Abstract
Local studies have shown that the distribution of red knots Calidris canutus across intertidal mudflats is consistent with the predictions of an ideal distribution, but not a free distribution. Here, we scale up the study of feeding distributions to their entire wintering area in western Europe. Densities of red knots were compared among seven wintering sites in The Netherlands, UK and France, where the available mollusc food stocks were also measured and from where diets were known. We tested between three different distribution models that respectively assumed (i) a uniform distribution of red knots over all areas, (ii) a uniform distribution across all suitable habitat (based on threshold densities of harvestable mollusc prey), and (iii) an ideal and free distribution (IFD) across all suitable habitats. Red knots were not homogeneously distributed across the different European wintering areas, also not when considering suitable habitats only. Their distribution was best explained by the IFD model, suggesting that the birds are exposed to interference and have good knowledge about their resource landscape at the spatial scale of NW Europe, and that the costs of movement between estuaries, at least when averaged over a whole winter, are negligible.
Keywords: dispersal, energetics, group living, intake rate, interference, spatial scale
1. Introduction
How animals distribute themselves across the globe over a continuum of temporal and spatial scales is a fundamental issue, and an applied one when species and habitat conservation is of concern [1]. Animal distributions have been studied at a wide variety of scales and levels of organization [2]. When explaining distributions, for simplicity, it is usually assumed that individuals have perfect knowledge of the quality of their habitats, so that distributions reflect multiple hierarchical processes of optimal habitat selection. This thinking was operationalized in the concept of the ideal free distribution (IFD) by Fretwell & Lucas [3]. Assuming equal competitive abilities, omniscience and no movement costs, IFD models predict that animals would move to those places where their rewards, expressed as intake rate, will be highest. Intake rate decreases as the number of competitors increases, owing to interference between foragers [4]. Therefore, IFD predicts that at equilibrium, animals distribute themselves in such ways that intake rates are equal across all occupied localities.
The assumptions and predictions of IFD models have been tested in many different ways, and IFD has met variable success [5]. In any case, the IFD can be considered a useful null model [6], deviations of which have been interpreted variously to reflect (i) a perceptual limit [7], (ii) the presence of despotic processes such as territorial behaviour [8] or unequal competitive abilities [9], and (iii) risk-sensitive foraging behaviour [10,11]. Whereas IFD predictions have often been tested over short time periods and small spatial scales, they have still been poorly tested over coarser scales [12–14].
In previous studies on the distribution of a molluscivorous shorebird, the red knot Calidris canutus [15], it was established that during their daily routines across tens of square kilometres of intertidal flats in the Dutch Wadden Sea they were behaving as ideal, but not as free, foragers. Here we aim to upscale the comparison from daily home ranges to the entire wintering area, across western Europe, of the islandica subspecies. This comparison is built on variously validated functional response equations for digestively constrained red knots foraging on hard-shelled mollusc prey [16,17], as well as estimates of the costs of activity [18] and outdoor living [19]. In order to predict IFDs, we included interference into these functional response models, expressed as the time lost to interactions with congeners during foraging. Although red knots are highly gregarious foragers, taking account of interference when modelling their intake rate is required because aggressive interactions have been observed in the field (P. van den Hout 2010, personal observation) and during small-scale indoor experiments [20,21]. Because we do not know the precise mechanism of interference, we tried out different interference models, both phenomenological and mechanistic ones, in order to predict the knots' IFD [22,23].
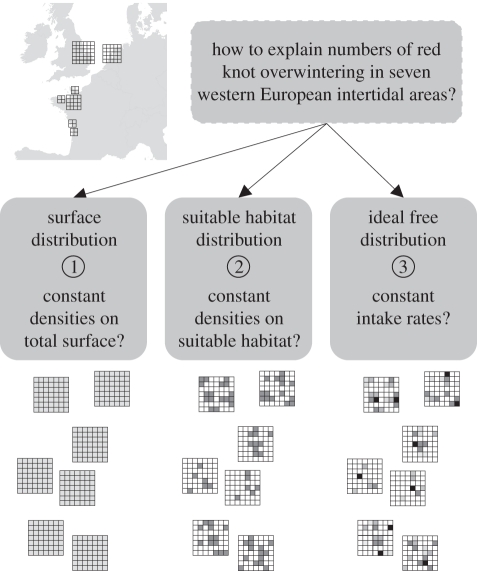
Predictions of IFD models were compared with two more simple, interference-free models: one model assumes knot numbers to vary in accordance with intertidal surface area only; the other model assumes knot numbers to scale with the surface of suitable feeding sites only, where a site is considered suitable when (interference-free) intake rate is sufficient to cover the daily energy expenditure (figure 1). These predictions were tested using data on food availability collected during two winters at seven main wintering sites in the UK, The Netherlands and France, thus covering much of their wintering range across western Europe (inset in figure 1).
Figure 1.
Basic overview of the three approaches used for explaining the distribution of knots overwintering in seven study sites across western Europe. (1) A null model, the ‘surface model’, which assumes a constant density across the total intertidal surface; (2) a suitable habitat model that assumes a constant density across the suitable surface; (3) an IFD model that assumes a constant density-dependent intake rate throughout suitable habitats. In all three models, the grey shading indicates the relative density of foragers (the highest densities at the darkest stations) across different sites that vary in food abundance.
2. Material and methods
(a). Study sites
The UK estuaries, the Dutch Wadden Sea and the French coastline harbour 64, 20 and 6 per cent, respectively, of the wintering islandica population [24]. Study sites were chosen to cover much of this winter range (electronic supplementary material, figure S1): the Wash (UK) and the western half of the Dutch Wadden Sea (The Netherlands). Five sites in France were also included: three northern sites along the English Channel coast (Bay des Veys, Mont Saint-Michel Bay and Saint-Brieuc Bay) and two more southern sites along the Atlantic coast (Aiguillon Bay and Marennes-Oléron Bay, both forming the southern limit of islandica's main wintering distribution). Across these seven intertidal sites, red knots encountered different food stocks [25] and weather conditions [19], thus yielding serious heterogeneity in habitat suitability.
