Abstract
Spheroid culture is a preferable cell culture approach for some cell types, including hepatocytes, as this type of culture often allows maintenance of organ-specific functions. In this study, we describe a spheroid microarray chip (SM chip) that allows stable immobilization of hepatocyte spheroids in microwells and that can be used to evaluate drug metabolism with high efficiency. The SM chip consists of 300-μm-diameter cylindrical wells with chemically modified bottom faces that form a 100-μm-diameter cell adhesion region surrounded by a nonadhesion region. Primary hepatocytes seeded onto this chip spontaneously formed spheroids of uniform diameter on the cell adhesion region in each microwell and these could be used for cytochrome P-450 fluorescence assays. A row of microwells could also be connected to a microchannel for simultaneous detection of different cytochrome P-450 enzyme activities on a single chip. The miniaturized features of this SM chip reduce the numbers of cells and the amounts of reagents required for assays. The detection of four cytochrome P-450 enzyme activities was demonstrated following induction by 3-methylcholantlene, with a sensitivity significantly higher than that in conventional monolayer culture. This microfabricated chip could therefore serve as a novel culture platform for various cell-based assays, including those used in drug screening, basic biological studies, and tissue engineering applications.
INTRODUCTION
Primary hepatocytes have been widely used for a number of different applications, including in vitro pharmacological assays1, 2 and clinical treatments such as bioartificial livers and cell transplantation.3, 4 However, primary hepatocytes isolated from the liver are known to undergo rapid alterations in their hepatic phenotype during conventional monolayer culture. For this reason, spheroid culture, in which hepatocytes form a three-dimensional (3D) spherical aggregate, has attracted much attention as a potential method for maintaining in vitro hepatocyte functions.5
Hepatocytes that form a spheroid have a cuboidal cell shapes and continue to express intercellular adhesion molecules that are required for cell-cell communication. They also stably maintain many metabolic functions, including those involved in drug metabolism.6, 7 Several culture vessels have been developed to induce the formation of spheroids, such as a low-cell adhesive surface,5 a porous substratum,8, 9 and an agitation tank.10 However, the drawbacks of these culture systems are the production of spheroids of inconsistent diameters, low throughput, and inability to immobilize the spheroids at a defined location. In some cases, cells within a large or coalesced spheroid will become necrotic owing to lack of oxygen, making it difficult to conduct reproducible assays.11, 12
Microfluidics and microscale technologies have begun to revolutionize cell-based assays.13, 14 Microfluidics technologies, in particular, are highly advantageous in a number of situations that require miniaturization, high throughput, and precise control over cellular microenvironments.15 For example, gradient generators with branched microchannels have been used to control the spatial distributions of soluble factors over spheroids immobilized within a microchannel.16 Microscale technologies such as microcontact printing have also found wide use in applications such as cell immobilization at a defined location.17, 18, 19 Most studies that have investigated microcontact printing have created two-dimensional cell patterns by accelerating or inhibiting cell attachment in predetermined regions.20 Under these types of culture conditions, hepatocytes typically spread out and form a monolayer and therefore do not maintain their normal functions. Coculture with nonparenchymal cells has improved the maintenance of functions of hepatocytes, but the improved function is generally limited to only those cells that are located at the heterogeneous cell-cell interfaces.21
In the present study, we introduce a microfabricated chip that enables spheroid size control, immobilization at a defined location, and use in simultaneous drug metabolism assays. In particular, we demonstrate that the chip can be used to estimate induction of drug metabolism pathways following chemical administration.
We use the alkoxyresorufin O-dealkylase (AROD) assay, which is a simple and useful method to evaluate drug metabolism.22 In this assay, four alkoxyresorufins (nonfluorescent) are converted to resorufin (a fluorescent product) by cytochrome P-450 isozymes. Thus, the activities of ethoxy-, methoxy-, pentoxy-, and benzyloxy-resorufin O-dealkylases (EROD, MROD, PROD, and BROD) can be evaluated by measuring the fluorescence intensity of resorufin.23 In rat hepatocytes, the activities of EROD, MROD, PROD, and BROD are related to CYP1A1, 1A2, 2B1, and 2B2 enzymes, respectively.7 We show that compared to hepatocytes grown in monolayer culture, spheroids are highly suitable for this assay owing to the maintenance of cytochrome P-450 enzyme activity and their thicker cell aggregate configuration. The presented spheroid culture chip could potentially be a useful tool for pharmacological assays.
