098/rspb.2007.0860) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dukas R., Real L. A. 1993. Learning constraints and floral choice behaviour in bumble bees. Anim. Behav. 46, 637–644 10.1006/anbe.1993.1240 (doi:10.1006/anbe.1993.1240) [DOI] [Google Scholar]
- 25.Chittka L. 1992. The colour hexagon: a chromaticy diagram based on photoreceptor excitations as a generalized representation of colour opponency. J. Comp. Physiol. A 170, 533–543 [Google Scholar]
- 26.Dyer A. G., Chittka L. 2004. Biological significance of distinguishing between similar colours in spectrally variable illumination: bumblebees (Bombus terrestris) as a case study. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 190, 105–114 10.1007/s00359-003-0475-2 (doi:10.1007/s00359-003-0475-2) [DOI] [PubMed] [Google Scholar]
- 27.Leadbeater E., Chittka L. 2007. The dynamics of social learning in an insect model, the bumblebee (Bombus terrestris). Behav. Ecol. Sociobiol. 61, 1789–1796 10.1007/s00265-007-0412-4 (doi:10.1007/s00265-007-0412-4) [DOI] [Google Scholar]
- 28.Worden B. D., Papaj D. R. 2005. Flower choice copying in bumblebees. Biol. Lett. 1, 504–507 10.1098/rsbl.2005.0368 (doi:10.1098/rsbl.2005.0368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 30.Crawley M. J. 2007. The R book. New York, NY: John Wiley [Google Scholar]
- 31.Scheiner S. M., Gurevitch J. 1993. Design and analysis of ecological experiments, 2nd edn New York, NY: Oxford University Press [Google Scholar]
- 32.Waser N. M. 1986. Flower constancy—definition, cause, and measurement. Am. Nat. 127, 593–603 10.1086/284507 (doi:10.1086/284507) [DOI] [Google Scholar]
- 33.Jacobs J. 1974. Quantitative measurement of food selection—a modification of the forage ratio and Ivlev's electivity index. Oecologia 14, 413–417 10.1007/BF00384581 (doi:10.1007/BF00384581) [DOI] [PubMed] [Google Scholar]
- 34.Heyes C. M., Ray E. D., Mitchell C. J., Nokes T. 2000. Stimulus enhancement: controls for social facilitation and local enhancement. Learn. Motiv. 31, 83–98 10.1006/lmot.1999.1041 (doi:10.1006/lmot.1999.1041) [DOI] [Google Scholar]
- 35.Thorpe W. H. 1956. Learning and instinct in animals. London, UK: Methuen [Google Scholar]
- 36.Chittka L., Spaethe J. 2007. Visual search and the importance of time in complex decison making by bees. Arthropod Plant Interact. 1, 37–44 10.1007/s11829-007-9001-8 (doi:10.1007/s11829-007-9001-8) [DOI] [Google Scholar]
- 37.Fletcher R. J. J. 2006. Emergent properties of conspecific attraction in fragmented landscapes. Am. Nat. 168, 207–219 10.1086/505764 (doi:10.1086/505764) [DOI] [PubMed] [Google Scholar]
- 38.Forsman J. T., Seppanen J. T., Mönkkönen M. 2002. Positive fitness consequences of interspecific interaction with a potential competitor. Proc. R. Soc. Lond. B 269, 1619–1623 10.1098/rspb.2002.2065 (doi:10.1098/rspb.2002.2065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monkkonen M., Hardling R., Forsman J. T., Tuomi J. 1999. Evolution of heterospecific attraction: using other species as cues in habitat selection. Evol. Ecol. 13, 91–104 10.1023/A:1006590215306 (doi:10.1023/A:1006590215306) [DOI] [Google Scholar]
- 40.Fletcher R. J. J. 2007. Species interactions and population density mediate the use of social cues for habitat selection. J. Anim. Ecol. 76, 598–606 10.1111/j.1365-2656.2007.01230.x (doi:10.1111/j.1365-2656.2007.01230.x) [DOI] [PubMed] [Google Scholar]
- 41.Forsman J. T., Hjernquist M. B., Taipale J., Gustafsson L. 2008. Competitor density cues for habitat quality facilitating habitat selection and investment decisions. Behav. Ecol. 19, 539–545 10.1093/beheco/arn005 (doi:10.1093/beheco/arn005) [DOI] [Google Scholar]
- 42.Parejo D., Danchin É., Avilés J. 2004. The heterospecific habitat copying hypothesis: can competitors indicate habitat quality. Behav. Ecol. 16, 96–105 10.1093/beheco/arh136 (doi:10.1093/beheco/arh136) [DOI] [Google Scholar]
- 43.Gegear R. J., Thomson J. D. 2004. Does the flower constancy of bumble bees reflect foraging economics? Ethology 110, 793–805 10.1111/j.1439-0310.2004.01010.x (doi:10.1111/j.1439-0310.2004.01010.x) [DOI] [Google Scholar]
- 44.Goulson D. 2000. Are insects flower constant because they use search images to find flowers? Oikos 88, 547–552 10.1034/j.1600-0706.2000.880311.x (doi:10.1034/j.1600-0706.2000.880311.x) [DOI] [Google Scholar]
- 45.Waser N. M. 1983. The adaptive nature of flower traits: ideas and evidence. In Pollination biology (ed. Real L. A.), pp. 241–285 Orlando, FL: Academic Press [Google Scholar]