(b). Sampling and treatment
At each intertidal study site, sampling grids (stations at 250 m intervals; see [26]) were used to map the abundance of the potential macrozoobenthic prey. (1) In the Wash, two sandflats were entirely sampled during mid-January 2004: Breast Sand in the south and Stubborn Sand in the east, which together cover 9 per cent of the entire intertidal surface area of the bay. (2) In the western Dutch Wadden Sea, 67 per cent of intertidal flats were sampled from late July to late October 2003. (3) In Normandy, we sampled the central part of the Bay des Veys (97% of the entire intertidal surface) in January 2006. In Brittany, we sampled (4) the Mont-Saint-Michel Bay (14% of the entire intertidal surface) in January 2004 and (5) Saint-Brieuc Bay (88%) in January 2006. The two sites in the south of France, (6) Aiguillon Bay (100%) and (7) Marennes-Oléron Bay (68%), were sampled between mid-February and mid-March 2004.
On each grid station, a sediment core of 0.0179 m2 was taken down to a depth of 20 cm. Cores were sieved on a 1 mm mesh. Because red knots cannot access benthic prey that are burrowed deeper than the 3.5 cm length of their bill [27], we separately sieved the upper, accessible 4 cm of the sample from the bottom, inaccessible part of the sample (4–20 cm). An additional core of 0.0037 m2 down to a depth of 3 cm was sieved over a 0.5 mm mesh in order to sample the abundant mudsnail, Hydrobia ulvae. Samples were frozen at −20°C until they were processed. In the laboratory, each potential macrozoobenthic prey item was counted (to estimate density) and its length was determined to the nearest millimetre (in order to determine ingestibility; see below). For each bivalve item, the flesh was separated from the shell, then both components were dried during three days at 55–60°C such that dry mass of the flesh and dry flesh of the indigestible ballast material (DMshell) could be determined. Finally, dried flesh was burned for 5 h at 550°C to determine ash-free dry mass (AFDMflesh). Shell and flesh parts of Hydrobia could not be measured separately and hence we determined AFDMtotal for this species. Under a microscope, however, we were able to separate flesh from shell (average ± s.e. length = 3.9 ± 0.7 mm; n = 40), which revealed that 40 per cent (s.e. = 2%) of AFDMtotal constitutes AFDMshell.
Diet reconstructions were made on the basis of the analysis of knot droppings collected at each site [28]. Six prey species made up only 98 per cent of the red knot's diet: five bivalves (the Baltic tellin Macoma balthica, the thin tellin Tellina tenuis, the edible cockle Cerastoderma edule and two Scrobicularidae: Scrobicularia plana and Abra tenuis) and one gastropod (the mudsnail Hydrobia ulvae [17]; G. Quaintenne & P. Bocher 2010, unpublished data). We restricted our analyses in this paper to these six prey species. Prey too large to be ingested were excluded, applying the upper length classes defined by Zwarts & Blomert [27]: 18 mm for Macoma, 16 mm for Cerastoderma and 15 mm for Scrobicularia (the latter according to droppings mainly collected in Pertuis Charentais). All size classes of Abra, Tellina and Hydrobia are considered ingestible.
(c). Modelling gross intake rate
Gross energy intake rate (mg AFDMflesh s−1) was predicted for each sampling station within each site using the so-called ‘digestive rate model’ (DRM) [16,29,30]. This model accurately predicted the diet of red knots, under both experimental and field conditions [16,17,31]. For mathematical details, we refer to Hirakawa [30] or van Gils et al. [16], but we will briefly review the basics here.
When multiple prey types are available, the DRM predicts the optimal diet composition that maximizes long-term average energy intake rate (Y in electronic supplementary material, table S1) under the constraints of finding, handling and digesting prey. Digestion is considered not to be mutually exclusive with searching and handling, but there is a certain ballast mass processing rate that cannot be exceeded in the long run (the digestive constraint, c in electronic supplementary material, table S1). For red knots that swallow hard-shelled prey whole, this rate is the maximum at which the digestive system is able to crush the shell and process the shell material, which in turn is determined by the size of the knot's gizzard [32,33]. When knots face this digestive constraint, they should be selective towards prey types that have a high flesh-to-shell-mass ratio [16,17,31]. The preference for a given prey type pi is thus optimal based on energy intake maximization. We refer to the graphical procedure of Hirakawa [30] and van Gils et al. [16] for the calculation of pi, where each prey is plotted according to its energy intake while handling this type (its profitability) against its ballast intake while handling this type.
In the DRM model, prey types were represented by 1 mm size classes of each of the six selected prey species (see electronic supplementary material, table S2 for parameter details). For each prey type, we used sampling-station-specific values for available density, energy content (AFDMflesh) and ballast mass (DMshell). Estimates for searching efficiency (m2 s−1) and handling time (s) were taken from van Gils et al. [16]. Digestive capacity was defined for a gizzard mass of 9 g (having a shell mass processing capacity c of 4.1 mg DMshell s−1 [33]), a mass that is in accordance with gizzard measurements during this time of year [32] and with diet compositions across all of our study sites that best fitted with optimal diet predictions that were based on this mass [17].
(d). Energy requirements and habitat suitability
Daily energy requirements were approximated (electronic supplementary material, tables S1 and S2) under the assumption that red knots devote 10 hours a day to active foraging and the rest of their daily time to resting and flying back and forth to the roost [32,34,35]. At each site, for each sampling station, we calculated the distance to the nearest roost. Locations of roosts were identified in the Wash according to Rehfisch et al. [36], in the Wadden Sea according to Spaans et al. [37], in Bay des Veys according to E. Caillot (2006, personal communication), in Mont-Saint-Michel Bay according to Le Dréan-Quénec'hdu et al. [38], in Saint-Brieuc Bay according to Annezo & Hamon [39], and in Aiguillon Bay and Marennes-Oléron Bay according to P. Bocher (2010, personal observation).
Using these station-specific flight distances, daily energy requirements were calculated as the sum of maintenance metabolism and activity costs. Maintenance metabolism, which is defined as the sum of basal metabolic rate and the cost of thermoregulation [19], can be predicted on the basis of knowledge on three standard climatic variables (air temperature, wind speed and global solar radiation), while taking account of the birds' microhabitat use (described in [19]). Hourly weather data, averaged on a monthly basis (December, January and February) over at least a period of 20 years, were used to define maintenance metabolism specific to each area: the Wash (British Atmospheric Data Centre and Met Office; from 1980 to 2000), Wadden Sea (KNMI; from 1981 to 2006), the English Channel coast and the two most southern sites (Meteo France; from 1980 to 2006).
