Abstract
The symptoms of attention-deficit/hyperactivity disorder (ADHD) involve impairments in prefrontal cortical top-down regulation of attention and behavior. All current pharmacological treatments for ADHD facilitate catecholamine transmission, and basic research suggests that these compounds have prominent actions in the prefrontal cortex (PFC). The dorsolateral PFC is especially sensitive to levels of norepinephrine and dopamine, whereby either too little or too much markedly impairs PFC function. Recent physiological studies have shown that norepinephrine strengthens PFC network connectivity and maintains persistent firing during a working memory task through stimulation of postsynaptic α2A-adrenoceptors on PFC neurons. Conversely, dopamine acts at D1 receptors to narrow spatial tuning, sculpting network inputs to decrease noise (i.e., stabilization of the representation). The stimulant medications and atomoxetine appear to enhance PFC function by indirectly increasing these catecholamine actions through blockade of norepinephrine and/or dopamine transporters. In contrast, guanfacine mimics the enhancing effects of norepinephrine at postsynaptic α2A-receptors in the PFC, strengthening network connectivity. Stronger PFC regulation of attention, behavior, and emotion likely contributes to the therapeutic effects of these medications for the treatment of ADHD.
Keywords: Atomoxetine, dopamine, guanfacine, methylphenidate, norepinephrine, working memory
As described in this special issue, many of the symptoms of attention-deficit/hyperactivity disorder (ADHD) are thought to arise from dysfunction of the prefrontal cortex (PFC) and its connections with cortical and subcortical brain regions. Prefrontal cortex dysfunction may arise from a wide variety of vulnerabilities, including a protracted developmental period (more than 2 decades) that increases susceptibility to both environmental and genetic insults (1). Prefrontal cortex networks are able to represent information in the absence of bottom-up external stimulation, but this fragile process is tremendously dependent on the correct neurochemical environment, whereby arousal pathways coordinate cognitive state with environmental events. As there are built-in mechanisms to take PFC offline during fatigue and stress, the PFC may be especially vulnerable to a wide variety of genetic insults in the arousal pathways (2,3). Current data suggest that the lateral PFC regions regulating attention and behavior are especially sensitive to the influence of norepinephrine (NE) and dopamine (DA) (4–7). These findings may explain why all currently approved medications for ADHD increase or mimic NE and/or DA signaling. The current review examines the roles of NE and DA on dorsolateral PFC network physiology and function with focus on working memory circuits, as the anatomy and physiology have been most richly studied for this cognitive domain. These studies have revealed that NE and DA have complementary effects on the strength of PFC network connections, with NE strengthening connectivity via α2A receptor stimulation and DA sculpting network inputs via D1 receptors. We are currently learning about the second messenger signaling pathways underlying NE and DA actions in PFC. This information may help us understand how genetic insults in signaling pathways can alter the integrity of PFC function and lead to symptoms of ADHD.
Top-Down Regulation by Prefrontal Cortex
The PFC intelligently regulates our thoughts, actions, and emotions through extensive connections with other brain regions, including projections to the association cortices for the regulation of sensory processing (8) and extensive projections to the basal ganglia and cerebellum for the regulation of motor, cognitive, and emotional responses (9) (Figure 1). The PFC creates a mental sketch pad through networks of neurons that maintain information in the absence of environmental stimulation (10). This process is sometimes referred to as working memory: the ability to keep in mind an event that has just occurred or bring to mind information from long-term storage and use this representational knowledge to regulate behavior, thought, and emotion (11). The PFC is able to protect these fragile representations from the interference of external or internal distractions and is key for inhibiting inappropriate actions and promoting task-relevant operations (so-called top-down regulation) (12–15). Prefrontal cortex operations allow the flexible regulation of behavior to properly respond to a changing environment, e.g., the ability to shift attentional set to new dimensions and to alter decision making as reward contingencies shift (16,17). The PFC also monitors errors, giving us the insight that we are incorrect and need to shift strategies (18). Important for ADHD symptoms, the PFC is needed for frustration tolerance, e.g., being able to inhibit responding immediately for a small reward and instead waiting for a larger reward (19,20). There are regional specializations for these functions, particularly within the large human PFC, with dorsolateral PFC regions often involved in the regulation of attention and the right inferior PFC being especially important for the inhibition of inappropriate behaviors (21,22). All of these abilities depend on proper PFC neuronal network connections, which are highly sensitive to their neurochemical environment.
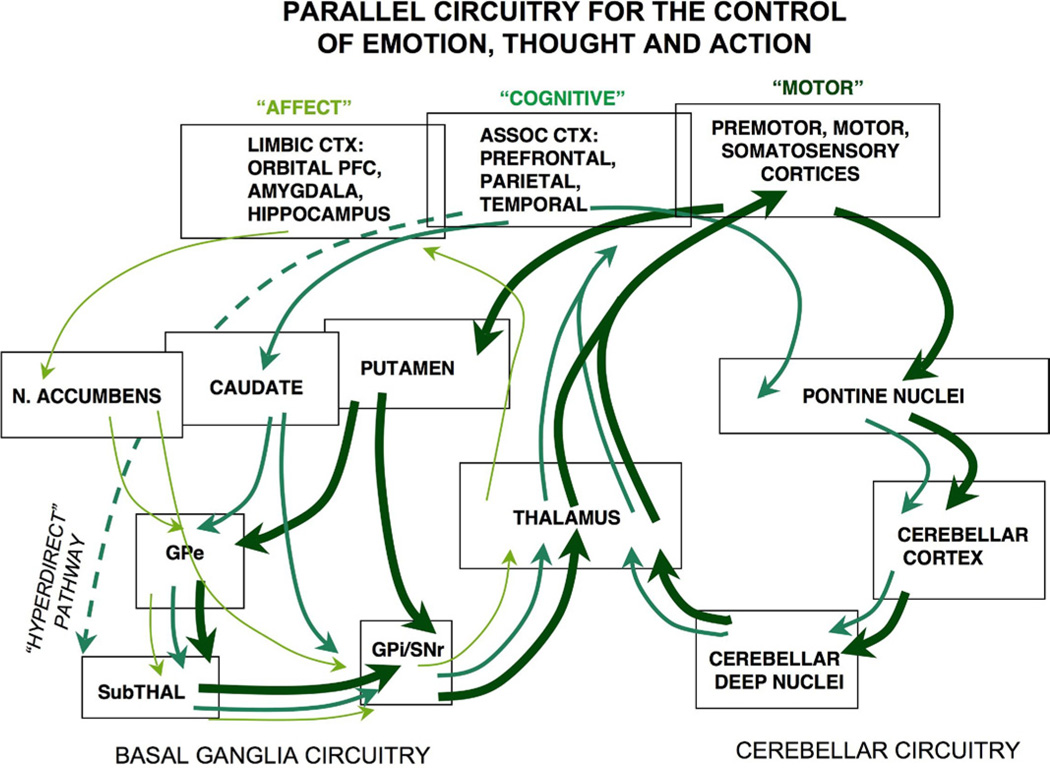
Figure 1.
Parallel basal ganglia and cerebellar loops for the control of behavior, thought, and emotion. The pioneering work of Middleton and Strick (9) has revealed parallel pathways regulating motor response, cognition, and emotion. One set of pathways projects from cortex through striatal structures in the basal ganglia to focus back on prefrontal cortex for the selection and planning of motor, cognitive, and emotional habits. A second set of pathways project through the pontine nuclei to the cerebellum; indeed, the majority of the cerebellar cortex in primates receives projections from nonmotor cortices. Please note that some prefrontal cortex regions also project directly to the subthalamic nucleus for rapid stopping of behavioral responses (not shown). Alterations in these pathways may often contribute to the symptoms of attention-deficit/hyperactivity disorder. ASSOC, association; CTX, cortex; GPe, globus pallidus external segment; GPi, globus pallidus internal segment; N. ACCUMBENS, nucleus accumbens; SNr, substantia nigra pars reticulata; SubTHAL, subthalamic nucleus.
