Abstract
Many free-living nematodes, including the laboratory model organisms Caenorhabditis elegans and Pristionchus pacificus, have a choice between direct and indirect development, representing an important case of phenotypic plasticity. Under harsh environmental conditions, these nematodes form dauer larvae, which arrest development, show high resistance to environmental stress and constitute a dispersal stage. Pristionchus pacificus occurs in a strong association with scarab beetles in the wild and remains in the dauer stage on the living beetle. Here, we explored the circumstances under which P. pacificus enters and exits the dauer stage by using a natural variation approach. The analysis of survival, recovery and fitness after dauer exit of eight P. pacificus strains revealed that dauer larvae can survive for up to 1 year under experimental conditions. In a second experiment, we isolated dauer pheromones from 16 P. pacificus strains, and tested for natural variation in pheromone production and sensitivity in cross-reactivity assays. Surprisingly, 13 of the 16 strains produce a pheromone that induces the highest dauer formation in individuals of other genotypes. These results argue against a simple adaptation model for natural variation in dauer formation and suggest that strains may have evolved to induce dauer formation precociously in other strains in order to reduce the fitness of these strains. We therefore discuss intraspecific competition among genotypes as a previously unconsidered aspect of dauer formation.
Keywords: Pristionchus pacificus, phenotypic plasticity, dauer larvae, natural variation, dauer pheromone, intraspecific competition
1. Introduction
Phenotypic plasticity—the formation of distinct phenotypes from the same genotype—represents an important phenomenon in evolutionary biology and provides a major response mechanism to changing environmental conditions [1–3]. Although phenotypic plasticity is best studied in insects and plants [4,5], it can be observed in nearly all multicellular taxa. In nematodes, for example, many species have a choice between two alternative developmental paths, representing a major case of phenotypic plasticity. Early in development, nematode larvae can either undergo direct development into reproducing adult individuals or enter indirect development via dauer formation. Direct development is favoured under mild and stable environmental conditions with ample food, while many free-living nematode species form arrested dauer larvae when exposed to harsh conditions, such as food shortage, high temperature or high population density [6–8]. Dauer larvae are non-feeding, have a closed mouth and are resistant to many environmental stresses. At the same time, dauer larvae constitute a dispersal stage because they can attach to other invertebrates, which carry them to new habitats [9].
Dauer formation has been intensively studied in the model organism Caenorhabditis elegans, largely by investigation of the genetic and molecular aspects of dauer regulation [7]. The analysis of C. elegans dauer formation represents an important model system for insulin, TGF-β and endocrine hormone signalling. Upstream of these signalling activities is the secretion of a dauer pheromone that induces dauer formation when sensed by conspecifics [10]. Work over the last few years has revealed that the dauer pheromone consists of a complex blend of ascarosides [11,12]. In contrast to the detailed knowledge of the developmental regulation of C. elegans dauer formation, surprisingly few studies have looked at dauer formation from an evolutionary and ecological perspective. However, some recent studies have indicated that there is variation in the reaction norm of dauer formation among C. elegans wild isolates [13]. Quantitative trait loci have been identified on two chromosomes in recombinant inbred line experiments [13,14].
The nematode Pristionchus pacificus has been established as a model organism in evolutionary developmental biology and evolutionary ecology [15,16]. Pristionchus pacificus has a self-fertilizing hermaphroditic propagation, which facilitates genetic analysis [17]. More recent studies have provided an ecological perspective by revealing that several Pristionchus species, including P. pacificus, are often found on scarab beetles [18–21]. All Pristionchus nematodes found on living beetles are in the dauer stage [22]. The dauer larvae stay associated with the beetle until the natural death of their host, after which they resume development and start feeding on microbes that grow on the beetle carcass. The discovery of this necromenic association with scarab beetles resulted in an interest in studying various aspects of the ecology of P. pacificus and the associated genetic processes, including feeding habits [16], formation of tooth-like denticles in the buccal cavity [23] and olfaction [24,25].
