Abstract
For prostate cancer patients, online image-guided (IG) radiotherapy has been widely used in clinic to correct the translational inter-fractional motion at each treatment fraction. For uncertainties that cannot be corrected online, such as rotation and deformation of the target volume, margins are still required to be added to the clinical target volume (CTV) for the treatment planning. Offline adaptive radiotherapy has been implemented to optimize the treatment for each individual patient based on the measurements at early stages of treatment process. It has been shown that offline adaptive radiotherapy can effectively reduce the required margin. Recently a hybrid strategy of offline adaptive replanning and online IG was proposed and the geometric evaluation was performed. It was found that the planning margins can be further reduced by 1–2 mm compared to online IG only strategy. The purpose of this study was to investigate the dosimetric benefits of such hybrid strategy on the target and organs at risk (OARs). A total of 420 repeated helical computed tomography (HCT) scans from 28 patients were included in the study. Both low-risk patients (LRP, CTV = prostate) and intermediate-risk patients (IRP, CTV = prostate + seminal vesicles, SV) were included in the simulation. Two registration methods, based on center-of-mass (COM) shift of prostate only and prostate plus SV, were performed for IRP. The intensity modulated radiotherapy (IMRT) was used in the simulation. Criteria on both cumulative dose and fractional doses were evaluated. Furthermore, the geometric evaluation was extended to investigate the optimal number of fractions necessary to construct the internal target volume (ITV) for the hybrid strategy. The dosimetric margin improvement was smaller than its geometric counterpart and was in the range of 0 mm to 1 mm. The optimal number of fractions necessary for the ITV construction is 2 for LRP and 3–4 for IRP in a hypofractionation protocol. A new cumulative index of target volume (CITV) was proposed for the evaluation of adaptive radiotherapy strategies, and it was found that it had the advantages over other indices in evaluating different adaptive radiotherapy strategies.
1. Introduction
External beam radiotherapy is one of the major treatment modalities for localized prostate cancer. Since the treatment course typically consists of many fractions and lasts several weeks, a major concern is the uncertainties from setup errors, inter-fractional and intra-fractional motion of prostate and organs at risk (OAR). If not taken into account properly, those uncertainties could produce insufficient dose coverage to the target and over-dose to the adjacent normal tissues and OARs.
The setup error correction has been covered by many studies (Stroom et al., 1999; van Herk et al., 2002), and can be implemented through either online or offline image guidance (IG) (Wu et al., 2006). In principle, online IG can correct both the random and systematic errors, but it may prolong the treatment time considerably. Offline correction can eliminate or significantly reduce the systematic setup error (Yan et al., 2000; Bel et al., 1996; Amer et al., 2001; de Boer and Heijmen, 2001) without adversely affecting treatment time at each fraction. However, the random setup error and the residuals have to be compensated by margins. In comparison, uncertainties caused by prostate motion are more complicated. Similar to setup error correction, both inter-fractional and intra-fractional motions can be managed through online corrections (Balter et al., 1995; Langen et al., 2008; Kitamura et al., 2002), or offline adaptive strategies (Yan et al., 2000; Adamson and Wu, 2010).
With the recent development of image guidance systems, many forms of image guidance techniques have been implemented. Image guidance, whether online or offline, can reduce different types of the uncertainties and allows significant reductions of the necessary margins. This in turn reduces the doses to the normal tissues and OARs while the dose coverage to the target is maintained. In general, the online IG uses population based margins and the offline IG uses patient-specific margins.
One advantage of the online IG over offline IG is that the setup error and translational organ motion are eliminated online. In offline IG, only the systematic components are removed. This can radically reduce the required margin in the treatment planning (Wu et al., 2006; Liang et al., 2009). For those uncertainties that cannot be accounted for by online IG, margins are still needed. However, the required margin is usually smaller than those by the offline IG. This is also the common form of IG implemented in many clinics. One common question is on the comparison of different IG strategies on some practical issues such as the efforts involved and the changes in the clinical workflow, as compared to the conventional radiotherapy without image guidance. With online IG only, there is no additional planning effort required. However, in each treatment session, additional procedures for image acquisition, registration, patient positioning correction and verification need to be added. They may prolong the treatment session time by a few minutes, which can add up considerably when all fractions are included, therefore, it is suitable for hypro-fractionated treatment. In contrast, the offline IG only will not add any burden to the online treatment process. All analysis and replanning are performed offline, this additional planning efforts need to be considered.
An interesting question is whether offline IG is necessary or how much improvement in margins it can achieve after online IG is used. In a recent study (Lei and Wu, 2010), cone-beam computed tomography (CBCT) based online IG patient data were analyzed. It was shown through statistical analyses that the target rotations, which were not commonly corrected in clinic during online IG, were systematic (i.e., different from zero); patient-specific (magnitude depends on the patient); persistent through the treatment fractions (i.e., if they exist before the treatment, they likely stay that after the treatment at each fraction). The rotations measured at early fractions were also representative of those at late fractions. In addition, a hybrid strategy combining online IG and offline adaptive replanning was proposed in that study and the benefit was quantitatively evaluated geometrically. Two residual uncertainties from online IG only, namely rotations and deformations, could be potentially accounted for in the hybrid technique. However, margin is still required to account for uncertainty from intra-fractional motion. Compared with online IG only strategy, the hybrid method is more complex. However, all the extra efforts and time (contouring, replanning, etc.) are spent offline, so there is no degradation on the online treatment efficiency, and it does not change the intra-fractional characteristics. It was found that the hybrid strategy was better than the standard online IG alone with further margin reductions of 1 mm for low-risk patients (LRP) and 2 mm for intermediate-risk patients (IRP). One shortcoming of that study is that the margin evaluation was limited in the geometric domain. The margin in radiation therapy is a dosimetric concept, and the benefit of the hybrid strategy should also be evaluated dosimetrically, which is more complex.