Activity costs, which are defined as the sum of flight costs and foraging costs, were calculated assuming direct flights at a speed of 54 km h−1 between roost and feeding stations [40]. Foraging costs are defined as the sum of the cost of walking, the cost of probing prey and the cost of digestive processing (i.e. the so-called ‘heat increment of feeding’) [18,32,41]. Thermoregulatory costs during foraging can be drastically reduced because of heat generated by walking and by heat increment of feeding. We assumed that all heat increment of feeding and 30 per cent of the heat generated by walking substitutes for thermoregulatory cost during foraging [32,41].
Sampling stations were assumed suitable for red knots when gross energy intake rate allows them to cover their daily energy requirements (we know that in winter, knots behave as satisficers, implying that they aim to balance energy expenditure and income [32]). From this, we calculated a site's suitable foraging area by multiplying its percentage of suitable stations by the total surface of intertidal flats.
(e). Distribution and red knot density
Numbers of red knot counted at high tide in the various areas were averaged from 2001/2002 to 2005/2006 using data from annual mid-winter counts of the Wetlands International midwinter counting programme that are performed in November in the Wash [42], and in January in both the Dutch Wadden Sea [26] and the French sites [43]. These numbers were then expressed in densities per overall surface unit of intertidal flat.
(f). Modelling interference
Because we do not know the precise mechanism of the interference process in red knots, we used different models of interference and selected the one that best predicted the distribution of knots across the seven estuaries. A total of seven models were tested, of which three can be considered ‘mechanistic’ (the Ruxton model [44], the Beddington model [45] and the Crowley–Martin model [46]), whereas the other four are more phenomenological (the Hassell–Varley model [47], the double-log model [48], the semi-log model [49] and the untransformed model [50]).
The models differ mainly in the way searching and handling foragers interfere with each other. The Crowley–Martin model considers that both handling and searching predators can interact with both searching and handling predators. For the Beddington model, only searching predators can interact with both searching and handling individuals. In the Ruxton model, searching predators interact only with searching predators. In a phenomenological way, the model of Hassell–Varley considers that interference affects only searching rates, whereas the double-log, semi-log, and untransformed models are based on empirical relationships between intake rate and predator density. Some models also differ because they consider the existence of threshold prey densities below which predators are absent (Ruxton, Beddington, Crowley–Martin and untransformed models), or because they assume some maximum predator density (double-log, semi-log and untransformed models).
For red knots, with their relatively short handling times [51], most interference would concern birds in searching states. Differences between functional response models regarding how handling predators are involved in interference interaction are minor for red knot when compared with birds with longer handling times, like oystercatchers Haematopus ostralegus [50]. Note, however, that small differences between functional response models can lead to major differences between predicted aggregative responses [22]. For example, the Ruxton model predicts that at low densities of prey (where predators are mostly in a searching state), birds are sensitive to interference while at high densities (where predators are mostly in a handling state), birds are insensitive to interference.
Interference-reduced intake rates were predicted by first modelling diet selection at each sampling station using the DRM procedure, then using the selected prey types as input for the different interference models. We refer to the electronic supplementary material, table S3, where we spell out the formulation of interference-depressed (gross) intake rates (mg AFDMflesh s−1) in the seven interference models under digestive constraint (as an example, the formulation of the Ruxton model is given in the legend of figure 3). In an IFD, it is assumed that foragers distribute in such a way that their interference-reduced intake rates are equalized across all sites. Given the fact that wintering knots are satisfiers, we assumed the equalized net intake rate to be 0 mg AFDMflesh s−1 (i.e. taking daily energy requirements over a 24 h period into account). Interference parameters were estimated by fitting each of the seven models to the data. We did so by calculating the slope of least-squares regression between observed and predicted knot densities in which the best fit was represented by a slope not significantly different from one.
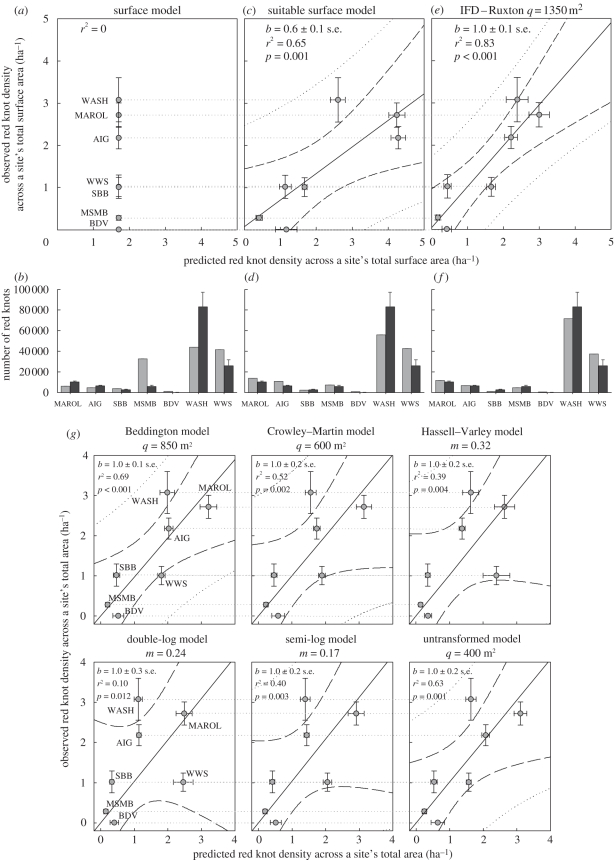
Figure 3.
(Opposite.) Comparison of mean (±s.e.) observed densities of red knots against predicted densities (±s.e.) of (a) surface model, (c) suitable habitat model and (e) the best-fitting IFD model: the Ruxton model for which q = 1350 m2. The Ruxton model predicts forager density p (m−2) assuming equalized net intake rates of 0 mg AFDMflesh s−1, where interference-reduced (gross) intake rate equals 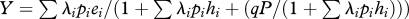 , with q being the interference area (m2), pi the probability that prey type i is accepted (following the DRM procedure), λi the encounter rate (s−1) with prey type i, hi(s) the handling time of prey type i and ei the energetic contents of prey type i (mg AFDMflesh). (c,e) Linear least-squares regression lines are given (solid lines, with slope b given in the upper left) with the 95% prediction intervals (dotted lines) and the 95% confidence intervals (dashed lines). The resulting site's total numbers of red knots predicted by each of the three models are given in the bottom histograms (b), (d) and (f). Predicted, grey bars; observed, black bars. (g) Results of the six other IFD models are given for their respective best-fitted values of interference parameters (figure 4). Site abbreviations are as given in table 1.