Pristionchus pacificus is the only known cosmopolitan species of the genus Pristionchus. The first P. pacificus strain PS312 was isolated in Pasadena (California, USA), and serves as a ‘wild-type’ strain for laboratory studies [17]. Since then, P. pacificus has been found in 17 countries on five continents. More recent work has concentrated on island biogeography and has identified P. pacificus as an abundant species on the island Réunion in the Indian Ocean, with more than 200 available isolates ([21]; R.J.S. 2010, unpublished data). Interestingly, P. pacificus is associated with several scarab beetles on that island, and the Réunion P. pacificus strains show a haplotype diversity that represents a substantial amount of the haplotype diversity known from around the world. These findings suggest that P. pacificus has invaded the island multiple times independently, most probably in association with different scarab beetles. The Réunion P. pacificus system is currently being developed as a model to work towards an integration of evolutionary ecology, population genetics and evo-devo [26]. In particular, the use of P. pacificus strains from Réunion allows the analysis of local natural variation in life-history traits on a microhabitat scale.
Given that most P. pacificus nematodes found in the wild are in the dauer stage, our goal was to investigate the ability of P. pacificus to enter and exit the dauer stage by using a natural variation approach. Dauer exit studies revealed that P. pacificus dauer larvae can survive for up to 1 year under experimental conditions. In a second experiment, we isolated dauer pheromones from 16 P. pacificus strains and tested for natural variation in pheromone production and sensitivity by analysing dauer formation in cross-reactivity assays. Surprisingly, 13 out of 16 strains produce a pheromone that induces the highest dauer formation in individuals of other genotypes, showing clear cross-preference as opposed to self-preference of the dauer pheromone.
2. Material and methods
(a). Nematode cultures
All worms were kept on nematode growth medium (NGM) agar plates with the Escherichia coli strain OP50 as food [27]. Sixteen P. pacificus strains were divided into two groups of eight global and eight local strains (electronic supplementary material, table S1). The eight global strains were used in the dauer exit experiments, while all 16 strains were used for the dauer pheromone assays. All P. pacificus strains are subsequently referred to by their respective strain numbers (e.g. PS312) according to nematode nomenclature.
(b). Dauer exit assays
Dauers were obtained by growing each strain in 500 ml of S-medium containing 0.002 per cent nystatin, 0.002 per cent streptomycin and 0.5 per cent OP50. After 5 days at 20°C, dauer larvae were purified from the liquid cultures using 20 per cent Ficoll and 50 per cent sucrose as described previously [23]. Dauers were resuspended in 250 ml of M9 buffer (containing 0.2% Triton) and stored in cell-culture bottles at 8°C (7.5 ml of dauers per cell-culture bottle). As a temperature control experiment, PS312 dauers were stored in cell-culture bottles at four additional temperatures (15°C, 20°C, 25°C and 30°C). To compensate for evaporation, M9 buffer was added to the cell-culture bottles as soon as the volume dropped below a level of 6 ml.
At the beginning of the assay, the volume of M9 buffer that contained approximately 100 dauers was determined for each strain. For each time point (0, 1, 2, 3, 4, 5, 6, 10, 14, 18, 22, 26, 30, 34, 38, 42, 46, 50, 54 and 58 weeks), this volume was taken as a sample from a cell-culture bottle and pipetted onto an NGM agar plate with OP50. We counted the number of live dauers (survival) and the number of dauers that developed into J4 larvae (recovery). Survival was calculated (in per cent) as the number of live dauers for each time point divided by the average number of live dauers of the first five time points (zero to four weeks). Therefore, survival sometimes exceeded 100 per cent. Recovery was defined as the percentage of surviving dauers that developed into J4 larvae. Survival and recovery were measured in two (0–34 weeks) or three (38–58 weeks) replicates. To calculate brood size, 10 J4 larvae were transferred to separate agar plates, and their progenies were counted as J2 or J3 larvae.
(c). Dauer pheromone assays
Dauer pheromone purification and dauer pheromone assays were performed as previously described [28], with some modifications. In brief, the dauer pheromone was purified from the supernatant of a liquid culture of each strain. To desalt the pheromone extract, 500 ml of supernatant was sterile-filtered and stirred for 1 h with 30 g of activated charcoal. After washing the charcoal with water, the dauer pheromone was eluted from the charcoal using 100 per cent ethanol. The eluate was centrifuged under vacuum to evaporate the ethanol, and the remaining yellow pheromone pellet was resuspended in 3 ml of water.