MATERIALS AND METHODS
Materials and reagents
The materials used for the fabrication of the SM chips were purchased from the following commercial resources: poly(methylmethacrylate) (PMMA) plates from Seiko, Fukuoka, Japan; poly(dimethylsiloxane) (PDMS) (Sylgard 184) from Dow Corning, MI, USA; polyethylene glycol with a thiol group (PEG-SH) (molecular weight: 5000) from Nektar Therapeutics, AL, USA; and RGD peptide (RGDSAAAAAC) from Sigma-Aldrich, Tokyo, Japan.
The reagents used for cell culture were purchased from the following commercial sources: collagenase from Wako Pure Chemical Industries, Osaka, Japan; Dulbecco’s modified Eagle medium from Invitrogen Corp., CA, USA; insulin from Sigma-Aldrich; hydrocortisone from Wako Pure Chemical Industries; epidermal growth factor from Biomedical Technologies Inc., MA, USA; proline from Wako Pure Chemical Industries; linoleic acid from Sigma-Aldrich; 4, 6-diaminodino-2-phenylindol (DAPI) from Wako Pure Chemical Industries; 3-methylcholantlene (3-MC) from Sigma-Aldrich; ethoxyresorufin, pentoxyresorufin, benzyloxyresorufin from Sigma-Aldrich; metoxyresorufin from Wako Pure Chemical Industries; and probenecid and dicumarol from Wako Pure Chemical Industries.
Chip fabrication
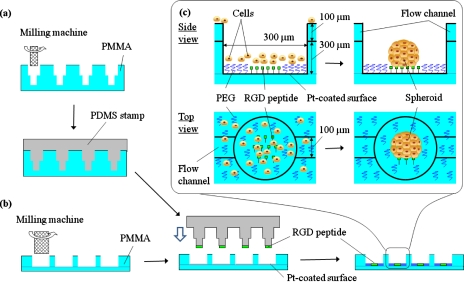
Figure 1 shows the schematics for the fabrication of SM chips using microfabrication and microcontact printing. In the present study, we fabricated two types of SM chip. One has individual microwells [batch-type SM chip, Fig. 2a], whereas the other has microwells connected to microchannels [flow-type SM chip, Fig. 2b]. We describe the details of only the flow-type SM chip since the fabrication processes are similar for both chip types and the details of the batch-type chip have been described elsewhere.24 Each chip is composed of two plates of PMMA (24 mm×24 mm). The upper side of one 400-μm-thick PMMA plate is fabricated with microchannels 100 μm wide and 100 μm deep using a programmable micromilling system (PMT Corp., Fukuoka, Japan). Holes 300 μm in diameter were then bored along with the microchannel. To configure the microwells, this plate was placed on a second 200-μm-thick PMMA plate and bonded by press heating at 110 °C for 6 h. Ten microwells were connected to each of the nine microchannels [Fig. 2b]. For the batch-type SM chip, 1575 microwells (300 μm diameter, 400 μm depth, and 330 μm pitch) were fabricated using two PMMA plates similar to those used for the flow-type SM chip [Fig. 2a].
Figure 1.
Schematics of the fabrication of a SM chip with microchannels. (a) Preparation of a stamp for microcontact printing. Concave wells of 100 μm head diameter were constructed in a PMMA plate, with which a PDMS stamp was molded. (b) Modification of microwells for cell culture. Microwells 300 μm in diameter and 400 μm in height were connected to channels 100 μm wide and 100 μm deep. The entire surface of the chip, including microwells, was coated with a thin platinum layer. The PDMS stamp was inked with 1 mM RGD peptide and microscopically contacted with the center of the bottom faces of the microwells to create cell adhesion regions. The chip was immersed in 5 mM PEG-SH in ethanol solution to obtain a cell nonadhesion region around the RGD peptide stamped regions. (c) Spheroid formation in a microwell.
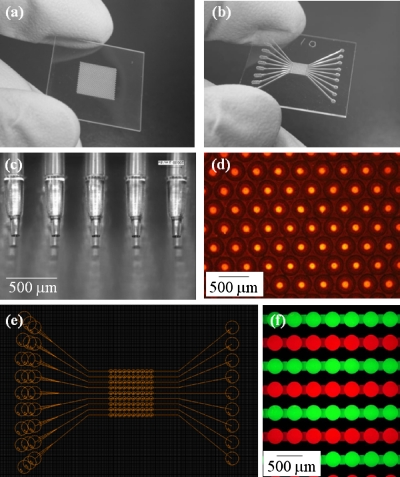
Figure 2.