This study was divided into three parts. First, dosimetric evaluation of margin benefit was performed for the hybrid strategy. Both cumulative and daily treatment dose distribution were investigated for LRP and IRP. Second, the hybrid strategy was optimized by searching for the optimal number of the early fractions to determine the patient-specific plan. Lastly, a new index based on cumulative target volume was proposed to evaluate different IG strategies and it was compared with other indices. The combination of geometric and dosimetric evaluation allows us to have an overall picture of the benefit of hybrid strategy.
2. Methods and Materials
2.1. Patient data and treatment planning
Twenty-eight prostate cancer patients with repeated helical computed tomography (HCT) images of the pelvic region were included in this study. Each patient had at least 1 planning HCT scan and 15 HCT scans with a conventional helical scanner during treatment course with 3 mm slice thickness. There was about a one week time interval between the planning CT and the first treatment fraction, and the last (15-th) treatment CT images were acquired between 49 and 70 days after the planning CT, with a mean value of 53.1 ± 4.7 days. All CT images were transferred to the treatment planning system (Pinnacle v8.0, Philips Radiation Oncology System, Madison, WI, USA) for further processing. To remove the setup error, all treatment CTs of the same patient were rigidly registered to the planning CT based on the bony structures. The contours for prostate, seminal vesicles (SVs), bladder and rectum were then delineated by experienced planners for each CT. The differences between the contours of the same organ on different days thus represent the inter-fraction motion (rigid and non-rigid) only. An alternative is to perform the contouring on each CT before bony registration. As explained later, the way of data preparation of these data does not affect the result. The treatment for both LRP and IRP were simulated. The clinical target volume (CTV) was prostate gland only for LRP, prostate plus seminal vesicles for IRP. Seven patients were excluded from the IRP analyses due to incomplete deformation data for seminal vesicles.
The original HCT image had the resolution of ~ 1 mm × 1 mm × 3 mm. While this is adequate for the treatment planning, the asymmetry in resolution among different axes can result in the loss of accuracy in the simulation of the image guidance, and complicated workaround had to be implemented (Liang et al., 2009). To circumvent this deficiency, we interpolated both CT images and contours in the superior-inferior direction (Lei and Wu, 2010), so the precision becomes ~ 1 mm in all directions. This is also the precision that can be achieved by typical image guidance systems, such as the treatment couch. All the following studies were based on the interpolated images and contours.
A hypofractionation protocol was used to simulate the treatment. The prescription dose was 3.9 Gy per fraction to the isocenter (in this study isocenter is the center of the CTV) over 15 fractions, for a total dose of 58.5 Gy. A five-field 15 MV photon beam arrangement was used for the intensity modulated radiotherapy (IMRT) plan, with gantry angles at 15, 90, 150, 220, 300 degree. A standard set of optimization objectives and constrains were used for all patients. The inverse planning objectives included the requirements of dose-volume-histograms (DVHs) for the planning target volume (PTV), bladder and rectum.
2.2. Online image guidance and offline adaptive planning
For simplicity the online IG was simulated as a translation of the center-of-mass (COM) of the CTVs in the treatment CT to that in the planning CT. It was found that results from COM method and normalized cross-correlation method, a more sophisticated and accurate technique for image registration within the same image modality, were very close (Liang et al., 2009). Since the CTV COM is determined from the contours, the method used in the data preparation has no effect on the results. For LRP, the online IG was simulated by matching the COM of the prostate for each treatment CT with those from the planning CT. For IRP, the CTV includes both the prostate gland and seminal vesicles, online correction becomes more challenging due to the independent motion of seminal vesicle relative to prostate. Similar to a previous study (Liang et al., 2009), two registration methods were simulated for IRP: registration based on entire CTV (prostate plus SVs, Type A), and prostate only (Type B). Registrations were limited to translations only in this study. Type A was used to simulate ideal registration assuming the entire CTVs are visible in the treatment CT images. Type B was used to simulate online image guidance based on implanted markers in prostate gland. These two types of registrations are illustrated in Figure 1. The slice was chosen to show both prostate and seminal vesicles, therefore, only the base of the prostate is shown. Typically, prostate gland has larger volume than seminal vesicles.
Figure 1.
Simulation of online image guidance on one axial helical CT slice for an intermediate-risk patient. (a) before online correction. Yellow contour is the CTV0 in reference planning CT, Red contour is the treatment CTVi. (b) after online correction based on COM. Yellow contour is the reference CTV0, Blue is the treatment CTVi after Type A registration, and Red is the treatment CTV after Type B registration. Red and Blue contours are slightly different from the CTVi in (a) because the registration were in 3D.
In the simulation of offline IG, the first five treatment fractions were used to construct a new target volume, the internal target volume (ITV), for each patient:
| (1) |
where TMi is the translational transformation matrix used in online IG of the i-th treatment fraction, i.e., the translation of the CTV COM; CTVi is CTV at the i-th fraction. The ITV concept was originally proposed to account for the intra-fractional respiratory motion, here it is used to account for the interfractional motion. Without the TMi, this formula becomes the one for pure offline IG. If no bony registrations were performed in the initial data preparation before the contouring, then the unions of these CTVi will include the setup errors, therefore will not reflect the setup correction procedure in the pure offline process. The ITV constructed in (1) did not include the initial planning CTV0, as was done in the standard offline IG. The reason for this was that CTV0 may not be a good representation of CTVs during the treatment course, the CTV0 was acquired about two weeks before the treatment, and some special procedures may be used in the acquisition of the CT0, such as contrast and enema. All these may cause the shape of CTV0 to be quite different from treatment CTVs. In our simulation, all treatment CTs were acquired on helical CT scanner with same quality as the initial planning CT. The construction of the ITV is illustrated in Figure 2.
Figure 2.
Construction of ITV5 on an axial CT slice for intermediate-risk patient. Dark blue is the ITV5, which is the composite of CTV from first five fractions. Red, purple, skyblue, green are subsequent CTVi in treatment CTs after online correction.
