Abstract
In humans, auditory perception reaches maturity over a broad age range, extending through adolescence. Despite this slow maturation, children are considered to be outstanding learners, suggesting that immature perceptual skills might actually be advantageous to improvement on an acoustic task as a result of training (perceptual learning). Previous non-human studies have not employed an identical task when comparing perceptual performance of young and mature subjects, making it difficult to assess learning. Here, we used an identical procedure on juvenile and adult gerbils to examine the perception of amplitude modulation (AM), a stimulus feature that is an important component of most natural sounds. On average, Adult animals could detect smaller fluctuations in amplitude (i.e. smaller modulation depths) than Juveniles, indicating immature perceptual skills in Juveniles. However, the population variance was much greater for Juveniles, a few animals displaying adult-like AM detection. To determine whether immature perceptual skills facilitated learning, we compared naïve performance on the AM detection task with the amount of improvement following additional training. The amount of improvement in Adults correlated with naïve performance: those with the poorest naïve performance improved the most. In contrast, the naïve performance of Juveniles did not predict the amount of learning. Those Juveniles with immature AM detection thresholds did not display greater learning than Adults. Furthermore, for several of the Juveniles with adult-like thresholds, AM detection deteriorated with repeated testing. Thus, immature perceptual skills in young animals were not associated with greater learning.
Keywords: auditory perception, development, detection, amplitude modulation, perceptual learning
INTRODUCTION
The time course for auditory perceptual development, based largely on human studies, suggests that maturation continues through late adolescence (Saffran et al., 2006). Despite this slow maturation, children are considered to be outstanding learners (Kuhl and Rivera-Gaxiola, 2008; Meltzoff et al., 2009). One hypothesis is that immature perception can facilitate learning because it permits experience to optimize the auditory system by refining undeveloped synaptic properties and broad receptive fields. In fact, computer simulations suggest that a low-resolution sensory system is better able to learn than a mature system (Jacobs and Dominguez, 2003). Alternatively, the development of learning may mature independently of perceptual skills. Although this hypothesis has not been assessed directly in previous studies, it is known that children display different rates of maturation for learning on declarative and procedural tasks (Wilhelm et al., 2008; Prehn-Kristensen et al., 2009). In this study, we were able to train both juvenile and adult gerbils on the same auditory detection task, which then allowed us to determine whether there is a developmental relationship between naïve perception and learning abilities. Specifically we asked whether or not naïve performance predicted the amount of improvement that animals displayed with repeated testing.
We tested the perceptual abilities of gerbils to detect a sinusoidally amplitude modulated (AM) noise stimulus, a temporal envelope cue that is elemental to animal communication sounds, including speech (Rosen, 1992; Shannon et al., 1995; Singh and Theunissen, 2003). Humans display a slow rate of maturation for the detection of temporal envelope cues, such as frequency and amplitude modulation, reaching adult levels of performance between 8–12 years (Hall and Grose, 1994; Banai et al., 2007; Dawes and Bishop 2008). The few quantitative studies in non-humans suggest that perception is quite immature initially. However, direct comparisons to adult performance are uncommon because adults do not display the same behavior used to assess juvenile abilities (e.g., approaching a maternal call) (Gray and Rubel, 1985; Kelly and Potash 1986; Gray, 1992). Here, we provide a direct comparison of nonhuman juvenile and adult auditory perceptual abilities using an identical behavioral procedure.
Once naïve performance on an auditory perceptual task has been determined, it is possible to examine whether this skill can be improved. In fact, auditory perceptual learning in adult humans, characterized by improvement as a result of sensory training, has been measured with many different acoustic tasks (Fine and Jacobs, 2002; Moore et al., 2009; Wright and Zhang, 2009). While auditory learning has been assessed in children, these studies generally examine only subjects with language-based learning impairments (for review, see Tallal, 2004). One study has measured auditory perceptual learning in normal children, and found that a subset improved with training, but only if their naïve thresholds were immature (Halliday et al., 2008). This suggests that a relationship exists between naïve performance and improvement such that immature abilities may be necessary for greater learning. The few studies exploring the influence of early experience in non-humans have considered only passive exposure to a relevant stimulus, such as imprinting (Gottlieb, 1975a, 1975b, 1978, 1980). Thus, the capacity for perceptual learning in juvenile animals, and its relationship to naïve perceptual abilities has not been explored.
With these factors in mind, we first determined whether AM detection is slow to mature in gerbils as is found in humans. Juvenile animals were trained on an AM detection task and performance was compared with adult animals trained under identical conditions. A comparison of naïve detection thresholds revealed that perception was immature in juvenile animals as compared with adults. We then asked whether juveniles displayed an improvement in performance with repeated testing, as found in adults. While adult improvement was correlated with naïve performance, juvenile improvement was quite variable, and many young animals failed to improve or worsened.
METHODS
Animals
All procedures relating to the maintenance and use of animals were in accordance with the “Institutional Animal Care & Use Committee Handbook” and were approved by the University Animal Welfare Committee at NYU. Male and female gerbil (Meriones unguiculatus) pups were weaned from commercial breeding pairs (Charles River) at postnatal day (P) 23–30. Males and females were caged separately and maintained in a 12-h light/12-h dark cycle. Data was obtained from three age groups comprised of animals from multiple litters. Two juvenile age groups were examined at ages that are several weeks prior to sexual maturation, which occurs between P70-85 for gerbils (Field and Sibold, 1999). The ‘Early Juvenile’ group (n=21) was trained and tested on the task from P25-P40, the earliest age at which animals could be weaned and placed on controlled water access. The ‘Late Juvenile‘ group (n=10), a second group of pre-sexually mature animals, were trained and tested from P40-P55. The performances of the juvenile groups were then compared with an ‘Adult’ group (n=16) which was trained and tested from P70-P85 (Figure 1A). All data reported in this study were obtained from animals at or beyond the age (postnatal day 30) at which cochlear thresholds are adult-like (Woolf and Ryan, 1984; McGuirt et al., 1995; Huang et al., 1995; McFadden et al., 1996; Overstreet et al., 2003).
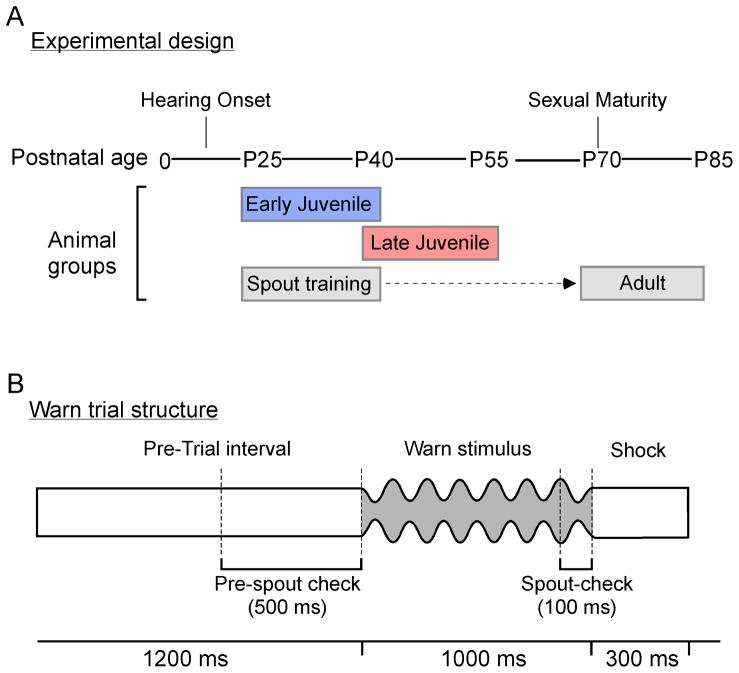
Figure 1.
A. Experimental Design. The timeline displays the age of hearing onset and sexual maturity for gerbils. The age range at which each experimental group was tested is shown below. Early Juveniles (blue box) were tested from P25-P40, Late Juveniles (red box) were tested from P40-P55, and Adults (gray box) were given spout training only from P25-P40 and were tested on the auditory task from P70-P85.