, with q being the interference area (m2), pi the probability that prey type i is accepted (following the DRM procedure), λi the encounter rate (s−1) with prey type i, hi(s) the handling time of prey type i and ei the energetic contents of prey type i (mg AFDMflesh). (c,e) Linear least-squares regression lines are given (solid lines, with slope b given in the upper left) with the 95% prediction intervals (dotted lines) and the 95% confidence intervals (dashed lines). The resulting site's total numbers of red knots predicted by each of the three models are given in the bottom histograms (b), (d) and (f). Predicted, grey bars; observed, black bars. (g) Results of the six other IFD models are given for their respective best-fitted values of interference parameters (figure 4). Site abbreviations are as given in table 1.
3. Results
(a). Surface areas, red knot densities and interference-free net intake rates
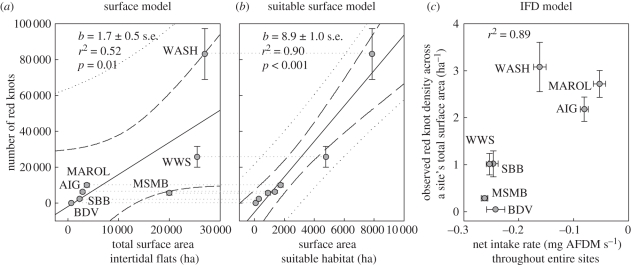
Study sites differed in terms of knot numbers, surface areas (both total and suitable) and maintenance costs (table 1). Numbers of overwintering red knots strongly correlated with the site's total surface area (figure 2a; r2 = 0.52, p = 0.012), but after taking account of the variable maintenance and activity costs, they correlated even more strongly with the surface area of the sites' suitable habitat (figure 2b; r2 = 0.90, p < 0.001). Across the total surface area of all sites, red knot density was 1.7 (±s.e. 0.5) individuals per hectare (slope in figure 2a), while across the suitable habitat only red knot density equalled 8.9 (±s.e. 1.0) birds per hectare (slope in figure 2b). As expected by the IFD model, observed red knot density across the sites' total surface areas correlated with the sites' mean net intake rates (calculated across each site's total surface, which explains the negative values; figure 2c; r2 = 0.65, p = 0.028).
Table 1.
Basic information per study site: total intertidal flat surface area, number of benthos samples collected, predicted maintenance cost, suitable habitat surface area and the number of overwintering red knots counted between 2001/2002 and 2005/2006.
| site | total surface intertidal flats, ha | samples n stations | maintenance metabolism, mg AFDMflesh s−1 | suitable habitat surface area, ha (%) | n red knots mean ± s.e. |
|---|---|---|---|---|---|
| the Wash (WASH) | 27 000 | 381 | 0.28 | 7866 (29) | 83 098 ± 14 120 |
| western Dutch Wadden Sea (WWS) | 25 500 | 2749 | 0.30 | 4777 (19) | 25 776 ± 5771 |
| Bay des Veys (BDV) | 650 | 101 | 0.26 | 90 (14) | 5 ± 2 |
| Mont Saint-Michel Bay (MSMB) | 20 000 | 440 | 0.26 | 909 (5) | 5656 ± 1084 |
| Saint-Brieuc Bay (SBB) | 2300 | 323 | 0.26 | 291 (13) | 2347 ± 633 |
| Aiguillon Bay (AIG) | 2870 | 459 | 0.24 | 1376 (48) | 6255 ± 751 |
| Marennes-Oléron Bay (MAROL) | 3700 | 405 | 0.24 | 1758 (48) | 10 060 ± 1063 |
Figure 2.
Mean number (±s.e.) of red knots overwintering in the different study areas plotted against (a) total intertidal surface area and (b) surface area of the suitable habitat. Based on linear least-squares regression (solid line with slope b), the surface model estimates an overall density of knots across total intertidal surface of 1.7 birds ha−1, whereas the suitable habitat model estimates a density of knots throughout suitable foraging areas of 8.9 birds ha−1. Dotted lines indicate the 95% prediction intervals and dashed lines the 95% confidence intervals of linear least-squares regression. (c) IFD models assume differences in overall knot densities to be related to differences in net intake rate throughout the whole area (both suitable and unsuitable area). Site abbreviations are as given in table 1.
(b). Predicted knot densities and comparison with observations
Based on the red knot density across the total surface area of all sites (slope b in figure 2a), the surface model assumes a density of 1.7 knots ha−1. Observed red knot densities across any site's total surface area did not live up to that expectation and were ranging from 3.1 to 0.1 bird per hectare (figure 3a; r2 = 0, p = 0.017). By multiplying the predicted density with a site's total surface area, we predicted a site's total number of red knots. Those predictions did not match with the observation either (figure 3b; G = 29.91, 6 d.f., p < 0.001). The fit between observed and predicted red knot densities strongly improved when considering the suitable habitat only (figure 3c; r2 = 0.65, p = 0.001, slope b = 0.65 ± 0.10 s.e.). This was also the case when comparing knot numbers (figure 3d; G = 9.32, p = 0.15).
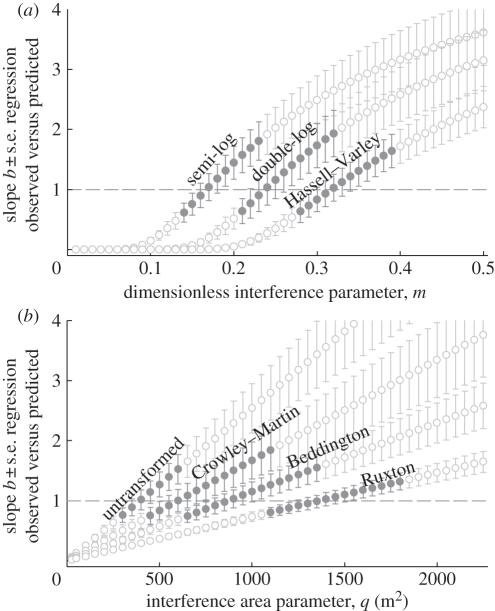
The best fit with the observed densities, however, was found for the IFD models (figure 3e, representing the Ruxton model; r2 = 0.83, p < 0.001, slope b = 0.99 ± 0.11 s.e.). The IFD predictions were also nearest to the observations for sites' total numbers of red knots (figure 3f, again reflecting the Ruxton model; G = 3.03, p = 0.80). We refer to electronic supplementary material, figure S2, where we exemplify the station-specific red knot densities predicted by the Ruxton IFD model. Among the seven interference models, the one by Ruxton et al. [44] performed best (based on r2 ranking), yielding an estimate for parameter q (interference area) of 1350 m2 (figure 4), which corresponds to an interference radius of 21 m. Among the six remaining interference models (figure 3g), most perform rather less well than the suitable habitat model, whereas only the Beddington model provides a better fit (r2 = 0.69). Goodness-of-fit for a range of parameter estimates for each of the seven models are given in figure 4.