SM chips. (a) Batch-type SM chip. (b) Flow-type SM chip. (c) PDMS stamp for microcontact printing. (d) Pattern configuration of cell adhesion regions constructed by microcontact printing in the batch-type SM chip, demonstrated using a red fluorescent paint. (e) Two-dimensional CAD drawing of the flow-type SM chip. (f) Microwells connected to microchannels in the flow-type SM chip, visualized with red and green fluorescent solutions.
The entire surface of the chip, including the microwells, was then coated with a 10-nm-thick layer of platinum in an ion sputter unit (Hitachi Science Systems, Ltd., Ibaragi, Japan). The master for preparing a stamp for microcontact printing was also fabricated by the micromilling system [Fig. 1a]. Concave wells with a 100 μm head diameter were constructed in a thick PMMA plate using a 300-μm-diameter end mill, followed by a 100-μm-diameter end mill. A PDMS stamp against the master was molded by casting the liquid PDMS prepolymer [Fig. 2c]. The PDMS stamp was briefly treated with oxygen plasma (Harrick Scientific Co., Ossining, NY) to increase its hydrophilicity and was then inked with 1 mM RGD peptide in phosphate buffer saline (PBS). The inked stamp was microscopically contacted with the center of the bottom face of the microwells to create cell adhesion regions [Fig. 1c]. This peptide contains cysteine at one end, and the thiol group allows the covalent bonding of the peptide onto a platinum-coated surface.25 The stamp was then carefully peeled off from the chip substrate. The chip was subsequently immersed in 5 mM PEG-SH in ethanol solution to create a cell nonadhesion region around the RGD peptide stamped islands [Fig. 1c]. The chip was then thoroughly rinsed with distilled, de-ionized water, and subsequently rinsed with 50% ethanol to remove the unattached PEG-SH and for sterilization. The chip was then immersed in Dulbecco’s modified Eagle medium and stored at 4 °C until use.
Hepatocyte isolation and culture medium
Hepatocytes were isolated from the whole liver of an adult Wistar rat (7–8-week-old male, weighing approximately 200 g) by liver perfusion using 0.05% collagenase. Cell viability was determined using the trypan blue exclusion method, and cells with over 85% viability were used for the culture. The culture medium used was Dulbecco’s modified Eagle medium supplemented with 10 μg∕ml insulin, 7.5 mg∕ml hydrocortisone, 50 ng∕ml epidermal growth factor, 60 mg∕l proline, 50 μg∕ml linoleic acid, 0.1 μM CuSO4⋅5H2O, 3 μg∕ml H2SeO3, 50 pM ZnSO4⋅7 H2O, 58.8 μg∕ml penicillin, and 100 μg∕ml streptomycin.
Cell seeding
The batch-type SM chip was placed in a 35 mm culture dish and then inoculated with isolated hepatocytes (1×106 cells) in 2 ml of culture medium. After 4 h of culture, the culture medium was replaced. For the flow-type SM chip, the entire surface of the chip, except the microwell region at the center, was covered with a PDMS substrate having a large square hole (3.5 mm×5 mm), which prevented trapping of cells within the microchannels. The chip with the PDMS substrate was placed in a 35 mm culture dish and inoculated with hepatocytes (2.5×105 cells) in 100 μl of culture medium in the microwell region of the square hole. After 4 h of culture, the PDMS substrate was removed and the culture medium was replaced with 2 ml of fresh culture medium. After 4 h of culture, both chips were cultured on a rotary shaker at 60 rpm. As a control experiment, hepatocytes (5×105 cells) in 2 ml of culture medium were inoculated onto a 35 mm type-I collagen-coated dish (Asahi Techno Glass, Tokyo, Japan) to obtain a conventional monolayer culture and were then cultured under stationary conditions. The medium was replaced 4 h after inoculation. Culture media in all the three cultures were replaced at 1 day interval. After 3 days of culture, the survival of hepatocytes inside the spheroids was evaluated by fixing the cells in 10% buffered formalin, embedding in paraffin, sectioning, and staining with hematoxylin and eosin or Masson trichrome stain.
