Abstract
A double-blind, randomized clinical trial was conducted to determine the effect of consumption of supplemental whey protein (WP), soy protein (SP), and an isoenergetic amount of carbohydrate (CHO) on body weight and composition in free-living overweight and obese but otherwise healthy participants. Ninety overweight and obese participants were randomly assigned to 1 of 3 treatment groups for 23 wk: 1) WP; 2) SP (each providing ~56 g/d of protein and 1670 kJ/d); or 3) an isoenergetic amount of CHO. Supplements were consumed as a beverage twice daily. Participants were provided no dietary advice and continued to consume their free-choice diets. Participants’ body weight and composition data were obtained monthly. Dietary intake was determined by 24-h dietary recalls collected every 10 d. After 23 wk, body weight and composition did not differ between the groups consuming the SP and WP or between SP and CHO; however, body weight and fat mass of the group consuming the WP were lower by 1.8 kg (P < 0.006) and 2.3 kg (P < 0.005), respectively, than the group consuming CHO. Lean body mass did not differ among any of the groups. Waist circumference was smaller in the participants consuming WP than in the other groups (P < 0.05). Fasting ghrelin was lower in participants consuming WP compared with SP or CHO. Through yet-unknown mechanisms, different sources of dietary protein may differentially facilitate weight loss and affect body composition. Dietary recommendations, especially those that emphasize the role of dietary protein in facilitating weight change, should also address the demonstrated clinical potential of supplemental WP.
Introduction
Dietary approaches for controlling unhealthy weight gain are becoming increasingly important and using dietary manipulations to control hunger is one potential means to control energy intake. Many investigations of dietary manipulations to modulate body weight, especially those with higher protein diets, include energy restriction during or subsequent to the dietary modulation (1–13). Results from these interventions suggest that body weight loss is greater while consuming higher protein diets and satiety may be a key factor (14). However, because these participants were in an energy deficit, it is difficult to separate the effects of the catabolic state from those of the dietary macronutrients.
In short-term studies with subjective assessment of hunger and satiety, dietary protein has been shown to be more satiating than isoenergetic intake of fat and carbohydrate (9, 15–17). Although results from these short-term studies can provide insight into energy intake regulation, it is unclear what effect any short-term response in food intake will have on long-term energy intake and body weight regulation, especially in a noncatabolic state. Thus, longer term dietary interventions with body weight or composition as outcomes may answer these questions.
Not all longer term dietary interventions of restricted energy intake concomitant with increased protein intake have demonstrated that these diets improve body weight or composition (18–20). In most interventions, the source of dietary protein is typically not described (3, 5, 7, 10, 11, 19) or is from mixed sources (6, 8, 12, 13). Protein source may be important to consider in understanding the success or failure of these interventions. For example, in a study of overweight and obese men fed isoenergetic diets, animal protein (pork) increased energy expenditure compared with a vegetable (soy) protein (21). Wistar rats (10 wk old) fed a high-protein diet with whey protein concentrate had a 4% reduction in weight gain and reduced visceral and subcutaneous fat deposition compared with rats fed a red meat-based protein (22). These results suggest that there might be differential effects among protein sources on energy intake or body weight regulation. However, the rat data are from young, growing animals whose physiological state might be much different from an adult human. Likewise, human studies investigating different sources of protein and body weight and composition have either been of very short duration or conducted with energy restriction, thus confounding the interpretation of the results.
The primary objective of the present study was to determine the effects of added supplemental protein to the habitual diet of free-living overweight and obese adults, without energy restriction, on body weight and composition. A secondary objective was to determine whether there are differential effects between protein sources on body weight and composition in a longer term intervention. Whey and soy are both readily available proteins and both have been implicated in regulating food intake. We hypothesized that supplementation of overweight and obese free-living individuals with whey protein (WP)3 would decrease body weight and fat compared with individuals supplemented with isonitrogenous soy protein (SP) or isoenergetic carbohydrate (CHO) and that insulin, insulin-like growth factor (IGF), ghrelin, and thyroid hormones would be affected by protein source.
Methods
Study design
A double-blind, randomized clinical trial was conducted to determine the effects of supplemental WP and SP and an isoenergetic amount of CHO on body weight and composition in free-living overweight and obese but otherwise healthy individuals for 23 wk. In addition, plasma glucose, insulin, ghrelin, IGF, and serum thyroid hormones were determined to evaluate metabolic and hormonal changes. Because dietary intake and physical activity can alter body weight and composition, these factors were monitored at frequent intervals throughout the intervention.