In the assay, each dauer pheromone of the eight global strains was tested with each global strain, and each dauer pheromone of the eight local strains was tested with each local strain. NGM agar plates containing 50 µl of dauer pheromone were spotted with 15 µl of kanamycin-killed OP50 (three replicates per pheromone). Per plate, four young adult hermaphrodites were allowed to lay eggs overnight at 20°C (three replicates per strain), giving rise to approximately 100 progeny. The number of dauers per plate was scored after 3 days. Dauer formation (the response of each strain to each pheromone) was calculated as the percentage of progeny that entered the dauer stage. Note that dauer formation was only measured in response to the dauer pheromone and not in response to other factors, such as high temperature or starvation (i.e. the assay was performed at 20°C, and the worms were given ample food). Dauer pheromone from the purification of one liquid culture (i.e. one batch of dauer pheromone) was used to test the dauer formation of all strains in one assay. For the eight global strains, both the pheromone purification and the pheromone assay were repeated, and showed similar results (data not shown). In addition, the dauer formation of PS312 and RS5134 was tested at three different pheromone concentrations (50, 100 and 500 µl). The values for dauer formation obtained using the described pheromone assay procedure could not be normalized owing to potential differences in the unknown molecular specifics of pheromone production and sensitivity among strains.
(d). Statistical analyses
All statistical analyses were performed using the program R (http://www.r-project.org). For the dauer exit assay data and the dauer pheromone assay data, 95 per cent confidence intervals were calculated. p-values less than 0.05 were considered statistically significant.
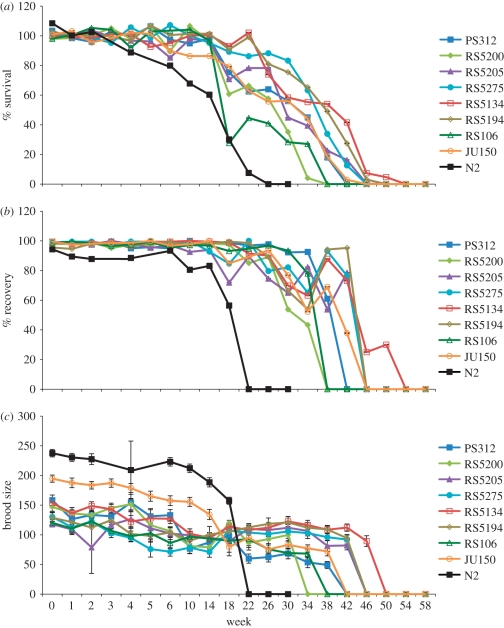
In the dauer exit assays, the total number of dauers in each cell-culture bottle is unknown. Each dauer has a certain chance of being taken up into the sample volume and a certain chance of surviving. Therefore, a Poisson distribution was assumed for the survival data, and confidence intervals were calculated using a Poisson test (figure 1a and electronic supplementary material, figure S1a). Each live dauer has an independent chance of recovering. Therefore, a binomial distribution was assumed for the recovery data, and confidence intervals were calculated using a binomial test (figure 1b and electronic supplementary material, figure S1b). For the brood-size data, a normal distribution was assumed, and confidence intervals were calculated as the standard deviation of the 10 replicates (figure 1c and electronic supplementary material, figure S1c). The values for survival, recovery and brood size of each strain after one week were compared with the values of the same strain after 30 weeks using a Poisson test, a binomial test and a t-test, respectively (electronic supplementary material, table S2). In the same manner, the values for survival, recovery and brood size of each strain after 30 weeks were compared with the values of all other strains after 30 weeks (electronic supplementary material, table S2). The average number of live dauers of the first five time points (zero to four weeks) was used as a time base for the Poisson tests.
Figure 1.
Natural variation in dauer exit. (a) Survival, (b) recovery and (c) brood size of dauers of eight P. pacificus strains stored at 8°C over a period of 58 weeks. Survival was calculated (in per cent) as the number of live dauers in the sample volume divided by the average number of live dauers of the first five time points (zero to four weeks). Recovery was measured as the percentage of survivors that developed into J4 larvae. Brood size is the average number of progeny of 10 J4 larvae. The C. elegans dataset was generated in the same way as for P. pacificus, but no measurements were made at weeks 3 and 5.