2.3. Dosimetric evaluation
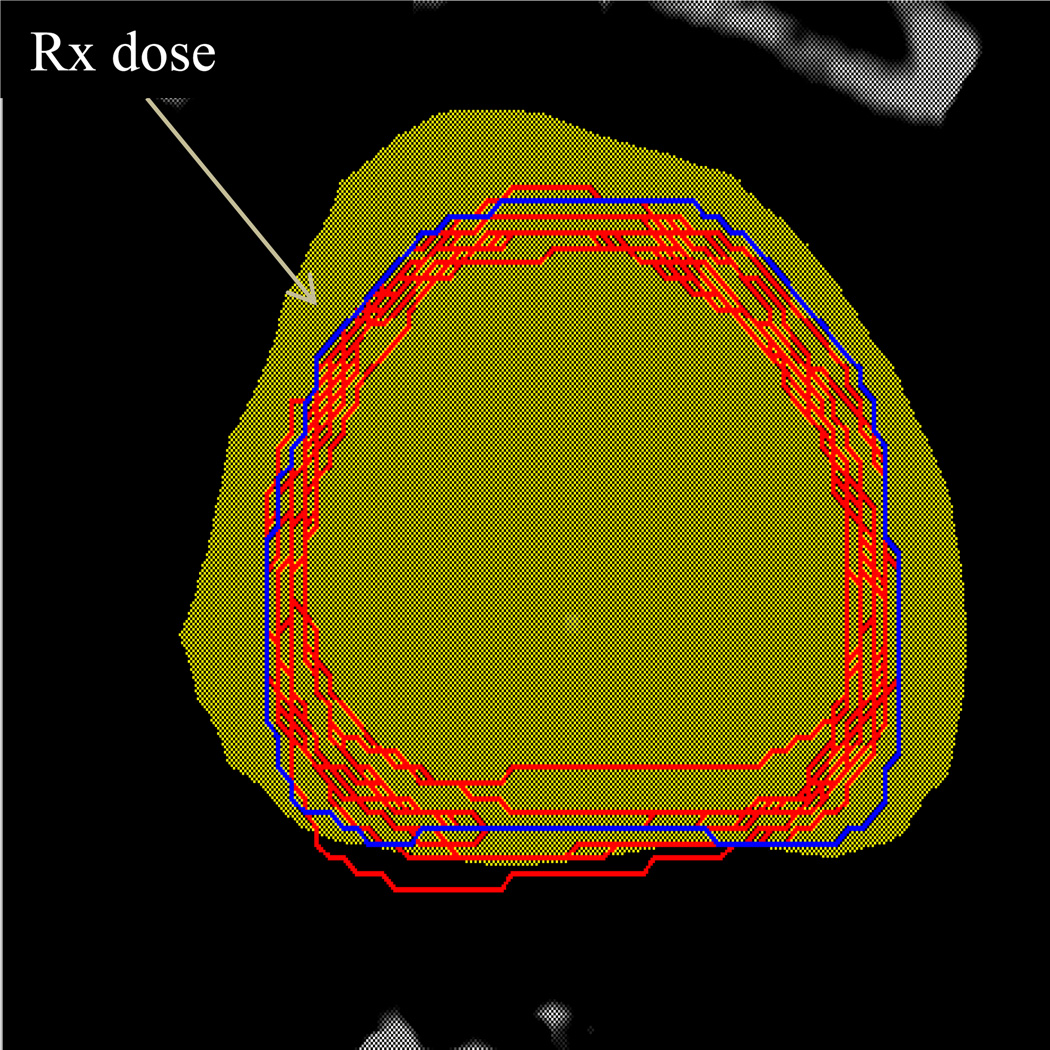
In this study, a uniform margin in all directions in the range of 0 to 5 mm with increment of 1 mm was added to the CTV0 to form the PTV for online IG only strategy. The CT image has the resolution of approximately 1 mm in all directions; an increment less than 1 mm will not generate meaningful results in the treatment planning system. Instead, the results were interpolated afterwards. In the standard offline IG, a population averaged margin was used to expand the initial CTV0 to the PTV for the first few treatment fractions. The setup errors and inter-fractional motion were measured and analyzed; a patient-specific margin was obtained for replanning for later treatment fractions. Figure 3 shows the typical dose distribution for a low-risk patient.
Figure 3.
Plan isodose distribution with zero ITV-to-PTV margin for one low-risk patient. Shaded areas are the 100% of prescription dose. Dark blue contour is the ITV5, Red contours are subsequent CTVi in treatment CTs after online correction.
To obtain the dose distribution at each fraction for each patient, the isocenter of the treatment plan was shifted based on the online correction, and the dose was recomputed. Two types of dose evaluations, daily and cumulative, were performed. For the cumulative dose comparison, in order to account for inter-fractional motion and shape changes, a deformable registration algorithm based on a finite element method was performed, and doses for target and critical organs (bladder and rectal wall) were accumulated (Yan et al., 1999; Wu et al., 2006). For daily dose comparison, deformation results were not necessary. The DVHs were generated for daily and cumulative dose distributions for target and critical organs. The dosimetric index used for the evaluation was D99 (dose to 99% of the volume) for CTV, and generalized equivalent uniform dose (gEUD) for OAR. The parameter of the gEUD was chosen as 10 for both bladder and rectal wall (Wu et al., 2002).
The dose evaluation was performed for both online IG and hybrid strategy. The optimal margins required depend on the criteria used. Various CTV(ITV)-to-PTV margins were studied with 1mm increment, and the minimum margin values were sought to satisfy this predefined criteria: the cumulative D99 (a representation of minimum dose) of the CTV should not be less than the D99 of the PTV in the original plan. The dose variation is defined as:
| (2) |
For target, the cumulative dose was calculated for the CTV, and the planning dose was for the PTV. For bladder and rectal wall, both the cumulative and planning doses were computed for the same organ. In order to do a fair comparison of the dose variation for bladder and rectal wall for different strategies, gEUD values were normalized as ratio to original plan of optimal margin of online IG.