Fluorescence-based sensing of cytochrome P-450 enzyme activities
The EROD activity with the batch-type SM chip was evaluated after 10 days of culture. The entire culture medium was replaced with fresh culture medium containing 3-MC at different concentrations, and the culture was continued for a further 24 h. The chip was washed twice with PBS solution. Subsequently, 2 ml of PBS solution containing 20 μM ethoxyresorufin, as well as 2 mM probenecid (an inhibitor of glucuronidation) and 25 μM dicumarol (an inhibitor of DT-diaphorase) to inhibit the secondary metabolism of resorufin, were added to the culture.26 The chip was incubated for 1 h and the extracellular solution containing resorufin was collected. The concentration of resorufin was measured using a fluorescence plate reader (excitation wavelength: 544 nm; emission wavelength: 590 nm; Fluoroskan Ascent, Thermo Electron, Vantaa, Finland). The number of cells was determined by a conventional fluorescence assay using DAPI (excitation wavelength: 355 nm; emission wavelength: 460 nm).27 A standard curve of cell number–fluorescence intensity was prepared using cell suspensions with densities ranging from 1.0×105 to 5.0×105 cells∕ml and used to convert fluorescence intensity to a cell number.
Four AROD activities were simultaneously evaluated using the flow-type SM chip. After 24 h of activation with 3-MC, using the same protocol mentioned above, a Kimwipe sheet was gently placed on the outlets of the microchannels to absorb the solution. A drop of PBS solution containing 20 μM ethoxyresorufin, metoxyresorufin, pentoxyresorufin, or benzyloxyresorufin was then introduced from the inlets to the outlets three times to completely replace the solution in the microwells. These four solutions were also supplemented with 2 mM probenecid and 25 μM dicumarol to inhibit the secondary metabolism of resorufin. After removing the Kimwipe, 1 μl of 20 μM ethoxyresorufin, metoxyresorufin, pentoxyresorufin, or benzyloxyresorufin solution was introduced into each microchannel from the inlets and the chip was incubated for 30 min. The chip was then washed twice with PBS solution containing 2 mM probenecid and 25 μM dicumarol. Subsequently, cells that contained the fluorescent product, resorufin, were detected with a confocal laser microscope (Bio-Rad, Hertfordshire, UK) with an argon laser light source and a 570-nm-long pass filter. The fluorescence intensity was analyzed using a Macintosh computer with the public domain NIH IMAGE program.
RESULTS AND DISCUSSION
SM chips
We have previously reported that microwells can be used to divide seeded cells into subgroups of a certain number, which then become involved in the formation of one spheroid, thereby facilitating control over the spheroid size.28 In the previous study, however, the microwells were fabricated directly at the bottom face of a conventional culture dish using a micromilling machine. Therefore, all cavities had considerable roughness at the bottom generated by the end mill, which made chemical modification difficult for the immobilization of spheroids and also interfered with fluorescent analysis of cellular functions by increasing the fluorescence background. In the present study, we improved the fabrication procedure and performed a fluorescence analysis with spheroids in microwells of both the batch-type [Fig. 2a] and the flow-type [Fig. 2b] SM chips.
Both chips types were composed of two PMMA plates. The batch-type SM chip was fabricated by bonding a plate containing 1575 through holes to a plain PMMA plate. The flow-type SM chip was fabricated by the same procedure except that microchannels were also constructed. The microchannels do not have an enclosed structure, and the upper side was open to air.
A PDMS stamp for microcontact printing was made by micromolding [Figs. 1a, 2c]. The center of the flat-bottomed surface was modified with RGD peptide by microcontact printing [Fig. 1b]. The pattern configuration of the cell adhesion regions was visualized using a solution containing red fluorescent marker [Fig. 2d]. Although this process was conducted manually under a microscope, no considerable technical hurdles were encountered, and spots could be generated in 1575 microwells in only ∼5 min. The chip was subsequently immersed in the PEG-SH solution to modify the entire surface of the chip, except the oligopeptide spots. Because the molecular weight of PEG-SH is approximately three times that of the peptide, our concern was that PEG-SH might insert itself into the adhesion region or replace the immobilized peptide during the immersion, resulting in the inhibition of cell adhesion on the cell adhesion region. However, this phenomenon was not observed. Adhesion was also confirmed by the formation of cell micropatterns on a flat substrate without microwells, as previously shown using collagen (data not shown).24
Spheroids formed within the SM chips
Hepatocytes seeded in the batch-type chip entered each microwell [Fig. 3a], aggregated at the center of the microwell after 12 h of culture and spontaneously formed spheroids on the cell adhesion regions within 2 days of culture [Figs. 3b, 3c]. More than 1500 spheroids of uniform diameter formed on a single chip. Similar spheroids were formed in the flow-type chip [Fig. 3d]. Initially, a few cells did not enter the microwells and remained on the upper surface or within the microchannels, between the microwells. However, these cells eventually entered the microwells during the rotational culture or were removed by medium exchange. The spheroids formed in both chips maintained their shape for at least 2 weeks.