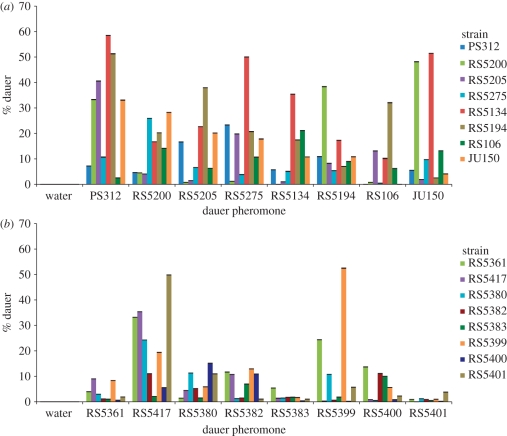
In the dauer pheromone assays, each worm has an independent chance of becoming a dauer. Therefore, a binomial distribution was assumed for the dauer formation data, and confidence intervals were calculated using a binomial test (figures 2 and 3). For each dauer pheromone, Fisher's exact test was performed to compare the response of the strain from which the pheromone was purified with the response of the strain that showed the highest (or second highest) dauer formation (electronic supplementary material, table S3). Fisher's exact test was also used to compare the dauer formation of PS312 and RS5134 at different pheromone concentrations (electronic supplementary material, table S4).
Figure 2.
Natural variation in dauer pheromone production and sensitivity. Sixteen P. pacificus strains were divided into two groups of (a) eight global and (b) eight local strains. Each dauer pheromone was tested with each strain in one group. Dauer formation was measured as the percentage of the worms of each strain that entered the dauer stage in response to each pheromone. For each dauer pheromone, the response of the strain from which the pheromone was isolated was compared with the response of the strain that showed the highest (or second-highest) dauer formation using Fisher's exact test (electronic supplementary material, table S3). All comparisons yielded p-values less than 0.05 (considered statistically significant) except the RS5380 pheromone.
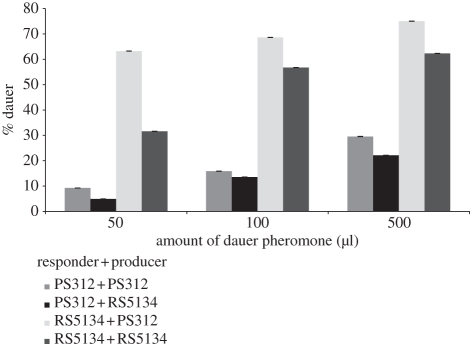
Figure 3.
Dauer formation of PS312 and RS5134 at different pheromone concentrations. Response of PS312 and RS5134 to their dauer pheromones at different pheromone concentrations. All combinations of strain + pheromone (responder + producer) were tested for each pheromone concentration. Dauer formation was measured as the percentage of PS312 and RS5134 worms that entered the dauer stage in response to 50, 100 and 500 µl of the PS312 and RS5134 pheromones.
3. Results
(a). Natural variation in dauer exit
Previous studies have indicated extensive natural variation regarding dauer entry in C. elegans [13], but little is known about the longevity of dauer larvae, the recovery and fitness of nematodes after dauer exit or the natural variation in these traits. We wanted to investigate the longevity of dauer larvae in a systematic manner under defined laboratory conditions. Therefore, we measured (i) dauer survival, (ii) dauer recovery after exposure to food and (iii) brood size as a fitness value of recovered dauers (see §2 for details). These three traits were first analysed in PS312 dauer larvae that were stored at five different temperatures (8°C, 15°C, 20°C, 25°C and 30°C). PS312 dauers stored at 8°C were able to survive for the longest period of time and showed the highest recovery after exposure to food (electronic supplementary material, figure S1). Interestingly, PS312 dauers stored at higher temperatures compensated for lower survival and recovery by increasing their brood size, suggesting an unexpected trade-off (electronic supplementary material, figure S1).
Based on these findings and aiming for maximum survival values, we selected the temperature 8°C to study natural variation among eight P. pacificus strains collected from different parts of the world (electronic supplementary material, table S1). These eight strains show a wide range of natural variation regarding dauer exit (figure 1). In all strains, survival and recovery stayed above 80 per cent for the first 14 weeks (figure 1a,b). Survival declined steadily over the following period, and two strains (RS5200 from India and RS106 from Poland) had no surviving dauers after 38 weeks (figure 1a). After 50 weeks, only a few dauers of one strain (RS5134 from Wooster, Ohio, USA) were still alive, indicating a maximum survival time of P. pacificus dauers of nearly 1 year under laboratory conditions (figure 1a). All strains were able to survive in the dauer stage for at least 30 weeks. However, the survival values of most strains after 30 weeks are significantly lower than after one week (electronic supplementary material, table S2), and there are some significant differences among strains after 30 weeks (electronic supplementary material, table S2). In all of the eight tested strains, over 50 per cent of the surviving dauers were still able to recover and reproduce after 30 weeks, and recovery remained high until almost no more dauers survived (figure 1b). The recovery values of most strains after 30 weeks are not significantly lower than after one week (electronic supplementary material, table S2). Surviving dauers that were able to recover usually still produced a large number (over 50) of progeny (figure 1c). This indicates that brood size is the most stable of the three analysed traits, although the brood-size values of most strains after 30 weeks are significantly lower than after one week (electronic supplementary material, table S2). RS5134 was able to remain in the dauer stage for the longest period of time (46 weeks), and showed 25 per cent recovery and a brood size of 90 (figure 1).
