Abstract
In socially monogamous animals, mate choice is constrained by the availability of unpaired individuals in the local population. Here, we experimentally investigate the physiological stress endured by a female (the choosy sex) when pairing with a non-preferred social partner. In two experimental contexts, female Gouldian finches (Erythrura gouldiae) socially paired with poor-quality mates had levels of circulating corticosterone that were three to four times higher than those observed in females that were paired with preferred mates. The elevated level of this stress hormone in response to partner quality was observed within 12 h of the experimental introduction and maintained over a period of several weeks. Our findings demonstrate the extent of intra-individual conflict that occurs when individuals are forced to make mate-choice decisions that are not perfectly aligned with mate-choice preferences. The elevated level of corticosterone also suggests a mechanistic route through which females might adaptively manage their responses to intersexual conflict over reproductive investment.
Keywords: mate choice, social monogamy, corticosterone, sexual conflict
1. Introduction
Many animals, including humans, form socially monogamous pair bonds that last through the course of at least one reproductive event. In birds, over 90 per cent of species breed in this way, with some social relationships between males and females persisting across many decades [1]. Within socially monogamous animals, the duration of the social partnership, and the extent of the bond between the individuals shows considerable variation, from a bond lasting just a few months (during the period of parental care), to lifelong bonds that are often cemented through highly ritualized courtship displays (e.g. the synchronized dance of the great crested grebe Podiceps cristatus; [2]) and frequent behavioural interactions between the partners, including allo-preening [3] and highly synchronized acoustic duets [4]. The underlying cause of social monogamy is believed to be the increased fitness of both parents that results from reproductive investment by both the male and female together—biparental care [5].
However, the allocation of biparental care itself does not necessarily require a high level of coordination or indeed a particularly strong level of social interaction between the male and female. For example, in the blue tit Cyanistes caeruleus, while the male and female may each visit the nest and deliver many hundreds of food items to their brood each day [6], they forage quite independently and any apparent correlation in their individual feeding rates is more likely to relate to individual responses to offspring signals of hunger rather than any direct communication or social interaction between the parents [7]. The pair share a common goal—the successful production of viable offspring—but can jointly achieve that even with a high degree of underlying evolutionary conflict. In birds particularly, the past couple of decades have seen the demonstration of a number of different, and often quite widespread, sources of evolutionary conflict at the heart of the socially monogamous pair bond. The most widely demonstrated conflict arises from the incidence of extrapair paternity, with an average of 19 per cent of broods, across surveyed socially monogamous species, containing offspring sired outside the pair bond [8]. The level of extrapair paternity within a species, and the incidence of divorce and re-pairing will determine the difference in the long-term evolutionary interests of the individual male and female in a socially monogamous partnership. In species in which individuals have a high prospect of a future reproductive event with an alternative partner, there will be a greater divergence in the evolutionary interests of the male and female and we may expect a more self-centred approach to the investment into current reproduction in lieu of future opportunities (e.g. differential allocation; [9,10]). This sexual conflict over investment can paradoxically reduce the overall level of investment by the two partners (e.g. [11]), as a result of the dynamics of the investment rules of the two competing parents, with each attempting to defer the higher workload onto the other [12].
Irrespective of the level of sexual conflict between the partners in any particular species, one common feature of all socially monogamous animals is that female mate choice is more constrained than in mating systems with a greater degree of reproductive skew, for example, highly polygynous lek mating systems where relatively few attractive males will monopolize most of the copulations [13]. In lek mating systems, where females gain no material benefits from males other than sperm, nearly all females will secure matings with a sexual partner of above-average quality, and males of below-average quality probably secure little to no matings [13,14]. By contrast, in socially monogamous systems, assuming an approximately even operational adult sex ratio, most individuals will secure a social partner, and inevitably a good proportion of the females in a population will be paired with males of below-average quality. The dynamics of pair formation in socially monogamous systems have a number of consequences for the evolution of sexual conflict and the mechanisms through which males and females can adaptively respond to their social situation. There are three probable scenarios:
— all individuals' pair assortatively with respect to quality and although the male partner may not match the females ideal preference he will be the closest that the female can achieve owing to the limited number of perfect males left available. Each female is ‘satisfied’ with her partner because he is the best that she will get;
— female preference is quality- or condition-dependent and some females choose to pair with the least dominant or unattractive males because they may confer different benefits than the more dominant or showy males [15,16]. For example, males with low testosterone may have reduced expression of ornamental traits but provide higher levels of paternal care to offspring [17]. Here, we expect even those females paired with the least ornamented or dominant males to be ‘satisfied’ with their partner as they are the result of active choice and match a female's preference; and
— all females would prefer to pair with the most attractive males and consequently those that lose out in the scramble competition for the best unpaired males will be under pressure to mate with a low-quality male or forego reproduction altogether. In this scenario, we might expect many females to be ‘dissatisfied’ with their social situation.