The Key Role of Prefrontal Cortical Networks in Representational Knowledge
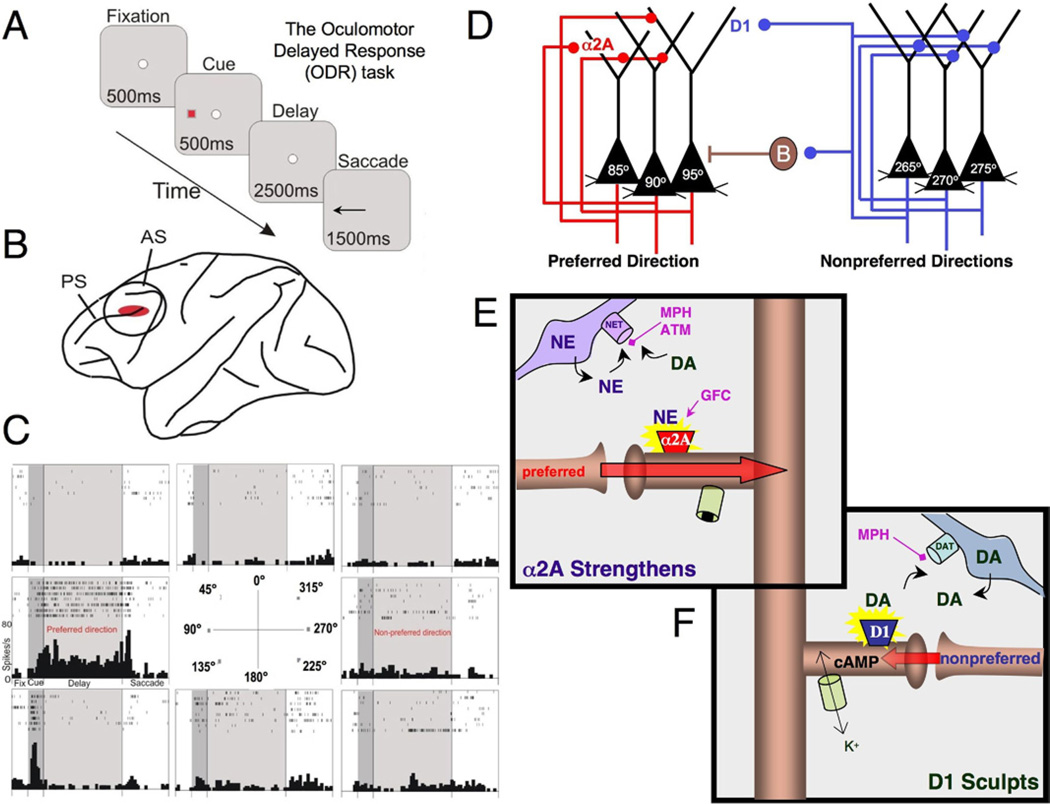
The PFC is able to represent information that is not currently in the environment through networks of pyramidal neurons that excite each other to maintain information in mind. The circuitry underlying representational knowledge in PFC has been most intensively studied in the visuospatial realm, in monkeys performing a spatial working memory task (Figure 2A). In this task, the monkey has to maintain the memory of a precise spatial location over a delay period before moving its eyes to the remembered location to receive a juice reward. Each session consists of hundreds of trials, with the correct spatial position constantly changing. Thus, the contents of working memory must be constantly updated. In monkeys, visuospatial information is initially mapped by the parietal association cortices and fed forward to the dorsolateral PFC surrounding the principal sulcus (Figure 2B). This region contains neurons that represent visuospatial position during the delay period, an example of which is shown in Figure 2C. This neuron fires to the spatial cue and maintains firing throughout the delay period if the cue had been at 90° but not other spatial locations. Thus, it provides a representation of the 90° location over the delay period when information is no longer available in the environment. For this neuron, 90° is said to be its preferred direction, while others are nonpreferred directions.
Figure 2.
Spatial working memory networks in the dorsolateral prefrontal cortex (PFC). (A) The oculomotor delayed response task is a spatial working memory task that is used to probe the physiological profiles of PFC neurons. The subject must remember the spatial position of the most recent cue over a delay period of several seconds and then indicate that position with a saccade to the memorized location. (B) The region of the monkey dorsolateral PFC (Walker’s area 46) where neurons show persistent, spatially tuned firing during the oculomotor delayed response task. (C) An example of a PFC neuron that shows persistent firing during the delay period if the cue had occurred at 90° (the preferred direction for this neuron) but not at other spatial locations. A nonpreferred direction opposite to the preferred direction is also labeled for reference to Figure 3 in which only the preferred and one nonpreferred direction are shown. Rasters show the firing of the neuron over seven trials at each spatial position. (D) A schematic drawing of the PFC microcircuits underlying spatial working memory as described by Goldman-Rakic (23). Layer III pyramidal cells (black) receive highly processed spatial information from parietal association cortices (not shown). Pyramidal cells with similar spatial characteristics engage in recurrent excitation to maintain persistent activity over the delay period (note that the subcellular localization of these excitatory connections is not currently known; they could be on the apical and/or basal dendrites). Gamma-aminobutyric acidergic interneurons help to spatially tune neurons through lateral inhibition; one of these is labeled as B (basket cell). Network synaptic inputs from isodirectional inputs (neurons with the same tuning profile) are shown in red, while inputs form cross-directional microcircuits (neurons with different tuning characteristics) are shown in blue. (E) A working model of norepinephrine (NE) actions at α2A receptors on PFC dendritic spines. Stimulation of α2A receptors inhibits cyclic adenosine monophosphate signaling, which closes nearby potassium channels and strengthens the efficacy of network connections onto the spine. The physiological data suggest that these actions occur on spines receiving preferred network inputs (Wang et al. [57]). Many of the drugs approved to treat attention-deficit/hyperactivity disorder block the NE transporter and increase NE (and dopamine [DA]) availability, e.g., medications such as methylphenidate and atomoxetine. In contrast, guanfacine mimics NE actions by directly stimulating the α2A receptor. (F) Physiological studies in monkeys performing working memory tasks indicate that an optimal level of DA D1 receptor stimulation weakens neuronal firing for nonpreferred inputs, thus enhancing the spatial tuning of the neuron (Vijayraghavan et al. [109]). Physiological recordings indicate that these sculpting actions involve increased cyclic adenosine monophosphate signaling, likely via opening of potassium channels. Stimulants such as methylphenidate block the DA transporter to increase DA availability; blockade of the NE transporter similarly increases DA availability in the PFC (see text). AS, arcuate sulcus; ATM, atomoxetine; B, basket cell; DA, dopamine; DAT, dopamine transporter; GFC, guanfacine; K+, potassium; MPH, methylphenidate; NE, norepinephrine; NET, norepinephrine transporter; ODR, oculomotor delayed response; PS, principal sulcus.
The work of Goldman-Rakic (23) revealed the microcircuitry in PFC that underlies spatially tuned, persistent firing during a spatial working memory task. Her work showed that layer III pyramidal cells excite each other through connections on spines to maintain persistent firing over the delay period (Figure 2D). Thus, a 90° neuron is excited by other 90° neurons, through connections on spines (Figure 2E). This network activity is spatially tuned by inhibitory gamma-aminobutyric acid (GABA)ergic interneurons (e.g., basket cells) through lateral inhibition (Figure 2D), e.g., the 270° neurons activate interneurons to suppress the firing of 90° neurons while remembering a 270° cue. Thus, the contents of working memory are specific and informative. Computational models have predicted that the persistent network firing during the delay period would require N-methyl-D-aspartate (NMDA) receptor stimulation to provide slow, stable excitation (24), and recent physiological data support this prediction (reviewed in [3]). Thus, insults to NMDA receptor signaling erode working memory performance (e.g., [25]). Spatially tuned, persistent firing also depends on the neurochemical environment, which powerfully regulates the strength of network connections (3).
The Strength of Network Connections Is Rapidly Altered by the Neurochemical Environment: Integrating Arousal and Cognition
The arousal pathways (e.g., norepinephrine, dopamine, acetylcholine, serotonin, histamine, and orexins) all project to the PFC, and it is likely that all influence PFC function (26). However, a long history of research on the catecholamines has advanced our knowledge of these mechanisms in particular. This research began with the landmark discovery that depletion of catecholamines from the dorsolateral PFC was as detrimental as ablation of the cortical tissue itself (4). It is now known that both NE and DA have an inverted-U dose effect on PFC function, whereby either too little (e.g., depletion or fatigue) or too much (e.g., uncontrollable stress) impairs PFC function, while moderate levels of catecholamine released when a subject is alert and interested strengthen and sculpt inputs to optimize PFC function based on environmental demands.
The NE and DA neurons in the brainstem change their firing rate according to our arousal state, as well as the relevance of events in the environment. Norepinephrine neurons in the locus coeruleus are silent during rapid eye movement sleep and have low tonic firing during slow wave sleep, moderate tonic firing and pronounced phasic firing to relevant stimuli during nonstressed waking, and high tonic firing with dysregulated phasic firing during stress (27). They fire to relevant stimuli during alert waking but can fire to distracters during fatigue or stress (27). Dopamine neurons have not been followed as methodically with regard to states of arousal but have been shown to fire related to prediction of reward (28). However, recent studies suggest that a subset of midbrain DA neurons can increase their firing to aversive stimuli (29), and these neurons may contribute to increased DA release in the PFC during stress. Biochemical studies in rats have consistently shown that both DA and NA release are increased in the medial PFC during acute stress exposure (30–32).