Figure 4.
Sensitivity analysis of two interference parameters with respect to the goodness-of-fit of the different interference models. In (a), we vary interference parameter m (included in the Hassel–Varley, double-log and semi-log models); in (b), we vary interference area q (m2) (included in the Ruxton, Beddington, Crowley–Martin and untransformed models). Goodness-of-fit of models are assessed from the slope b ± s.e. of the linear regression between the observed and predicted knot densities (with closed dots representing slopes not significantly different from one and open dots slopes significantly different from one).
4. Discussion
In this study, we applied three basic distribution models to explain numbers of red knots overwintering at seven western European estuaries. The two models that performed best included the knots' energetic requirements and interference-free functional response parameters, which enabled us to define the critical threshold of food densities for red knots to cover their daily energetic requirements. Among these two models, the best model additionally took account of interference between individual birds, suggesting that red knots distribute themselves ideally and freely across western Europe. The three mechanistic models were among the four best-fitting IFD models, with Ruxton et al.'s [44] model yielding the best fit (figure 3). Some realistic mechanisms involved in the Ruxton model fit well with the observed behaviour of red knots: (i) red knots have relatively short handling times [16,52] and interference seems to occur only between searching birds [21]; (ii) red knots often strongly aggregate in the best food patches, in which they can often tolerate very high densities of conspecifics—this often occurs in midwinter when feeding on extremely high densities of mudsnail Hydrobia ulvae [19,20].
With respect to the ideal assumption, the good fit with the IFD model suggests that red knots are well-informed about the spatial distribution of their potential food throughout northwestern Europe. On much finer spatial scales, foraging red knots can quickly assess the density of food using their sensitive bill tip, which is able to detect pressure gradients in the sediment [51], and this information is updated effectively in a Bayesian manner [53]. Besides this form of personal information about their resource landscape, red knots have plenty of access to public information as they live in large groups at their wintering sites. For example, red knots may exchange information about the quality of feeding sites when at their roost during high tide [54]; also, knots can use the location of conspecifics on intertidal flats as an indicator of patch quality [15]. Furthermore, knots wintering in the Dutch Wadden Sea can easily fly to the Wash once or twice in a single winter, and thereby track the continuously changing quality of an estuary, as evidenced by the resightings of colour-ringed individuals [37].
With respect to the ‘free’ assumption, it suggests that the cost of travelling between the northwestern European estuaries would be negligible. Perhaps this is not so surprising given the timescale of a full winter that we considered here. Even if red knots were to switch from one estuary to another each month, their travel costs would still make up only 1 per cent of the total monthly energy expenditure. This conclusion contrasts with an earlier study on red knots in which, on a much finer temporal and spatial scale, knots did pay a significant travel cost and were therefore not distributed ‘freely’ [15]. This is because on the smaller temporal scale of a single day, travelling to the roost (which occurs four times a day) does significantly affect the energy expenditure.
IFD may not only be driven by birds moving between alternative estuaries that differ in short-term energetic gains in the course of winter, but also by population dynamic processes, if birds in different areas are totally site-faithful but show different birth and death rates [55–57]. However, red knots are a migratory species that typically show rather large dispersal tendencies during the non-breeding season and little site-faithfulness [37,58]. For this reason, we argue that dispersal would be the predominant mechanism underlying the establishment of the IFD in red knots.
Red knots being a social species, it is actually quite remarkable that the interference-based IFD model fits best. Outside the breeding season, red knots typically forage and roost together in large and dense flocks [59]. Indeed, in another study on red knots, it was revealed that not so much the food, but conspecific attraction to other knots, explained their distribution best [60]. This is an idea that has also been proposed recently for other bird species [61,62]. However, the study by Folmer et al. [60] was performed at the fine temporal scale of single tides. In our study, we analysed knot numbers that were averaged across five full winters at the scale of a continental coastline. At any moment, red knots may prefer the presence of conspecifics, even if these conspecifics do not occupy the best patch. As previously documented [52,59,63], in the course of time, the accumulated distribution of red knot flocks will eventually make a good match with the distribution of their food (as documented early on in [59]).
To the best of our knowledge, this is the first time the IFD predictions have been tested and verified at this geographical scale. Our approach of predicting animal numbers on the basis of food distributions, known metabolic cost functions and interference-based functional responses should be applicable to other systems. For example, an understanding of the ecological underpinning of the distribution of large herbivores in, for example, the Serengeti or Kruger National Park [64,65] is based on correlative exercises (plotting presence on indirect measures of habitat suitability) rather than the kind of thoroughly understood mechanistic reasoning presented here. There is also a substantial body of work using large-scale surveys of marine mammals to model their habitat selection. Most of these models are based on point occurrence data related to the geographical information of biotic and abiotic variables without really being given insight into the mechanistic processes determining cetacean distributions (see review of Redfern et al. [66] on cetacean habitat models). The mechanistic approach developed for red knots, with obvious worldwide relevance for this particular species [67], would enable rather precise estimation of expected distributions in our changing world. Such insights can have huge dividends when developing whole-landscape, or even continental, perspectives on nature conservation.
Acknowledgements
The study would not have been possible without the dataset collected with the precious help of numerous people from the three countries involved. We would like to acknowledge the many people who have been involved in bird counts organized by LPO, ONCFS, RNF, BTO, SOVON and Wetlands International. We are grateful to P. Wiersma for information on energy expenditure and weather. This work was financially supported by the Dutch–French Van Gogh programme administered by the Netherlands Organization for Scientific Research (NWO), the French Ministry of Foreign Affairs and the Conseil Général de la Charente-Maritime. We appreciate the feedback on drafts by Gavin Thomas and an anonymous reviewer.