Although each scenario above is possible, at least one recent high profile study [18] has assumed that in a socially monogamous system, female mate preference can be measured by assessing the phenotype of her partner—i.e. that each female pairs with a male that matches her ideal choice of partner. As social monogamy constrains each male to pair with just one female the outcome of this logic would be akin to assuming that only women as attractive as Angelina Jolie find a man such as Brad Pitt attractive. The reality in that case is that only one very high-quality woman is able to socially pair with Brad Pitt. So more realistically, mate choice (the partner paired with) is a resolution of preference function (the ideal phenotype that we aspire to) and the reality that free choice is severely constrained in a socially monogamous system where most individuals are not available as social partners [19].
Here, in a socially monogamous finch in which good- and poor-quality partners can be readily identified, we presented males to females in two different contexts to experimentally assess the direct effects of partner quality on the level of female stress. This allowed the first examination, in a socially monogamous animal, of the extent of female ‘satisfaction’ with her social partner. In addition to illuminating which of the three scenarios above is most probable, our study may also provide some insight into the mechanisms that could mediate the behaviours that females use to adaptively respond to poor-quality partners.
2. Material and methods
(a). Species and approach
Previous work on the polymorphic Gouldian finch, Erythrura gouldiae, has demonstrated that the genetically discrete red and black head-colour morphs are partially genetically incompatible with sons and daughters produced by mixed pairings suffering about 40 and 80 per cent higher mortality, respectively, than offspring produced by pairs of the same head-colour morph [20]. This genetic incompatibility results in strong preferences by both males and females for partners of the same head colour as themselves [21,22]. The red and black morphs occur at a relatively stable frequency both spatially and temporally across the species distribution in northern Australia, but the frequency of males and females of each morph differs [23]. Therefore, in the wild, assortative pairing by head colour is not possible for all individuals in a population, and as a result individuals will often face the choice of breeding with an incompatible partner or not breeding at all—both likely to be stressful situations.
To assess a female's ‘satisfaction’ with her social partner, we considered a behavioural response—her willingness to breed with him—and a physiological response—the level of circulating corticosterone. In birds, as in most other vertebrates, corticosterone is the major glucocorticoid released in response to stressors [24], and the level of corticosterone circulating in the blood is a good measure of an individual's level of stress, over both the short and long term.
We adopted two experimental approaches to examine the effect of partner availability and the quality on female physiological state—a free-choice aviary experiment that replicated mate choice in natural populations and a forced-pair experiment that controlled for differences in individual quality. The breeding experiments were conducted between January and April 2009.
(b). Free-choice aviary experiment
Twenty to twenty-eight unfamiliar birds (i.e. half males and half females) were introduced into each of six large flight aviaries (15 m long × 5.5 m wide × 3.8 m high) and allowed to breed. Two replicates (each with a different set of individuals) of six different social environments were created by altering the relative frequency of red and black birds in each of the six populations (table 1). The variation in the population size and frequency of red and black birds across the different aviaries was not particularly systematic and designed only to provide a high degree of variation in the social environment experienced by individual females. At the time of the introduction, all of the females were unknown to all of the males (and vice versa). In each aviary, the birds were provided ad libitum with dry seed mix and freshly sprouted seed to stimulate reproduction. Twelve nest-boxes were erected in each aviary, and the nesting material was provided for nesting and to stimulate courtship behaviour by males. The adults were introduced into the aviary on day 0 of the experiment and nest-boxes were subsequently monitored for the appearance of the first egg laid by each female. Latency to breed was the number of days after the experimental introduction of birds into the aviary before the first egg was laid. Nest ownership and the social partnership were identified by the unique combination of colour bands on the birds legs and also remotely by using passive integrated transponder (PIT) tags (that had been glued onto the colour bands) and decoders coils fitted around the nest-box entrance to record all birds entering and exiting the nest-box [25]. On the day that a female laid her second egg (which was predicted after the nest checks on the previous day had found the first egg), she was captured by either using a hand net (n = 13) or removing her directly from her nest (n = 71) and a blood sample was taken for hormone analysis (see below). This was done first thing in the morning before disturbance in the aviary for husbandry or nest checks and on average, it took 1.32 min (±0.47) between entering the aviary to catch a particular female and the sampling of the blood. As females were very asynchronous in their breeding times within an aviary we never had to sample more than two females from a single aviary on a single morning. In total, 84 breeding females across the different social environments were caught and bleed during egg laying (table 1).
Table 1.