The levels of catecholamine release in PFC may rapidly alter the strength of PFC network connections to coordinate cognitive state with physiological demands. We have proposed that weakening of PFC network connections could conserve energy during states of fatigue when energy is scarce (3), particularly as recurrent network excitation is an energy-intensive operation (33). Conversely, high levels of catecholamine release during stress can rapidly take PFC offline in response to danger, to switch control of behavior to more primitive brain regions (e.g., amygdala, striatum) that mediate instinctive reactions. Under optimal arousal conditions, phasic catecholamine release appears to regulate the strength and breadth of network inputs in a manner that is essential to PFC cognitive function. Thus, precise regulation of NE and DA is needed for appropriate PFC regulation.
The Underappreciated Role of Norepinephrine
Although much of the previous research on ADHD and PFC has focused on DA mechanisms, recent data indicate that NE mechanisms are just as important and may even have more utility for the development of medications because of distinct NE actions at adrenergic receptors. Norepinephrine has the highest affinity for α2 adrenergic receptors and lower affinity for α1 and β receptors (34). Therefore, the type of receptor engaged may be determined by the amount of NE release.
Data from studies of monkey dorsolateral PFC demonstrate an inverted-U dose-response: moderate levels of NE released during alert, nonstressed waking improve working memory performance by engaging postsynaptic, α2A receptors (35,36), whereas high levels of NE released during stress impair PFC function via stimulation of lower affinity α1 (37) and β1 receptors (38). Norepinephrine α1 and β1 receptor stimulation can facilitate glucocorticoid detrimental actions (39), suggesting a coordinated stress response. This inverted-U has been seen at both the behavioral and cellular levels in dorsolateral PFC (40).
A variety of behavioral evidence indicates that NE stimulation of α2A receptors in dorsolateral PFC is critical for working memory. Either depletion of NE (35) or blockade of α2A receptors in PFC (36) impairs working memory performance in monkeys, suggesting that endogenous NE plays a large role in strengthening working memory performance. Conversely, stimulation of postsynaptic α2A receptors in dorsolateral PFC, e.g., with guanfacine infusions directly into this region, improves working memory performance (35,41–43). Systemic administration of α2A agonists to monkeys also improves performance of tasks that depend on dorsolateral PFC, including spatial working memory under distracting conditions (44,45). Systemic guanfacine administration in humans also can improve dorsolateral PFC functions, such as working memory and planning ([46,47], although Muller et al. [48]). Guanfacine has also been shown to improve working memory performance in patients with epilepsy (47) and schizotypal disorder (49).
In contrast to α2A receptors, infusion of an α1 agonist directly into the dorsolateral PFC impairs working memory performance (42). Blockade of α1 or β receptors in dorsolateral PFC has no effect on working memory performance under basal conditions (36), suggesting that these receptors are not sufficiently engaged under nonstress conditions when there are moderate levels of NE release. Similar effects are observed with systemic administration of α1 compounds. Systemic administration of an α1 agonist that crosses the blood brain barrier also impairs working memory performance, while administration of an α1 antagonist, e.g., prazosin, can protect working memory performance during stress (37,50,51). These actions may contribute to prazosin’s beneficial effects in treating posttraumatic stress disorder and drug addiction (52–54).
Although high levels of α1 and β receptor stimulation impair working memory performance, emerging data suggest they may improve other PFC cognitive operations. Recent studies in rats indicate that stop signal performance in rats is mediated by β receptors (55) and attentional set shifting may involve α1 receptors (7,56). It is interesting to consider that these cognitive operations may be part of an orchestrated stress response. The stop signal task requires stopping an ongoing movement (what Aron calls reactive inhibition, see his article in this special issue [21]), and this may involve different circuits and mechanisms than the more thoughtful forms of behavioral inhibition (what Aron calls proactive inhibitory control [21]), e.g., as occurs in the go/no-go task. Similarly, facilitated shifting of attention may be adaptive under stressful conditions. Thus, there may be a coordinated modulation of PFC operations by NE α2A versus α1/β receptors based on their relevance to survival under safe versus stressful arousal conditions.
Electrophysiological recordings from PFC neurons in monkeys performing a working memory task demonstrate a similar inverted-U dose-response as that seen in behavioral tasks (Figure 3A) and have revealed some of the intracellular signaling pathways underlying NE actions. Stimulation of α2A receptors increases delay-related firing of PFC neurons via inhibition of cyclic adenosine mono-phosphate (cAMP) (57), while stimulation of α1 receptors decreases delay-related firing via activation of phosphatidylinositol protein kinase C signaling (58). The latter finding may explain why lead poisoning can mimic the symptoms of ADHD (59), as extremely low concentrations of lead activate protein kinase C (60). Thus, environmental insults that disrupt proper PFC neuromodulation may have powerful effects on cognitive control of attention, behavior, and emotion.
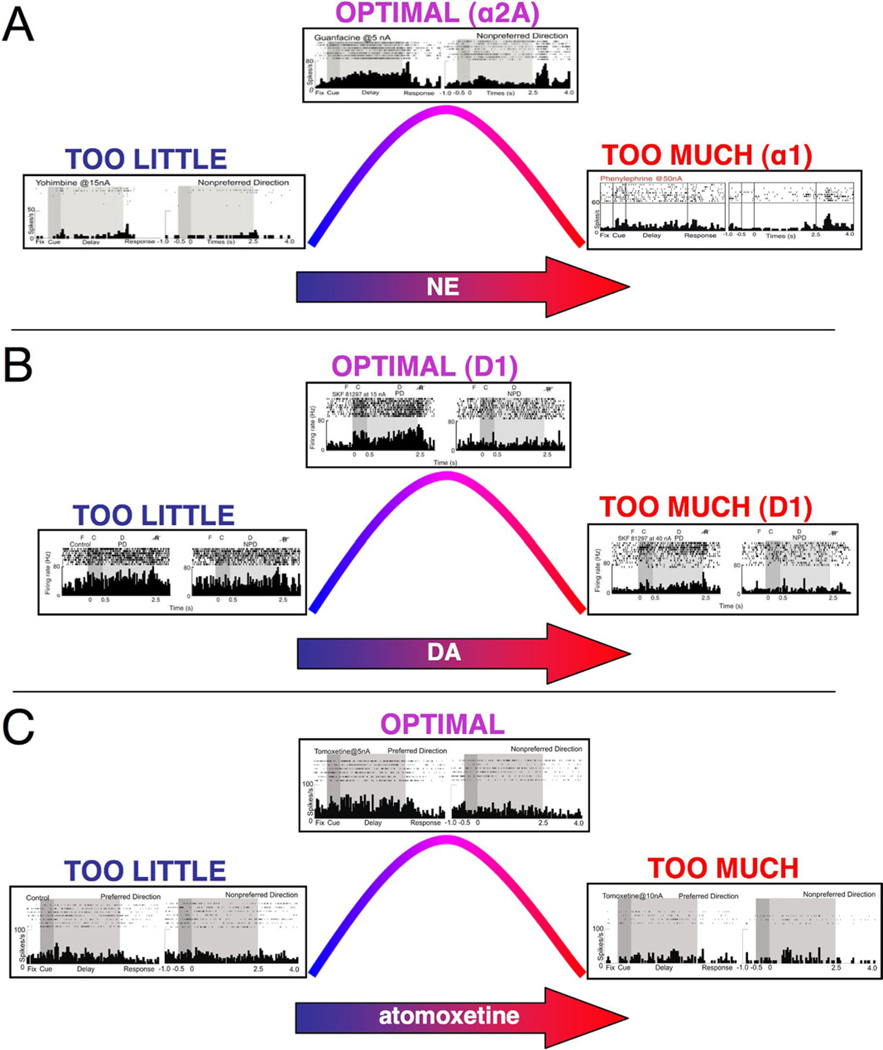
Figure 3.