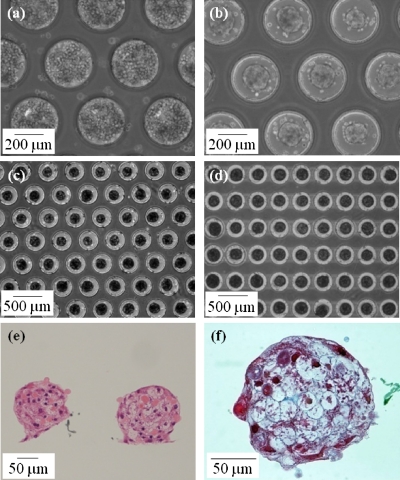
Figure 3.
Spheroid formation in the SM chips. (a) Primary hepatocytes seeded in the batch-type SM chip. (b) Hepatocytes spontaneously formed spheroids with a uniform diameter at the center of each microwell during 2 days of rotational culture. Hepatocyte spheroids in the batch-type (c) and the flow-type (d) SM chips during 7 days of rotational culture. Hematoxylin and eosin staining of vertical cross sections (e) and Masson trichrome staining of a horizontal cross section (f) of representative spheroids in the batch-type SM chip during 3 days of rotational culture.
Because cell necrosis often occurs within the core of large spheroids owing to limited oxygen supply, the regulation of uniform spheroid size is very important. The diameter of a spheroid at the survival limit has been mathematically and experimentally calculated to be in the range of 100–200 μm.29, 30, 31 Our chip system allows us to regulate the size of spheroid diameters by changing system parameters such as the microwell diameter, stamp diameter, and inoculation cell number. In the present study, we successfully controlled the spheroid diameter within the survival limit. The survival conditions of hepatocytes in the spheroids were investigated by hematoxylin and eosin staining of vertical sections [Fig. 3e]. The spheroids exhibited a hemispherical shape with a diameter of ∼150 μm. They consisted of viable nucleated cells that were observed even in the core of spheroids. Masson trichrome staining of the horizontal section demonstrated the round shape and the presence of collagen fibrils [blue stain in Fig. 3f].
Some cell types such as hepatocytes, pancreatic cells, neural stem cells, and embryonic stem cells require 3D culture environments to maintain their metabolism and growth activity and for induction of differentiation.32, 33, 34 These 3D culture modes are called “spheroid,” “neurosphere,” or “embryoid body.” Hepatocytes in spheroids had a cuboidal cell shape, unlike the conventional monolayer culture, in which cells exhibit an extended shape. The shape of hepatocytes is closely related to the maintenance of their functions in vitro.35 In the previous study, we demonstrated that hepatocytes that formed spheroids were able to stably maintain several important functions, such as albumin secretion, urea cycle enzyme activities, and expression of liver-enriched transcription factors (HNF-4 and C∕EBP-β) and cell adhesion molecules (connexin 32, E-cadherin, and claudin 3).24 In the current study, we focused on drug metabolism functions.
Fluorescent sensing of cytochrome P-450 enzyme activities on the chips
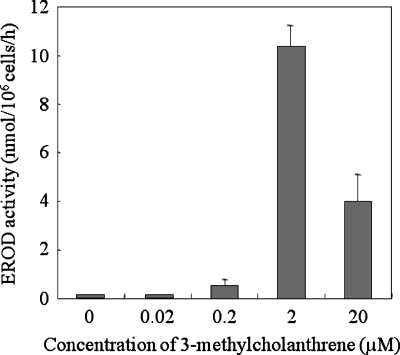
Changes in cytochrome P-450 enzyme activities of hepatocyte spheroids exposed to 3-MC, a carcinogen of polycyclic aromatic hydrocarbons, were detected using the AROD activity assay.26 Because 3-MC is known primarily to activate EROD activity, the dependence of EROD activity on the concentration of 3-MC was initially measured using the batch-type SM chip. The activity was determined by the fluorescence intensity of resorufin eluted from the cells into the culture medium. The activation of EROD activity was dependent on the concentration of 3-MC (Fig. 4). However, at the highest 3-MC concentration (20 μM), the activity decreased. Although the mechanism responsible for the decrease is unclear, the results are consistent with those of a previous study that used human primary hepatocytes, in which a clear peak of the activation by 3-MC was observed at a concentration of 2.5 μM.36 The decrease of 3-MC at higher doses was apparently not due to the cytotoxic effects of 3-MC. In the present study as well, we found no significant changes in the shape of spheroids after the evaluation (data not shown).