References
- 1.Jonzén N. 2008. Habitat selection: implications for monitoring, management, and conservation. Isr. J. Ecol. Evol. 54, 459–471 10.1560/ijee.54.3-4.459 (doi:10.1560/ijee.54.3-4.459) [DOI] [Google Scholar]
- 2.Morris D., Brown J. 1992. The role of habitat selection in landscape ecology. Evol. Ecol. 6, 357–359 10.1007/BF02270697 (doi:10.1007/BF02270697) [DOI] [Google Scholar]
- 3.Fretwell S. D., Lucas H. L. 1970. On territorial behavior and other factors influencing habitat distribution in birds. I. Theoretical development. Acta Biotheor. 19, 16–36 10.1007/BF01601953 (doi:10.1007/BF01601953) [DOI] [Google Scholar]
- 4.Goss-Custard J. D. 1980. Competition for food and interference amongst waders. Ardea 68, 31–52 [Google Scholar]
- 5.Tregenza T. 1995. Building on the ideal free distribution. Adv. Ecol. Res. 26, 253–302 10.1016/S0065-2504(08)60067-7 (doi:10.1016/S0065-2504(08)60067-7) [DOI] [Google Scholar]
- 6.Sutherland W. J. 1996. From individual behaviour to population ecology. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Abrahams M. V. 1986. Patch choice under perceptual constraints: a cause for departures from an ideal free distribution. Behav. Ecol. Sociobiol. 19, 409–415 10.1007/BF00300543 (doi:10.1007/BF00300543) [DOI] [Google Scholar]
- 8.Fretwell S. D. 1972. Populations in a seasonal environment. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 9.Houston A. I., McNamara J. M. 1988. The ideal free distribution when competitive abilities differ: an approach based on statistical mechanisms. Anim. Behav. 36, 166–174 10.1016/S0003-3472(88)80260-4 (doi:10.1016/S0003-3472(88)80260-4) [DOI] [Google Scholar]
- 10.Caraco T., Martindale S., Pulliam H. R. 1980. Avian flocking in the presence of a predator. Nature 285, 400–401 10.1038/285400a0 (doi:10.1038/285400a0) [DOI] [Google Scholar]
- 11.McNamara J. M., Houston A. I. 1992. Risk-sensitive foraging: a review of theory. Bull. Math. Biol. 54, 355–378 10.1007/BF02464838 (doi:10.1007/BF02464838) [DOI] [Google Scholar]
- 12.Doncaster C. P. 2000. Extension of ideal free resource use to breeding populations and metapopulations. Oikos 89, 24–36 10.1034/j.1600-0706.2000.890103.x (doi:10.1034/j.1600-0706.2000.890103.x) [DOI] [Google Scholar]
- 13.Haugen T. O., Winfield I. J., Vøllestad L. A., Fletcher J. M., James J. B., Stenseth N. C. 2006. The ideal free pike: 50 years of fitness-maximizing dispersal in Windermere. Proc. R. Soc. B 273, 2917–2924 10.1098/rspb.2006.3659 (doi:10.1098/rspb.2006.3659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris D. W. 2006. Ecology: moving to the ideal free home. Nature 443, 645–646 10.1038/443645a (doi:10.1038/443645a) [DOI] [PubMed] [Google Scholar]
- 15.van Gils J. A., Spaans B., Dekinga A., Piersma T. 2006. Foraging in a tidally structured environment by red knots (Calidris canutus): ideal, but not free. Ecology 87, 1189–1202 10.1890/0012-9658(2006)87[1189:FIATSE]2.0.CO;2 (doi:10.1890/0012-9658(2006)87[1189:FIATSE]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 16.van Gils J. A., de Rooij S. R., van Belle J., van der Meer J., Dekinga A., Piersma T., Drent R. 2005. Digestive bottleneck affects foraging decisions in red knots Calidris canutus. I. Prey choice. J. Anim. Ecol. 74, 105–119 10.1111/j.1365-2656.2004.00903.x (doi:10.1111/j.1365-2656.2004.00903.x) [DOI] [Google Scholar]
- 17.Quaintenne G., van Gils J. A., Bocher P., Dekinga A., Piersma T. 2010. Diet selection in a molluscivore shorebird across Western Europe: does it show short- or long-term intake rate maximization? J. Anim. Ecol. 79, 53–62 10.1111/j.1365-2656.2009.01608.x (doi:10.1111/j.1365-2656.2009.01608.x) [DOI] [PubMed] [Google Scholar]
- 18.Piersma T., Dekinga A., van Gils J. A., Achterkamp B., Visser G. H. 2003. Cost–benefit analysis of mollusc eating in a shorebird. I. Foraging and processing costs estimated by the doubly labelled water method. J. Exp. Biol. 206, 3361–3368 10.1242/jeb.00545 (doi:10.1242/jeb.00545) [DOI] [PubMed] [Google Scholar]
- 19.Wiersma P., Piersma T. 1994. Effects of microhabitat, flocking, climate and migratory goal on energy expenditure in the annual cycle of red knots. Condor 96, 257–279 10.2307/1369313 (doi:10.2307/1369313) [DOI] [Google Scholar]
- 20.van Gils J. A., Piersma T. 2004. Digestively constrained predators evade the cost of interference competition. J. Anim. Ecol. 73, 386–398 10.1111/j.0021-8790.2004.00812.x (doi:10.1111/j.0021-8790.2004.00812.x) [DOI] [Google Scholar]
- 21.Vahl W. K., van der Meer J., Weissing F. J., van Dullemen D., Piersma T. 2005. The mechanisms of interference competition: two experiments on foraging waders. Behav. Ecol. 16, 845–855 10.1093/beheco/ari073 (doi:10.1093/beheco/ari073) [DOI] [Google Scholar]
- 22.van der Meer J., Ens B. J. 1997. Models of interference and their consequences for the spatial distribution of ideal and free predators. J. Anim. Ecol. 66, 846–858 10.2307/6000 (doi:10.2307/6000) [DOI] [Google Scholar]
- 23.Collazo J. A., Gilliam J. F., Miranda-Castro L. 2010. Functional response models to estimate feeding rates of wading birds. Waterbirds 33, 33–40 10.1675/063.033.0104 (doi:10.1675/063.033.0104) [DOI] [Google Scholar]
- 24.Stroud D. A., Davidson N. C., West R., Scott D. A., Haanstra L., Thorup P., Ganter B., Delany S. 2004. Status of migratory wader populations in Africa and Western Eurasia in the 1990s. Int. Wader Stud. 15, 1–259 [Google Scholar]