Catecholamine influences on prefrontal cortex (PFC) physiology and function. Both norepinephrine (NE) and dopamine (DA) have inverted U-shaped influences on PFC physiology and cognition, whereby either too little or too much of the neurotransmitter impairs PFC function. There are increasing levels of catecholamine release according to arousal state, with low levels during fatigue; phasic release under alert, nonstress conditions; and high levels during uncontrollable stress exposure. These figures show examples of NE and DA influences on the pattern of dorsolateral PFC neuronal firing in monkeys performing the oculomotor delayed response spatial working memory task, as outlined in Figure 2. (A) The NE inverted U. Inadequate α2A receptor stimulation because of iontophoresis of yohimbine (15 nA) leads to weak neuronal firing (left). In contrast, iontophoresis of the α2A agonist, guanfacine (5 nA), increases memory-related firing during the delay period for the neuron’s preferred direction (top). With high levels of NE release during stress, NE engages lower affinity α1 receptors. Stimulation of α1 receptors via iontophoresis of phenylephrine (50 nA) reduces PFC neuronal firing (right). (B) The DA D1/5 inverted U. A neuron with noisy neuronal firing to all spatial directions under control conditions is seen in this figure (left). This noisy firing pattern can also be induced in a highly tuned neuron by iontophoresis of a moderate dose of a D1/5 antagonist (not shown). Iontophoresis of an optimal dose of the D1/5 agonist, SKF81297 (15 nA), to a noisy neuron selectively decreases memory-related firing for the neuron’s nonpreferred directions, thus sharpening spatial tuning (top). In contrast, high levels of D1 receptor stimulation (40 nA) reduce PFC neuronal firing for all directions (right). (C) The inverted U observed with iontophoresis of atomoxetine. A neuron with relatively low levels of memory-related firing under control conditions (left) shows enhanced firing for the memory of the neuron’s preferred direction with iontophoresis of a low dose of atomoxetine (5 nA; top). Iontophoresis of a higher dose of atomoxetine (15 nA) suppresses neuronal firing, consistent with excessive NE and/or DA release in PFC. NE, norepinephrine.
Physiological studies are beginning to reveal how α2A inhibition of cAMP signaling can improve PFC function. Iontophoresis of an α2A agonist such as guanfacine onto PFC neurons enhances neuronal firing during the delay period for the neurons’ preferred direction (Figure 3A), while blockade of α2A receptors markedly reduces PFC neuronal firing during the delay period (indeed, high doses can silence a neuron) (57,61). Stimulation of α2A receptors strengthens firing for the neuron’s preferred direction by inhibiting cAMP production, which, in turn, reduces cAMP opening of potassium channels, increasing the efficacy of nearby synaptic inputs (Figure 2E). Support for this working model comes from several lines of research. Multiple label immunoelectron microscopy has demonstrated α2A receptors next to hyperpolarization-activated cyclic nucleotide-gated (HCN) channels on the dendritic spines of pyramidal cells in the superficial layers of monkey dorsolateral PFC (57). These channels increase their open probability in the presence of cAMP. Physiological recordings have shown that guanfacine enhancement of delay-related firing for the neuron’s preferred direction is mediated by inhibition of cAMP-HCN signaling, while increases in cAMP-HCN channel signaling markedly weaken persistent neuronal firing (57). Similarly, behavioral studies have shown that increased cAMP-HCN signaling weakens working memory performance (57), while α2A receptor stimulation in PFC improves working memory performance through inhibition of these pathways (43). Thus, the strong network connections needed for working memory critically depend on α2A receptor stimulation.
Although the physiological studies of α2A receptor actions have been focused on spatial working memory paradigms in dorsolateral PFC, behavioral data indicate that these enhancing effects extend to other cognitive operations and additional subregions of the PFC (summarized in Table 1). For example, infusions of guanfacine into the ventrolateral PFC—a region altered in ADHD—improved response flexibility and conditional associative motor learning in monkeys (62). Guanfacine has also been shown to improve performance of a task that relies on orbital PFC, object reversal (63). Improvement on object reversal has also been seen with drugs that block the NE(butnotDA)transporter (64).Asorbital circuits are important for the control of aggression (65,22), guanfacine strengthening of orbital function may underlie the reduced aggression observed in monkeys (66) and ADHD patients (67) taking this medication.
Table 1.
Alpha-2A Adrenergic Influences on Prefrontal Cortical Functions in Primates
| Prefrontal Cortex Operation | References |
|---|---|
| Working Memory | |
| Improve | (36,46,47,49,136,137) |
| No effect | (48) |
| Attention Regulation | |
| Reduce distractibility | (44,45) |
| Improve sustained attention | (95) |
| Increase alerting | (138) |
| Strengthen Behavioral Inhibition Cognitive: | |
| Stroop interference | (139) |
| No-go performance | (69) |
| Response flexibility | (62,140) |
| Emotional: | |
| Reward reversal | (63) |
| High Order Associative Learning | (62,140) |
| Planning and Organization | (46) |
Importantly, blockade of α2 receptors selectively within the monkey PFC can recreate many of the key symptoms of ADHD. Iontophoresis of yohimbine onto dorsolateral PFC neurons markedly suppresses PFC cell firing in monkeys (57,61). Infusions of yohimbine into this same region induce locomotor hyperactivity (68), errors of commission on no-go trials in a go/no-go task (69), and impaired working memory performance (36). These data suggest that genetic insults that similarly weaken α2A receptor signaling may also impair PFC regulation of attention and behavior.
Genetic studies of NE signaling have revealed some interesting relationships to ADHD (70). For example, a polymorphism in the promoter region of the gene encoding for dopamine β hydroxylase leads to inadequate levels of dopamine β hydroxylase enzyme and reduced levels of NE synthesis (71). This genetic insult is associated with ADHD (72–74) and has been associated with features associated with ADHD, including poor sustained attention (75), weaker impulse control (76), and impaired performance on PFC tests of executive function (77). Genetic alterations in the α2A receptor also have been associated with ADHD (73,78 – 80) and with impaired PFC executive function (81). Thus, NE stimulation of α2A receptors is needed for strong PFC regulation in both animals and humans.
NE Mechanisms and ADHD Medications
All medications currently approved for the treatment of ADHD influence NE transmission (Figure 2E), and all can improve at least some aspects of PFC function (82).A common misconception is that methylphenidate is a selective DA transporter blocker, when in actuality it blocks both NE and DA transporters. Indeed, methylphenidate has more potent effects on NE than DA in the rat PFC (83) (84). Behavioral data in rats and monkeys indicate that methylphenidate can improve working memory performance by indirectly enhancing both NE α2A receptor and DA D1 receptor actions (40). Methylphenidate has also been shown to improve working memory and stop signal performance in both normal volunteers (85,86) and patients with ADHD (87–89). Methylphenidate also enhances PFC physiological responses in rats (84).
Like methylphenidate, atomoxetine also blocks the NE transporter (Figure 2E), and as the NE transporter clears both NE and DA in the PFC, this increases extracellular levels of both catecholamines (90). Physiological recordings in monkeys show that an iontophoresis of an optimal dose of atomoxetine can enhance PFC firing (Figure 3C), in part via increased NE stimulation of α2 receptors (40). Atomoxetine also has D1 actions at the cellular level in dorsolateral PFC (see below). However, higher doses of atomoxetine reduce dorsolateral PFC firing during working memory (Figure 3C), which may contribute to the variability in response to this medication. Atomoxetine has been shown to improve stop signal performance in normal volunteers (91) (although, see [86]), as well as in patients with ADHD (92). Importantly, atomoxetine (40 mg) has been shown to increase right inferior PFC activity during performance of this task (93), a brain region often shown to be underactive in patients with ADHD. However, a much higher dose of atomoxetine impaired go/no-go performance (94). It is possible that atomoxetine’s effects on PFC functions will be dose-related, with low doses producing moderate NE release that engages α2A receptors, improving working memory and go/no-go performance (proactive inhibition), while higher doses produce greater NE release that engages β receptors, improving stop signal reactive inhibition. This hypothesis could be tested in future research.
In contrast to stimulants and atomoxetine, guanfacine directly mimics NE stimulation of α2A receptors, as described above (Figure 2E). Guanfacine administration to children with ADHD and tics improved performance of a Conners’ Continuous Performance Test task that requires sustained attention, working memory, and behavioral inhibition (95). These data are consistent with those in monkeys showing beneficial effects of α2A receptor stimulation on a variety of PFC functions.
The Complex Roles of DA
It has long been appreciated that DA is essential to the working memory functions of the PFC (4). New research is now revealing the contribution of DA to orbital PFC functions as well, which appears to differ from serotonergic actions (96) (26). The current article focuses on DA actions in dorsolateral PFC during spatial working memory, given this has been best studied at the cellular level.
There are two families of DA receptors: the D1 (D1 or D5) and D2 (D2, D3, D4) families of receptors. The D1 receptor family is found in both superficial and deep layers of monkey dorsolateral PFC (97), while D2 receptors are focused in layer V (98). The D1 receptors are concentrated on dendritic spines near network synapses (99), although likely in a separate set of spines than those containing α2A receptors (100). In contrast, D5 receptors are found near the cell body in sites where they can regulate calcium release from the endoplasmic reticulum (101). The D2 receptors are found in a variety of subcellular localizations (102), while D4 receptors are concentrated on GABAergic interneurons (103). It is important to note that D4 receptors have exceptionally high affinity for NE and thus should be considered a catecholamine receptor rather than a DA receptor (104). This review will focus on D1 receptor influences on PFC function, as these are best understood, with only a brief discussion of D2 receptor influences. Please note that all currently availableD1drugs act at both D1 and D5 receptors, and thus pharmacological studies do not differentiate these mechanisms. Thus, they will be referred to here as D1/5 receptors as appropriate.