B. Trial Structure. A single ‘Warn’ trial is illustrated above a timeline (ms). The Pre-Trial period (1200ms) contained unmodulated noise. The trial continued only when animals remained in contact with the waterspout during >50% of the 500ms Pre-Trial Spout Check period. The Warn stimulus (AM noise at a 5Hz modulation frequency) was presented for 1000ms. During the final 100ms of the Warn stimulus, a Spout Check determined whether the gerbil was correctly off the spout (Hit) or incorrectly on the spout (Miss). A 300ms current was delivered through the waterspout immediately after the Warn stimulus as the aversive unconditioned stimulus. ‘Safe’ trials were identical in timing, although there was no AM stimulus or shock. During Safe trials, False alarms were determined from an identically positioned Spout Check interval.
All animals that entered the protocol were included in the analyses. No selection criteria were imposed, as is common in adult behavioral studies. Therefore, poor performers were not eliminated during any phase of the procedure, allowing us to compare both mean performance and between-animal variability within and across age groups.
Training and Testing
Experimental Environment
Gerbils were placed in a small acoustically transparent wire cage in a room lined with echo-attenuating material, and observed in a separate room via a closed circuit monitor. The test cage contained a stainless steel drinking spout and floor plate. When the animal contacted both the plate and spout, a circuit was completed that initiated water delivery via a syringe pump (Yale Apparatus). A PC computer, connected to a digital I/O interface (Tucker-Davis Technologies, TDT) controlled the timing of acoustic stimuli, water delivery (0.5 mL/min), and a small current delivered at the end of warning trials. Auditory stimuli were generated by the TDT system and delivered via a calibrated tweeter (KEF Electronics) positioned 1 m in front of the test cage. Sound level at the test cage was measured with a spectrum analyzer (Bruel & Kjaer 3550) via a ¼ ” free-field condenser microphone positioned at the head location when in contact with the spout. The metal waterspout was similar in appearance to that within the home cage.
Training
All animals were trained on a classical conditioning task (Heffner and Heffner, 1995; Kelly et al., 2006). Animals were initially placed on controlled water access for 48 hours prior to introduction into the experimental cage. For the duration of training and testing, body weight was monitored daily to ensure that it remained at >80% of the initial value. Furthermore, animals were allowed to drink until sated on each day of training or testing. Upon introduction to the experimental cage, animals were trained to obtain water from the spout (‘spout-training’). This was done in the presence of an unmodulated noise stimulus while contact with the waterspout was monitored. Once animals recognized the source of water (‘spout training’), they were trained to withdraw from the spout when an acoustic cue (AM) was present. To train the withdrawal response, a low AC current (0.5–1.0 mA, 300 ms; Lafayette Instruments) was delivered through the waterspout immediately after the AM signal. Since both humans and animals display large between-subject variability in pain sensitivity (Nielsen et al., 2009; Wasner and Brock, 2008; Mogil, 1999), it is important to note that the strength of the shock varied between animals. The shock level for each animal was chosen to reliably produce withdrawal from the spout, but not so great as to dissuade an animal from approaching the spout on subsequent trials. The animals’ behavior during training was monitored constantly to ensure that the level was set correctly. To train animals on the procedure, warning trials (AM noise; 100% modulation depth) were presented until performance reached a minimum criterion of 70% correct over 10 consecutive trials. The Adult animal group obtained ‘spout training’ only from P25-40: animals were placed in the cage where they drank from the water-spout in the presence of a noise background. This occurred at the same age during which the Early Juveniles were trained and tested on the auditory task. Adults were not trained on, or exposed to, the AM stimuli or conditioning procedure until they reached adulthood (Figure 1A).
A schematic of a single warning trial is shown in Figure 1B. Each trial was 2500 ms in total length. The stimulus was broadband noise with a low frequency falloff of 25 dB at 3.5 kHz and a high frequency falloff of 25 dB at 20 kHz. The level is given as dB sound pressure spectrum level (SPSL) and remained constant (45 dB SPSL) during the pre-trial and warn intervals to exclude the use of an energy cue. Each trial contained 1200 ms of unmodulated noise (‘pretrial’) within which the spout was monitored for contact over a 500 ms interval (‘pretrial spout check’). The trial proceeded only when the animal remained in contact with the spout for >250 ms during this pretrial spout check. A warning trial consisted of 1000 ms of noise, sinusoidally amplitude modulated at 5 Hz and of varying depths. The warning trial was followed immediately by an aversive unconditioned stimulus (‘shock’, a 300 ms electrical current delivered via the spout). To determine whether the animal detected the warning stimulus, contact with the spout was monitored during the final 100 ms of the warn stimulus (‘spout check’); for warn trials, a contact time of <50 ms was scored as a hit (H). For safe trials, the entire 2500 ms duration consisted of unmodulated noise, and a contact time of <50 ms during the spout check was scored as a false alarm (FA). Warn trials always occurred at the end of a block of 2–4 safe trials, randomized to avoid temporal conditioning.
Once animals reached criterion, we obtained naïve detection thresholds by testing animals on a broad range of AM depths (10–100%, divided up evenly into steps of 10% depth) presented in a randomized order, and the same order was delivered to each animal (‘randomized trials’). Each depth was presented a total of 10 times, and a psychometric function was constructed from performance on these randomized trials. On days following the completion of the ‘randomized trials’, a smaller range of AM depths (5 in total, divided evenly into steps of 10% depth) was repeatedly presented in descending order (‘descending limits trials’), bracketing each animal’s naïve detection threshold. For every subsequent day of ‘descending limits trials’, an animal’s performance on the previous day determined the range of depths on which it was tested (i.e., always bracketing the previous threshold). Animals were tested with ‘descending limits’ on 4 consecutive days to determine whether performance on AM detection changed with repeated testing. In total, animals received 10–12 days of training and testing on the task (including testing on both randomized and descending limits stimuli). The training period was limited to minimize the effect of development per se, and observe an effect of auditory training.
Data Analysis
A performance value, d′ = z(false alarm) – z(hit), was obtained for z scores that corresponded to the right-tail p values (Swets, 1996), and was calculated for each AM depth. Thresholds were defined as the AM depth at which performance reached a d′=1. Psychometric functions were constructed from each day of testing. To determine the ability of animals to improve with repeated testing, the naïve threshold (using ‘randomized trials’) was compared with the average of the last 2 days of performance (using ‘descending limits trials’). We also assessed the FA rate at each age. All values are given as mean ± standard error of the mean (SEM).
RESULTS
Initial training on the behavioral task
Behavioral data was obtained from animals in 3 age groups: Early Juveniles were tested from P25-40, Late Juveniles were tested from P40-55, and Adults were tested from P70-85 (Figure 1A; see Methods). During the initial procedural training period, animals were presented with trials of AM at 100% depth until each animal responded correctly in seven out of ten consecutive trials (70%). Each animal reached this criterion level of performance within 1–2 training sessions (1 session per day). The average number of trials that it took to reach this criterion did not differ significantly between any of the three groups (Adults: 27.5 ± 3.8; Early Juvenile: 34.5 ± 3.4; Late Juvenile: 22.2 ± 3.0; ANOVA: p = 0.08, df=2, F=2.65). In addition, the d′ obtained from these final ten trials did not differ among the 3 age groups (Adult: 2.1 ± 0.1; Early Juvenile: 2.0 ±0.2; Late Juvenile: 2.1 ± 0.2; ANOVA: p = 0.76, df = 2, F = 0.27). Since there was no difference in the number of initial training trials or in the performance on these trials, animals were assumed to be equally proficient at the task prior to testing with a broad range of AM depths.