Participants.
Ninety participants were recruited and randomly assigned (stratified by sex, BMI, and age) to 1 of 3 groups: WP, SP, or an isoenergetic amount of CHO (maltodextrin). The sample size was selected to determine a 3% change in body weight (P < 0.05) with 90% power among each treatment comparison (23–25). Participants were stratified to treatment based on gender and BMI.
Inclusion into the study was for nonsmokers having a BMI (in kg/m2) >28 and <38, fasting glucose <7 mmol/L, blood pressure <160/100 mm Hg, and total cholesterol <6.2 mmol/L. Exclusion criteria included: history or presence of kidney, gastrointestinal, liver, or thyroid disease, gout, certain cancers, or type 2 diabetes; recent weight loss; recently following a high-protein diet or using antiobesity medications or supplements; and consuming a WP or SP supplement. Medical history, routine blood chemistry indexes, complete blood count, urine analysis, and a physical examination were used to evaluate each participant’s eligibility for inclusion in the study. The protocol and consent form were reviewed and approved by the Institutional Review Board of Medstar Research Institute. Participants provided written informed consent and received $800 for successful participation.
Intervention.
Each treatment supplement was specifically formulated and manufactured for this study (Innovative Food Processors) and was provided in 3 flavors in serving sizes of 52 g/packet (Table 1). The source of WP was WP concentrate-80, the source of SP was an isoflavone-free SP isolate (Prolisse, Cargill), and the source of CHO was maltodextrin (Maltrin M180, Grain Processing). The WP concentrate-80 was from a cheese-derived source and was not hydrolyzed. An isoflavone-free SP isolate was selected to minimize the impact of nonprotein compounds and focus on the biological effects of the macronutrient component. Participants were instructed to consume 1 pack immediately prior to, during, or immediately after breakfast and dinner. The total amount of energy from the treatments was 1670 kJ/d. Participants were provided with information on the energy content of the products but with minimal instruction from a registered dietician on how to make dietary alterations to incorporate these products. Participants completed a questionnaire each day to record the time the treatment was consumed and general health questions. Participants were provided a daily vitamin and mineral supplement (Os-cal Ultra; GlaxoSmithKline Consumer Healthcare) to standardize supplemental calcium intake.
TABLE 1.
Chemical composition of the carbohydrate (CHO), whey protein (WP), and soy protein (SP) treatment supplements12
| CHO | WP | SP | |
| g/packet | |||
| Weight | 52 | 51 | 52 |
| Protein | 0.6 | 27.5 | 28.1 |
| Moisture | 1.7 | 2.2 | 1.8 |
| Fat, acid hydrolysis | 0.7 | 1.5 | 2.0 |
| Ash | 1.0 | 1.4 | 2.7 |
| Total carbohydrate | 48.0 | 18.4 | 17.4 |
| Calcium | 0.20 | 0.22 | 0.25 |
| para-Aminobenzoic acid | 0.2 | 0.2 | 0.2 |
| mg/packet | |||
| l-Aspartic acid | 36.4 | 3060 | 3200 |
| l-Threonine | 18.2 | 1850 | 945 |
| l-Serine | 23.4 | 1570 | 1480 |
| l-Glutamic acid | 62.4 | 4860 | 5340 |
| l-Proline | 26.0 | 1690 | 1000 |
| l-Glycine | 18.2 | 541 | 1150 |
| l-Alanine | 15.6 | 1370 | 1150 |
| l-Cystine | 10.4 | 694 | 319 |
| l-Valine | 20.8 | 1590 | 1310 |
| l-Methionine | 10.4 | 592 | 354 |
| l-Isoleucine | 13.0 | 1730 | 1310 |
| l-Leucine | 26.0 | 3060 | 2190 |
| l-Tyrosine | 26.0 | 820 | 997 |
| l-Phenylalanine | 18.2 | 918 | 1440 |
| l-Histidine | 10.4 | 530 | 716 |
| l-Lysine | 15.6 | 2470 | 1690 |
| l-Arginine | 26.0 | 726 | 2070 |
| l-Tryptophan | 10.4 | 607 | 400 |
Participants consumed 2 treatment packets/d, 1 with breakfast and the evening meal, along with their typical diet.
Chemical composition was determined by Covance Laboratories.
Compliance.
Compliance was determined by counting the number of packets distributed and recounting those not consumed and by measuring para amino benzoic acid (PABA) in urine samples collected at random, unannounced times monthly to determine whether PABA was present in the urine. The PABA was added to each treatment packet at a concentration of 0.24 mg/kJ. The half-life of PABA is very short (<18 h); it was decided that ≥3 of 5 urine samples without PABA would be a criterion for noncompliance.