D1/5 Receptor Mechanisms
Dopamine has an inverted U-shaped influence on working memory abilities via actions at the D1 receptor family in both animals (100) and humans (105). Cognitive studies in animals have shown that either blockade of D1/5 receptors or excessive stimulation of D1/5 receptors in the PFC impairs spatial working memory (106–108). This inverted U-shaped relationship can be observed at the cellular level (Figure 3B), where an optimal level of D1/5 receptor stimulation enhances spatial tuning by suppressing delay-related firing to the neurons’ nonpreferred directions (109). Iontophoresis of very high doses of D1/5 antagonists on PFC neurons stop all neuronal firing (110) (not shown in Figure 3B), while more moderate doses of a D1/5 antagonist induce noisy firing to both preferred and nonpreferred directions (Figure 3B, left side). A modest dose of D1/5 agonist enhanced spatial tuning by selectively inhibiting firing to nonpreferred directions (Figure 3B, top). Thus, DA and NE have complementary roles: NE α2A receptor stimulation enhances network firing for shared, preferred inputs (i.e., increasing signals [57]), whereas D1 receptor stimulation sculpts neuronal firing by decreasing firing to nonpreferred inputs (i.e., decreasing noise [109]). In contrast to moderate D1/5 receptor stimulation, high doses of D1/5 agonist suppress all neuronal firing (Figure 3B, right side of curve). These actions likely contribute to impaired working memory abilities during uncontrollable stress exposure, when high levels of DA are released in PFC. The potential mechanisms underlying this inverted U are briefly discussed below. For a more lengthy discussion, please see Arnsten et al. (100).
The marked loss of firing when high doses of D1/5 antagonist are applied to monkey PFC neurons may arise from blockade of fundamental excitatory DA D1/5 actions. These actions are likely saturated in the in vivo animal recordings and thus are best observed through in vitro slice preparations of rat medial PFC. Early investigations showed D1/5-mediated excitatory effects on prefrontal pyramidal cells (111). A more recent study showed that D1/5 receptor stimulation appears to increase the evoked excitability of pyramidal cells while reducing spontaneous excitability by acting in concert on the persistent sodium current, high-threshold dendritic calcium spikes, and a slowly inactivating potassium current (112). The D1/5 agonists increased NMDA receptor-mediated synaptic currents while slightly reducing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents, leading to enhancement of sustained excitatory postsynaptic potential trains (113). The D1/5 receptor agonists also appear to shift the activation curve of the persistent sodium current to more hyperpolarized potentials, thus contributing further to signal-to-noise improvements (114,115). Intracellular recordings from layer II/III PFC pyramidal neurons have shown that D1/5 receptor stimulation may promote excitation by increasing the amplitude of excitatory postsynaptic currents through calcium, protein kinase A, and calcium/calmodulin-dependent protein kinase II mechanisms (116). Thus, a variety of mechanisms may contribute to D1/5 excitatory actions.
Lower dose iontophoretic application of D1/5 receptor agonists and antagonists revealed important sculpting actions that likely occur under normal physiological conditions. Thus, a low dose of D1/5 antagonist produced noisy cell firing, while an optimal dose of D1/5 agonist enhanced spatial tuning by suppressing firing for only the nonpreferred directions (Figure 3B, top of curve). These sculpting actions may contribute to the stabilization of representations by preventing responses to distracting, irrelevant events (117, for a detailed discussion of these actions in human subjects). The D1/5 sculpting actions resemble the lateral inhibition observed with GABAergic interneurons (Figure 2D) and indeed may involve D1/5 enhancing GABAergic actions (118,119). However, in vivo recordings in monkeys showed only suppression of fast-spiking cells (presumed interneurons) following D1/5 agonist application. This may be due to the predominant influence of pyramidal cell network firing in the cognitively engaged monkey; thus, suppression of pyramidal cell firing reduces excitatory drive on interneurons. The sculpting effects of D1/D5 agonists may also involve engagement of presynaptic D1 receptors on network terminals, decreasing glutamate release (120), as well as engagement of D1 receptors on spines, gating network inputs via increased cAMP signaling (Figure 2F) (3,100,109). In this way, arousal state and the predicted rewarding properties of events in the environment can sharpen neuronal tuning. These D1 sculpting actions are beneficial for cognitive operations requiring a narrow range of network inputs (e.g., spatial working memory for a small location in space) but are harmful if a broader range of inputs is required (e.g., during attentional set shifting [121]). Under optimal conditions, DA release may be dynamically regulated according to momentary cognitive demands (for an extensive review, see [100]).
However, at high levels of D1 receptor stimulation, firing is suppressed for all directions, and the neuron loses both its spatial tuning and its level of responsiveness (109) (Figure 3B). Indeed, PFC neurons will sometimes stop firing altogether following higher dose D1 agonist application. These suppressive actions are prevented by inhibition of cAMP-protein kinase A signaling (109), consistent with the working model shown in Figure 2F. High levels of D1 suppressive actions likely occur with stress exposure but may also contribute to the feeling of being a “zombie” (e.g., reports of empty thoughts) with an excessive dose of stimulant medication.
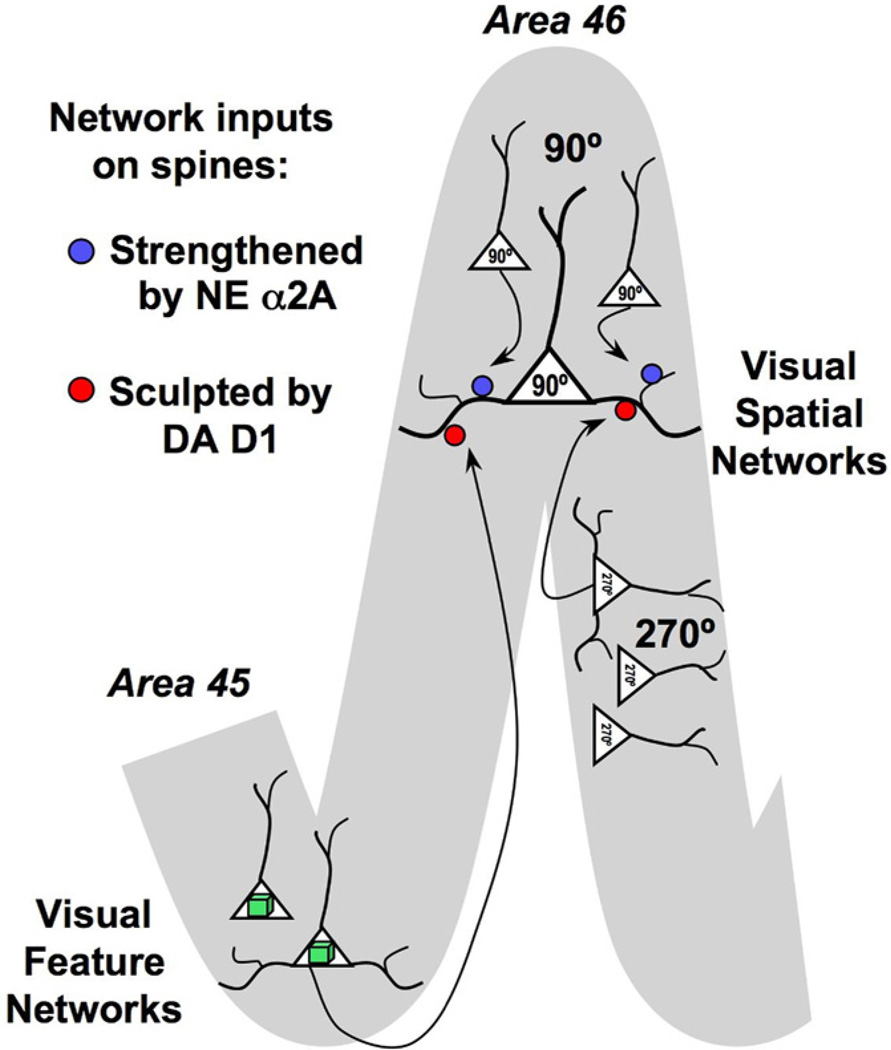
We have hypothesized that D1 receptors may have a general role in gating network inputs to PFC neurons, as schematically depicted in Figure 4. The D1 receptors on the spines of an area 46 pyramidal cell may gate both nonpreferred spatial inputs from other neurons in area 46, as well as visuofeature inputs from area 45. According to this working model, D1 receptor stimulation in area 46 would be particularly detrimental when a neuron is trying to maintain broad inputs, e.g., both spatial and feature information, in working memory. The ability to dynamically regulate the width of network inputs according to the affective significance of stimuli in the environment may have great relevance to survival, e.g., narrowing inputs on stimuli predicting reward, disconnecting PFC networks in response to a threat. However, it is unlikely that a D1 agonist can mimic the dynamic range of dopaminergic response needed to effectively coordinate cognition with arousal state, which may limit the therapeutic utility of these agents.