Developmental emergence of naïve AM detection thresholds
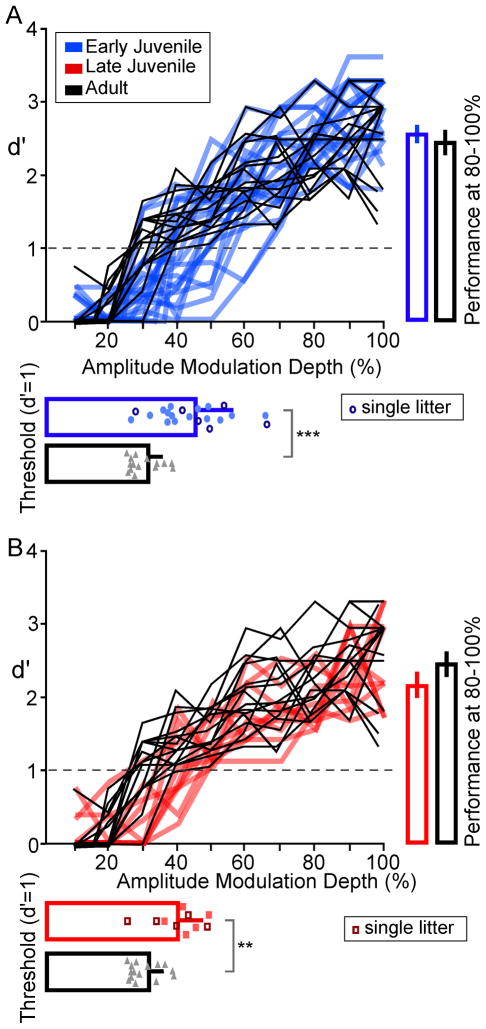
In order to determine whether AM detection was immature in juvenile gerbils as compared with adults, each animal was first tested on a broad range of AM depths (10–100%) presented in a randomized order. This naïve performance was obtained immediately after each gerbil reached criterion on procedural training (above) with the intent of minimizing an effect of training. Figure 2 shows each animal’s psychometric function obtained from the ‘randomized stimuli’ trials. For clarity, the Adult psychometric functions are plotted twice: once compared with Early Juveniles (Figure 2A) and again compared with Late Juveniles (Figure 2B). Detection threshold was defined as the modulation depth at which an animal’s sensitivity was d′=1 (plotted below the psychometric functions in Figure 2). The mean naïve AM detection threshold for Adults was 30.7 ± 1.2 %. In contrast, both Early and Late Juveniles displayed significantly higher thresholds (ANOVA: p < 0.001, df = 2, F = 12.8; t-test: Early Juvenile: 44.6 ± 2.3 %; p < 0.001, df = 29, t = 5.34; Late Juvenile: 40.9 ± 2.3 %, p < 0.002, df = 13, t = 3.96). Between-subject variance was much greater for both Juvenile groups, as compared to Adults (Test for Homogeneity of Variance; Levene: F = 4.44, p < 0.05, df = 2) (see distribution of individual thresholds with each bar graph of Figure 2). Thus, while some Juvenile animals displayed adult-like thresholds, most performed worse than the poorest adult.
Figure 2.
Developmental emergence of naïve AM detection thresholds. A, B. Individual psychometric functions are shown for the naïve AM detection thresholds, as determined with random stimulus presentation (see Methods). In panel A, Early Juveniles (thick, blue lines) are compared with naïve Adults (thin, black lines). In panel B, Late Juveniles (thick, red lines) are compared with naïve Adults (thin, black lines). All animal groups display a similar ability to perform the task at large AM depths (80–100%), as shown by bars on right of both graphs. However, detection thresholds at d′=1 were significantly higher and more variable for Early Juveniles and Late Juveniles, compared with Adults, as shown by data points below the curves. The bars placed over these data points represent the mean and SEM of AM detection (Early Juveniles, blue circles; Late Juveniles, red squares; Adults, gray triangles; ***, p<0.001; **, p<0.002). For both Early and Late Juveniles, data points with a dark outline represent data from animals within a single litter. This illustrates that one litter did not bias the group mean.
It is possible that the developmental differences in AM detection threshold (Figure 2A and B) were due to factors other than sensory characteristics. First, despite an attempt made to obtain criterion performance during initial training (above), the Juveniles might not have been equally proficient at the task as compared with Adults. To address this issue, the average d′ value for the three largest AM depths (80–100%) was obtained from each psychometric function. As shown in Figure 2 (bars to the right of each plot), there were no significant differences in asymptotic performance (ANOVA: p = 0.17, df = 2, F = 1.83; Adults: 2.49 ± 0.18; Early Juveniles: 2.63 ± 0.12; Late Juveniles: 2.35 ± 0.18). This suggests that the between-group differences in AM detection thresholds were not related to differences in performing this auditory task.
Second, it is possible that differences in attention could account for group differences in AM detection threshold. As an indirect measure of attention, we quantified the magnitude of within-subject performance variability as the difference between AM depth detection thresholds from the first to second half of trials. Using this measure we found no significant difference between age groups (ANOVA: p = 0.27, df = 2, F = 1.36; Adult: 10.2 ± 1.7 %; Early Juveniles: 10.8 ± 2.0 %; Late Juveniles: 15.4 ± 2.7 %).
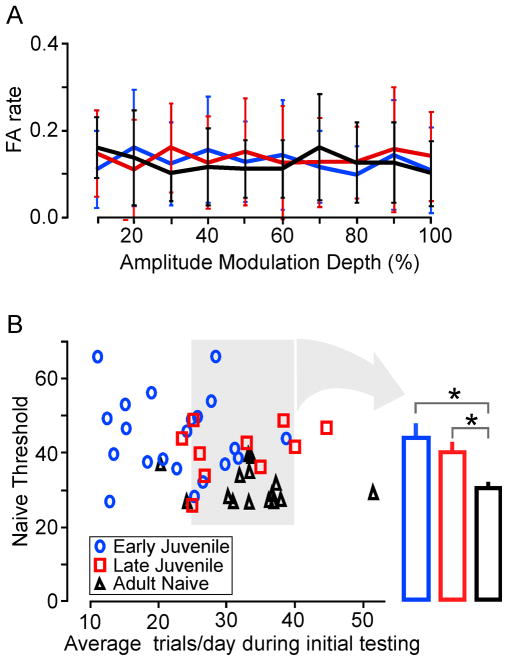
Third, the differences in AM detection threshold could result from alternate strategies used to perform the task. Therefore, we assessed the false alarm rate (the rate at which animals broke contact with the spout during safe trials). Since our performance measure, d′, takes false alarms into account, a high false alarm rate would result in poor performance (low d′). Figure 3A shows that the average false alarm rates across AM depth did not display group differences (ANOVA: p = 0.89, df = 2, F = 0.12; Mean false alarm rate; Adult: 0.12 ± 0.01; Early Juveniles: 0.13 ± 0.02: Late Juveniles: 0.14 ± 0.02).
Figure 3.
Developmental emergence of AM detection threshold is not due to differences in strategies used or in the number of trials performed per day. A. Average False Alarm rate across AM depth was similar for each of the age groups (Early Juveniles, blue; Late Juveniles, red; Adults, black).
B. Average number of trials performed by each animal during initial testing was compared to the naïve AM detection threshold. Early Juveniles (blue circles) displayed the greatest variability in threshold for a given number of trials/day. The gray box on the left contains animals that fall into the Adult range of trials/day. The bar graph (arrow) displays the average naïve threshold of animals within the corresponding gray box (those animals that performed a similar number of average daily trials). The average detection threshold of both Early Juveniles and Late Juveniles remained poorer than those of Adults after controlling for average daily trials (*, p<0.05).
Fourth, since juvenile body weight was smaller, they may have consumed less water, and thus completed fewer trials per day. To determine whether fewer trials performed daily could explain the age group differences in performance, the AM detection thresholds were examined with respect to the average number of trials per day. Figure 3B shows that the average trials per day were greater in Adults than in Juveniles. However, there were several animals at each age with similar numbers of daily trials (i.e., 25–40 trials per day, shaded area of Figure 3B). When AM detection thresholds were compared across age for this subset of animals, the group differences remained significant (Adult: 30.6 ± 1.4%; Early Juveniles: 44.0 ± 3.5%; Late Juveniles: 39.7 ± 2.8%; ANOVA: p < 0.005, df = 2, F = 7.85; t-test: P30 vs Adult: p < 0.005, df = 12, t = 3.56; P40 vs. Adult: p < 0.05, df = 11, t = 2.96). This indicates that developmental improvement in AM detection threshold was independent of the number of trials per day.