The social environment for the finches used in this study as determined by the proportion of red and black males and females. Two replicates of 20–28 birds per aviary were used (total numbers across both replicates are provided). Although a number of compatible and incompatible pairs bred in each environment, only subsamples of females breeding in each environment were sampled.
| number of birds placed in each environment |
number of breeding birds blood sampled |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| social environment | red female | black female | red male | black male | red female/red male | red female/black male | black female/red male | black female/black male | compatibly-paired females | incompatibly-paired females |
| A | 20 | 0 | 20 | 0 | 12 | 0 | 0 | 0 | 12 | 0 |
| B | 0 | 24 | 0 | 24 | 0 | 0 | 0 | 10 | 10 | 0 |
| C | 8 | 16 | 16 | 8 | 3 | 1 | 3 | 6 | 9 | 4 |
| D | 16 | 12 | 12 | 16 | 5 | 5 | 6 | 0 | 5 | 11 |
| E | 4 | 20 | 20 | 4 | 0 | 0 | 13 | 4 | 4 | 13 |
| F | 24 | 0 | 0 | 24 | 0 | 16 | 0 | 0 | 0 | 16 |
(c). Forced-pairing experiment
In total, 50 red females were paired with either a red (n = 25) or black male (n = 25), and 50 black females were paired with either a red (n = 25) or black male (n = 25). The pairs were allocated at random and all birds were 1 year old virgins, which had not previously encountered the individual with whom they were paired. All pairs were placed in single, visually isolated breeding cages (1.2 m3) with nest-boxes (details as in [25]). The pair was allowed to breed and then at the completion of the first brood (when the offspring became independent at 60 days after hatching), the adult male and offspring were removed from the cage. After an isolation period of 18 days in her cage, a new male of the alternate morph was put into the cage and they were then allowed to pair and breed. Consequently, each female (n = 100) was paired with a partner of their own phenotype (compatible pair) and a partner of a different phenotype (incompatible pair), but the order in which the different males were presented was randomized. Overall, this experimental design ensured that any effects of variation in maternal hormonal responses were not confounded by differences between females in their genetics or their initial condition or quality. To preserve the conservative within-individual experimental design, only breeding attempts where a female bred with both male morphs were included in analyses (n = 86; 43 compatible and 43 incompatible pairs). Latency to breed with the allocated male was measured as the number of days between the introduction of the male and when the first egg was laid. In both experimental rounds, a blood sample was taken 12 h after the introduction of the male to measure a female's initial response to her new partner, and a second sample was taken on the day that she laid her second egg. This work was conducted under the authority of an approval by the Macquaire University Animal Ethics Committee (2008/037).
(d). Blood sampling and corticosterone assay
To examine individual variation in plasma corticosterone levels of females, we took blood samples in the early morning (05.00–06.00). Because corticosterone increases rapidly in birds after capture, we took blood samples (ca 75 µl) always within 2 min of capture (average 49.4 ± 5.9 seconds) to ensure we measured baseline measures. There were no differences in capture times between females paired with compatible and incompatible males (F1,171 = 0.39, p = 0.71) and there was no detectable effect of time to sample after capture on plasma corticosterone levels (F1,171 = 0.53, p = 0.59). However, similar to findings for Gouldian finch males [26], corticosterone levels of non-breeding and non-experimental females held for longer periods (10 females bled 60 min after catching) increased dramatically (within 2 min: 17.8 ± 9.3 ng ml−1; after 60 min: mean ± s.d. = 173.4 ± 78.4 ng ml−1), demonstrating that corticosterone secretion is stimulated during stress in these birds. Blood samples were centrifuged and plasma removed and stored at −20°C. Corticosterone was measured in duplicate from plasma samples using a Cayman Enzyme Immunoassay kit (no. 500 651; Ann Arbor, MI, USA). Kit instructions were followed, but each plasma sample was also initially spiked with corticosterone (Amersham [1,2,6,7-3H]) to determine percentage recovery after steroid extraction in dichloromethane. After extraction, samples were reconstituted in buffer at dilutions (1 : 40) optimized for the standard curve. Final hormone values were corrected for individual sample recovery (mean recovery was 86.3 ± 1.3%). Intra-assay variation was 7.2 per cent and inter-assay variation was 9.8 per cent, assessed using pooled chicken plasma as a reference.
(e). Statistical analyses
Behavioural and hormonal data were analysed in GENSTAT 9 (Rothamsted Experimental Station, Harpendon, UK) using generalized linear models (GLMs) with a Poisson distribution and logarithmic link function. For within-female responses, female identity was included and retained as the random repeated subject, but did not constitute a significant random component in any model (p > 0.25). Potential explanatory (fixed) terms were entered into each GLM using the stepwise forward technique until the model only included those terms for which elimination would have significantly reduced the explanatory power of the model. The significance of these predictor terms was tested by the change in deviance of the different models using a χ2 approximation. For all models, all possible effects, combinations and interactions were initially modelled. Second-order Akaike's information criterion weights were calculated for each model and used to objectively compare different models. Only final models (and significant interactions) are reported. Significant probability values are derived from having all significant terms fitted in the final model together. Probability values of non-significant fixed terms are obtained from having all significant terms in the model and each non-significant term fitted individually. Means ± s.e.m. are presented throughout.