Figure 4.
Working model of catecholamine regulation of cortical network inputs on spines. Schematic diagram of prefrontal cortex (PFC) network inputs to a layer III PFC pyramidal cell in area 46, the brain region specialized for spatial working memory. The preferred spatial direction for this neuron is 90°. The neuron receives extensive excitatory inputs from other 90° neurons in area 46, from the same column as well as more distant columns. These network inputs are strengthened by stimulation of α2A receptors on the spines receiving the preferred inputs, shown in red. In contrast, network inputs from neurons with different spatial tuning, e.g., 45°, are gated by dopamine D1 receptors, shown in blue. These sculpting actions sharpen spatial tuning. We hypothesize that D1 receptors may also gate network inputs from other neurons with nonpreferred characteristics, e.g., synaptic inputs from neurons in area 45 that process visuofeature information such as faces. In this way, D1 may dynamically alter the breadth of network inputs to a PFC pyramidal cell.
D2 Receptor Mechanisms
The D2 receptors are less prevalent in monkey dorsolateral PFC and are concentrated on layer V neurons, the layer that projects to striatum and the superior colliculus (98,122,123). Iontophoresis of D2 drugs onto PFC neurons has no effect on delay cell firing during the delay period in a working memory task but has marked effects on response cell firing in relationship to the eye movement response (124). Thus, D2 agonists increased the amplitude and speeded the timing of response-related firing. Importantly, the D2 antagonist, raclopride, decreased the amplitude and slowed the response-related firing, indicating that endogenous DA stimulation of D2 receptors normally enhances response-related firing. These findings are consistent with results from imaging studies, where the D2 agonist, bromocriptine, also increases response-related blood oxygenation level-dependent signals in PFC (125). In monkey physiology studies, the timing of the neuronal response is accurate enough to reveal response neurons that fire in anticipation of the motor response (likely mediating the action), as well as those that fire during the response. Firing during the motor response may be involved in clearing the mental sketchpad for the next trial, an updating function (e.g., as suggested by [126]). Neuronal firing during the saccadic motor response also may be providing corollary discharge, the mental tag that informs the brain that an event is self-initiated (127). The D2 drugs were observed to alter both early and late-firing response cells, suggesting that D2 mechanisms may regulate the amplitude of PFC communication with subcortical structures mediating motor responses, as well as pathways involved in updating (126) or in corollary discharge. Disruptions in corollary discharge may contribute to hallucinations (128), which can be a side effect of D2 medications (129). Thus, D2 agonists likely remain suboptimal therapeutic agents for treating PFC dysfunction.
The microcircuitry contributing to response cell firing is less well understood than delay cells, and it is not known if or how GABA interneurons influence the firing of response cells. Research in rodent medial PFC has shown that D2 receptor stimulation can reduce GABAergic inputs to pyramidal cells (130). It is not known if this mechanism contributes to the increased firing of response cells following D2 agonist iontophoresis in primates and/or whether it arises from direct D2 actions on pyramidal cells, e.g., D2-mediated increases in excitatory transient receptor potential channel currents (131).
DA Mechanisms and ADHD Medications
Positron-emission tomography imaging studies have shown that therapeutic doses of stimulant medications increase endogenous DA stimulation of D2 receptors in the striatum (132) and are particularly effective under conditions when DA neurons would be activated, i.e., in response to a salient stimulus (133). Unfortunately, positron-emission tomography imaging is not able to detect DA interactions with receptors in the PFC, but biochemical studies in rats suggest that methylphenidate is even more effective in increasing DA in PFC than in striatal structures (83). Animal studies have also shown that the enhancing effects of methylphenidate and atomoxetine on working memory performance involve D1/5 receptor stimulation, as well as the NE α2 receptor actions described above. However, higher doses of these agents impaired working memory, consistent with the D1 inverted-U response. The impairment in PFC function with higher doses of stimulants observed in animals may also impede cognitive flexibility in patients with ADHD (134). The physiological recordings from monkeys described above also suggest that even lower, therapeutic doses of stimulants may constrict creativity or other tasks that require broad network inputs through D1 narrowing of cortical network inputs. On the other hand, stimulants likely enhance motivation and other striatal functions not engaged by NE medications (135). Thus, optimal medication strategies depend on individual symptoms and needs.
Conclusions
There has been remarkable progress in our understanding of PFC circuits and their relevance to ADHD. Studies of catecholamine actions on dorsolateral PFC microcircuitry have revealed intricate and powerful mechanisms to regulate the strength of persistent neuronal firing and the degree of neuronal tuning. Given the complexity and precision of these mechanisms, it is not surprising that problems with PFC regulation are prevalent in many neuropsychiatric disorders. As NE and DA mediate the strength of PFC network connections, they may be especially important in disorders where there are developmental errors in connectivity. Insight into the intracellular mechanisms underlying catecholamine actions in PFC may give rise to a deeper understanding of the etiology of ADHD and its treatment.
Acknowledgments
Much of the research cited in this review has been supported by MERIT Award AG06036 and by P50MH068789, PO1AG030004, and RL1AA017536 as part of U54RR024350, as well as the Kavli Neuroscience Institute at Yale and a National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award to AFTA.
AFTA and Yale University receive royalties from Shire Pharmaceuticals for the sale of guanfacine extended release (Intuniv) for the treatment of pediatric attention-deficit/hyperactivity disorder and related disorders. Royalties are not received for the sale of immediate release guanfacine.
References
- 1.Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. J Am Acad Child Adolesc Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnsten AF. Stress signaling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnsten AFT, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten AF, Berridge CW, Segal DS. Stress produces opioid-like effects on investigatory behavior. Pharmacol Biochem Behav. 1985;22:803–809. doi: 10.1016/0091-3057(85)90531-3. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AC, Salvia MA, Wilkinson LS, Collins P, Muir JL, Everitt BJ, Robbins TW. 6-Hydroxydopamine lesions of the prefrontal cortex in monkeys enhance performance on an analog of the Wisconsin Card Sort Test: Possible interactions with subcortical dopamine. J Neurosci. 1994;14:2531–2544. doi: 10.1523/JNEUROSCI.14-05-02531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17 suppl 1:i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
- 8.Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.08.008. XX:XXX–XXX. [DOI] [PubMed] [Google Scholar]
- 9.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 10.Fuster JM. The Prefrontal Cortex. 4th ed. San Diego: Academic Press; 2008. [Google Scholar]
- 11.Goldman-Rakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 12.Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, et al. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- 13.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 15.Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D’Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb Cortex. 2007;17 suppl 1:i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robbins TW. From arousal to cognition: The integrative position of the prefrontal cortex. Prog Brain Res. 2000;126:469–483. doi: 10.1016/S0079-6123(00)26030-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee D, Seo H. Mechanisms of reinforcement learning and decision making in the primate dorsolateral prefrontal cortex. AnnNY Acad Sci. 2007;1104:108–122. doi: 10.1196/annals.1390.007. [DOI] [PubMed] [Google Scholar]
- 18.Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J Neurosci. 2008;28:14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Hwang J, Lee D. Prefrontal coding of temporally discounted values during intertemporal choice. Neuron. 2008;59:161–172. doi: 10.1016/j.neuron.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.07.005. XX:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.07.024. XX:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.09.023. XX:XXX–XXX. [DOI] [PubMed] [Google Scholar]
- 23.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang XJ. Synaptic basis of cortical persistent activity: The importance of NMDA receptors to working memory. J Neurosci. 1999;19:9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buccafusco JJ, Terry AVJ. A reversible model of the cognitive impairment associated with schizophrenia in monkeys: Potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochem Pharmacol. 2009;78:852–862. doi: 10.1016/j.bcp.2009.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts AC. The importance of serotonin for orbitofrontal function. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.12.037. XX:XXX–XXX. [DOI] [PubMed] [Google Scholar]