2.4. Optimal number of treatment fractions for ITV construction in hybrid strategy
With a pure offline IG protocol, it was found that the optimal number of fractions required to achieve the specified dosimetric criterion is 4–5 (Yan et al., 2000). Similar conclusions were reached by using a simple statistical models (Bortfeld et al., 2002), and with a method based on principal component analysis (Sohn et al., 2005). Since the main advantage of the hybrid over online IG only strategy is its capability of handling deformation, and also the number of total treatment fractions is quite different for hypofractionation and standard fractionated radiation therapy, an interesting question is how many fractions are necessary for the ITV construction under a hypofractionation protocol for the hybrid strategy. More measurements from early fractions will result in better estimate of the ITV and subject the patient to treat with relative large margins at the early treatment course, and too few measurements will result in less accurate estimate of the ITV. The first five fractions was used in the proposal of hybrid strategy to investigate the margin benefit geometrically (Lei and Wu, 2010). In order to find the optimal number of fractions needed in the hybrid strategy, similar geometric analysis was performed with varied number n of treatment fractions to construct the ITV:
| (3) |
The procedure of the evaluation was described in detail elsewhere (Lei and Wu, 2010). Here only a brief summary is given. The benefit in geometric margin reduction was similarly compared to the standard online IG only strategy. For a fair comparison, the volume difference of ITVn and CTV0 was taken into account by defining the equivalent margin, M0, for ITVn and CTV0: Volume(ITVn) = Volume(CTV0+M0). The volume overlap index (OI) was used for the evaluation and a range of uniform margins were studied. The margins required for ITVn and CTV0 (MI,0.99 and MC,0.99) to achieve 99% of average overlap index were interpolated from the relations between the overlap index and margin added. Then the equivalent margin difference ΔM can be obtained as the difference between MC,0.99 and MI,0.99 after subtracting M0: ΔM = MC,OIi −MI,OIi −M0.
2.5. Cumulative index of target volume to evaluate IGRT strategy
As mentioned above, it can be quite cumbersome to perform a fair comparison between different IG strategies. Comparing the margins alone can lead to incorrect impression because the volume of the ITV is different from the CTV0 and is usually larger. For a fair comparison, the M0 needs to be included. We propose a new index in this study, cumulative index for target volume, or
| (4) |
where N is the total number of treatment fractions. For online IG, let M1 be the margin required to achieve OI = 99%, the PTV is constructed by expanding the CTV0 with margin M1: PTV = CTV0 + M1. The cumulative index of target volume thus becomes:
| (5) |
Similarly, for the hybrid strategy, we have PTV1 = CTV0 + M1 for i ≤ n (n=2,3,4,5 is the number of fractions used to construct the ITV) and PTV2 = ITVn + M2 for i > n. The CITV can be written as
| (6) |
M2 is the margin required to obtain OI = 99% for the hybrid strategy.
3. Results
3.1. Dosimetric evaluation of the hybrid strategy
The average dose differences between the cumulative and the initial plan as a function of CTV(ITV)-to-PTV margin are listed in Table 1 for both online IG and hybrid strategies. The criterion in this study was the cumulative D99 of the CTV to be greater than the D99 of the PTV in the original plan.
Table 1.
Average dose differences (percent) between the cumulative dose and initial IMRT plan as a function of CTV(ITV)-to-PTV margin for low-risk and intermediate-risk patients. Abbreviations: CTV = clinical target volume; ITV = internal target volume; PTV = planning target volume; Online IG = Online image guidance only strategy; hybrid = online image guidance and offline adaptive replanning strategy; LRP = Low-risk patients; IRP(A) = Intermediate-risk patients with online IG based on prostate + SVs; IRP(B) = Intermediate-risk patients with online IG based on prostate only; D99 = dose to 99% of the volume, a representation of minimum dose. The criterion is the cumulative D99 of the CTV should not be less than the D99 of the PTV in the original plan.
| Group | Dose index | Margin (mm) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| LRP | Online IG δD99 (%) | −2.2±3.4 | −0.2±2.0 | 1.0±1.6 | 2.2±1.2 | 2.8±1.4 | 3.5±1.7 |
| Hybrid δD99 (%) | 0.7±1.3 | 2.0±1.6 | 2.9±1.7 | 3.8±2.0 | 4.3±2.2 | 4.8±2.5 | |
| IRP(A) | Online IG δD99 (%) | −4.3±3.6 | −1.0±2.5 | 0.9±2.1 | 2.7±1.3 | 3.9±1.1 | 5.1±1.3 |
| Hybrid δD99 (%) | 1.0±1.9 | 2.9±1.8 | 4.0±1.7 | 5.3±1.8 | 6.4±2.1 | 7.1±2.2 | |
| IRP(B) | Online IG δD99 (%) | −6.0±4.7 | −2.3±3.3 | −0.2±3.0 | 2.0±2.1 | 3.3±1.5 | 4.7±1.3 |
| Hybrid δD99 (%) | −0.2±1.6 | 2.1±1.6 | 3.4±1.4 | 4.4±1.4 | 5.6±1.5 | 6.4±2.0 | |
At small margins, the average dose variations on D99 were negative for online IG. Since the positioning uncertainties were assumed to be removed by the online IG with COM shift, the main reason led to negative values was due to rotations, deformations or other factors. As the margin increased, the dose variation on D99 turned toward positive values.
With the hybrid strategy, dose coverage for the target improved significantly. For example, at zero margin for LRP, the average dose variation on D99 improved from (−2.2±3.4) % for Online IG to (0.7±1.2) % for the hybrid strategy (p < 0.00001 from paired Student’s t-test). As margin increase, the magnitude of improvement decreases as expected.
For IRP, two types of online IG were simulated: entire CTV (Type A) and prostate alone (Type B). The number of patients failed the criteria, Nfailed, is in general less for Type A than for Type B registration. This indicates that Type A registration is better than Type B registration, and Margin A is also better. This agrees with our previous study (Liang et al., 2009).