Figure 4.
Dependence of EROD metabolism of hepatocyte spheroids on the concentration of 3-methylcholanthrene in a batch-type SM chip during 10 days of rotational culture. The values and error bars represent the mean and standard deviation, respectively, of three independent experiments.
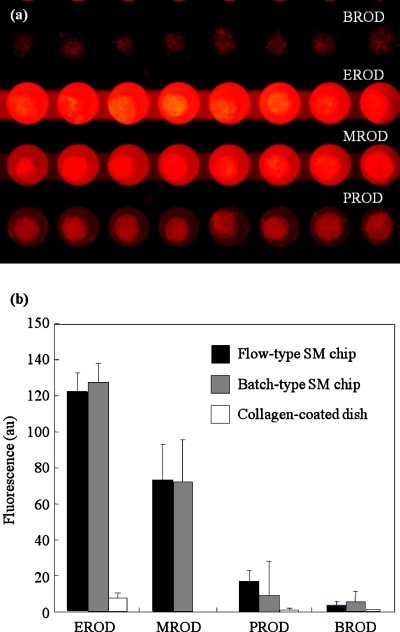
We evaluated the simultaneous changes in four AROD activities with the flow-type SM chip. This chip contained ten microwells connected to a microchannel and a total of nine microchannels were fabricated on a single chip [Fig. 2e]. The microchannel was easily flushed and the solution could be replaced following repeated washing of the channel [Fig. 2f]. Simultaneous evaluation of the AROD activities was conducted after activating hepatocytes with 2 μM of 3-MC and replacing the solution. Figure 5a shows a representative image of the red fluorescence of resorufin that was generated from ethoxy-, methoxy-, pentoxy-, and benzyloxy-resorufin by cytochrome P-450 enzymes. The cell adhesion spots and microwells prevented any washout of the spheroids during solution replacements.
Figure 5.
Four AROD activities measured simultaneously with the flow-type SM chip. (a) Representative fluorescence microscopy image. The red fluorescence of resorufin, which was obtained by the conversion of ethoxy-, methoxy-, pentoxy-, and benzyloxy-resorufin, was detected on a single chip. (b) Comparison of four AROD activities. The values and error bars represent the mean and standard deviation, respectively, of three independent experiments.
Similar experiments were also conducted with the batch-type SM chip and a collagen-coated dish. As shown in Fig. 5b, equivalent fluorescence intensities were obtained with both types of SM chips, and EROD and MROD were significantly induced by 3-MC, as described in the previous literature.23 In contrast, fluorescence intensity was very weak or undetectable in hepatocytes cultured in a monolayer in a collagen-coated dish. Although this type of culture has typically been used for primary hepatocytes, the cytochrome P-450 enzyme activities may be lost after 10 days of culture. In addition, since spheroids have a cell layer thicker than a monolayer, fluorescence detection can be improved. The spheroid culture described here is therefore very suitable for fluorescence-based assays.
CONCLUSIONS
A combination of microfabrication and microcontact printing was used to construct two types of hepatocyte culture chips. The resulting batch- and flow-type SM chips allowed spontaneous formation and immobilization of hepatocyte spheroids at the bottom of microwells. These spheroids stably maintained cytochrome P-450 enzyme activities, which could be detected on the transparent chips by measuring fluorescence following conversion of alkoxyresorufin to resorufin. Four alkoxyresorufin O-dealkylase activities were simultaneously measured on the flow-type chip. This capability is especially important when comparative evaluations are required based on fluorescent intensity. Our next objective is temporary sealing of the upper side of the microchannels on the flow-type chip using a flat substrate. This will allow continuous perfusion of solutions containing different drugs for simultaneous evaluation of their effects following the activation of a single AROD activity on a single chip. Our spheroid microarray chip could potentially be a fundamental tool for studying drug metabolism and other liver-specific functions in a miniaturized and stable manner.