3. Results
When pairs were allowed to form naturally in the aviaries, the females that paired with a male of the same head colour (i.e. paired assortatively) laid their first egg nearly a month earlier than those females that paired disassortatively (F1,83 = 52.32, p < 0.001; good-quality partner = 25 days ± 1.8; poor-quality partner = 54 days ± 2.4). This effect was independent of head-colour morph (red or black: F1,83 = 0.40, p = 0.69). Although latency to breed varied across the different aviaries (F5,78 = 6.75, p < 0.001), this was a result of the relative proportion of compatible and incompatible pairs (range from 0 to 100%) in each environment. There was no difference in latency to breed between compatible and incompatible pairs in each aviary (aviary × pair: F8,75 = 1.31, p = 0.26); thus, within each of the different social environments, incompatible pairs took much longer to breed than compatible pairs.
Similarly, in the forced-pairing experiment, the within-individual analysis revealed that females laid their first egg significantly faster when paired with a compatible male than an incompatible male (F1,43 = 33.43, p < 0.001; compatible partner = 20 days ± 1.2; incompatible partner = 42 days ± 2.4). There were no treatment-order effects (compatible mate first or second; F1,43 = 0.29, p = 0.78) or morph-specific differences in the response to compatible or incompatible males by black or red females (F1,43 = 0.56, p = 0.57).
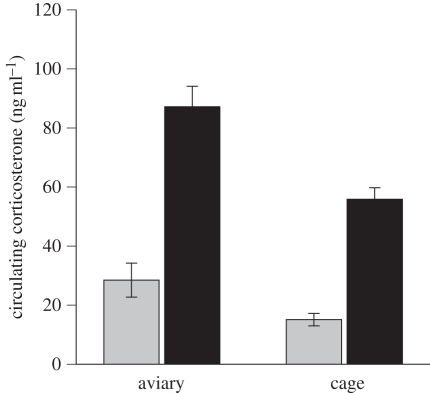
In the aviaries, where females chose their own partners (albeit under the constraints of diminishing male availability), females paired with incompatible males had significantly higher levels of circulating corticosterone at egg laying than those paired with compatible partners (F1,83 = 8.08, p = 0.005; figure 1). Although the level of circulating corticosterone in females during egg laying differed between the aviaries (F1,83 = 5.65, p < 0.001), this was a result of the relative number of compatible and incompatible pairs in each aviary (table 1), and corticosterone levels were similar for both compatible and incompatible pairs in the different environments (aviary × pair: F8,75 = 0.71, p = 0.49; table 2).
Figure 1.
The mean difference (±s.e.m.) in the level of corticosterone circulating in females on the day they laid their second egg when paired with a compatible (grey shaded) or incompatible (black shaded) male in the between-female aviary or within-female forced-pair cage experiment.
Table 2.
Response of females to mate quality across the different social environments with respect to latency to lay and the level of circulating corticosterone (mean values ± standard error).
| aviary | latency to lay (compatibly-paired females) | latency to lay (incompatibly-paired females) | level of corticosterone (compatibly-paired females) | level of corticosterone (incompatibly-paired females) | N compatibly-paired females | N incompatibly-paired females |
|---|---|---|---|---|---|---|
| A | 26.0 ± 3.8 | n.a. | 33.5 ± 12.3 | n.a. | 12 | 0 |
| B | 23.0 ± 4.1 | n.a. | 3.1 ± 0.9 | n.a. | 10 | 0 |
| C | 28.0 ± 4.7 | 43.5 ± 7.0 | 45.9 ± 14.0 | 67.4 ± 21.0 | 9 | 4 |
| D | 23.2 ± 7.3 | 51.1 ± 4.9 | 20.3 ± 34.8 | 46.9 ± 9.8 | 5 | 11 |
| E | 23.5 ± 7.0 | 58.2 ± 3.9 | 47.6 ± 19.1 | 102.4 ± 10.6 | 4 | 13 |
| F | n.a. | 54.3 ± 3.7 | n.a. | 107.5 ± 10.5 | 0 | 16 |
We found similar results in the within-female forced-pair design, with females having a significantly higher level of corticosterone when paired with an incompatible partner than when paired with a compatible partner (F1,43 = 32.17, p < 0.001; figures 1 and 2). There was no effect of the order in which a female was paired with the compatible or incompatible partner (F1,43 = 0.52, p = 0.47), nor any effect of female morph (F1,43 = 0.01, p = 0.75). The corticosterone levels at egg-laying (and the effect of mate compatibility) can be compared between the aviary experiment and the within-female cage experiment (figure 1) and were very consistent; in both, the level of corticosterone was between three and four times higher with incompatible partners.
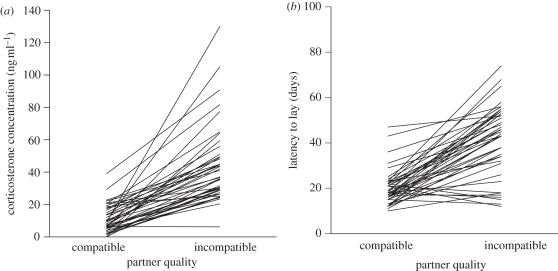
Figure 2.