To obtain a meaningful margin, Figure 4 shows the criteria expressed as pass rate or confidence level as a function of the required margin for different groups of patients and registrations for both online IG and hybrid strategies. The dosimetric margins required can be easily obtained through the interpolation from this figure. For example, at 95% confidence level, i.e., only 5% of patients are allowed to fail the criteria, for IRP with Type A registration, a 2.8 mm margin is required for online IG; and 1 mm margin is required for hybrid strategy. In this study, the recommended margins were chosen based on 100% pass rate.
Figure 4.
Pass rate (in percent) as a function of required margin. (a) low-risk patients, (b) intermediate-risk patients with Type A registration, and (c) intermediate-risk patients with Type B registration.
In simulations of the hybrid strategy, two types of margins were used in the first 5 fractions: (1) a fixed value taken from the recommended margins obtained from online IG strategy and (2) the same value for all fractions. As expected, the number of patients failed the criteria is fewer in margin (1) than (2) for smaller margins. However, the difference diminishes as the margin increases. The recommended margin for (2) is slightly larger. Therefore, only the margins (2) were presented in this paper.
To compare the doses to bladder and rectal wall between the hybrid strategy and Online IG, plans at recommended margins obtained from the above dosimetric evaluation of the target were used. In this study all patients were required to pass the pre-defined criteria. In order to have a fair comparison of different image guidance strategies, the optimized margin of online IG only strategy (3mm for LRP and IRP(Type A), 4mm for IRP(Type B)) was used as the reference. As shown in Figure 5, the gEUD values for each specific IG strategy at its recommended margins were expressed as a ratio of cumulative to the corresponding values in the reference plan. The paired student’s t-tests were applied to evaluate the significance of the comparison. The doses to bladder and rectal wall were significantly lower in the hybrid strategy than online IG for LRP with p < 0.05. For IRP, statistically significant difference only exists in the doses to rectal wall for IRP(Type B) between online IG and the hybrid strategy.
Figure 5.
Dose indices (gEUD) of critical organs for online IG and hybrid strategies. The gEUD values are expressed as a ratio of cumulative dose to plan dose at optimized margin for online IG. (a) bladder; (b) rectal wall.
In addition to the cumulative dose comparison, the evaluation was also performed based on daily dose information. Daily DVHs were generated directly from the planning system, and deformation registration was not required for this study. The total number of treatment fractions for all patients was 420 for LRP and 315 for IRP. The results obtained depend on dosimetric criteria, which is different from those for cumulative doses. Here, we chose D99 of CTV for each treatment fraction should not be less than 98% of PTV D99 in the original plan. The percentage of treatment fractions failed the pre-defined criteria is shown as a function of margin in Figure 6. For example, at a 95% confidence level for LRP, no margin is required for hybrid strategy and 3 mm is necessary for online IG only strategy.
Figure 6.
The percentage of fractions that failed the criteria of 2% PTV dose reduction of CTV0 and ITV5 as a function of the margin for low-risk and intermediate-risk patients.
3.2. Geometric evaluation of the hybrid strategy of online and offline image guidance
The margins required to have an average overlap index OI = 0.99 can be calculated from the relationship between the OI and margins added to the CTV (or ITV), and they are summarized in Table 2 for both LRP and IRP. Also shown in this table are the average equivalent margins M0 for CTV0 to have the equal volume as ITVn, and the corresponding geometric margin benefit ΔM for the hybrid strategy over online IG only strategy. With the hybrid strategy, the planning margins can be reduced for both LRP and IRP, compared with the standard online IG only strategy. For IRP, margin benefit ΔM is smaller for Type A registration than Type B registration, which indicates online correction based on the entire CTV is better. The trend of ΔM change as a function of n also suggests that the optimal number of fractions for the ITV construction is 2 for LRP and 3–4 for IRP.
Table 2.
The uniform margins needed to obtain an OIi of 0.99 for CTV0 (MC,0.99) and ITVn (MI,0.99), the equivalent uniform margin M0 for CTV0 to have the equal volume as ITVn, and the corresponding equivalent margin differences ΔM.
| Group | No. of fractions | MC,0.99 (mm) | MI,0.99 (mm) | M0 (mm) | ΔM (mm) |
|---|---|---|---|---|---|
| LRP | 2 | 2.6 ± 0.9 | 1.7 ± 0.6 | 0.2 ± 0.5 | 0.7 ± 0.5 |
| 3 | 2.6 ± 0.9 | 1.1 ± 0.5 | 0.5 ± 0.6 | 1.0 ± 0.6 | |
| 4 | 2.6 ± 0.9 | 0.9 ± 0.5 | 0.7 ± 0.6 | 1.0 ± 0.6 | |
| 5 | 2.5 ± 0.9 | 0.6 ± 0.5 | 0.9 ± 0.6 | 1.0 ± 0.7 | |
| IRP(A) | 2 | 4.1 ± 1.7 | 2.8 ± 1.3 | 0.3 ± 0.5 | 0.9 ± 1.2 |
| 3 | 4.1 ± 1.7 | 1.9 ± 1.0 | 0.7 ± 0.6 | 1.5 ± 1.0 | |
| 4 | 4.0 ± 1.5 | 1.5 ± 0.9 | 0.9 ± 0.7 | 1.6 ± 1.0 | |
| 5 | 4.0 ± 1.7 | 1.1 ± 0.6 | 1.1 ± 0.7 | 1.7 ± 1.0 | |
| IRP(B) | 2 | 4.4 ± 1.7 | 3.2 ± 1.4 | 0.3 ± 0.6 | 1.0 ± 1.3 |
| 3 | 4.4 ± 1.7 | 2.1 ± 1.0 | 0.7 ± 0.6 | 1.6 ± 1.0 | |
| 4 | 4.4 ± 1.7 | 1.7 ± 0.9 | 1.0 ± 0.7 | 1.8 ± 1.1 | |
| 5 | 4.3 ± 1.7 | 1.3 ± 0.8 | 1.2 ± 0.7 | 1.8 ± 1.1 |
The average volumes for CTV0 are (48.7 ± 19.3) cc for LRP and (67.7 ± 21.7) cc for IRP. Figure 7 shows the average volumes of CTV0, ITVn (n=2,3,4,5), and also the PTV volumes with recommended margin. Since the ITV was constructed by union of first few treatment fractions, the average volume of ITVn is about 20% larger than CTV0 volume. The ITV volumes increase with the increasing number of fractions used in the ITV construction. Even though the volume of the ITV is larger than the volume of the CTV0, the PTV constructed from the ITV is significantly smaller. One way repeated measure ANOVA shows that the PTV volumes are significantly different between PTVCTV0 and PTVITVn as a function of number of fractions, both LRP and IRP saturates at n=4. This implies that the total irradiated target volumes with the hybrid strategy are smaller than the online IG only strategy. We noticed that the average volumes of PTV were not the same for Type A and Type B registrations of IRP for online IG strategy. This was due to the fact that margins required to achieve 99% of overlap index were slightly different for Type A and Type B registrations.