Finally, we repeated the dauer exit assay with the C. elegans strain N2. Caenorhabditis elegans was able to survive in the dauer stage for 18 weeks at 8°C (figure 1). We speculate that the much higher dauer longevity of P. pacificus compared with C. elegans could be due to the different ecologies of these two nematode species (see §4 for details).
(b). Natural variation in dauer pheromone production and sensitivity
We investigated natural variation in pheromone production and sensitivity by analysing the dauer-inducing capacities of dauer pheromones isolated from 16 P. pacificus strains (divided into two groups of eight global and eight local strains; electronic supplementary material, table S1) in cross-reactivity assays. Specifically, we tested dauer formation in response to the eight pheromones in each group (see §2 for details). To study global natural variation in pheromone production and sensitivity, we selected eight global strains from countries in different parts of the world. The eight local strains were chosen from the Réunion strain collection to study local natural variation on a microhabitat scale.
The dataset shown in figure 2 indicates enormous natural variation in pheromone production and/or pheromone sensitivity among both global and local P. pacificus strains. Three major results can be concluded from these data. First, 13 out of 16 strains show superior cross-reactivity (i.e. they produce a pheromone that induces the highest dauer formation in other strains; figure 2 and electronic supplementary material, table S3). For example, PS312 forms 7 per cent dauers in response to its own pheromone, while RS5134 forms nearly 60 per cent dauers in response to the PS312 pheromone (figure 2a). One likely explanation for these results is variability in pheromone sensitivity. It is important to note that all pheromones can induce dauer formation in the strains from which they were isolated, but often with higher thresholds than for other strains.
Second, the only three strains that show superior self-reactivity are RS5134, RS5399 (from Trois Bassins on Réunion) and RS5401 (also from Trois Bassins). They produce a pheromone that acts best on individuals of their own genotype (figure 2 and electronic supplementary material, table S3). However, it does not necessarily follow that these three strains respond to their own pheromone with a higher dauer formation than to the pheromone of other strains. For example, the comparison of PS312 with RS5134 indicates that RS5134 forms more dauers in response to the PS312 pheromone than in response to its own pheromone. This effect has been observed at three independent pheromone concentrations, although the level of dauer formation is concentration-dependent (figure 3). In response to the PS312 pheromone, dauer formation of RS5134 is significantly higher than dauer formation of PS312 at all three tested concentrations (electronic supplementary material, table S4). These data might best be explained by assuming differences in both pheromone production and sensitivity.
Third, the enormous natural variation in dauer formation is not restricted to strains of global origin but is also observed among local strains from Réunion (figure 2b), even among strains isolated from the same beetle (electronic supplementary material, table S1). For example, RS5399 forms 52 per cent dauers in response to its own pheromone, while RS5400 (from Trois Bassins) and RS5401 show a very low dauer formation in response to their own pheromone and that of RS5399 (figure 2b). These results provide the first evidence for extreme natural variation in ecologically relevant nematode traits on a microhabitat scale.
4. Discussion
Our goal was to investigate natural variation in dauer entry and dauer exit among P. pacificus strains. To accomplish this, we analysed survival, recovery and brood size after dauer exit, and performed dauer pheromone cross-reactivity assays. In order to study the effects of dauer pheromones in the most accurate manner, all other environmental factors that might also influence dauer formation (such as temperature) were kept at constant levels throughout the dauer pheromone assays.
The analysis of survival, recovery and brood size after dauer exit provides the first quantitative data for the longevity of P. pacificus dauer larvae under experimental conditions. While dauer survival and recovery in the wild might differ from the results observed under laboratory conditions, our data clearly indicate that P. pacificus dauers can survive for up to 1 year. We performed an additional analysis of our data to exclude the possibility that by measuring natural variation in dauer longevity at 8°C, we were actually measuring natural variation in resistance to cold stress. We found no correlation between dauer longevity at 8°C and average coldest temperature at the locations from which the strains were collected (data not shown). This analysis suggests that the ability to survive in the dauer stage for a certain period of time is not adapted to the temperature in the natural habitat.