Improvement of AM detection thresholds with repeated testing: the effect of age
The first paradigm assessed naïve performance for which we sought to minimize the effect of training. Since many adult perceptual skills improve over the course of training, we next determined whether AM detection thresholds improved with repeated testing in Juveniles and Adults. Following testing with ‘randomized’ stimuli (above) each animal was tested with a small range of AM depths, presented in descending order. On the first day of testing, the stimulus values bracketed the detection threshold obtained with randomized stimuli for each individual animal. On each subsequent day of testing, the AM stimuli bracketed the previous day’s threshold (Methods). Daily thresholds were obtained and used to track improvement in performance.
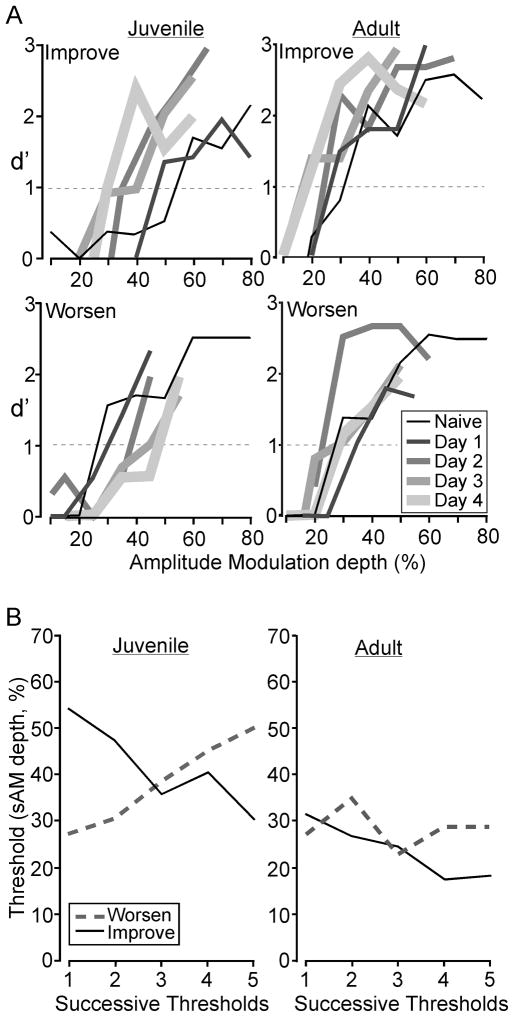
The progression of 2 Early Juveniles and 2 Adults during the testing period is illustrated in Figure 4. Psychometric functions for naïve performance and each subsequent day of testing with ‘descending limits’ are shown for one animal that improved (Figure 4A, top), and one animal that worsened (Figure 4A, bottom). Figure 4B plots detection threshold (at d′=1) as a function of testing day for each animal. One of the Early Juveniles improved by 24% AM depth, while the other worsened by 23% AM depth. One of the Adults improved by 13% AM depth, while the other worsened by 2% AM depth.
Figure 4.
Tracking improvement in performance across days. A. Psychometric functions are shown for naïve performance and each day of repeated testing for two individual Juvenile and Adult animals. (Top) Example Juvenile and Adult animals are shown that each displayed improvement in performance from initial testing (i.e., using randomized trials) through the end of repeated testing (i.e., using descending limits). Each line weight and width indicates a specific day of testing (see key in figure). (Bottom) Example Juvenile and Adult animals are shown that each displayed a decrement in performance from initial testing through the end of repeated testing.
B. The detection thresholds (d′=1) from each set of psychometric functions in panel A are plotted across testing session. Solid lines represent the animals that improved over the course of testing. Dashed lines represent the animals that worsened over the course of testing.
To quantify improvement, the mean detection threshold obtained during the final two days of testing was subtracted from the naïve threshold (i.e., the thresholds shown in Figure 2). Therefore, positive values signified improvement and negative values signified a decline in performance. There were animals at each age that displayed either improvement or a decline in performance. On average, there was no significant difference in the magnitude of improvement displayed at any of the three ages (Adults: 4.5 ± 2.0 %; Early Juveniles: 4.2 ± 2.8 %; Late Juveniles: 3.5 ± 3.9 %. ANOVA: p = 0.976, df = 2, F = 3.21).
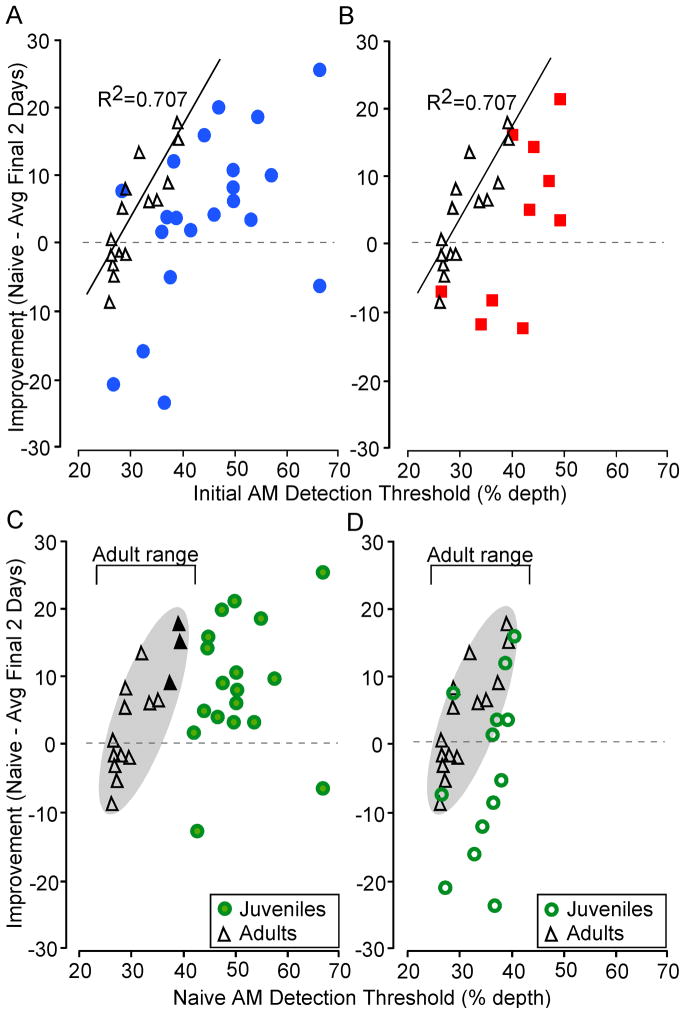
A primary goal of the study was to determine whether there existed a relationship between naïve performance and improvement over the course of repeated testing. The Adult group displayed a significant correlation between the naïve detection threshold and improvement (R2 = 0.707, b= 1.38, p < 0.001), indicating that animals with the poorest naïve thresholds improved the most with repeated testing (Figure 5). In contrast, the Early Juvenile group did not display a significant correlation between naïve threshold and improvement (Figure 5A; R2 = 0.264, p = 0.23). Figure 5B illustrates that the Late Juvenile group displayed a similar pattern as the Early Juveniles, although a trend was apparent (R2 = 0.397, p = 0.08). For both Juvenile groups, a subset of animals displayed an Adult-like pattern of improvement, while another subset displayed poor naïve thresholds that remained constant or worsened with testing. We assessed whether there was a difference in the relationship (initial threshold vs improvement) for Juvenile and Adult groups using a one-tailed z-test. Early Juveniles differed from Adults (Fishers r to r′ transformation and z-test: p < 0.05, z = 1.77), whereas Late Juveniles did not (p = 0.16, z = 1.01).
Figure 5.
Improvement of AM detection thresholds with repeated testing. A, B. The naïve detection threshold (x-axis) is plotted against improvement in performance (y-axis: naïve minus final two detection thresholds) for Adults (black triangles) as compared to either Early Juveniles (blue circles, panel A) or Late Juveniles (red squares, panel B). For Adults, improvement was significantly correlated with the naïve detection threshold. This correlation was not observed in either Early or Late Juveniles, suggesting that learning is not yet mature.