Dietary intake assessment.
Usual dietary intake was assessed every 10 d using the USDA’s Automated Multiple-Pass Method (26). All 24-h recalls were completed in person and were performed in the morning on all days of the week.
Subjective satiety measures.
Subjective satiety and hunger were assessed daily for 23 wk, before consumption of the treatment and evening meal by means of 4 visual analogue scale (VAS) questions that described hunger, desire to eat, the amount of food that could be eaten, and stomach fullness. The VAS were all 100 mm in length and were anchored at either end with terms indicating opposite descriptors (27).
Physical activity assessment.
Physical activity was measured semimonthly with an activity monitor (accelerometer) for 7 consecutive days (Actigraph MTI AM 7164–1.2; Manufacturing Technology). Activity monitors were attached to a snuggly fitting belt worn around the waist (28).
Body weight and composition.
Prior to the start of the intervention and then monthly, body weight and composition were measured by air-displacement plethysmography (BodPod 2000A, BodPod 2.0 Software, Life Measurement). Measurements were made according to the manufacturer’s guidelines. Participants fasted for at least 12 h before the measurements and refrained from exercise. Thoracic lung volume was automatically estimated. The Siri formula was used to calculate percent body fat (29).
Anthropometry.
Prior to the start of the intervention and then monthly, waist circumference was measured above the right ilium on the midaxillary line. Hip circumference was measured at the level of the maximum extension of the buttocks. Measurements were made with a fiberglass tape measure by 2 trained individuals following a written protocol with almost 90% of the measures performed by 1 of the 2 individuals.
Biological sample collection and analysis.
Five times during the study (before the intervention and after 12, 16, 20, and 23 wk of the intervention), blood was collected after a 12-h fast. Plasma and serum samples were collected after centrifugation and frozen at −80°C. Plasma insulin concentrations were measured by ELISA (LINCOplex; LINCO Research). Plasma glucose concentrations were measured enzymatically (Smith-Kline Beecham Laboratories). Plasma concentrations of total ghrelin were measured by RIA (LINCO Research). Plasma IGF-I and IGF binding protein (IGFBP)-3 concentrations were measured by ELISA (R&D Systems). Plasma IGFBP-1 concentration was measured by ELISA (LINCOplex; LINCO Research). Serum free thyroxine (T4) concentrations and triiodothyronine (T3) uptake were analyzed with an enzyme-multiplied immunoassay (Siemens; Centaur). Urine samples were collected monthly. Urine was collected and frozen at −80°C until analysis for PABA by HPLC (30–32).
Statistical analyses.
Prior to ANOVA, each variable was evaluated for normality and homogeneity of variance within groups. A log transformation was performed for glucose and insulin so that these data would not violate the homogeneity of variance assumption needed to perform the ANOVA. Repeated-measures analyses (MIXED procedure in SAS; SAS Institute) were used to evaluate changes over time. The model included treatment, sex, time, 2-way interactions, and the 3-way interaction as fixed effects. Pretreatment values were included as covariates. Results were interpreted first through the 3-way interaction. If this interaction was significant (P < 0.05), within time, treatment effects were evaluated. If this interaction was not significant, the 2-way interactions were investigated. Within-time and within-sex treatment effects were investigated. If no interactions were significant, the main effect of treatment was evaluated. If the treatment effect was significant for any of the interactions or main effect evaluations, the outcome for WP was compared with the CHO and SP values by using the slice option to compare the least-squares means. Values reported are means ± SE.
Results
Participants and compliance.
Seventy-three participants completed the intervention. Participant characteristics prior to the start of the intervention (i.e. baseline) of those who completed the entire protocol are presented in Table 2.
TABLE 2.