- 27.Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 28.Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto M, Hikosaka O. Excitatory and inhibitory responses of midbrain dopamine neurons to cues predicting aversive stimuli. Soc Neurosci Abstr. 2008;691:24. [Google Scholar]
- 30.Roth RH, Tam S-Y, Ida Y, Yang J-X, Deutch AY. Stress and the mesocorticolimbic dopamine systems. Ann NY Acad Sci. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- 31.Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- 32.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: Effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 33.Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci. 1994;14:2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnsten AFT. Through the looking glass: Differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnsten AFT, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- 36.Li B-M, Mei Z-T. Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 37.Birnbaum SG, Gobeske KT, Auerbach J, Taylor JR, Arnsten AFT. A role for norepinephrine in stress-induced cognitive deficits: Alpha-1- adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 38.Ramos B, Colgan L, Nou E, Ovadia S, Wilson SR, Arnsten AFT. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci U S A. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gamo NJ, Wang M, Arnsten AFT. Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai JX, Ma Y, Xu L, Hu X. Reserpine impairs spatial working memory performance in monkeys: Reversal by the alpha 2-adrenergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- 42.Mao Z-M, Arnsten AFT, Li B-M. Local infusion of an alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- 43.Ramos B, Stark D, Verduzco L, van Dyck CH, Arnsten AFT. Alpha2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnsten AFT, Contant TA. Alpha-2 adrenergic agonists decrease distractibility in aged monkeys performing the delayed response task. Psychopharmacology (Berl) 1992;108:159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- 45.O’Neill J, Fitten LJ, Siembieda DW, Ortiz F, Halgren E. Effects of guanfacine on three forms of distraction in the aging macaque. Life Sci. 2000;67:877–885. doi: 10.1016/s0024-3205(00)00681-0. [DOI] [PubMed] [Google Scholar]
- 46.Jakala P, Riekkinen M, Sirvio J, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology. 1999;20:460–470. doi: 10.1016/S0893-133X(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 47.Swartz BE, McDonald CR, Patel A, Torgersen D. The effects of guanfacine on working memory performance in patients with localization-related epilepsy and healthy controls. Clin Neuropharmacol. 2008;31:251–260. doi: 10.1097/WNF.0b013e3181633461. [DOI] [PubMed] [Google Scholar]
- 48.Muller U, Clark L, Lam ML, Moore RM, Murphy CL, Richmond NK, et al. Lack of effects of guanfacine on executive and memory functions in healthy male volunteers. Psychopharmacology (Berl) 2005;182:205–213. doi: 10.1007/s00213-005-0078-4. [DOI] [PubMed] [Google Scholar]
- 49.McClure MM, Barch DM, Romero MJ, Harvey PD, Siever LJ. The effects of guanfacine on context processing abnormalities in schizotypal personality disorder. Biol Psychiatry. 2007;61:1157–1160. doi: 10.1016/j.biopsych.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Arnsten AFT, Jentsch JD. The alpha-1 adrenergic agonist, cirazoline, impairs spatial working memory performance in aged monkeys. Pharmacol Biochem Behav. 1997;58:55–59. doi: 10.1016/s0091-3057(96)00477-7. [DOI] [PubMed] [Google Scholar]
- 51.Arnsten AFT, Mathew R, Ubriani R, Taylor JR, Li B-M. Alpha-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 52.Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22:82–85. doi: 10.1097/00004714-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 53.Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson C, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 54.Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, et al. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 55.Eagle DM, Davies KR, Towse BW, Keeler JF, Theobald DE, Robbins TW. Beta-adrenoceptor-mediated action of atomoxetine during behavioral inhibition on the stop-signal task in rats. Soc Neurosci Abstr. 2010;508:510. [Google Scholar]
- 56.Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:913–923. doi: 10.1016/j.pnpbp.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang M, Ramos B, Paspalas C, Shu Y, Simen A, Duque A, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Birnbaum SB, Yuan P, Wang M, Vijayraghavan S, Bloom A, Davis D, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 59.Krowchuk Hv. Neuro-behavioral effects of childhood lead exposure. Annu Review Nurs Res. 1995;13:87–114. [PubMed] [Google Scholar]
- 60.Marcovac J, Goldstein G. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988;334:71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- 61.Li B-M, Mao Z-M, Wang M, Mei Z-T. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 62.Wang M, Tang ZX, Li BM. Enhanced visuomotor associative learning following stimulation of alpha 2A-adrenoceptors in the ventral prefrontal cortex in monkeys. Brain Res. 2004;1024:176–182. doi: 10.1016/j.brainres.2004.07.062. [DOI] [PubMed] [Google Scholar]
- 63.Steere JC, Arnsten AFT. The alpha-2A noradrenergic receptor agonist, guanfacine, improves visual object discrimination reversal performance in aged rhesus monkeys. Behav Neurosci. 1997;111:883–891. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- 64.Seu E, Lang A, Rivera RJ, Jentsch JD. Inhibition of the norepinephrine transporter improves behavioral flexibility in rats and monkeys. Psychopharmacology (Berl) 2009;202:505–519. doi: 10.1007/s00213-008-1250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Cortico-limbic function in impulsive aggressive behavior. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.02.032. XX:XXX–XXX. [DOI] [PubMed] [Google Scholar]
- 66.Macy JDJ, Beattie TA, Morgenstern SE, Arnsten AF. Use of guanfacine to control self-injurious behavior in two rhesus macaques (Macaca mulatta) and one baboon (Papio Anubis) Comp Med. 2000;50:419–425. [PubMed] [Google Scholar]
- 67.Connor DF, Findling RL, Kollins SH, Sallee F, López FA, Lyne A, Tremblay G. Effects of guanfacine extended release on oppositional symptoms in children aged 6–12 years with attention-deficit hyperactivity disorder and oppositional symptoms: A randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24:755–768. doi: 10.2165/11537790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 68.Ma C-L, Arnsten AFT, Li B-M. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Biol Psychiatry. 2005;57:192–195. doi: 10.1016/j.biopsych.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Ma C-L, Qi X-L, Peng J-Y, Li B-M. Selective deficit in no-go performance induced by blockade of prefrontal cortical alpha 2-adrenoceptors in monkeys. Neuroreport. 2003;14:1013–1016. doi: 10.1097/01.wnr.0000070831.57864.7b. [DOI] [PubMed] [Google Scholar]
- 70.Barnes JJM, Dean AJ, Nandam LS, O’Connell RG, Bellgrove MA. The molecular genetics of executive function: role of monoamine system genes. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.12.040. XX:XXX–XXX. [DOI] [PubMed] [Google Scholar]
- 71.Kopecková M, Paclt I, Goetz P. Polymorphisms of dopamine-beta-hydroxylase in ADHD children. Folia Biol (Praha) 2006;52:194–201. doi: 10.14712/fb2006052060194. [DOI] [PubMed] [Google Scholar]
- 72.Comings DE, Gade-Andavolu R, Gonzalez N, MacMurray JP. Additive effect of three noradrenergic genes (ADRA2A, ADRA2C, DBH) on attention-deficit hyperactivity disorder and learning disabilities in Tourette syndrome subjects. Clin Genet. 1999;55:160–172. doi: 10.1034/j.1399-0004.1999.550304.x. [DOI] [PubMed] [Google Scholar]
- 73.Daly G, Hawi Z, Fitzgerald M, Gill M. Mapping susceptibility loci in attention deficit hyperactivity disorder: Preferential transmission of parental alleles at DAT1, DBH and DRD5 to affected children. Mol Psychiatry. 1999;4:192–196. doi: 10.1038/sj.mp.4000510. [DOI] [PubMed] [Google Scholar]
- 74.Roman T, Schmitz M, Polanczyk GV, Eizirik M, Rohde LA, Hutz MH. Further evidence for the association between attention-deficit/hyperactivity disorder and the dopamine-beta-hydroxylase gene. Am J Med Genet. 2002;114:154–158. doi: 10.1002/ajmg.10194. [DOI] [PubMed] [Google Scholar]
- 75.Greene CM, Bellgrove MA, Gill M, Robertson IH. Noradrenergic genotype predicts lapses in sustained attention. Neuropsychologia. 2009;47:591–594. doi: 10.1016/j.neuropsychologia.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Hess C, Reif A, Strobel A, Boreatti-Hümmer A, Heine M, Lesch KP, Jacob CP. A functional dopamine-beta-hydroxylase gene promoter polymorphism is associated with impulsive personality styles, but not with affective disorders. J Neural Transm. 2009;116:121–130. doi: 10.1007/s00702-008-0138-0. [DOI] [PubMed] [Google Scholar]
- 77.Kieling C, Genro JP, Hutz MH, Rohde LA. The -1021 C/T DBH polymorphism is associated with neuropsychological performance among children and adolescents with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:485–490. doi: 10.1002/ajmg.b.30636. [DOI] [PubMed] [Google Scholar]
- 78.Roman T, Schmitz M, Polanczyk GV, Eizirik M, Rohde LA, Hutz MH. Is the alpha-2A adrenergic receptor gene (ADRA2A) associated with attention-deficit/hyperactivity disorder? Am J Med Genet B Neuropsychiatr Genet. 2003;120:116–120. doi: 10.1002/ajmg.b.20018. [DOI] [PubMed] [Google Scholar]
- 79.Park L, Nigg JT, Waldman ID, Nummy KA, Huang-Pollock C, Rappley M, Friderici KH. Association and linkage of alpha-2A adrenergic receptor gene polymorphisms with childhood ADHD. Mol Psychiatry. 2005;10:572–580. doi: 10.1038/sj.mp.4001605. [DOI] [PubMed] [Google Scholar]
- 80.Deupree JD, Smith SD, Kratochvil CJ, Bohac D, Ellis CR, Polaha J, et al. Possible involvement of alpha-2A adrenergic receptors in attention deficit hyperactivity disorder: Radioligand binding and polymorphism studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:877–884. doi: 10.1002/ajmg.b.30371. [DOI] [PubMed] [Google Scholar]