(a) Level of circulating corticosterone 12 h after the experimental male (compatible or incompatible) was introduced into her cage, and (b) the number of days after the experimental introduction of the experimental male before she laid her first egg. Lines connect the data points for the two conditions from individual females.
In the forced-pair cage experiment, females responded rapidly to male quality. Females had significantly higher corticosterone levels 12 h after they were paired with incompatible males than when they were paired with compatible males (F1,43 = 25.67, p < 0.001). Controlling for clutch initiation date, there were consistent within-female differences in corticosterone responses between initial introduction and subsequent egg production (F1,85 = 13.76, p < 0.001), which were dependent on mate quality (F1,85 = 12.84, p < 0.001; mate quality × corticosterone measure: F2,84 = 6.99, p = 0.009). Females generally had a higher level of circulating corticosterone the day the second egg was laid than at the time of the male introduction although the increase between the two samples was much greater for those females paired with incompatible males; compatible (t = 2.65, p = 0.009), and incompatible pairs (t = 9.29, p < 0.001; figure 3). In naturally formed pairs in the aviaries, there was a correlation between the latency to lay and the level of circulating corticosterone in females at the time of egg laying (Rs = 0.56, n = 84, p < 0.0001). In the forced-pair cage experiments however, in a more conservative within-female test, the difference in corticosterone at egg-laying between the breeding attempts with the incompatible and compatible males was not correlated with the difference in the time that the female took to lay eggs for the two different males (Rs = 0.11, n = 43, p > 0.40).
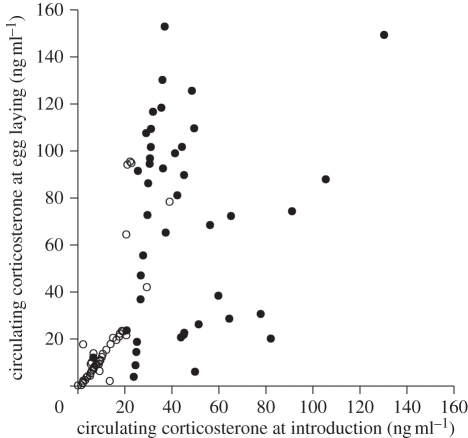
Figure 3.
The relationships between circulating corticosterone at the time of the experimental male introduction (after 12 h), and the time of egg laying the second egg (several weeks later). The two data series represent repeated measures of the same females, when breeding with compatible (open circles) and incompatible males (filled circles). There were consistent differences between females in their corticosterone responses to the different types of male. Corticosterone levels from initial introduction were correlated with subsequent levels at second egg production for females paired with compatible males (Spearman rank correlation Rho = 0.89, p < 0.0001; y = −6.49 + 2.38x), but not when paired with incompatible males (Rho = 0.17, p > 0.27; y = 4.17x −17.57).
4. Discussion
We used two experimental pairing contexts in the socially monogamous Gouldian finch to demonstrate the following: (i) Females socially paired with low-quality males delay the onset of reproduction and have a higher level of circulating corticosterone. (ii) The physiological response to partner quality is the same regardless of whether the female chooses her own partner (from a limited pool in the aviary) or has no choice at all. (iii) The physiological stress response to partner quality was both rapid (within hours of receiving a partner) and prolonged (sustained for many weeks). The speed of the response suggests that the hormonal response was not driven by indirect effects of male behaviour on the female, but rather by her initial perception of him.
The physiological response to partner quality that we have observed demonstrates that in this system many females are not satisfied with the partner that they paired with, even in the aviary context where all but the last female to pair chose their own partner. Although the aviary context in which we studied these individuals represented a subsample of a real population, it is likely to fairly accurately reflect mate choice for socially monogamous individuals in the wild. In most situations, the choosy sex (usually females) will only have available a relatively constrained pool of males to choose. In many socially monogamous species, individuals are typically paired up throughout the year, and often for the lifetime of the partners [5]. In such species, the only available partners at any one time are the few individuals that become available either at the point of sexual maturity, or through the death of a partner. In species with such long-term and continuous pair bonds, the ecology of mate choice is difficult to study, as it is temporally and spatially difficult to address. By contrast, the study of mate choice in seasonally breeding migrants is more tractable as typically partnerships across a whole population are formed within the space of a few weeks as females settle down onto territories to breed. Perhaps, the most complete view of mate choice in such a system is provided by studies of the European pied and collared flycatchers (Ficedula hypoleuca and Ficedula albicollis). In these species, males arrive back to the breeding grounds a few weeks before females and when females arrive there is essentially a scramble competition by females to secure partners that are holding territories and are still available. Female selection of a partner is most strongly influenced by the quality of territory and nest site that he holds [27], and is made typically after rather a limited search of potential options, with females visiting fewer than 10 males and pairing up within 2 days of arrival at the breeding ground [28–30]. As the season progresses, given the strong phenological selection on the timing of nestling hatching, female choice becomes more relaxed to the point where late arriving females will pair with the first available male they encounter [31], and some females even pair with a member of the wrong species [32]. The rather limited initial search for a partner and the increasingly relaxed criteria by which females accept social partners in these flycatchers represents a prudent adaptive decision by these individuals, which is predicted by theory. For many individuals, the costs of competing or delaying pairing in the hope of getting a better partner is likely to be far higher than simply taking what is currently on offer and making the best of that situation [33,34]. This theoretical framework also explains recent empirical demonstrations of the condition dependence of female choice [35,36], whereby poor-quality females will relax or modify their preference functions.