ACKNOWLEDGMENTS
This research was supported by the Ministry of Education, Culture, Sports, Science and Technology via the Kitakyushu Innovative Cluster Project and by the Grant-in-Aid for Young Scientists (A) (Contract No. 20686056).
References
- Khetani S. R. and Bhatia S. N., Nat. Biotechnol. 26, 120 (2008). 10.1038/nbt1361 [DOI] [PubMed] [Google Scholar]
- Shitara Y., Horie T., and Sugiyama Y., Eur. J. Pharm. Sci. 27, 425 (2006). 10.1016/j.ejps.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Langer R. and Vacanti J. P., Science 260, 920 (1993). 10.1126/science.8493529 [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Ijima H., Fukuda J., Sakiyama R., Yamashita Y., Shimada M., Shirabe K., Tsujita E., Sugimachi K., and Funatsu K., Int. J. Artif. Organs 25, 51 (2002). [DOI] [PubMed] [Google Scholar]
- Koide N., Sakaguchi K., Koide Y., Asano K., Kawaguchi M., Matsushima H., Takenami T., Shinji T., Mori M., and Tsuji T., Exp. Cell Res. 186, 227 (1990). 10.1016/0014-4827(90)90300-Y [DOI] [PubMed] [Google Scholar]
- Peshwa M. V., Wu F. J., Sharp H. L., Cerra F. B., and Hu W. S., In Vitro Cell. Dev. Biol.: Anim. 32, 197 (1996). 10.1007/BF02722946 [DOI] [PubMed] [Google Scholar]
- Sakai Y., Tanaka T., Fukuda J., and Nakazawa K., J. Biosci. Bioeng. 109, 395 (2010). 10.1016/j.jbiosc.2009.10.001 [DOI] [PubMed] [Google Scholar]
- Ijima H., Matsushita T., Nakazawa K., Fujii Y., and Funatsu K., Tissue Eng. 4, 213 (1998). 10.1089/ten.1998.4.213 [DOI] [Google Scholar]
- Fukuda J., Sakiyama R., Nakazawa K., Ijima H., Yamashita Y., Shimada M., Shirabe K., Tsujita E., Sugimachi K., and Funatsu K., Int. J. Artif. Organs 24, 799 (2001). [PubMed] [Google Scholar]
- Sakai Y., Naruse K., Nagashima I., Muto T., and Suzuki M., Int. J. Artif. Organs 19, 294 (1996). [PubMed] [Google Scholar]
- Curcio E., Salerno S., Barbieri G., De Bartolo L., Drioli E., and Bader A., Biomaterials 28, 5487 (2007). 10.1016/j.biomaterials.2007.08.033 [DOI] [PubMed] [Google Scholar]
- Anada T., Masuda T., Honda Y., Fukuda J., Arai F., Fukuda T., and Suzuki O. Sens. Actuators B 147, 376 (2010). 10.1016/j.snb.2010.01.065 [DOI] [Google Scholar]
- El-Ali J., Sorger P. K., and Jensen K. F., Nature (London) 442, 403 (2006). 10.1038/nature05063 [DOI] [PubMed] [Google Scholar]
- Khademhosseini A., Bettinger C., Karp J. M., Yeh J., Ling Y. B., Borenstein J., Fukuda J., and Langer R., J. Biomater. Sci., Polym. Ed. 17, 1221 (2006). 10.1163/156856206778667488 [DOI] [PubMed] [Google Scholar]
- Whitesides G. M., Nature (London) 442, 368 (2006). 10.1038/nature05058 [DOI] [PubMed] [Google Scholar]
- Okuyama T., Yamazoe H., Seto Y., Suzuki H., and Fukuda J., J. Biosci. Bioeng. 110, 230 (2010). 10.1016/j.jbiosc.2010.02.001 [DOI] [PubMed] [Google Scholar]
- Kane R. S., Takayama S., Ostuni E., Ingber D. E., and Whitesides G. M., Biomaterials 20, 2363 (1999). 10.1016/S0142-9612(99)00165-9 [DOI] [PubMed] [Google Scholar]
- Fukuda J., Khademhosseini A., Yeh J., Eng G., Cheng J. J., Farokhzad O. C., and Langer R., Biomaterials 27, 1479 (2006). 10.1016/j.biomaterials.2005.09.015 [DOI] [PubMed] [Google Scholar]