Baseline characteristics of the overweight or obese adult men and women who completed the study protocol1
| Treatment | Age | Height | Weight | BMI | n |
| y | m | kg | kg/m2 | ||
| Women | |||||
| CHO | 50 ± 10 | 1.6 ± 0.0 | 84.0 ± 9.3 | 31.2 ± 2.8 | 13 |
| WP | 45 ± 9 | 1.7 ± 0.1 | 87.3 ± 11.7 | 31.4 ± 2.4 | 13 |
| SP | 53 ± 9 | 1.7 ± 0.1 | 86.5 ± 9.2 | 30.8 ± 2.3 | 13 |
| Men | |||||
| CHO | 51 ± 7 | 1.8 ± 0.1 | 99.7 ± 13.1 | 30.9 ± 2.2 | 12 |
| WP | 55 ± 7 | 1.8 ± 0.0 | 95.3 ± 6.4 | 30.5 ± 1.9 | 10 |
| SP | 54 ± 9 | 1.8 ± 0.1 | 102.7 ± 11.8 | 31.1 ± 2.4 | 12 |
| All | |||||
| CHO | 51 ± 9 | 1.7 ± 0.1 | 91.5 ± 13.7 | 31.1 ± 2.5 | 25 |
| WP | 49 ± 9 | 1.7 ± 0.1 | 90.8 ± 10.4 | 31.0 ± 2.2 | 23 |
| SP | 53 ± 9 | 1.7 ± 0.1 | 90.3 ± 13.2 | 30.9 ± 2.3 | 25 |
All values are means ± SEM.
The mean number of supplement packets consumed over the intervention was 2 per day, which was the prescribed amount. However, PABA analysis of urine samples revealed 2 participants with undetectable PABA concentrations in 4 of 5 random samples. Their data were excluded from all the analyses.
At breakfast time, most packets were consumed immediately before or during the meal (44 and 41%, respectively) and fewer were consumed immediately after the meal (15%). At dinner time, over one-half of the packets (52%) were consumed immediately prior to the meal. The remainder dinner time packets were consumed with the meal (20%) or immediately after the meal (28%).
Dietary intake.
Dietary data are reported from 1060 dietary recalls for 73 participants who completed the study. Mean energy intake (including supplements) was 9060 ± 560, 9140 ± 510, and 9490 ± 460 kJ/d for the CHO, WP, and SP groups, respectively, and did not differ among treatment groups. Mean protein intake was 76 ± 3, 131 ± 6, and 135 ± 3 g/d for the CHO, WP, and SP treatment groups, respectively. Mean percent of energy intake from protein was 14 ± 1, 24 ± 2, and 24 ± 2% for the CHO, WP, and SP treatment groups, respectively. Mean percent of energy intake from CHO was 58 ± 2, 49 ± 2, and 48 ± 1% for the CHO, WP, and SP treatment groups, respectively. The percentages of energy intake from fat were 28 ± 2, 27 ± 2, and 28 ± 1% for the CHO, WP, and SP treatment groups, respectively. Protein intakes were 1.4 g/kg of body weight for the protein treatments and 0.8 g/kg of body weight for the CHO treatment groups. Energy and macronutrient intakes were higher for men than for women (P < 0.0001), with no detectable effect of treatment on changes in energy, protein, carbohydrate, or fat intake during the course of the intervention. Between the initial and final recall, there was a decrease in carbohydrate intake in the group consuming the WP supplement (P < 0.04).
Subjective satiety.
VAS questions were used to evaluate the subjective satiety responses of the participants before the evening meal for 23 wk. The dietary treatments did not affect hunger (P = 0.11), desire to eat (P = 0.11), prospective consumption (P = 0.38), or stomach fullness (P = 0.62). No significant treatment × sex or treatment × time interactions were found, indicating that the treatment effects were not influenced by sex or time (data not shown).
Physical activity.
Physical activity did not differ between treatment groups during the intervention period. Time of use compliance of the monitors was >72%, with an mean wear of 17.4 ± 2.6 h/d.
Body weight and composition.
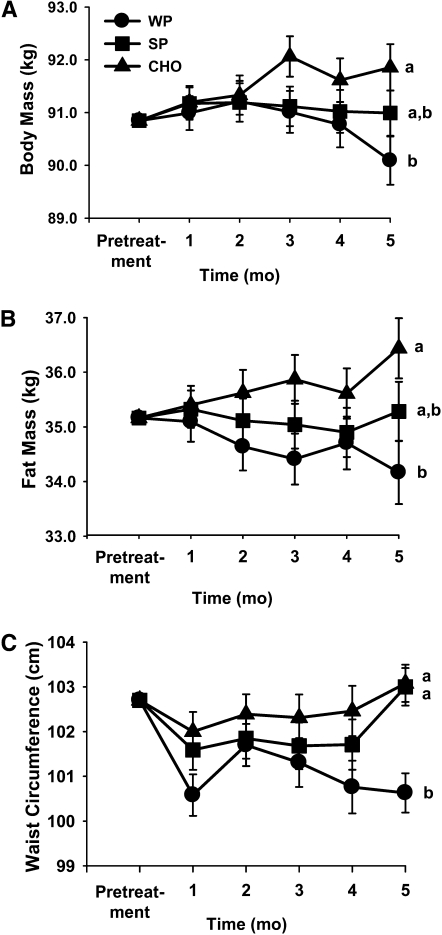
There were no differences between treatment groups at baseline. A significant interaction existed between treatment and time; i.e. at the last measurement time, the treatment means were different. At the end of the intervention (after 23 wk), body weight of the group consuming WP was 1.8 kg (2%) lower than that of the group consuming the CHO treatment (P < 0.006) (Fig. 1A). Body weight did not differ between the groups consuming SP and WP (0.9-kg difference) or between the groups consuming SP and CHO (0.9-kg difference). The treatment × sex interaction was not significant, indicating that the effect of treatment was not different for men and women.