C. Relationship between naïve detection thresholds and improvement of only those Early and Late Juveniles (filled green circles) with naïve thresholds that were above the Adult range (black triangles, gray oval). While most of these Juveniles displayed improvement, for about half of them, improvement magnitude was less than the 3 adults with the worst initial performance (solid black triangles).
D. Relationship between naïve detection thresholds and improvement of only those Early and Late Juveniles (open green circles) with naïve thresholds within the Adult range (black triangles). Several Juveniles were found within the Adult cluster (gray oval), indicating Adult-like improvement. However, many Juveniles displayed much less improvement or worsening, despite having Adult-like naïve perceptual skills.
Adult animals with the worst initial thresholds displayed the greatest improvement. Therefore, we asked whether juvenile animals with the poorest initial performance would also display the most improvement. Figure 5C illustrates that at least half of the Juveniles in this subgroup of poor initial performers (green circles) did not display as much improvement as the 3 adults with the worst initial performance (solid black triangles). Furthermore, the average improvement displayed by this subgroup was not significantly greater than the Adults (Immature Juveniles: 9.2 ± 2.3 %; Adults: 4.5 ± 2.0 %. t-test: p = 0.13, df = 31, t = 1.55), suggesting that immature naïve thresholds were not associated with greater learning.
A second possibility is that immature auditory perceptual skills, as displayed in Juveniles (Figure 2), limit the animal’s ability to improve on a task during repeated testing. This hypothesis would predict that those Juveniles with adult-like naïve thresholds would display adult-like improvements. Therefore, the improvement measured in Adults was compared with the improvement measured in only those Juveniles that displayed adult-like naïve thresholds. Figure 5D shows that given a similar naïve threshold, many Juveniles did not improve as much as Adults. In fact, the performance of half of these Juveniles deteriorated with additional testing. The results demonstrate a trend for greater improvement displayed by Adults when compared with Adult-like Juveniles (Adult-like Juveniles: −3.3 ± 3.4%; Adults: 4.5 ± 2.0%. t-test: p = 0.06, df = 20, t = −1.95). Together, these analyses indicate that perceptual skill in Juveniles neither impeded nor enhanced their perceptual learning.
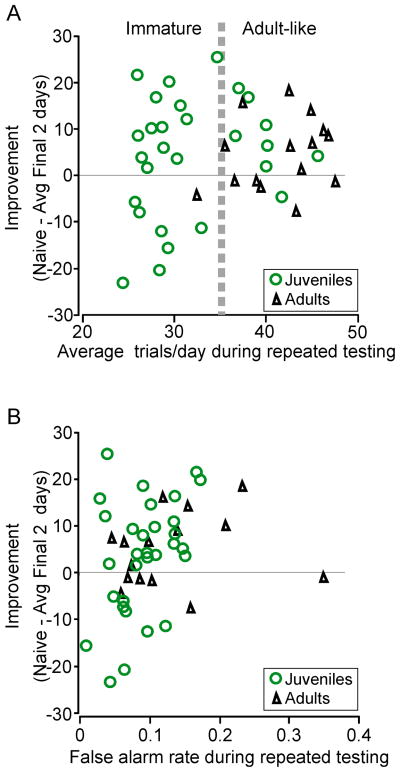
To assess whether the amount of experience an animal received was correlated with learning, we examined the relationship between trials performed during repeated testing and improvement. For this comparison, we re-grouped all Juveniles (both Early and Late) based on the average number of trials performed and compared these groups with Adults (Figure 6A). No between group differences were found (ANOVA: p = 0.492, df = 2, F = 0.73). That is, the animals with the largest number of trials did not consistently display the greatest improvement. Juvenile animals with fewer trials could improve as much as Adults with many more trials. This indicated that the amount of experience during repeated testing could not, in itself, explain the developmental differences.
Figure 6.
Developmental differences in improvement with repeated testing are not due to the number of trials performed per day or alternate strategies. A. Average number of trials per day (x-axis) is plotted against performance improvement with repeated testing (y-axis; naïve minus final two detection thresholds). A vertical black line separates Juveniles (open green circles) based on the number of trials performed: Juveniles that performed a similar number of trials as Adults and Juveniles that performed a fewer number of trials than Adults. A comparison between the Adults and both Juvenile subgroups indicate no significant difference in improvement based on number of daily trials performed.
B. Average false alarm rate (x-axis) is plotted against improvement in performance with repeated testing (y-axis; naïve minus final two detection thresholds). There are no patterns of improvement based on false alarm rate in either Juveniles (green circles) or Adults (black triangles).
Another possibility is that a decrease in attentiveness during the course of repeated testing may have led to a decline in performance. To assess this, we compared each animal’s average false alarm rate during the four days of repeated testing to its improvement measure (Figure 6B). Neither Adults nor Juveniles displayed a significant relationship between false alarm rate and improvement (Adults: R2 = 0.033, p = 0.52; Juveniles: R2 = 0.14, p = 0.09; ANOVA: p = 0.101, df = 2, F = 2.42). That is, the animals that displayed the highest false alarm rate did not consistently improve or worsen the most. A second way in which we assessed attention was to compare the within-subject variability across the third and fourth days of repeated testing. By quantifying the magnitude of the difference between the thresholds of the final two testing days, we found that there was no group difference in this measure of variability (ANOVA: p = 0.82, df = 2, F = 0.20 Adults: 7.7 ± 1.7%; Early Juveniles: 6.4 ± 1.3%; Late Juveniles: 6.4 ± 2.8%).
DISCUSSION
This study asked whether juvenile and adult animals, tested with an identical procedure, displayed an equivalent level of naïve performance on an auditory detection task, and whether they improved equivalently with repeated testing. Both Early and Late Juvenile animals displayed immature sensitivity to AM detection, as compared with the Adults (Figure 2). A specific issue addressed by these measures was whether immature perception in Juveniles influenced the amount of perceptual learning. Juveniles displayed high between-subject variability in their improvement with repeated testing. The majority of Juveniles initially displayed immature AM detection thresholds, and their learning was no greater than that displayed by Adults (Figure 5C). The subset of Juveniles that initially displayed adult-like AM detection thresholds displayed little improvement or became worse (Figure 5D). These data lead us to conclude that AM detection displayed a prolonged maturation in gerbils, as shown previously in humans (Hall and Grose, 1994; Banai et al., 2007). Furthermore, the pattern of perceptual learning in Juveniles is not influenced by their naïve perceptual skills (Figures 2 and 5).
Maturation of AM detection
One limiting factor in the maturation of AM detection could be the auditory periphery. A response to airborne sound is first obtained in P12 gerbils, and adult sensitivity occurs at ≈ P30 (Woolf and Ryan, 1984; McGuirt et al., 1995; Huang et al., 1995; McFadden et al., 1996; Overstreet et al., 2003). Compound action potential (CAP) latency and endocochlear potential also mature by ≈P30 (Huang et al., 1995; McGuirt et al., 1995). Therefore, cochlear threshold alone does not explain Juveniles’ performance. However, the CAP amplitude at 10 dB above threshold matures between P30-54, depending on frequency (Huang et al., 1995). For high intensities, the maximum CAP amplitude matures until sexual maturation (McGuirt et al., 1995). Although we used a broadband stimulus at a relatively low intensity (45 dB SPL), it is possible that a limited cochlear dynamic range contributed to the Juveniles’ immature performance compared with Adults. However, since a subset of Juveniles displayed Adult-like detection thresholds (Figure 5D), they would presumably have Adult cochlear function, whereas others in the same litter would not (Figure 2).
A second limitation could involve immature central coding properties in Juvenile animals. In general, physiology studies show that frequency selectivity improves during a relatively brief period, due to both peripheral and central maturation (reviews, Fitzgerald and Sanes, 2001; Sanes and Bao, 2009). However, temporal receptive field properties may emerge over a longer duration (Pienkowski and Harrison, 2005). In humans, the auditory brainstem response to speech sounds is still immature at 3–4 years (Johnson et al., 2008). Consistent with this finding, the responses of single neurons to time-varying sounds, including amplitude and frequency modulation, are reported to mature over a prolonged period of development (Heil et al., 1995; Thornton et al., 1999; Razak and Fuzessery, 2007). This latter set of studies suggests that the prolonged perceptual development demonstrated here (Figure 2) could be explained by an immature CNS coding of modulation. However, these comparisons must be tentative because all developmental physiology studies have been performed on anesthetized animals.