- 25.Bocher P., Piersma T., Dekinga A., Kraan C., Yates M. G., Guyot T., Folmer E. O., Radenac G. 2007. Site- and species-specific distribution patterns of molluscs at five intertidal soft-sediment areas in northwest Europe during a single winter. Mar. Biol. 151, 577–594 10.1007/s00227-006-0500-4 (doi:10.1007/s00227-006-0500-4) [DOI] [Google Scholar]
- 26.Kraan C., van Gils J. A., Spaans B., Dekinga A., Bijleveld A. I., van Roomen M., Kleefstra R., Piersma T. 2009. Landscape-scale experiment demonstrates that Wadden Sea intertidal flats are used to capacity by molluscivore migrant shorebirds. J. Anim. Ecol. 78, 1259–1268 10.1111/j.1365-2656.2009.01564.x (doi:10.1111/j.1365-2656.2009.01564.x) [DOI] [PubMed] [Google Scholar]
- 27.Zwarts L., Blomert A. M. 1992. Why knot Calidris canutus take medium-sized Macoma balthica when 6 prey species are available. Mar. Ecol. Prog. Ser. 83, 113–128 10.3354/meps083113 (doi:10.3354/meps083113) [DOI] [Google Scholar]
- 28.Dekinga A., Piersma T. 1993. Reconstructing diet composition on the basis of faeces in a mollusc-eating wader, the knot Calidris canutus. Bird Study 40, 144–156 10.1080/00063659309477140 (doi:10.1080/00063659309477140) [DOI] [Google Scholar]
- 29.Verlinden C., Wiley R. H. 1989. The constraints of digestive rate: an alternative model of diet selection. Evol. Ecol. 3, 264–273 10.1007/BF02270727 (doi:10.1007/BF02270727) [DOI] [Google Scholar]
- 30.Hirakawa H. 1995. Diet optimization with a nutrient or toxin constraint. Theor. Popul. Biol. 47, 331–346 10.1006/tpbi.1995.1015 (doi:10.1006/tpbi.1995.1015) [DOI] [PubMed] [Google Scholar]
- 31.van Gils J. A., Dekinga A., Spaans B., Vahl W. K., Piersma T. 2005. Digestive bottleneck affects foraging decisions in red knots Calidris canutus. II. Patch choice and length of working day. J. Anim. Ecol. 74, 120–130 10.1111/j.1365-2656.2004.00904.x (doi:10.1111/j.1365-2656.2004.00904.x) [DOI] [Google Scholar]
- 32.van Gils J. A., Piersma T., Dekinga A., Dietz M. W. 2003. Cost–benefit analysis of mollusc-eating in a shorebird. II. Optimizing gizzard size in the face of seasonal demands. J. Exp. Biol. 206, 3369–3380 10.1242/jeb.00546 (doi:10.1242/jeb.00546) [DOI] [PubMed] [Google Scholar]
- 33.van Gils J. A., Piersma T., Dekinga A., Battley P. F. 2006. Modelling phenotypic flexibility: an optimality analysis of gizzard size in red knots Calidris canutus. Ardea 94, 409–420 [Google Scholar]
- 34.Alerstam T., Gudmundsson G. A., Johannesson K. 1992. Resources for long distance migration: intertidal exploitation of Littorina and Mytilus by knots Calidris canutus in Iceland. Oikos 65, 179–189 10.2307/3545008 (doi:10.2307/3545008) [DOI] [Google Scholar]
- 35.Rogers D. I., Piersma T., Hassell C. J. 2006. Roost availability may constrain shorebird distribution: exploring the energetic costs of roosting and disturbance around a tropical bay. Biol. Conserv. 133, 225–235 10.1016/j.biocon.2006.06.007 (doi:10.1016/j.biocon.2006.06.007) [DOI] [Google Scholar]
- 36.Rehfisch M. M., Clark N. A., Langston R. H. W., Greenwood J. D. 1996. A guide to the provision of refuges for waders: an analysis of 30 years of ringing data from the Wash, England. J. Appl. Ecol. 33, 673–687 10.2307/2404939 (doi:10.2307/2404939) [DOI] [Google Scholar]
- 37.Spaans B., Brugge M., Dekinga A., Horn H., van Kooten L., Piersma T. 2009. Space use of red knots Calidris canutus in the Dutch Wadden Sea. Limosa 82, 113–121 [Google Scholar]
- 38.Le Dréan-Quénec'hdu S., Borer P., Mahéo R. 1995. Mont Saint Michel Bay: spatial distribution of major wader species. Wader Study Group Bull. 77, 55–61 [Google Scholar]
- 39.Annezo J. P., Hamon D. 1989. Prédation par les limicoles de la macrofaune intertidale en Baie de Saint-Brieuc. Brest, France: IFREMER [Google Scholar]
- 40.Nudds R., Bryant D. 2000. The energetic cost of short flights in birds. J. Exp. Biol. 203, 1561–1572 [DOI] [PubMed] [Google Scholar]
- 41.Bruinzeel L. W., Piersma T., Kersten M. 1999. Low costs of terrestrial locomotion in waders. Ardea 87, 199–205 [Google Scholar]
- 42.Musgrove A. J., Collier M. P., Banks A. N., Calbrade N. A., Hearn R. D., Austin G. E. 2007. Waterbirds in the UK 2005/06: the wetland bird survey. Thetford, UK: BTO/WWT/RSPB/JNCC [Google Scholar]
- 43.Mahéo R. 2008. Limicoles séjournant en France (Janvier) 1977–2008. Rennes, France: Wetlands International/ONCFS/Université de Rennes [Google Scholar]
- 44.Ruxton G. D., Gurney W. S. C., Deroos A. M. 1992. Interference and generation cycles. Theor. Popul. Biol. 42, 235–253 10.1016/0040-5809(92)90014-K (doi:10.1016/0040-5809(92)90014-K) [DOI] [Google Scholar]
- 45.Beddington J. R. 1975. Mutual interference between parasites or predators and its effect on searching efficiency. J. Anim. Ecol. 44, 331–340 10.2307/3866 (doi:10.2307/3866) [DOI] [Google Scholar]
- 46.Crowley P. H., Martin E. K. 1989. Functional responses and interference within and between year classes of a dragonfly population. J. N. Am. Benthol. Soc. 8, 211–221 10.2307/1467324 (doi:10.2307/1467324) [DOI] [Google Scholar]
- 47.Hassell M. P., Varley G. C. 1969. New inductive population model for insect parasites and its bearing on biological control. Nature 223, 1133–1137 10.1038/2231133a0 (doi:10.1038/2231133a0) [DOI] [PubMed] [Google Scholar]