Figure 7.
The average volumes and the margin added volumes of CTV0, ITVn (n=2,3,4,5) versus number of fractions to construct the ITVn. (a) Low-risk patients; (b) Intermediate-risk patients with Type A registration; (c) Intermediate-risk patients with Type B registration. The symbols on the left are for online IG and the rest for hybrid.
The cumulative index of target volume is shown in Figure 8 for both online IG and hybrid strategies for both groups of patients. The CITV for hybrid is less than that of online IG for both LRP and IRP, indicating that the hybrid strategy is better than the online IG. For same n with hybrid strategy, CITV(IRP, Type B) > CITV(IRP, Type A) > CITV(LRP), which means cumulative relative target volumes get irradiated are smaller for LRP compare to that of IRP, primarily due to the irregular shape of the CTV in IRP. In addition, Type A registration is significantly better than Type B for IRP with lower CITV values (student t-test p < 0.003). One way repeated measure ANOVA shows that there are significant differences in the CITV values between online IG and hybrid strategies. However, as the n increase, the improvement diminishes. The optimal n is 2 for LRP, and 3 for IRP with both Type A and B registrations.
Figure 8.
Cumulative index of target volume (CITV) for online IG and hybrid strategies. The symbols on the left are for online IG and the rest for hybrid.
4. Discussions and Conclusions
4.1. Dosimetric evaluation
In this study, we investigated the dosimetric benefits of the hybrid strategy combining online image guidance and offline adaptive replanning for prostate cancer radiotherapy, specifically the margin reductions over the online image guidance only protocol. In order to conduct a fair comparison between the online IG only and hybrid strategies, an additional equivalent margin needs to be taken into consideration for the volume difference of CTV0 and ITV. An estimate can be made from the M0 from Table 2. For example, if a confidence level of 95% is specified (only 5% of patients were allowed to fail the criteria) for LRP, then a 2.8 mm margin is required for online IG only strategy, and 1.3 mm margin is necessary for the hybrid strategy (From Figure 4). By subtracting the additional margin of 0.9 mm come from the volume difference of CTV0 and ITV, the dosimetric margin benefit of hybrid over online IG only strategy is 0.6 mm. The dosimetric margin benefits of hybrid over online IG only strategy are summarized in Table 4 for LRP and IRP at confidence levels of 95% and 100%.
Table 4.
Dosimetric margin benefit of the hybrid compare with online IG only strategy. LRP = Low-risk patients; IRP(A) = Intermediate-risk patients with prostate + SV registration; IRP(B) = Intermediate-risk patients with prostate only registration. The criterion is cumulative D99 of the clinical target volume to be more than the D99 of planning target volume in the plan.
| Group | Confidence Level | ΔM (mm) |
|---|---|---|
| LRP | 95% | 0.6 |
| 100% | 0.1 | |
| IRP (A) | 95% | 0.8 |
| 100% | 0.0 | |
| IRP (B) | 95% | 1.0 |
| 100% | 0.8 |
The margin in radiation therapy is a complicated and basically a dosimetric concept (Langen and Jones, 2001). While the dosimetric evaluation is in principle more important than the geometric evaluation, it is usually not straightforward to compare the margin benefit from both evaluations. As performed in this study, the dosimetric margins are usually smaller than its geometric counterpart. This is because dosimetric margin is dependent on many other factors such as the choice of photon energies, the beam arrangement and the modality of the treatment (three-dimensional conformal radiotherapy or IMRT). The dosimetric margin also depends on the pre-defined criteria we chose for the evaluation. The criteria we chose in this paper should be reasonable if not optimal for every clinic. However, the pass rate as a function of margin was provided in Figure 4, so readers should be able to interpolate valued from that figure based on their own criteria. These factors may undermine the dosimetric margin dependence on the underlining motion uncertainties, so the benefit of the hybrid strategy may appear smaller than its actual value. In addition, because of the online IG, the residual organ deformation is likely of random nature, voxels of CTV that were under-dosed at one fraction is unlikely to be under-dosed at all fractions, i.e., they are compensated by other fractions. The net effect is that the minimum cumulative dose is likely larger than the daily dose. In comparison, there is no cumulative effect in either geometrical evaluation or daily dose evaluation. Another reason is that significant margin reductions on the order of a few mm have been realized through either the online IG only or offline IG only, the improvement brought by the hybrid strategy is likely in the second order and only incremental. The current systems, including online IG, CT images in treatment planning system, have a finite resolution on the order of 1 mm, could not discern such a small difference. As shown in this paper, the margin used for evaluation in the process has the resolution of 1 mm, any fractional margins were derived from interpolations. Therefore, for dosimetric evaluation of different image guidance strategies, new concepts or indices other than margins may be necessary for the comparisons to be meaningful. We would like to point out that the dosimetric margin obtained from the evaluation is the minimum margin required because we assume perfect online image guidance. In actual clinical protocols, addition margins are still required to account for the residual uncertainties, such as imperfect registrations, deformation, and intra-fractional motion.