FIGURE 1.
Effect of supplemental carbohydrate (CHO), whey protein (WP), and soy protein (SP) on body mass (A), fat mass (B), and waist circumference (C) in overweight or obese adult men and women. All values are least squares means ± SEM, n = 73 (39 women, 34 men); n = 25 (CHO), 23 (WP), or 25 (SP). Means without a common letter differ at the final measure, P < 0.05.
At the end of the intervention, body fat mass was 2.3 kg lower in the group consuming the WP than in the group consuming the CHO treatment (P < 0.005) (Fig. 1B). Body fat mass of the group consuming SP was not different from that of the group consuming the WP (1.1-kg difference), nor was it significantly different from the group consuming the CHO treatment (1.2-kg difference). Lean body mass did not significantly differ among groups.
Anthropometry.
There was no effect of treatment on waist circumference before the last measurement. At the last measurement time, waist circumference was 2.4 cm lower in the group supplemented with WP than in the other 2 groups (Fig. 1C). The effect of treatment was not different for men and women. Hip circumference was not affected by treatment.
Biological samples.
Fasting blood glucose concentrations were unaffected by treatment; however, circulating insulin concentrations were lower for participants consuming the whey and SP treatments than for participants consuming the CHO treatments (Table 3). There was no effect of treatment over time and the effect of treatment was similar for both genders. Participants consuming WP had lower ghrelin concentrations compared with participants consuming the SP (P = 0.04) and CHO (P = 0.007) treatments. Participants consuming the SP compared with CHO treatment showed no treatment effects on ghrelin (P = 0.31). Circulating IGF-I concentrations were higher in the group consuming the SP supplement than in the groups supplemented with WP or CHO, whereas IGFBP-3 concentrations were lower in the group supplemented with WP than in the other 2 groups. The IGFBP-1 concentration was not affected by treatment. T3 uptake was lower in the group supplemented with WP compared with the group supplemented with SP; the group supplemented with CHO did not differ from either protein group. Free T4 concentrations were lower in the groups supplemented with WP and CHO than in the group supplemented with SP.
TABLE 3.
Glucoregulatory biomarkers, insulin growth factors, and thyroid function after consumption of the carbohydrate (CHO), whey protein (WP), or soy protein (SP) supplement in overweight or obese adult men and women12
| Treatment |
|||
| CHO | WP | SP | |
| Glucose, log(mmol/L) | 0.255 ± 0.001 | 0.255 ± 0.001 | 0.255 ± 0.001 |
| Insulin, log(pmol/L) | 18.3 ± 0.3b | 16.3 ± 0.6a | 17.2 ± 0.3a |
| Ghrelin, ng/L | 870 ± 23b | 752 ± 36a | 837 ± 23b |
| IGF-I, μg/L | 77.8 ± 1.4a | 81.5 ± 2.1a | 87.0 ± 1.3b |
| IGFBP-1, ng/L | 721 ± 6 | 717 ± 11 | 719 ± 6 |
| IGFBP-3, mg/L | 1.98 ± 0.03b | 1.82 ± 0.05a | 2.04 ± 0.03b |
| T3 uptake, % | 31.4 ± 0.4ab | 30.9 ± 0.5a | 32.5 ± 0.4b |
| Free T4,pmol/L | 14.1 ± 0.1a | 13.7 ± 0.1a | 14.5 ± 0.3b |
All values are least squares means ± SEM, = 73 (39 women, 34 men). Means without a common letter differ.
Plasma glucose, insulin, ghrelin, IGF-I, IGFBP-1, IGFBP-3, and serum T3 uptake and T4.