Relationship to previous studies of auditory perceptual development
The literature in non-humans suggests that auditory perception is quite immature initially (Kelly and Potash 1986; Gray and Rubel, 1985; Gray, 1992). However, the testing procedures measured a behavior exhibited only by young animals (e.g., approaching a maternal call), making a direct comparison to adults unfeasible. In contrast, there are many auditory perceptual studies in humans, which often use similar testing procedures across age. These studies show that detection thresholds for frequency modulation are mature by ≈8 years, whereas amplitude modulation thresholds improve through 12 years (Hall and Grose, 1994; Banai et al., 2007; Dawes and Bishop, 2008). In contrast, performance on duration discrimination and gap detection tasks mature by ≈5 years (Morrongiello and Trehub, 1987; Wightman et al. 1989; Werner et al., 1992). In humans, peripheral development occurs between 1 and 4 years (Moore and Linthicum, 2007), which corresponds to the maturation of tone thresholds and frequency resolution (Spetner and Olsho, 1990; Trehub et al., 1980; Olsho et al., 1988; Werner and Boike, 2001), and suggests that envelope detection may be associated with central nervous system maturation. Thus, perceptual development in gerbils and humans display similar characteristics.
Are developmental differences in perception due to cognitive factors?
Between-subject variability was relatively large in Juveniles (Figure 2), and this has been attributed to fluctuations in auditory attention (Moore et al., 2008). Thus, it is important to consider whether cognitive factors, such as attention, could explain immature AM detection thresholds in Juveniles. In children, asymptotic performance is used as an indirect measure of attention (Bargones et al., 1995). If this is a valid measure, then Figure 2 indicates that Juvenile animals did not differ from Adults, as asymptotic performance was identical. False alarm rate has also been used as a measure of sustained attention in children, and this measure suggests that attention on these specific tasks matures by ≈5 years (Lin et al., 1999; Kanaka et al., 2008). If false alarm rate is a valid measure of attention, then Figure 3A demonstrates that Juvenile animals did not differ from Adults, as average false alarm rate was identical across AM depths. Another indicator of attention, as utilized by Moore et al. (2008) is within-subject variability. Within-subject variability was measured in this study by comparing the magnitude of performance consistency between both halves of the random stimuli as well as between the final two days of repeated testing. Both measures revealed no group differences in performance variability, suggesting that inherent attention in Juveniles was not different than that of Adults. It remains possible that differences in attention influenced the results, but our data suggests that this was not an overriding factor in determining performance.
A separate hypothesis for between-subject variability is that animals of the same chronological age may be at quite different positions along a neurodevelopmental axis. That is, chronological age does not correlate adequately with nervous system (and therefore behavioral) development. Thus, as subjects get older, the late-developing animals would tend to catch up, and the group variance would therefore decline. Since the variance of the Late Juvenile group is intermediate between that of the Early Juvenile and Adult groups (Figure 2), we favor this explanation for between-subject variability.
It is possible that the number of trials per session influenced the performance measures. In general, Juveniles performed fewer trials per session during the initial testing period as compared to Adults, presumably due to their small size and diminished water requirement. To control for this, performance was compared between animals that completed a similar number of trials per day. The developmental differences in AM detection were observed even when the analysis was limited to this subset of animals (Figure 3B).
AM detection thresholds improve with repeated testing: the effect of age
All studies assessing the effects of auditory training on performance have been performed on adults. In adult humans, performance can improve across many acoustic dimensions (Demany 1985; Turner and Nelson,1982; Wright et al., 1997; Ari-Even Roth et al., 2003; Mossbridge et al., 2006; Wassenhove and Nagarajan, 2007; Amitay et al., 2005, 2006a, 2006b; Fitzgerald and Wright, 2005; Wright and Fitzgerald, 2001). Similarly, training in non-human adults leads to improvements in perceptual skills (Kacelnik et al., 2006; Sakai and Kudoh, 2005; Recanzone et al., 1993; Schulze and Scheich, 1999). This is consistent with a broad literature in other sensory systems (reviews, Fine and Jacobs, 2002; Fahle, 2005).
The goal of this study was not to determine whether young animals can learn; they do. Rather, a primary motivation for this study was to determine whether training developing animals on the same task used for Adults would also lead to improved performance. The few developmental learning studies in non-humans have all used paradigms that cannot be, or have not yet been, employed in adults. For example, neonatal chicks display auditory learning of an individual maternal call (Lickliter and Hellewell, 1992). Similarly, one-day-old rats can be trained to turn their heads in a specific direction to receive a period of platform heating (Flory et al., 1997). However, a series of studies on learning in juvenile rats have established several important developmental principles. In general, sensory stimuli are able to elicit reflexive responses prior to the time when they can be used for associative learning; this holds for gustatory, visual, and auditory stimuli (Vogt and Rudy, 1984; Hyson and Rudy, 1984; Rudy and Hyson, 1984; Moye and Rudy, 1985). Additionally, there is a developmental improvement in the ability to associate events separated by longer periods of time (Moye and Rudy, 1987). Furthermore, there are maturational changes in the type of associations that can be made. For example, contextual fear conditioning and latent inhibition both emerge after fear conditioning to an auditory conditioned stimulus (Rudy, 1993; Rudy, 1994). Therefore, many forms of learning display fundamental transformations prior to the age range examined in this study.
Having established that naïve AM sensitivity was immature in both Early and Late Juveniles (Figure 2), we asked whether daily testing improved performance in an equivalent manner for Juvenile and Adult animals. For Adults, the amount of improvement was predicted by naïve performance: animals with poor naïve thresholds improved the most. However, while many Juveniles with poor naïve AM sensitivity did improve with testing, the amount of improvement was not greater than those adults with the worst initial performance (Figure 5C). In contrast, many of the Juvenile animals with the best naïve AM sensitivity displayed no improvement or worsened with practice (Figure 5D). Because Juveniles with immature naïve thresholds did not display greater improvement than those Adults with the worst initial performances, we concluded that immature perception in Juveniles was not advantageous to learning. What remains unclear, however, is whether Juveniles would have shown adult-like improvement patterns if given more training. For example, infants are estimated to travel about 29 football fields per day when learning to walk (Adolph et al., 2003).
Auditory developmental learning studies in humans have primarily examined children with speech or language impairments (Moore et al., 2009, review). However, Halliday et al. (2008) measured perceptual learning of a frequency discrimination task in normal subjects. While most of them did not improve with repeated testing, a subgroup of children with immature naïve thresholds did learn. Our findings (Figure 5) are in general agreement with this human study.
Our results indicated that detection of amplitude modulation displayed a prolonged period of maturation in gerbils, similar to that reported in humans (Hall and Grose, 1994; Banai et al., 2007). However, naïve performance did not predict the magnitude of perceptual learning in Juveniles, as it did in Adults. Developing animals are typically thought to have a larger capacity for plasticity, and one might have expected Juveniles to display the greatest improvement with repeated testing. In fact, it has recently been reported that 11-year-old humans do not display perceptual learning, as adults do, on an interval discrimination task (Huyck and Wright, 2008). Therefore, one possibility is that the effects of auditory training in young animals may be most advantageous to their future performance, and evident only when it is assessed in adulthood.
Acknowledgments
This work was supported by DC009237 (DHS) and DC9729 (ECS). We thank Jack Kelly, Merri Rosen, Beverly Wright, Kiriana Cowansage, Brad Buran, and Vibhakar Kotak for many helpful ideas, discussions, and/or comments on the manuscript.