In this study, the primary goal of the image guidance is to ensure proper dose coverage of the target and reduce margins safely. The online corrections and offline planning were focused on the target and no special considerations were taken to reduce the doses to surrounding normal tissues and OARs. The position and shape changes for the bladder and rectal wall were not corrected even though they were the source of the most uncertainties in prostate radiotherapy. Therefore, the dose improvement in the rectal wall and bladder were small. Some of them were statistically significant, mainly due to the reduction of the margin in the planning.
Dose evaluation based on daily dose information was also studied. The minimum margin necessary can be obtained from the relationship between margin and the number of fractions failed the pre-defined criteria. At a 95% confidence level, for LRP, 2.8mm is required for the online IG only strategy, and 1.2 mm is required for the hybrid strategy. For IRP with Type B registration, about 3 mm margin is necessary for online IG strategy, no margin or very small margin is needed for hybrid strategy. Minimum margin required with type B registration is slightly larger compare with that with Type A registration for IRP. We should emphasis that the results obtained here depend on the criteria, which we chose D99 of CTV for each treatment fraction should not be less than 98% of PTV D99 in the original plan. As mentioned above, since there is no accumulation in the daily dose evaluation, under-dose in one fraction is not compensated by other fractions, the margins determined are therefore larger.
Besides IMRT, the commonly used three-dimensional conformal radiotherapy (3D-CRT) was also simulated but the results were not shown. IMRT required relative larger margins than 3D-CRT, however, IMRT provided highly conformed dose distributions to the target volume, and doses to OARs were actually significantly lower. Similar as IMRT, the dosimetric margin benefit of the hybrid over online IG strategy for 3D-CRT varied from 0 mm to 1 mm.
4.2.Geometric evaluation and new index CITV
The average volume of the ITVn was larger than that of the CTV0 and increased with the number of fractions (n) used in the ITV construction. However, the volumes of the PTV constructed from the ITV decreased and tended to saturate with the increasing n for both LRP and IRP. CITV(Hybrid) < CITV(Online IG) for both LRP and IRP, this means the hybrid strategy has smaller cumulative irradiated target volume than the online IG only strategy. The geometric evaluation of the hybrid strategy indicates the optimal number of fractions necessary to construct the ITV is 2 for LRP and 3–4 for IRP.
We proposed a new concept, the cumulative index of target volume, or CITV, for the evaluation of different image guidance strategies. It is geometric in nature. It has a few advantages over other indices.
Comparing geometric margin alone can lead to incorrect impression due to the volumetric difference of CTV0 and ITV. The CITV has simple scheme than the geometric margin because it combines the equivalent margin (M0) and the margin difference (ΔM) into one, and it covers the effect of the entire treatment process.
The CITV is based on volume which is three dimensional, unlike the margin which is only one dimensional. Therefore, it can be expanded to compare strategies using non-uniform margins.
The CITV is less susceptible to the precision of current image guidance system, while the margin, as explained earlier, is currently not suitable for sub mm comparisons.
The CITV has the effect of accumulation built into its definition, which most geometrical indices do not. Therefore, both the effect of adaptive replanning with multiple plans (PTVs) and the effect of target volume changes during the treatment course can be accounted by CITV. This is an important aspect when image guidance strategies are compared with adaptive radiotherapy.
The CITV is not in dosimetry domain. Therefore, it does not depend on the parameters used in the treatment planning, such as photon energies, number of beams, beam angles, or treatment modalities. It is only a function of the underlining patient motion uncertainties. Therefore, it can be generalized and easily adaptable to different clinics where the treatment planning process can be quite different. We believe it is more suitable for comparisons of clinical image guidance protocols at different institutions than other indices used today.
The optimal number of fractions necessary for the construction of the ITV depends on the index and the criteria used. Based on CITV, it was found to be 2 for LRP, 3 for IRP. This is slightly smaller than the numbers from previous papers on the pure offline IG using principal component analysis which is 5 (Sohn et al., 2005). The main reason, we believe, is that the hybrid strategy has the online IG already built in. So the dominant uncertainty that is compensated is the deformation, which explains why the number of fractions is higher for IRP than LRP due to higher deformation in IRP. The magnitude of margin obtained here to compensate the deformation is consistent with previous study by using a magnetic resonance imaging technique (Nichol et al., 2007). We also tried to quantify the deformation using CITV in this paper.
An alternative to the hybrid method proposed in this study is the online adaptive planning (de la Zerda et al. 2007). One assumption in that paper and many others on the topic is that target contours were available online. However, this is not practical due to many factors. For example, the current CBCT (both MV and kV) does not have adequate image quality in soft tissue contrast so that the prostate can be contoured accurately in the short time online. Secondly, the infrastructure for online planning is not yet setup yet, we do not anticipate that the clinician will be available at the treatment console at each treatment fraction to contour and approve the structures in the near future. In addition, contouring and online replanning will prolong the treatment time considerably. As a consequence, the uncertainty from intra-fractional motion magnitude will increase. On the other hand, the feasibility of contouring prostate on CBCT images in an offline adaptive environment has been investigated and found that to be satisfactory (Wang et al. 2010). The hybrid method is more complex when compared with online IG only strategy. However, this is similar to the pure offline adaptive radiotherapy (Yan et al, 2000), which had been implemented clinically. All the extra efforts (contouring, replanning, etc.) are spent offline, so there is no adverse effect on the online treatment efficiency, and it does not change the intra-fractional motion characteristics. Therefore, it is clinically practical.
In conclusion, we performed dosimetric evaluation of the proposed hybrid strategy of online image guidance and offline adaptive replanning, and compared it with the current standard online image guidance only protocol. The margin benefit was evaluated for both 3D-CRT and IMRT plans and they varied from 0 mm to 1 mm. For intermediate-risk patients, registration base on the entire CTV is better than that based on prostate only. The optimal number of fractions necessary for the ITV construction is 2–3 for LRP and 3–4 for IRP in a hypofractionation protocol. We also proposed a new cumulative index of target volume for evaluations of adaptive radiotherapy and it has some advantages over other commonly used indices.