The physiological stress response to a poor-quality partner that we demonstrated here is consistent with theoretical predictions and empirical observations that suggest that in a socially monogamous situation, even though a large proportion of females may choose when, and with whom they settle, it is naive to assume that females are settling with a partner that fulfils their criteria of a ‘perfect’ social partner. It is therefore reasonable to assume that most females will pair with a partner who is not perfectly aligned with their ideal phenotypic partner (on the basis of their preference function). The stress response we observed in female Gouldian finches is perhaps understandable, given that partner quality has such a strong influence on offspring fitness in this species [20] and that with a relatively short lifespan (less than 3 years) and only one or two opportunities to breed in a year [37], the quality of the current social partner has a significant bearing on lifetime reproductive success. Thus, females that pair with poor-quality partners do so on their own choosing, but the stress response we have demonstrated here reflects the internal conflict that underlies their decision—these females are making the best of a bad situation and are dissatisfied with their partner although he does represent a better option than not breeding at all.
If females are taking a prudent approach and pragmatically settling for a suboptimal social partner, it begs the question of why they should be physiologically stressed about it. We found that females with poor-quality partners had circulating corticosterone levels three to four times higher than females paired with good-quality partners, and this was maintained for many weeks. An interesting possibility is that while a raised corticosterone profile will have some deleterious effects on female health, it may also provide a very useful mechanism for moderating an individual adaptive response to breeding with a suboptimal mate, through a number of post-pairing investment decisions and alternate reproductive behaviours.
After choosing a social partner and forming a pair-bond with a particular male, a female bird still has additional scope for optimizing her lifetime fitness. Firstly, through either pre- or post-copulatory processes she may select alternative, extrapair fathers for some of her offspring—a widespread reproductive strategy in birds [8]. In their study of sympatric Ficedula flycatchers, Veen et al. [32] found that females that had formed heterospecific social partnerships were more likely to have extrapair offspring in their broods, ameliorating the costly mistake of pairing socially with an incompatible male. Females also appear to have considerable scope in differentially altering their investment with respect to the quality of their social partner. Such differential investment that may relate to sex ratio, egg size, clutch size or the provision of parental care, or all traits simultaneously (e.g. [10]). While there is continuing debate over whether such differential investment is driven by an adaptive process of differential allocation (e.g. [9]), or compensatory investment (e.g. [38]), maternal effects such as these are widespread in birds and can affect offspring phenotype and fitness in the short and long term (reviewed in [39]). The level of circulating corticosterone offers a tangible mechanism through which a female could moderate her sexual behaviour (the degree to which she seeks polyandrous matings), and her reproductive investment. Increased stress (often socially induced through overcrowding) is known to inhibit reproduction [24,40], and could account for the reduced investment (in egg size, clutch size) that females make when stressed by the poor quality of their partner.
Our study provides insight into the constraints that exist over mate choice in socially monogamous animals, demonstrating the extent to which many females are physiologically stressed in response to the quality of the male that they are socially bonded to. While this important component of social stress may have some deleterious effects on the female, the level of circulating corticosterone in females is a probable mechanism through which females may adaptively respond to the quality of her social partner by indulging in alternative mating strategies and differential reproductive investment, in an effort to optimize individual fitness and that of her offspring.
Acknowledgements
We thank Colin Cortie for assistance with the hormone assays and the Save the Gouldian Fund for access to their research facility. The work was supported by an Australian Research Council Linkage grant to S.C.G., S.R.P. and W.A.B.; and Australian Research Council Discovery grants to S.C.G. and S.R.P.