Discussion
This randomized, controlled clinical trial evaluated the effects of supplementation with WP, SP, or an isoenergetic amount of CHO on body weight and composition in free-living overweight and obese adults. At the end of the intervention, in the group consuming supplemental WP compared with those consuming supplemental CHO, there was a 1.8-kg difference in body mass and a 2.3-kg difference in fat mass, with the CHO-supplemented group being heavier than the protein-supplemented group. By contrast, in the group consuming supplemental SP compared with the group consuming supplemental CHO, there was no difference in body mass or composition. Similarly, the groups consuming the 2 protein sources did not differ. Based on the length of the treatment and the daily energy provided from the supplement, we would estimate that weight gain would exceed ~10 kg without any compensation for the additional energy of the supplement. Given the observed changes in weight, it appears that the energy compensation occurred for all treatments. The difference in body weight and composition at the end of the intervention likely is related to better compensation among the group consuming the whey treatment compared with the CHO treatment. These differences among treatments in body weight and composition may be a result of subtle effects of CHO and protein on satiety. Changes in energy intake in the range of only 170–210 kJ/d could account for the observed modest change in body weight during this 23-wk intervention. These changes are so subtle that they may not be detectible with the 24-h recall methodology. Additionally, consuming WP resulted in a significantly smaller waist circumference compared with the group consuming supplemental CHO. This finding is important, because the amount of intra-abdominal adipose tissue is more significantly correlated with metabolic complications in obese individuals than is subcutaneous fat (33, 34). During energy restriction, higher protein diets consumed ad libitum facilitate weight loss, and improved satiety is a presumed contributing mechanism (14). In this study in which energy restriction was not part of the intervention, changes in body weight and composition were small but nevertheless suggest that habitual consumption of supplemental protein may result in improved body composition and incremental, but ultimately significant, weight loss. These data suggest that supplemental dietary protein may reduce the risk of unhealthy weight gain observed in many populations (i.e. 500–1000 g/y).
Although there were no differences among treatments with respect to total (background diet + treatment) energy intake, there was a decrease in the CHO intake of the background diet between the initial and final dietary recall in the participants consuming the WP treatment. Consumption of supplemental WP decreased concentrations of the orexigenic peptide ghrelin. Ghrelin may serve as a hunger signal; it strongly increases food intake in both animals and humans (35). In 1 study (36), there was a decrease in the ghrelin concentration at 2 and 3 h following acute ingestion of 55 g of whey or casein compared with ingestion of 56 g of glucose or lactose. These results are similar to our findings from samples collected after a 12-h fast. In a second study, Bowen et al. (37) found a decrease in ghrelin after ingestion of 50 g of soy, whey, or gluten protein compared with glucose. However, in contrast to our finding, they did not detect a difference among the protein sources. Study design differences could account for the observed differences in response; our observations are from samples collected after a 12-h fast and longer intervention, whereas Bowen et al. (37) collected samples 2 or 3 h after ingestion of the foods, without prior exposure. Further, protein consumption may reduce body fat by stimulating the release of hormones affecting metabolic rate. Thyroid hormone concentrations (T3 and T4) can increase in participants consuming a high-protein diet compared with a high-carbohydrate diet (38). Protein source did affect free T4 and T3 uptake concentrations; however, consuming SP increased these concentrations more than did consuming WP. Further research is required to gain a better understanding of the long-term effects of differing protein sources on thyroid function.
Strengths and limitations of the study design warrant consideration. Based on the number of participants who completed the intervention, this study was well powered to detect a small change in body weight. To better ensure participant compliance with treatment (beyond measuring disappearance of packets), we qualitatively measured urinary excretion of an internal treatment marker at random time points during the study. Assessment of dietary intake and physical activity was performed on a regular and frequent schedule. To assess dietary intake throughout the study, we used a method that is designed to estimate current dietary intake and strives to minimize the problem of misreporting (39). However, this methodology is not sensitive enough to detect the subtle changes in energy intake that take place to result in the small yet significant differences in weight seen in this study (1.8 kg between WP and CHO groups). This study did not include a placebo control group (no intervention) to maintain a double-blind standard. Protein and carbohydrate were selected for the intervention, because they both provide similar metabolizable energy intake for a given mass. In this intervention, participants were instructed to consume their product immediately before, after, or during their meal. Much previous research has used macronutrient interventions as a preload to a meal. Preloading participants might have resulted in greater treatment differences.