- 81.Waldman ID, Nigg JT, Gizer IR, Park L, Rappley MD, Friderici K. The adrenergic receptor alpha-2A gene (ADRA2A) and neuropsychological executive functions as putative endophenotypes for childhood ADHD. Cogn Affect Behav Neurosci. 2006;6:18–30. doi: 10.3758/cabn.6.1.18. [DOI] [PubMed] [Google Scholar]
- 82.Chamberlain SR, Robbins TW, Winder-Rhodes S, Müller U, Sahakian BJ, Blackwell AD, et al. Translational approaches to frontostriatal dysfunction in attention-deficit/hyperactivity disorder using a computerized neuropsychological battery. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.08.019. XX:XXX–XXX. [DOI] [PubMed] [Google Scholar]
- 83.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AFT, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: The prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.06.023. XX:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000;20:RC651–RC656. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nandam LS, Hester R, Wagner J, Cummins TD, Garner K, Dean AJ, et al. Methylphenidate but not atomoxetine or citalopram modulates inhibitory control and response time variability. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.11.014. [published online ahead of print December 28] [DOI] [PubMed] [Google Scholar]
- 87.Bedard AC, Ickowicz A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R. Selective inhibition in children with attention-deficit hyperactivity disorder off and on stimulant medication. J Abnorm Child Psychol. 2003;31:315–327. doi: 10.1023/a:1023285614844. [DOI] [PubMed] [Google Scholar]
- 88.Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 89.Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: Relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. doi: 10.1111/j.1469-7610.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 90.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 91.Chamberlain SR, Muller U, Blackwell AD, Clark L, Robbins TW, Sahakian BJ. Neurochemical modulation of response inhibition and probabilistic learning in humans. Science. 2006;311:861–863. doi: 10.1126/science.1121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chamberlain SR, Del Campo N, Dowson J, Müller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Chamberlain SR, Hampshire A, Müller U, Rubia K, Del Campo N, Craig K, et al. Atomoxetine modulates right inferior frontal activation during inhibitory control: A pharmacological functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 94.Graf H, Abler B, Freudenmann R, Beschoner P, Schaeffeler E, Spitzer M, et al. Neural correlates of error monitoring modulated by atomoxetine in healthy volunteers. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.10.018. [published online ahead of print December 16] [DOI] [PubMed] [Google Scholar]
- 95.Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–1074. doi: 10.1176/appi.ajp.158.7.1067. [DOI] [PubMed] [Google Scholar]
- 96.Walker SC, Robbins TW, Roberts AC. Differential contributions of dopamine and serotonin to orbitofrontal cortex function in the marmoset. Cereb Cortex. 2009;19:889–898. doi: 10.1093/cercor/bhn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lidow MS, Wang F, Cao Y, Goldman-Rakic PS. Layer V neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse. 1998;28:10–20. doi: 10.1002/(SICI)1098-2396(199801)28:1<10::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 99.Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: Predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci U S A. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arnsten AFT, Vijayraghavan S, Wang M, Gamo NJ, Paspalas CD. Dopamine’s influence on prefrontal cortical cognition: Actions and circuits in behaving primates. In: Bjorklund A, Dunnett S, Iversen L, Iversen S, editors. Dopamine Handbook. Oxford, UK: Oxford University Press; 2009. pp. 230–249. [Google Scholar]
- 101.Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J Neurosci. 2004;24:5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paspalas CD, Rakic P, Goldman-Rakic PS. Internalization of D2 dopamine receptors is clathrin-dependent and select to dendro-axonic appositions in primate prefrontal cortex. Eur J Neurosci. 2006;24:1395–1403. doi: 10.1111/j.1460-9568.2006.05023.x. [DOI] [PubMed] [Google Scholar]
- 103.Mrzljak L, Bergson C, Pappy M, Levenson R, Huff R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- 104.Van Tol HHM, Bunzow JR, Guan H-C, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 105.Gibbs SE, D’Esposito M. A functional magnetic resonance imaging study of the effects of pergolide, a dopamine receptor agonist, on component processes of working memory. Neuroscience. 2006;139:359–371. doi: 10.1016/j.neuroscience.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 106.Arnsten AFT, Goldman-Rakic PS. Stress impairs prefrontal cortex cognitive function in monkeys: Role of dopamine. Soc Neurosci Abstr. 1990;16:164. [Google Scholar]
- 107.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 108.Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 110.Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- 111.Penit-Soria J, Audinat E, Crepel F. Excitation of rat prefrontal cortical neurons by dopamine: An in vitro electrophysiological study. Brain Res. 1987;425:263–274. doi: 10.1016/0006-8993(87)90509-9. [DOI] [PubMed] [Google Scholar]
- 112.Yang CR, Seamans JK. Dopamine D1 receptor actions in layers V–VI rat prefrontal cortex neurons in vitro: Modulation of dendriticsomatic signal integration. J Neurosci. 1996;16:1922–1935. doi: 10.1523/JNEUROSCI.16-05-01922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci U S A. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gorelova NA, Yang CR. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol. 2000;84:75–87. doi: 10.1152/jn.2000.84.1.75. [DOI] [PubMed] [Google Scholar]
- 115.Franceschetti S, Taverna S, Sancini G, Panzica F, Lombardi R, Avanzini G. Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurones. J Physiol. 2000;528:291–304. doi: 10.1111/j.1469-7793.2000.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gonzalez-Islas C, Hablitz JJ. Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci. 2003;23:867–875. doi: 10.1523/JNEUROSCI.23-03-00867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cools R, D’Esposito M. Inverted-U shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.03.028. XX:XXX–XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gorelova NA, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 119.Kroner S, Krimer LS, Lewis DA, , Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- 120.Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci U S A. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Crofts HS, Dalley JW, Collins P, van Denderen JCM, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 122.Lidow M, Goldman-Rakic P, Rakic P, Innis R. DopamineD2receptors in the cerebral cortex: Distribution and pharmacological characterization with [3H]raclopride. Proc Natl Acad Sci U S A. 1989;86:6412–6416. doi: 10.1073/pnas.86.16.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- 124.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 125.Gibbs SE, D’Esposito M. A functional MRI study of the effects of bromocriptine, a dopamine receptor agonist, on component processes of working memory. Psychopharmacology (Berl) 2005;180:644–653. doi: 10.1007/s00213-005-0077-5. [DOI] [PubMed] [Google Scholar]
- 126.Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 127.Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci. 2008;31:317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- 129.Arnsten AFT, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: The effects of quinpirole on memory and motor performance in monkeys. J Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hannan MA, Kabbani N, Paspalas CD, Levenson R. Interaction with dopamine D2 receptor enhances expression of transient receptor potential channel 1 at the cell surface. Biochim Biophys Acta. 2008;1778:974–982. doi: 10.1016/j.bbamem.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: Results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 133.Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: New model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 134.Tannock R, Schachar R. Methylphenidate and cognitive perseveration in hyperactive children. J Child Psychol Psychiatry. 1992;33:1217–1228. doi: 10.1111/j.1469-7610.1992.tb00940.x. [DOI] [PubMed] [Google Scholar]
- 135.Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 136.Arnsten AFT, Cai JX, Goldman-Rakic PS. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: Evidence for alpha-2 receptor subtypes. J Neurosci. 1988;8:4287–4298. doi: 10.1523/JNEUROSCI.08-11-04287.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav. 1996;55:415–422. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- 138.Clerkin SM, Schulz KP, Halperin JM, Newcorn JH, Ivanov I, Tang CY, Fan J. Guanfacine potentiates the activation of prefrontal cortex evoked by warning signals. Biol Psychiatry. 2009;66:307–312. doi: 10.1016/j.biopsych.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taylor FB, Russo J. Comparing guanfacine and dextroamphetamine for the treatment of adult attention deficit-hyperactivity disorder. J Clin Psychopharmacol. 2001;21:223–228. doi: 10.1097/00004714-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 140.Jakala P, Sirvio J, Riekkinen M, Koivisto E, Kejonen K, Vanhanen M, Riekkinen P., Jr Guanfacine and clonidine, alpha 2-agonists, improve paired associates learning, but not delayed matching to sample, in humans. Neuropsychopharmacology. 1999;20:119–130. doi: 10.1016/S0893-133X(98)00055-4. [DOI] [PubMed] [Google Scholar]