References
- 1.Lack D. L. 1968. Ecological adaptations for breeding in birds. London, UK: Methuen & Co. Ltd [Google Scholar]
- 2.Huxley J. S. 1914. The courtship-habits of the great crested grebe (Podiceps cristatus); with an addition to the theory of sexual selection. Proc. Zool. Soc. Lond. 1914, 491–562 [Google Scholar]
- 3.Spoon T. R., Millam J. R., Owings D. H. 2006. The importance of mate behavioural compatibility in parenting and reproductive success by cockatiels, Nymphicus hollandicus. Anim. Behav. 71, 315–326 10.1016/j.anbehav.2005.03.034 (doi:10.1016/j.anbehav.2005.03.034) [DOI] [Google Scholar]
- 4.Elie J. E., Mariette M. M., Soula H. A., Griffith S. C., Mathevon N., Vignal C. 2010. Vocal communication at the nest between mates in wild zebra finches: a private vocal duet? Anim. Behav. 80, 597–605. (doi:10.1016/j.anbehav.2010.06.003) [Google Scholar]
- 5.Gowaty P. A. 1996. Battle of the sexes and origins of monogamy. In Partnerships in birds: the study of monogamy (ed. Black J. M.), pp. 21–52 Oxford, UK: Oxford University Press [Google Scholar]
- 6.Perrins C. 1979. British tits. London, UK: Collins [Google Scholar]
- 7.Wright J., Cuthill I. 1989. Manipulation of sex differences in parental care. Behav. Ecol. Sociobiol. 25, 171–181 10.1007/BF00302916 (doi:10.1007/BF00302916) [DOI] [Google Scholar]
- 8.Griffith S. C., Owens I. P. F., Thuman K. A. 2002. Extra-pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212 10.1046/j.1365-294X.2002.01613.x (doi:10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 9.Burley N. 1986. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 127, 415–445 10.1086/284493 (doi:10.1086/284493) [DOI] [Google Scholar]
- 10.Pryke S. R., Griffith S. C. 2009. Genetic incompatibility drives sex allocation and maternal investment in a polymorphic finch. Science 323, 1605–1607 10.1126/science.1168928 (doi:10.1126/science.1168928) [DOI] [PubMed] [Google Scholar]
- 11.Royle N. J., Hartley I. R., Parker G. A. 2002. Sexual conflict reduces offspring fitness in zebra finches. Nature 416, 733–736 10.1038/416733a (doi:10.1038/416733a) [DOI] [PubMed] [Google Scholar]
- 12.McNamara J. M., Houston A. I., Barta Z., Osorno J. L. 2003. Should young ever be better off with one parent than with two? Behav. Ecol. 14, 301–310 10.1093/beheco/14.3.301 (doi:10.1093/beheco/14.3.301) [DOI] [Google Scholar]
- 13.Widemo F., Owens I. P. F. 1995. Lek size, male mating skew and the evolution of lekking. Nature 373, 148–151 10.1038/373148a0 (doi:10.1038/373148a0) [DOI] [Google Scholar]
- 14.Alatalo R. V., Burke T., Dann J., Hanotte O., Höglund J., Lundberg A., Moss R., Rintamäki P. T. 1996. Paternity, copulation disturbance and female choice in lekking black grouse. Anim. Behav. 52, 861–873 10.1006/anbe.1996.0234 (doi:10.1006/anbe.1996.0234) [DOI] [Google Scholar]
- 15.Griffith S. C., Owens I. P. F., Burke T. 1999. Female choice and annual reproductive success favour less-ornamented male house sparrows. Proc. R. Soc. Lond. B 266, 765–770 10.1098/rspb.1999.0703 (doi:10.1098/rspb.1999.0703) [DOI] [Google Scholar]
- 16.Qvarnström A., Forsgren E. 1998. Should females prefer dominant males? Trends Ecol. Evol. 13, 498–501 10.1016/S0169-5347(98)01513-4 (doi:10.1016/S0169-5347(98)01513-4) [DOI] [PubMed] [Google Scholar]
- 17.Hegner R. E., Wingfield J. C. 1987. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. Auk 104, 462–469 [Google Scholar]
- 18.Qvarnström A., Brommer J. E., Gustafsson L. 2006. Testing the genetics underlying the co-evolution of mate choice and ornament in the wild. Nature 441, 84–86 10.1038/nature04564 (doi:10.1038/nature04564) [DOI] [PubMed] [Google Scholar]
- 19.Postma E., Griffith S. C., Brooks R. 2006. Evolutionary genetics—evolution of mate choice in the wild. Nature 444, E16–E16 10.1038/nature05501 (doi:10.1038/nature05501) [DOI] [PubMed] [Google Scholar]
- 20.Pryke S. R., Griffith S. C. 2009. Postztgotic genetic incompatibility between sympatric color morphs. Evolution 63, 793–798 10.1111/j.1558-5646.2008.00584.x (doi:10.1111/j.1558-5646.2008.00584.x) [DOI] [PubMed] [Google Scholar]
- 21.Pryke S. R. 2010. Sex chromosome linkage of mate preference and color signal maintains assortative mating between interbreeding finch morphs. Evolution 64, 1301–1310 [DOI] [PubMed] [Google Scholar]