In general, P. pacificus shows a much higher dauer longevity than C. elegans, which could be due to the different ecologies of these two nematodes. One hypothesis would be that P. pacificus has evolved under conditions where dauers have to spend most of their existence in the dauer stage waiting for a suitable beetle host. While many scarab beetles have life cycles of 1 year or more, the adult beetle lives for only a few weeks, and the beetles spend most of their life in the soil as grubs or pupae [29]. Previous studies have indicated that Pristionchus dauer larvae can indeed be found on scarab beetle grubs and pupae [18]. However, field and olfaction studies suggest a strong preference of Pristionchus dauers for later beetle stages, which are usually short-lived [18,24]. These observations are consistent with P. pacificus dauers being adapted to spending long periods of time waiting for a beetle host. Those dauers that are able to survive long enough until finally encountering a beetle would then not have to wait too long for the death of the beetle, given the short lifespan of adult beetles.
In C. elegans, it has so far been assumed that dauer formation serves two major functions: survival under harsh environmental conditions and dispersal [6,13,30]. Natural variation of dauer formation among wild isolates is considered to represent an adaptation by which dauer pheromone production and sensitivity are optimized in such a way that each population responds most strongly to its own pheromone to favour the survival of individuals of the same genotype [13]. Alternatively, natural variation in dauer formation might be the result of non-adaptive forces or might even be the effect of intraspecific competition. To distinguish among these three possibilities—adaptation, neutrality and intraspecific competition—dauer pheromones must be isolated from multiple strains and cross-reactivity assays (as described here for 16 P. pacificus strains) must be performed. To our knowledge, no similar experiments have been carried out using C. elegans wild isolates.
If natural variation in dauer formation represented an adaptation to enhance the fitness of a genotype, pheromone cross-reactivity assays should result in self-preference of dauer pheromones. The near absence of P. pacificus strains that have the highest dauer-inducing capacity on their own genotype argues against a simple adaptation model and favours the importance of other forces. In theory, natural variation in dauer formation might be neutral, as long as a given genotype can still respond to a certain concentration of its own pheromone. Indeed, all 16 tested P. pacificus strains were capable of initiating dauer formation in response to their own pheromone, but often to a lesser extent than to the pheromones of other strains. Alternatively, our results might provide the first evidence for the involvement of intraspecific competition in nematode dauer formation. Such a scenario would result in an evolutionary arms race [31] to induce dauer formation precociously in individuals of other genotypes. Intraspecific competition might occur naturally for necromenic nematodes, such as P. pacificus. When these nematodes exit the dauer stage to feed on microbes on the scarab beetle carcass, they find themselves in a typical saprobiontic environment that is short-lived and patchy in distribution. The number of generations for which a particular genotype can feed on the decaying organic matter strongly influences the survival chances of a particular population. Members of a population could gain an advantage if they were able to use food sources longer than individuals of other populations. Differences in the threshold for pheromone recognition could serve this function. The typical brood size of P. pacificus is between 100 and 200 individuals, while the infestation rate of scarab beetles with Pristionchus nematodes is usually very low (mostly less than 10 individuals; R.J.S. 2010, unpublished data [22]). Therefore, intraspecific competition might have a double effect. A genotype A that could force a genotype B into early dauer formation would be able to produce one further generation of its own genotype and would thereby multiply by a factor of 100 or more. At the same time, forcing genotype B into the dauer stage would reduce the number of dauer larvae of the opposing genotype B. Such a scenario would be consistent with our observation that P. pacificus haplotypes found on the same Oryctes borbonicus beetle individual at Trois Bassins on Réunion [21] do indeed differ with respect to dauer formation.
While the results of our experiments do not support a simple adaptation model for natural variation in dauer formation, we are currently unable to distinguish between neutrality and intraspecific competition. One experimental set-up to test predictions derived from the cross-preference data would be competition experiments, such as described in C. elegans [32]. In such an experiment, worms of competing strains would be driven into dauer development and would be allowed to recover after a given time. After a total of 10 generations, differences in relative fitness might be observable. We are currently in the process of generating single-nucleotide polymorphisms that would allow differentiation among strains.