- 48.Zwarts L., Drent R. H. 1981. Prey depletion and the regulation of predator density: oystercatchers (Haematopus ostralegus) feeding on mussels (Mytilus edulis). In Feeding and survival strategies of estuarine organisms (eds Jones N. V., Wolff W. J.), pp. 193–216 London, UK: Plenum Publishing Co [Google Scholar]
- 49.Ens B. J., Goss-Custard J. D. 1984. Interference among oystercatchers, Haematopus ostralegus, feeding on mussels, Mytilus edulis, on the Exe estuary. J. Anim. Ecol. 53, 217–231 10.2307/4353 (doi:10.2307/4353) [DOI] [Google Scholar]
- 50.Goss-Custard J. D., Durell S. E. A. 1987. Age-related effects in oystercatchers, Haematopus ostralegus, feeding on mussels, Mytilus edulis. III. The effect of interference on overall intake rate. J. Anim. Ecol. 56, 549–558 10.2307/5067 (doi:10.2307/5067) [DOI] [Google Scholar]
- 51.Piersma T., van Aelst R., Kurk K., Berkhoudt H., Maas L. R. M. 1998. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proc. R. Soc. Lond. B 265, 1377–1383 10.1098/rspb.1998.0445 (doi:10.1098/rspb.1998.0445) [DOI] [Google Scholar]
- 52.Piersma T., van Gils J. A., de Goeij P., van der Meer J. 1995. Holling's functional response model as a tool to link the food-finding mechanism of a probing shorebird with its spatial distribution. J. Anim. Ecol. 64, 493–504 10.2307/5652 (doi:10.2307/5652) [DOI] [Google Scholar]
- 53.van Gils J. A., Schenk I. W., Bos O., Piersma T. 2003. Incompletely informed shorebirds that face a digestive constraint maximize net energy gain when exploiting patches. Am. Nat. 161, 777–793 10.1086/374205 (doi:10.1086/374205) [DOI] [PubMed] [Google Scholar]
- 54.Bijleveld A. I., Egas M., van Gils J. A., Piersma T. 2010. Beyond the information centre hypothesis: communal roosting for information on food, predators, travel companions and mates? Oikos 119, 277–285 10.1111/j.1600-0706.2009.17892.x (doi:10.1111/j.1600-0706.2009.17892.x) [DOI] [Google Scholar]
- 55.Křivan V., Cressman R., Schneider C. 2008. The ideal free distribution: a review and synthesis of the game-theoretic perspective. Theor. Popul. Biol. 73, 403–425 10.1016/j.tpb.2007.12.009 (doi:10.1016/j.tpb.2007.12.009) [DOI] [PubMed] [Google Scholar]
- 56.Palmqvist E., Lundberg P., Jonzén N. 2000. Linking resource matching and dispersal. Evol. Ecol. 14, 1–12 10.1023/a:1011092401084 (doi:10.1023/a:1011092401084) [DOI] [Google Scholar]
- 57.Cressman R., Křivan V. 2006. Migration dynamics for the ideal free distribution. Am. Nat. 168, 384–397 10.1086/506970 (doi:10.1086/506970) [DOI] [PubMed] [Google Scholar]
- 58.Rehfisch M. M., Insley H., Swann B. 2003. Fidelity of overwintering shorebirds to roosts on the Moray Basin, Scotland: implications for predicting impacts of habitat loss. Ardea 91, 53–70 [Google Scholar]
- 59.Piersma T., Hoekstra R., Dekinga A., Koolhaas A., Wolf P., Battley P., Wiersma P. 1993. Scale and intensity of intertidal habitat use by knots Calidris canutus in the Western Wadden Sea in relation to food, friends and foes. Neth. J. Sea Res. 31, 331–357 10.1016/0077-7579(93)90052-T (doi:10.1016/0077-7579(93)90052-T) [DOI] [Google Scholar]
- 60.Folmer E. O., Olff H., Piersma T. 2010. How well do food distributions predict spatial distributions of shorebirds with different degrees of self-organization? J. Anim. Ecol. 79, 747–756 10.1111/j.1365-2656.2010.01680.x (doi:10.1111/j.1365-2656.2010.01680.x) [DOI] [PubMed] [Google Scholar]
- 61.Campomizzi A. J., Butcher J. A., Farrell S. L., Snelgrove A. G., Collier B. A., Gutzwiller K. J., Morrison M. L., Wilkins R. N. 2008. Conspecific attraction is a missing component in wildlife habitat modeling. J. Wildl. Manag. 72, 331–336 10.2193/2007-204 (doi:10.2193/2007-204) [DOI] [Google Scholar]
- 62.Ahlering M. A., Faaborg J. 2009. Avian habitat management meets conspecific attraction: if you build it, will they come? Auk 123, 301–312 10.1642/0004-8038(2006)123[301:ahmmca]2.0.co;2 (doi:10.1642/0004-8038(2006)123[301:ahmmca]2.0.co;2) [DOI] [Google Scholar]
- 63.Piersma T., Verkuil Y., Tulp I. 1994. Resources for long-distance migration of knots Calidris canutus islandica and C. c. canutus: how broad is the temporal exploitation window of benthic prey in the western and eastern Wadden Sea. Oikos 71, 393–407 10.2307/3545827 (doi:10.2307/3545827) [DOI] [Google Scholar]
- 64.Anderson T. M., Hopcraft J. G. C., Eby S., Ritchie M., Grace J. B., Olff H. 2010. Landscape-scale analyses suggest both nutrient and antipredator advantages to Serengeti herbivore hotspots. Ecology 91, 1519–1529 10.1890/09-0739.1 (doi:10.1890/09-0739.1) [DOI] [PubMed] [Google Scholar]
- 65.de Knegt H. J., et al. 2011. The spatial scaling of habitat selection by African elephants. J. Anim. Ecol. 80, 270–281 10.1111/j.1365-2656.2010.01764.x (doi:10.1111/j.1365-2656.2010.01764.x) [DOI] [PubMed] [Google Scholar]
- 66.Redfern J. V., et al. 2006. Techniques for cetacean habitat modeling. Mar. Ecol. Prog. Ser. 310, 271–295 10.3354/meps310271 (doi:10.3354/meps310271) [DOI] [Google Scholar]
- 67.Piersma T. 2007. Using the power of comparison to explain habitat use and migration strategies of shorebirds worldwide. J. Ornithol. 148, S45–S59 10.1007/s10336-007-0240-3 (doi:10.1007/s10336-007-0240-3) [DOI] [Google Scholar]