- 22.Pryke S. R., Griffith S. C. 2007. The relative role of male vs. female mate choice in maintaining assortative pairing among discrete colour morphs. J. Evol. Biol. 20, 1512–1521 10.1111/j.1420-9101.2007.01332.x (doi:10.1111/j.1420-9101.2007.01332.x) [DOI] [PubMed] [Google Scholar]
- 23.Gilby A. J., Pryke S. R., Griffith S. C. 2009. The historical frequency of head colour morphs in the Gouldian finch Erythrura gouldiae. Emu 109, 222–229 10.1071/MU09013 (doi:10.1071/MU09013) [DOI] [Google Scholar]
- 24.Sapolsky R. M., Romero L. M., Munck A. U. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory and preparative actions. Endocr. Rev. 21, 55–89 10.1210/er.21.1.55 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 25.Pryke S. R., Griffith S. C. 2010. Maternal adjustment of parental effort in relation to mate compatibility affects offspring fitness. Behav. Ecol. 21, 226–232 10.1093/beheco/arp180 (doi:10.1093/beheco/arp180) [DOI] [Google Scholar]
- 26.Pryke S. R., Astheimer L. B., Buttemer W. A., Griffith S. C. 2007. Frequency-dependent physiological trade-offs between competing colour morphs. Biol. Lett. 3, 494–497 10.1098/rsbl.2007.0213 (doi:10.1098/rsbl.2007.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alatalo R. V., Lundberg A., Glynn C. 1986. Female pied flycatchers choose territory quality and not male characteristics. Nature 323, 152–153 10.1038/323152a0 (doi:10.1038/323152a0) [DOI] [Google Scholar]
- 28.Dale S., Amundsen T., Lifjeld J. T., Slagsvold T. 1990. Mate sampling behaviour of female pied flycatchers: evidence for active mate choice. Behav. Ecol. Sociobiol. 27, 87–91 10.1007/BF00168450 (doi:10.1007/BF00168450) [DOI] [Google Scholar]
- 29.Dale S., Rinden H., Slagsvold T. 1992. Competition for a mate restricts mate search of female pied flycatchers. Behav. Ecol. Sociobiol. 30, 165–176 10.1007/BF00166699 (doi:10.1007/BF00166699) [DOI] [Google Scholar]
- 30.Slagsvold T., Lifjeld J. T., Stenmark G., Breiehagen T. 1988. On the cost of searching for a mate in female pied flycatchers Ficedula hypoleuca. Anim. Behav. 36, 433–442 10.1016/S0003-3472(88)80013-7 (doi:10.1016/S0003-3472(88)80013-7) [DOI] [Google Scholar]
- 31.Dale S., Slagsvold T. 1990. Random settlement of female pied flycatchers, Ficedula hypoleuca, significance of male territory size. Anim. Behav. 39, 231–243 10.1016/S0003-3472(05)80867-X (doi:10.1016/S0003-3472(05)80867-X) [DOI] [Google Scholar]
- 32.Veen T., Borge T., Griffith S. C., Sætre G.-P., Bures S., Gustafsson L., Sheldon B. C. 2001. Hybridization and adaptive mate choice in flycatchers. Nature 411, 45–50 10.1038/35075000 (doi:10.1038/35075000) [DOI] [PubMed] [Google Scholar]
- 33.Fawcett T. W., Johnstone R. A. 2003. Mate choice in the face of costly competition. Behav. Ecol. 14, 771–779 10.1093/beheco/arg075 (doi:10.1093/beheco/arg075) [DOI] [Google Scholar]
- 34.Härdling R., Kokko H. 2005. The evolution of prudent choice. Evol. Ecol. Res. 7, 697–715 [Google Scholar]
- 35.Holveck M.-J., Riebel K. 2010. Low-quality females prefer low-quality males when choosing a mate. Proc. R. Soc. B 277, 153–160 10.1098/rspb.2009.1222 (doi:10.1098/rspb.2009.1222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunt J., Brooks R., Jennions M. D. 2005. Female mate choice as a condition-dependent life-history trait. Am. Nat. 166, 79–92 10.1086/430672 (doi:10.1086/430672) [DOI] [PubMed] [Google Scholar]
- 37.Brazill-Boast J., Pryke S. R., Griffith S. C. 2010. Nest-site utilisation and niche overlap in two sympatric, cavity-nesting finches. Emu 110, 170–177 10.1071/MU09045 (doi:10.1071/MU09045) [DOI] [Google Scholar]
- 38.Bolund E., Schielzeth H., Forstmeier W. 2009. Compensatory investment in zebra finches: females lay larger eggs when paired to sexually unattractive males. Proc. R. Soc. B 276, 707–715 10.1098/rspb.2008.1251 (doi:10.1098/rspb.2008.1251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffith S. C., Buchanan K. 2010. Maternal effects in the zebra finch: a model mother reviewed. Emu 110, 251–267 10.1071/MU10006 (doi:10.1071/MU10006) [DOI] [Google Scholar]
- 40.Wasser S., Barash D. 1983. Reproductive suppression among female mammals: implications for biomedicine and sexual selection theory. Q. Rev. Biol. 58, 513–538 10.1086/413545 (doi:10.1086/413545) [DOI] [PubMed] [Google Scholar]