In conclusion, our experiments help to provide a more comprehensive basis for the evolution and ecology of P. pacificus dauer larvae and dauer formation. It is likely that related phenomena also exist in other nematodes. Therefore, our findings might offer an additional function for the most important form of developmental arrest in nematodes, and would provide novel aspects for the evolution and maintenance of life-history traits.
Acknowledgements
We are grateful to Dr. A. Ogawa for help with dauer pheromone purification and dauer pheromone assays, and to Dr. R. Neher for suggestions regarding statistical analyses. We thank G. Bento, A. Sinha, Drs M. Herrmann, A. McGaughran, K. Morgan, A. Ogawa and E. Ragsdale for critically reading the manuscript, and other members of the Sommer laboratory for discussions.
References
- 1.Schlichting C. D., Pigliucci M. 1993. Control of phenotypic plasticity via regulatory genes. Am. Nat. 142, 366–370 10.1086/285543 (doi:10.1086/285543) [DOI] [PubMed] [Google Scholar]
- 2.Beldade P., Brakefield P. M. 2002. The genetics and evo-devo of butterfly wing patterns. Nat. Rev. Genet. 3, 442–452 10.1038/nrg818 (doi:10.1038/nrg818) [DOI] [PubMed] [Google Scholar]
- 3.West-Eberhard M. J. 2005. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102, 6543–6549 10.1073/pnas.0501844102 (doi:10.1073/pnas.0501844102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitman D. W., Ananthakrishnan T. N. 2009. Phenotypic plasticity of insects: mechanisms and consequences. Enfield, NH: Science Publishers [Google Scholar]
- 5.Sultan S. E. 2003. Phenotypic plasticity in plants: a case study in ecological development. Evol. Dev. 5, 25–33 10.1046/j.1525-142X.2003.03005.x (doi:10.1046/j.1525-142X.2003.03005.x) [DOI] [PubMed] [Google Scholar]
- 6.Cassada R. C., Russell R. L. 1975. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46, 326–342 10.1016/0012-1606(75)90109-8 (doi:10.1016/0012-1606(75)90109-8) [DOI] [PubMed] [Google Scholar]
- 7.Hu P. J. 2007. Dauer. WormBook 8, 1–19 10.1895/wormbook.1.144.1 (doi:10.1895/wormbook.1.144.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa A., Sommer R. J. 2009. Strategies to get arrested. Science 326, 944–945 10.1126/science.1183272 (doi:10.1126/science.1183272) [DOI] [PubMed] [Google Scholar]
- 9.Poinar G. O. 1983. The natural history of nematodes. Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- 10.Golden J. W., Riddle D. L. 1982. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 218, 578–580 10.1126/science.6896933 (doi:10.1126/science.6896933) [DOI] [PubMed] [Google Scholar]
- 11.Jeong P. Y., et al. 2005. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433, 541–545 10.1038/nature03201 (doi:10.1038/nature03201) [DOI] [PubMed] [Google Scholar]
- 12.Butcher R. A., Fujita M., Schroeder F. C., Clardy J. 2007. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 3, 420–422 10.1038/nchembio.2007.3 (doi:10.1038/nchembio.2007.3) [DOI] [PubMed] [Google Scholar]
- 13.Viney M. E., Gardner M. P., Jackson J. A. 2003. Variation in Caenorhabditis elegans dauer larva formation. Dev. Growth Differ. 45, 389–396 10.1046/j.1440-169X.2003.00703.x (doi:10.1046/j.1440-169X.2003.00703.x) [DOI] [PubMed] [Google Scholar]
- 14.Harvey S. C., Shorto A., Viney M. E. 2008. Quantitative genetic analysis of life-history traits of Caenorhabditis elegans in stressful environments. BMC Evol. Biol. 8, 15–30 10.1186/1471-2148-8-15 (doi:10.1186/1471-2148-8-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong R. L., Sommer R. J. 2006. Pristionchus pacificus: a well-rounded nematode. BioEssays 28, 651–659 10.1002/bies.20404 (doi:10.1002/bies.20404) [DOI] [PubMed] [Google Scholar]