This intervention study reported on the effects of long-term consumption of supplemental WP, SP, and CHO in a free-living overweight and obese population without imposed energy restriction. However, most studies examining the effects of increased dietary protein have used mixed sources of proteins (dairy, vegetable, meat, and soy) in conjunction with weight loss; therefore, future research should target whether specific dietary proteins may elicit beneficial effects on body composition during energy restriction. Future research should also target the dose of specific proteins necessary for beneficial effects on weight and body composition and the interaction of dose and time needed to observe any effects.
In conclusion, this study suggests that after 6 mo of supplementation, there was a difference in body weight and fat mass between overweight and obese adults who consumed supplemental WP compared with those who consumed isoenergetic supplemental CHO. The difference in body weight was associated with a decrease in fat without an effect on lean mass. Supplemental SP compared with CHO did not alter body weight or composition, nor were there differences in body weight or composition between soy and WP sources. Although there were differences in food intake between males and females, the effects of the intervention were consistent between males and females. Short-term weight loss requires energy restriction and higher protein diets may assist in this acute weight reduction; however, protein supplementation, particularly WP, in overweight and obese individuals may assist in long-term maintenance of body weight without energy restriction.
Acknowledgments
D.J.B., B.A.C., and W.V.R. designed research; D.J.B., K.S.S., D.R.P., and W.V.R. conducted research; D.J.B. analyzed data; D.J.B. and K.S.S. wrote the paper; and D.J.B. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the USDA and the US Whey Protein Research Consortium. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Abbreviations used: CHO, carbohydrate; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; PABA, para amino benzoic acid; SP, soy protein; T3, triiodothyronine; T4, thyroxine; VAS, visual analogue scale; WP, whey protein.
Literature Cited
- 1.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–8 [DOI] [PubMed] [Google Scholar]
- 2.Clifton PM, Keogh JB, Noakes M. Long-term effects of a high-protein weight-loss diet. Am J Clin Nutr. 2008;87:23–9 [DOI] [PubMed] [Google Scholar]
- 3.Due A, Toubro S, Skov AR, Astrup A. Effect of normal-fat diets, either medium or high in protein, on body weight in overweight subjects: a randomised 1-year trial. Int J Obes Relat Metab Disord. 2004;28:1283–90 [DOI] [PubMed] [Google Scholar]
- 4.Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr. 2008;87:44–55 [DOI] [PubMed] [Google Scholar]
- 5.Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Williams T, Williams M, Gracely EJ, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med. 2003;348:2074–81 [DOI] [PubMed] [Google Scholar]
- 6.Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med. 2004;140:769–77 [DOI] [PubMed] [Google Scholar]
- 7.Brehm BJ, Seeley RJ, Daniels SR, D’Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–23 [DOI] [PubMed] [Google Scholar]
- 8.Volek J, Sharman M, Gomez A, Judelson D, Rubin M, Watson G, Sokmen B, Silvestre R, French D, et al. Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women. Nutr Metab (Lond). 2004;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lejeune MP, Westerterp KR, Adam TC, Luscombe-Marsh ND, Westerterp-Plantenga MS. Ghrelin and glucagon-like peptide 1 concentrations, 24-h satiety, and energy and substrate metabolism during a high-protein diet and measured in a respiration chamber. Am J Clin Nutr. 2006;83:89–94 [DOI] [PubMed] [Google Scholar]
- 10.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–77 [DOI] [PubMed] [Google Scholar]
- 11.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41 [DOI] [PubMed] [Google Scholar]
- 12.Skov AR, Toubro S, Ronn B, Holm L, Astrup A. Randomized trial on protein vs carbohydrate in ad libitum fat reduced diet for the treatment of obesity. Int J Obes Relat Metab Disord. 1999;23:528–36 [DOI] [PubMed] [Google Scholar]
- 13.Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–30 [DOI] [PubMed] [Google Scholar]
- 14.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41 [DOI] [PubMed] [Google Scholar]
- 15.Blom WA, Lluch A, Stafleu A, Vinoy S, Holst JJ, Schaafsma G, Hendriks HF. Effect of a high-protein breakfast on the postprandial ghrelin response. Am J Clin Nutr. 2006;83:211–20 [DOI] [PubMed] [Google Scholar]