References
- Adolph KE, Vereijken B, Shrout PE. What changes in infant walking and why. Child Development. 2003;74:475–497. doi: 10.1111/1467-8624.7402011. [DOI] [PubMed] [Google Scholar]
- Amitay S, Hawkey DJ, Moore DR. Auditory frequency discrimination learning is affected by stimulus variability. Percept Psychophys. 2005;67:691–698. doi: 10.3758/bf03193525. [DOI] [PubMed] [Google Scholar]
- Amitay S, Irwin A, Moore DR. Discrimination learning induced by training with identical stimuli. Nat Neurosci. 2006a;9:1446–1448. doi: 10.1038/nn1787. [DOI] [PubMed] [Google Scholar]
- Amitay S, Irwin A, Hawkey DJC, Cowan JA, Moore DR. A comparison of adaptive procedures for rapid and reliable threshold assessment and training in naive listeners. J Acoust Soc Am. 2006b;119:1616–1625. doi: 10.1121/1.2164988. [DOI] [PubMed] [Google Scholar]
- Ari-Even Roth D, Kishon-Rabin L, Hildesheimer M. Auditory backward masking in normal hearing children. J Basic Clin Physiol Pharmacol. 2003;13:105–115. doi: 10.1515/jbcpp.2002.13.2.105. [DOI] [PubMed] [Google Scholar]
- Banai K, Sabin AT, Kraus N, Wright BA. The development of sensitivity to amplitude and frequency modulation follow distinct time courses. ARO. 2007:Abstract 283. [Google Scholar]
- Bargones JY, Werner LA, Marea GC. Infant psychometric functions for detection: Mechanisms of immature sensitivity. J Acoust Soc Am. 1995;98:99–111. doi: 10.1121/1.414446. [DOI] [PubMed] [Google Scholar]
- Dawes P, Bishop DVM. Maturation of visual and auditory temporal processing in school-aged children. J Speech Lang and Hear Res. 2008;51:1002–1015. doi: 10.1044/1092-4388(2008/073). [DOI] [PubMed] [Google Scholar]
- Demany L. Perceptual learning in frequency discrimination. J Acoust Soc Am. 1985;78:1118–1120. doi: 10.1121/1.393034. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: specificity versus generalization. Curr Opin Neurobiol. 2005;15:154–160. doi: 10.1016/j.conb.2005.03.010. review. [DOI] [PubMed] [Google Scholar]
- Field KJ, Sibold AL. Husbandry, Breeding. In: Suckow MA, editor. The Laboratory Hamster and Gerbil. CRC Press, LLC; 1999. pp. 43–46. [Google Scholar]
- Fine I, Jacobs RA. Comparing perceptual learning across tasks: A review. J Vis. 2002;2:190–203. doi: 10.1167/2.2.5. review. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KK, Sanes DH. The development of stimulus coding in the auditory system. In: Jahn A, Santos-Sacchi J, editors. Physiology of the Ear. 2 2001. [Google Scholar]
- Fitzgerald MB, Wright BA. A perceptual learning investigation of the pitch elicited by amplitude-modulated noise. J Acoust Soc Am. 2005;118:3794–3803. doi: 10.1121/1.2074687. [DOI] [PubMed] [Google Scholar]
- Flory GS, Langley CM, Pfister JF, Alberts JR. Instrumental learning for a thermal reinforcer in 1-day-old-rats. Dev Psychobiol. 1997;30:41–47. doi: 10.1002/(sici)1098-2302(199701)30:1<41::aid-dev4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: I. Nature of perceptual deficit caused by embryonic auditory deprivation. J Comp Physiol Psychol. 1975a;89:387–399. doi: 10.1037/h0077068. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: II. Experimental prevention of perceptual deficit caused by embryonic auditory deprivation. J Comp Physiol Psychol. 1975b;89:675–684. doi: 10.1037/h0077067. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: IV. Change in species-specific perception caused by auditory deprivation. J Comp Physiol Psychol. 1978;92:375–387. doi: 10.1037/h0077473. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Development of species identification in ducklings: VI. Specific embryonic experience required to maintain species-typical perception in ducklings. J Comp Physiol Psychol. 1980;94:579–587. doi: 10.1037/h0077691. [DOI] [PubMed] [Google Scholar]
- Gray L. An auditory psychometric function from newborn chicks. J Acoust Soc Am. 1992;91:1606–1615. doi: 10.1121/1.402441. [DOI] [PubMed] [Google Scholar]
- Gray L, Rubel EW. Development of absolute thresholds in chickens. J Acoust Soc Am. 1985;77:1162–1172. doi: 10.1121/1.392180. [DOI] [PubMed] [Google Scholar]
- Hall JW, Grose JH. Development of temporal-resolution in children as measured by the temporal modulation transfer function. J Acoust Soc Am. 1994;96:150–154. doi: 10.1121/1.410474. [DOI] [PubMed] [Google Scholar]
- Halliday LF, Taylor JL, Edmondson-Jones AM, Moore DR. Frequency discrimination learning in children. J Acoust Soc Am. 2008;123:4393–4402. doi: 10.1121/1.2890749. [DOI] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Conditioned avoidance. In: Klump GM, Dooling RJ, Fay RR, Stebbins WC, editors. Methods in comparative psychoacoustics. Academic Press; Basel: 1995. [Google Scholar]
- Heil P, Schulze H, Langner G. Ontogenetic development of periodicity coding in the inferior colliculus of the Mongolian gerbil. Aud Neurosci. 1995;1:363–383. [Google Scholar]
- Huang JM, Berlin CI, Cullen JK, Jr, Wickremasinghe AR. Development of the VIIIth nerve compound action potential evoked by low-intensity tone pips in the Mongolian gerbil. Hear Res. 1995;88:14–18. doi: 10.1016/0378-5955(95)00094-k. [DOI] [PubMed] [Google Scholar]
- Huyck JJ, Wright BA. Development of temporal-interval discrimination learning during adolescence. Association for Research in Otolaryngology Abstracts. 2008;31:1296. [Google Scholar]
- Hyson RL, Rudy JW. Ontogenesis of learning: II. Variation in the rat’s reflexive and learned responses to acoustic stimulation. Dev Psychobiol. 1984 May;17:263–283. doi: 10.1002/dev.420170307. [DOI] [PubMed] [Google Scholar]
- Jacobs RA, Dominguez M. Visual development and the acquisition of motion velocity sensitivities. Neural Comput. 2003;15:761–781. doi: 10.1162/08997660360581895. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Nicol T, Zecker SG, Kraus N. Developmental plasticity in the human auditory brainstem. J Neurosci. 2008;28:4000–4007. doi: 10.1523/JNEUROSCI.0012-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacelnik O, Nodal FR, Parsons CH, King AJ. Training-Induced Plasticity of Auditory Localization in Adult Mammals. PLoS Bio. 2006;4:0627–0638. doi: 10.1371/journal.pbio.0040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaka, et al. Measurement of development of cognitive and attention functions in children using continuous performance test. Psych Clin Neurosci. 2008;62:135–141. doi: 10.1111/j.1440-1819.2008.01746.x. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Cooke JE, Gilbride PC, Mitchell C, Zhang H. Behavioral limits of auditory temporal resolution in the rat: amplitude modulation and duration discrimination. J Comp Psychol. 2006;120:98–105. doi: 10.1037/0735-7036.120.2.98. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Potash M. Directional responses to sounds in young gerbils (Meriones unguiculatus) J Comp Psych. 1986;100:37–45. [PubMed] [Google Scholar]
- Kuhl P, Rivera-Gaxiola M. Neural substrates of language acquisition. Annu Rev Neurosci. 2008;31:511–534. doi: 10.1146/annurev.neuro.30.051606.094321. [DOI] [PubMed] [Google Scholar]
- Lickliter R, Hellewell TB. Contextual determinants of auditory learning in bobwhite quail embryos and hatchlings. Dev Psychobiol. 1992;25:17–31. doi: 10.1002/dev.420250103. [DOI] [PubMed] [Google Scholar]
- Lin CC, Hsiao CK, Chen WJ. Development of sustained attention assessed using the continuous performance test among children 6–15 years of age. J Abnorm Child Psychol. 1999;27:403–412. doi: 10.1023/a:1021932119311. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Walsh EJ, McGee J. Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus) Hear Res. 1996;100:68–79. doi: 10.1016/0378-5955(96)00108-6. [DOI] [PubMed] [Google Scholar]