- 16.Rae R., Riebesell M., Dinkelacker I., Wang Q., Herrmann M., Weller A. M., Dieterich C., Sommer R. J. 2008. Isolation of naturally associated bacteria of necromenic Pristionchus nematodes and fitness consequences. J. Exp. Biol. 211, 1927–1936 10.1242/jeb.014944 (doi:10.1242/jeb.014944) [DOI] [PubMed] [Google Scholar]
- 17.Sommer R. J., Carta L. K., Kim S.-Y., Sternberg P. W. 1996. Morphological, genetic and molecular description of Pristionchus pacificus sp. n. (Nematoda, Diplogastridae). Fundam. Appl. Nematol. 19, 511–521 [Google Scholar]
- 18.Herrmann M., Mayer W. E., Sommer R. J. 2006. Sex, bugs and Haldane's rule: the nematode genus Pristionchus in the United States. Front. Zool. 3, 14–28 10.1186/1742-9994-3-14 (doi:10.1186/1742-9994-3-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrmann M., Mayer W. E., Sommer R. J. 2006. Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology 109, 96–108 10.1016/j.zool.2006.03.001 (doi:10.1016/j.zool.2006.03.001) [DOI] [PubMed] [Google Scholar]
- 20.Herrmann M., Mayer W. E., Hong R. L., Kienle S., Minasaki R., Sommer R. J. 2007. The nematode Pristionchus pacificus (Nematoda: Diplogastridae) is associated with the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae) in Japan. Zoolog. Sci. 24, 883–889 10.2108/zsj.24.883 (doi:10.2108/zsj.24.883) [DOI] [PubMed] [Google Scholar]
- 21.Herrmann M., Kienle S., Rochat J., Mayer W. E., Sommer R. J. 2010. Haplotype diversity of the nematode Pristionchus pacificus on Réunion in the Indian Ocean suggests multiple independent invasions. Biol. J. Linn. Soc. 100, 170–179 10.1111/j.1095-8312.2010.01410.x (doi:10.1111/j.1095-8312.2010.01410.x) [DOI] [Google Scholar]
- 22.Weller A. M., Mayer W. E., Rae R., Sommer R. J. 2010. Quantitative assessment of the nematode fauna present on Geotrupes dung beetles reveals species-rich communities with a heterogenous distribution. J. Parasitol. 96, 525–531 10.1645/GE-2319.1 (doi:10.1645/GE-2319.1) [DOI] [PubMed] [Google Scholar]
- 23.Bento G., Ogawa A., Sommer R. J. 2010. Co-option of the hormone-signalling module dafachronic acid-DAF-12 in nematode evolution. Nature 466, 494–497 10.1038/nature09164 (doi:10.1038/nature09164) [DOI] [PubMed] [Google Scholar]
- 24.Hong R. L., Svatos A., Herrmann M., Sommer R. J. 2008. Species-specific recognition of beetle cues by the nematode Pristionchus maupasi. Evol. Dev. 10, 273–279 10.1111/j.1525-142X.2008.00236.x (doi:10.1111/j.1525-142X.2008.00236.x) [DOI] [PubMed] [Google Scholar]
- 25.Hong R. L., Witte H., Sommer R. J. 2008. Natural variation in Pristionchus pacificus insect pheromone attraction involves the protein kinase EGL-4. Proc. Natl Acad. Sci. USA 105, 7779–7784 10.1073/pnas.0708406105 (doi:10.1073/pnas.0708406105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer R. J. 2009. The future of evo-devo: model systems and evolutionary theory. Nat. Rev. Genet. 10, 416–422 10.1038/nrg2567 (doi:10.1038/nrg2567) [DOI] [PubMed] [Google Scholar]
- 27.Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa A., Streit A., Antebi A., Sommer R. J. 2009. A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Curr. Biol. 19, 67–71 10.1016/j.cub.2008.11.063 (doi:10.1016/j.cub.2008.11.063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dechambre R.-P., Lachaume G. 2001. Dynastidae, the genus Oryctes. Beetles of the World, vol. 27 Canterbury, UK: Hillside Books [Google Scholar]
- 30.Kiontke K., Sudhaus W. 2006. Ecology of Caenorhabditis species. WormBook 9, 1–14 10.1895/wormbook.1.37.1 (doi:10.1895/wormbook.1.37.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawkins R., Krebs J. R. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511 10.1098/rspb.1979.0081 (doi:10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 32.Walker D. W., McColl G., Jenkins N. L., Harris J., Lithgow G. J. 2000. Natural selection: evolution of lifespan in C. elegans. Nature 405, 296–297 10.1038/35012693 (doi:10.1038/35012693) [DOI] [PubMed] [Google Scholar]