- Fukuda J., Khademhosseini A., Yeo Y., Yang X. Y., Yeh J., Eng G., Blumling J., Wang C. F., Kohane D. S., and Langer R., Biomaterials 27, 5259 (2006). 10.1016/j.biomaterials.2006.05.044 [DOI] [PubMed] [Google Scholar]
- Mrksich M., Dike L. E., Tien J., Ingber D. E., and Whitesides G. M., Exp. Cell Res. 235, 305 (1997). 10.1006/excr.1997.3668 [DOI] [PubMed] [Google Scholar]
- Bhatia S. N., Balis U. J., Yarmush M. L., and Toner M., FASEB J. 13, 1883 (1999). [DOI] [PubMed] [Google Scholar]
- Behnia K., Bhatia S., Jastromb N., Balis U., Sullivan S., Yarmush M., and Toner M., Tissue Eng. 6, 467 (2000). 10.1089/107632700750022125 [DOI] [PubMed] [Google Scholar]
- Burke M. D., Thompson S., Weaver R. J., Wolf C. R., and Mayer R. T., Biochem. Pharmacol. 48, 923 (1994). 10.1016/0006-2952(94)90363-8 [DOI] [PubMed] [Google Scholar]
- Fukuda J., Sakai Y., and Nakazawa K., Biomaterials 27, 1061 (2006). 10.1016/j.biomaterials.2005.07.031 [DOI] [PubMed] [Google Scholar]
- Ulman A., An Introduction to Ultrathin Organic Films: From Langmuir-Blodgett to Self-Assembly (Academic, Boston, 1991). [Google Scholar]
- Hsiao C. C., Wu J. R., Wu F. J., Ko W. J., Remmel R. P., and Hu W. S., Tissue Eng. 5, 207 (1999). 10.1089/ten.1999.5.207 [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Izumi Y., Fukuda J., and Yasuda T., J. Biomater. Sci., Polym. Ed. 17, 859 (2006). 10.1163/156856206777996853 [DOI] [PubMed] [Google Scholar]
- Fukuda J. and Nakazawa K., Tissue Eng. 11, 1254 (2005). 10.1089/ten.2005.11.1254 [DOI] [PubMed] [Google Scholar]
- Glicklis R., Merchuk J. C., and Cohen S., Biotechnol. Bioeng. 86, 672 (2004). 10.1002/bit.20086 [DOI] [PubMed] [Google Scholar]
- Fukuda J., Okamura K., Nakazawa K., Ijima H., Yamashita Y., Shimada M., Shirabe K., Tsujita E., Sugimachi K., and Funatsu K., Cell Transplant. 12, 51 (2003). 10.3727/000000003783985151 [DOI] [PubMed] [Google Scholar]
- Fukuda J., Mizumoto H., Nakazawa K., Kajiwara T., and Funatsu K., Int. J. Artif. Organs 27, 1091 (2004). [DOI] [PubMed] [Google Scholar]
- Inaba R., Khademhosseini A., Suzuki H., and Fukuda J., Biomaterials 30, 3573 (2009). 10.1016/j.biomaterials.2009.03.045 [DOI] [PubMed] [Google Scholar]
- Karp J. M., Yeh J., Eng G., Fukuda J., Blumling J., Suh K. Y., Cheng J., Mahdavi A., Borenstein J., Langer R., and Khademhosseini A., Lab Chip 7, 786 (2007). 10.1039/b705085m [DOI] [PubMed] [Google Scholar]
- Sakai Y., Yoshida S., Yoshiura Y., Mori R., Tamura T., Yahiro K., Mori H., Kanemura Y., Yamasaki M., and Nakazawa K., J. Biosci. Bioeng. 110, 223 (2010). 10.1016/j.jbiosc.2010.01.021 [DOI] [PubMed] [Google Scholar]
- Berthiaume F., Moghe P. V., Toner M., and Yarmush M. L., FASEB J. 10, 1471 (1996). [DOI] [PubMed] [Google Scholar]
- Delescluse C., Ledirac N., de Sousa G., Pralavorio M., Botta-Fridlund D., Letreut Y., and Rahmani R., Toxicol. In Vitro 11, 443 (1997). 10.1016/S0887-2333(97)00077-5 [DOI] [PubMed] [Google Scholar]