- McGuirt JP, Schmiedt RA, Schulte BA. Development of cochlear potentials in the neonatal gerbil. Hear Res. 1995;84:52–60. doi: 10.1016/0378-5955(95)00015-v. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN, Kuhl PK, Movellan J, Sejnowski TJ. Foundations for a new science of learning. Science. 2009;325:284–288. doi: 10.1126/science.1175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci USA. 1999;96:7744–7751. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Halliday LF, Amitay S. Use of auditory learning to manage listening problems in children. Phil Trans R Soc B. 2009;364:409–420. doi: 10.1098/rstb.2008.0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Ferguson MA, Halliday LA, Riley A. Frequency discrimination in children: Perception, learning and attention. Hear Res. 2008;238:147–154. doi: 10.1016/j.heares.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Moore JK, Linthicum FH., Jr The human auditory system: a timeline of development. Int J Audiol. 2007;46:460–478. doi: 10.1080/14992020701383019. [DOI] [PubMed] [Google Scholar]
- Morrongiello BA, Trehub SE. Age-related changes in auditory temporal perception. J Exp Child Psychol. 1987;44:413–426. doi: 10.1016/0022-0965(87)90043-9. [DOI] [PubMed] [Google Scholar]
- Mossbridge JA, Fitzgerald MB, O’Connor ES, Wright BA. Perceptual-learning evidence for separate processing of asynchrony and order tasks. J Neurosci. 2006;26:12708–12716. doi: 10.1523/JNEUROSCI.2254-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of learning: VI. Learned and unlearned responses to visual stimulation in the infant hooded rat. Dev Psychobiol. 1985 Sep;18:395–409. doi: 10.1002/dev.420180505. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of trace conditioning in young rats: dissociation of associative and memory processes. Dev Psychobiol. 1987 Jul;20:405–414. doi: 10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- Nielsen CS, Staud R, Price DD. Individual differences in pain sensitivity: measurement, causation, and consequences. J Pain. 2009;10:231–237. doi: 10.1016/j.jpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Olsho LW, Koch EG, Carter EA, Halpin CF, Spetner NB. Pure-tone sensitivity of human infants. J Acoust Soc Am. 1988;84:1316–1324. doi: 10.1121/1.396630. [DOI] [PubMed] [Google Scholar]
- Overstreet EH, III, Richter CP, Temchin AN, Cheatham MA, Ruggero MA. High-frequency sensitivity of the mature gerbil cochlea and its development. Audiol Neurootol. 2003;8:19–27. doi: 10.1159/000067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkowski M, Harrison RV. Tone frequency maps and receptive fields in the developing chinchilla auditory cortex. J Neurophysiol. 2005;93:454–466. doi: 10.1152/jn.00569.2004. [DOI] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Göder R, Chirobeja S, Bressmann I, Ferstl R, Baving L. Sleep in children enhances preferentially emotional declarative but not procedural memories. J Exp Child Psychol. 2009;104:132–139. doi: 10.1016/j.jecp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Development of inhibitory mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. J Neurosci. 2007;27:1769–1781. doi: 10.1523/JNEUROSCI.3851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S. Temporal information in speech: acoustic, auditory and linguistic aspects. Philos Trans R Soc Lond B Biol Sci. 1992;336:367–373. doi: 10.1098/rstb.1992.0070. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Hyson RL. Ontogenesis of learning: III. Variation in the rat’s differential reflexive and learned responses to sound frequencies. Dev Psychobiol. 1984;17:285–300. doi: 10.1002/dev.420170308. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behav Neurosci. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Ontogeny of context-specific latent inhibition of conditioned fear: implications for configural associations theory and hippocampal formation development. Dev Psychobiol. 1994;27:367–379. doi: 10.1002/dev.420270605. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Werker J, Werner L. The infant’s auditory world: Hearing, speech, and the beginnings of language. In: Siegler R, Kuhn D, editors. Handbook of Child Development. New York: Wiley; 2006. pp. 58–108. [Google Scholar]
- Sakai M, Kudoh M. Characteristics of sound discrimination enhancement after sound exposure in adult rats. Behav Neurosci. 2005;119:961–973. doi: 10.1037/0735-7044.119.4.961. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr Opinion Neurobio. 2009;19:188–199. doi: 10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze H, Scheich H. Discrimination learning of amplitude modulated tones in Mongolian gerbils. Neurosci Lett. 1999;261:13–16. doi: 10.1016/s0304-3940(98)00991-4. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Singh NC, Theunissen FE. Modulation spectra of natural sounds and ethological theories of auditory processing. J Acoust Soc Am. 2003;114:3394–3411. doi: 10.1121/1.1624067. [DOI] [PubMed] [Google Scholar]
- Smith DI, Kraus N. Postnatal development of the auditory brainstem response (ABR) in the unanesthetized gerbil. Hear Res. 1987;27:157–164. doi: 10.1016/0378-5955(87)90016-5. [DOI] [PubMed] [Google Scholar]
- Spetner NB, Olsho LW. Auditory frequency resolution in human infancy. Child Dev. 1990;61:632–652. [PubMed] [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nature. 2004;5:721–728. doi: 10.1038/nrn1499. review. [DOI] [PubMed] [Google Scholar]
- Thornton S, Semple MN, Sanes DH. Development of auditory motion processing in the gerbil inferior colliculus. Eur J Neurosci. 1999;11:1414–1420. doi: 10.1046/j.1460-9568.1999.00558.x. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Schneider BA, Endman M. Developmental changes in infants’ sensitivity to octave-band noises. J Exper Child Psychol. 1980;29:282–293. doi: 10.1016/0022-0965(80)90020-x. [DOI] [PubMed] [Google Scholar]
- Turner CW, Nelson DA. Frequency discrimination in regions of normal and impaired sensitivity. J Speech Hear Res. 1982;25:34–41. doi: 10.1044/jshr.2501.34. [DOI] [PubMed] [Google Scholar]
- Vogt MB, Rudy JW. Ontogenesis of learning: I. Variation in the rat’s reflexive and learned responses to gustatory stimulation. Dev Psychobiol. 1984 Jan;17:11–33. doi: 10.1002/dev.420170103. [DOI] [PubMed] [Google Scholar]
- Wasner GL, Brock JA. Determinants of thermal pain thresholds in normal subjects. Clin Neurophys. 2008;119:2389–2395. doi: 10.1016/j.clinph.2008.07.223. [DOI] [PubMed] [Google Scholar]
- Wassenhove V, Nagarajan SS. Auditory cortical plasticity in learning to discriminate modulation rate. J Neurosci. 2007;27:2663–2672. doi: 10.1523/JNEUROSCI.4844-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner LA, Marean GC, Halpin CF, Spetner NB, Gillenwater JM. Infant auditory temporal acuity: gap detection. Child Dev. 1992;63:260–272. [PubMed] [Google Scholar]
- Werner LA, Boike K. Infants’ sensitivity to broadband noise. J Acoust Soc Am. 2001;109:2103–2111. doi: 10.1121/1.1365112. [DOI] [PubMed] [Google Scholar]
- Wightman F, Allen P, Dolan T, Kistler D, Jamieson D. Temporal resolution in children. Child Dev. 1989;60:611–624. [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learn Mem. 2008;15:373–377. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- Wright BA, Buonomano DV, Mahncke HW, Merzenich MM. Learning and generalization of auditory temporal-interval discrimination in humans. J Neurosci. 1997;17:3956–3963. doi: 10.1523/JNEUROSCI.17-10-03956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Fitzgerald MB. Different patterns of human discrimination learning for two interaural cues to sound-source location. Proc Natl Acad Sci, USA. 2001;98:12307–12312. doi: 10.1073/pnas.211220498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BA, Zhang Y. A review of the generalization of auditory learning. Phil Trans R Soc B. 2009;364:301–311. doi: 10.1098/rstb.2008.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF. The development of auditory function in the cochlea of the mongolian gerbil. Hear Res. 1984;13:277–283. doi: 10.1016/0378-5955(84)90081-9. [DOI] [PubMed] [Google Scholar]