- 16.Latner JD, Schwartz M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite. 1999;33:119–28 [DOI] [PubMed] [Google Scholar]
- 17.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53:495–502 [DOI] [PubMed] [Google Scholar]
- 18.Noakes M, Keogh JB, Foster PR, Clifton PM. Effect of an energy-restricted, high-protein, low-fat diet relative to a conventional high-carbohydrate, low-fat diet on weight loss, body composition, nutritional status, and markers of cardiovascular health in obese women. Am J Clin Nutr. 2005;81:1298–306 [DOI] [PubMed] [Google Scholar]
- 19.Lecheminant JD, Gibson CA, Sullivan DK, Hall S, Washburn R, Vernon MC, Curry C, Stewart E, Westman EC, et al. Comparison of a low carbohydrate and low fat diet for weight maintenance in overweight or obese adults enrolled in a clinical weight management program. Nutr J. 2007;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMillan-Price J, Petocz P, Atkinson F, O’Neill K, Samman S, Steinbeck K, Caterson I, Brand-Miller J. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med. 2006;166:1466–75 [DOI] [PubMed] [Google Scholar]
- 21.Mikkelsen PB, Toubro S, Astrup A. Effect of fat-reduced diets on 24-h energy expenditure: comparisons between animal protein, vegetable protein, and carbohydrate. Am J Clin Nutr. 2000;72:1135–41 [DOI] [PubMed] [Google Scholar]
- 22.Belobrajdic DP, McIntosh GH, Owens JA. A high-whey-protein diet reduces body weight gain and alters insulin sensitivity relative to red meat in wistar rats. J Nutr. 2004;134:1454–8 [DOI] [PubMed] [Google Scholar]
- 23.Rumpler WV, Rhodes DG, Baer DJ, Conway JM, Seale JL. Energy value of moderate alcohol consumption by humans. Am J Clin Nutr. 1996;64:108–14 [DOI] [PubMed] [Google Scholar]
- 24.Basiotis PP, Thomas RG, Kelsay JL, Mertz W. Sources of variation in energy intake by men and women as determined from one year’s daily dietary records. Am J Clin Nutr. 1989;50:448–53 [DOI] [PubMed] [Google Scholar]
- 25.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–7 [DOI] [PubMed] [Google Scholar]
- 26.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–32 [DOI] [PubMed] [Google Scholar]
- 27.Raben A, Tagliabue A, Astrup A. The reproducibility of subjective appetite scores. Br J Nutr. 1995;73:517–30 [DOI] [PubMed] [Google Scholar]
- 28.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siri W. Body composition from fluid space and density. : Brozek J, Hanschel A, Techniques for measuring body composition. Washington, DC: National Academy of Science; 1961. p. 223–44 [Google Scholar]
- 30.Bruno MJ, Hoek FJ, Delzenne B, van Leeuwen DJ, Schteingart CD, Hofmann AF, Tytgat GN. Simultaneous assessments of exocrine pancreatic function by cholesteryl-[14C]octanoate breath test and measurement of plasma p-aminobenzoic acid. Clin Chem. 1995;41:599–604 [PubMed] [Google Scholar]
- 31.Russell K, Craig ID, Rawlings JM, Millward DJ, Harper EJ. The use of P-aminobenzoic acid and chromic oxide to confirm complete excreta collection in a carnivore, Felis silvestris catus. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130:339–45 [DOI] [PubMed] [Google Scholar]
- 32.Jakobsen J, Ovesen L, Fagt S, Pedersen AN. Para-aminobenzoic acid used as a marker for completeness of 24 hour urine: assessment of control limits for a specific HPLC method. Eur J Clin Nutr. 1997;51:514–9 [DOI] [PubMed] [Google Scholar]
- 33.Whey protein. Monograph. Altern Med Rev. 2008;13:341–7 [PubMed] [Google Scholar]
- 34.Despres JP. Intra-abdominal obesity: an untreated risk factor for Type 2 diabetes and cardiovascular disease. J Endocrinol Invest. 2006;29:77–82 [PubMed] [Google Scholar]
- 35.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84 [DOI] [PubMed] [Google Scholar]
- 36.Bowen J, Noakes M, Trenerry C, Clifton PM. Energy intake, ghrelin, and cholecystokinin after different carbohydrate and protein preloads in overweight men. J Clin Endocrinol Metab. 2006;91:1477–83 [DOI] [PubMed] [Google Scholar]
- 37.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91:2913–9 [DOI] [PubMed] [Google Scholar]
- 38.Khalil DA, Lucas EA, Juma S, Smith BJ, Payton ME, Arjmandi BH. Soy protein supplementation increases serum insulin-like growth factor-I in young and old men but does not affect markers of bone metabolism. J Nutr. 2002;132:2605–8 [DOI] [PubMed] [Google Scholar]
- 39.Raper N, Perloff B, Ingwersen L, Steinfeldt L, Anand J. An overview of the USDA’s Dietary Intake Data System. J Food Compost Anal. 2004;17:545–55 [Google Scholar]