Acknowledgments
This study is supported by grant CA118037 from the National Institute of Health. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
References
- Adamson J, Wu Q. Prostate intrafraction motion assessed by simultaneous kV fluoroscopy at MV delivery II: adaptive strategies. Int J Radiat Oncol Biol Phys. 2010;78:1323–1330. doi: 10.1016/j.ijrobp.2009.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer AM, Mackay RI, Roberts SA, Hendry JH, Williams PC. The required number of treatment imaging days for an effective off-line correction of systematic errors in conformal radiotherapy of prostate cancer--a radiobiological analysis. Radiother Oncol. 2001;61:143–150. doi: 10.1016/s0167-8140(01)00440-6. [DOI] [PubMed] [Google Scholar]
- Balter JM, Lam KL, Sandler HM, Littles JF, Bree RL, Ten Haken RK. Automated localization of the prostate at the time of treatment using implanted radiopaque markers: technical feasibility. Int J Radiat Oncol Biol Phys. 1995;33:1281–1286. doi: 10.1016/0360-3016(95)02083-7. [DOI] [PubMed] [Google Scholar]
- Bel A, Vos PH, Rodrigus PT, Creutzberg CL, Visser AG, Stroom JC, Lebesque JV. High-precision prostate cancer irradiation by clinical application of an offline patient setup verification procedure, using portal imaging. Int J Radiat Oncol Biol Phys. 1996;35:321–332. doi: 10.1016/0360-3016(95)02395-x. [DOI] [PubMed] [Google Scholar]
- Bortfeld T, van Herk M, Jiang S. When should systematic patient positioning errors in radiotherapy be corrected? Phys Med Biol. 2002;47:N297–N302. doi: 10.1088/0031-9155/47/23/401. [DOI] [PubMed] [Google Scholar]
- de Boer HC, Heijmen BJ. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys. 2001;50:1350–1365. doi: 10.1016/s0360-3016(01)01624-8. [DOI] [PubMed] [Google Scholar]
- de la Zerda A, Armbruster B, Xing L. Formulating adaptive radiation therapy (ART) treatment planning into a closed-loop control framework. Phys Med Biol. 2007;52:4137–4153. doi: 10.1088/0031-9155/52/14/008. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Shirato H, Seppenwoolde Y, Onimaru R, Oda M, Fujita K, Shimizu S, Shinohara N, Harabayashi T, Miyasaka K. Three-dimensional intrafractional movement of prostate measured during real-time tumor-tracking radiotherapy in supine and prone treatment positions. Int J Radiat Oncol Biol Phys. 2002;53:1117–1123. doi: 10.1016/s0360-3016(02)02882-1. [DOI] [PubMed] [Google Scholar]
- Langen KM, Jones DT. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001;50:265–278. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- Langen KM, Willoughby TR, Meeks SL, Santhanam A, Cunningham A, Levine L, Kupelian PA. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–1090. doi: 10.1016/j.ijrobp.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Lei Y, Wu Q. A hybrid strategy of offline adaptive planning and online image guidance for prostate cancer radiotherapy. Phys Med Biol. 2010;55:2221–2234. doi: 10.1088/0031-9155/55/8/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Wu Q, Yan D. The role of seminal vesicle motion in target margin assessment for online image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73:935–943. doi: 10.1016/j.ijrobp.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol AM, Brock KK, Lockwood GA, Moseley DJ, Rosewall T, Warde PR, Catton CN, Jaffray DA. A magnetic resonance imaging study of prostate deformation relative to implanted gold fiducial markers. Int J Radiat Oncol Biol Phys. 2007;67:48–56. doi: 10.1016/j.ijrobp.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Sohn M, Birkner M, Yan D, Alber M. Modelling individual geometric variation based on dominant eigenmodes of organ deformation: implementation and evaluation. Phys Med Biol. 2005;50:5893–5908. doi: 10.1088/0031-9155/50/24/009. [DOI] [PubMed] [Google Scholar]
- Stroom JC, de Boer HC, Huizenga H, Visser AG. Inclusion of geometrical uncertainties in radiotherapy treatment planning by means of coverage probability. Int J Radiat Oncol Biol Phys. 1999;43:905–919. doi: 10.1016/s0360-3016(98)00468-4. [DOI] [PubMed] [Google Scholar]
- van Herk M, Remeijer P, Lebesque JV. Inclusion of geometric uncertainties in treatment plan evaluation. Int J Radiat Oncol Biol Phys. 2002;52:1407–1422. doi: 10.1016/s0360-3016(01)02805-x. [DOI] [PubMed] [Google Scholar]
- Wang W, Wu Q, Yan D. Quantitative evaluation of cone-beam computed tomography in target volume definition for offline image-guided radiation therapy of prostate cancer. Radiother Oncol. 2010;94:71–75. doi: 10.1016/j.radonc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Ivaldi G, Liang J, Lockman D, Yan D, Martinez A. Geometric and dosimetric evaluations of an online image-guidance strategy for 3D-CRT of prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1596–1609. doi: 10.1016/j.ijrobp.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Wu Q, Mohan R, Niemierko A, Schmidt-Ullrich R. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. Int J Radiat Oncol Biol Phys. 2002;52:224–235. doi: 10.1016/s0360-3016(01)02585-8. [DOI] [PubMed] [Google Scholar]
- Yan D, Jaffray DA, Wong JW. A model to accumulate fractionated dose in a deforming organ. Int J Radiat Oncol Biol Phys. 1999;44:665–675. doi: 10.1016/s0360-3016(99)00007-3. [DOI] [PubMed] [Google Scholar]
- Yan D, Lockman D, Brabbins D, Tyburski L, Martinez A. An off-line strategy for constructing a patient-specific planning target volume in adaptive treatment process for prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48:289–302. doi: 10.1016/s0360-3016(00)00608-8. [DOI] [PubMed] [Google Scholar]