Abstract
Twin studies suggest that global and regional brain volumes are highly heritable. However, estimates of heritability vary across development. Given that all twin studies are open to the potential criticism of non-generalizability due to differences in intrauterine environment between twins and singletons, these age effects may reflect the influence of perinatal environmental factors which are unique to twins and which may be especially evident early in life. To address this question, we compared brain volumes and the relationship of brain volumes to gestational age in 136 singletons (67 male, 69 female) and 154 twins (75 male, 79 female; 82 DZ, 72 MZ) who had received high resolution MRI scans of the brain in the first month of life. Intracranial volume, total white matter, and ventricle volumes did not differ between twins and singletons. However, cerebrospinal fluid and frontal white matter volume was greater in twins compared to singletons. While gray matter volumes at MRI did not differ between groups, the slope of the relationship between total and cortical gray matter and gestational age at the MRI scan was steeper in MZ twins compared to DZ twins. Post-hoc analyses suggested that gray matter development is delayed in MZ twins in utero and that they experience “catch-up” growth in the first month of life. These differences should be taken into account when interpreting and designing studies in the early postnatal period.
INTRODUCTION
Twin studies have provided important insights into the genetic basis of a wide range of complex traits. Comparing the resemblance of MZ twins to DZ twins allows researchers to estimate the proportion of phenotypic variance attributable to genetic factors, shared environment, and unique environment. There are a number of large, well-established twin registries with carefully collected phenotypic and environmental data, often longitudinal. In addition to their use in classical twin study designs, these registries hold great potential for studying genotype-environment interactions and for evaluating the contribution of specific polymorphisms to genetic variance (Boomsma et al., 2002). However, all twin studies are potentially limited by non-generalizability due to differences in intrauterine and family environment between twins and singletons (Pol et al., 2002). In this paper, we examine the comparability of twins and singletons in neonatal brain structure and development.
Global brain volumes in adults, including total intracranial volume (ICV), total gray matter, and total white matter, are reported to be highly heritable, often with heritability estimates greater than 0.8 or 0.9 (reviewed in (Peper et al., 2007; Schmitt et al., 2007)). However, heritability may vary with the age of the study population. A sizeable study of twin children (aged 5–19 years) reported that while heritability of global brain volumes was, in general, very similar to those reported in adults (Wallace et al., 2006), heritability of total gray matter decreased with increasing age in late childhood and adolescence. In contrast, heritability of white matter increased over time. A recent study of the heritability of brain volumes during the neonatal period further demonstrates the importance of age effects. Gilmore et al. (2010) reported that the heritability of total gray matter in neonates was less than that observed in older children or adults (0.56 in neonates versus 0.82 (Wallace et al., 2006) and 0.77 (Peper et al., 2008) in children and 0.82 (Baare et al., 2001) in adults). A similar pattern was observed for the cerebellum which showed very low heritability (0.17) in neonates compared to earlier reports in children (0.49, (Wallace et al., 2006)) and adults (>0.60, (Wright et al., 2002)). In contrast, heritability of total white matter in neonates was very similar to that reported in older populations (0.85 for neonates versus 0.85/0.84 in children (Peper et al., 2008; Wallace et al., 2006) and 0.87 in adults (Baare et al., 2001)). It has been hypothesized that these age effects reflect “canalization”, that is the idea that as multiple genetic programs act over development heritability increases, resulting in a mature phenotype which is highly heritable, but a developmental trajectory which is shaped by the environment (Lenroot & Giedd, 2008). However, the reduced heritability of these structures in early life may also reflect the influence of prenatal environmental factors which are unique to twin pregnancies, the effects of which are limited to early life. We addressed this question by comparing total and regional brain volumes in a large sample of twin and singleton neonates.
MATERIALS AND METHODS
Subjects
136 singleton neonates (67 male, 69 female) and 154 twin (75 male, 79 female) neonates who had T1 and T2/proton density scans that were free of major motion and produced high quality tissue-segmentation results. There were 82 DZ (43 male, 39 female) and 72 MZ (32 male, 40 female) twins. Mothers were recruited during the second trimester of pregnancy from the outpatient obstetrics and gynecology clinics at UNC hospitals. Exclusion criteria at enrollment were the presence of abnormalities on fetal ultrasound or major medical or psychotic illness in the mother. Additional exclusion criteria for this analysis included premature birth (defined as birth at less than 32 weeks, n = 21) and major perinatal or postnatal complications such as sepsis, pneumonia, or respiratory distress requiring intubation (n = 13). All scans were also reviewed by a neuroradiologist (J.K.S.). Children with a significant CNS abnormality on MRI were also excluded (n = 2). 40 subjects had small incidental subdural hematomas or other small intracranial bleeds, which are present in ≈25% of vaginal births (Looney et al., 2007); these subjects were included in the analysis. Demographic data of the sample is found in Table 1. This study was approved by the Institutional Review Board of the University of North Carolina (UNC) School of Medicine.
Table 1.
Demographic characteristics of twins and singletons in this sample
| Factor | Twin or Singleton | |||
|---|---|---|---|---|
| Singleton | Twin | Overall | ||
| Maternal Ethnicity | White N (%) | 117 (86.03) | 68 (74.73) | 185 (81.5) |
| Black | 17 (12.50) | 19 (20.88) | 36 (15.86) | |
| Asian | 2 (1.47) | 4 (4.40) | 6 (2.64) | |
| Gender | Male N (%) | 67 (49.26) | 45 (49.45) | 112 (49.34) |
| Female | 69 (50.74) | 46 (50.55) | 115 (50.66) | |
| Gestational age at birth(days)* | Mean (SD) | 276 (10) | 254 (11) | 267 (15) |
| Gestational age at MRI(days)* | Mean (SD) | 299 (11) | 290 (19) | 295 (16) |
| Gestational age birth to MRI(days)* | Mean (SD) | 23 (9) | 36 (20) | 28 (16) |
| Age of mothers at time of birth(years) | Mean (SD) | 31 (5) | 30 (6) | 30 (5) |
| Average Birth Weight per twin pair(gr)* | Mean (SD) | 3406 (484) | 2515 (410) | 3051 (631) |
| Greater Birth Weight between twin pair(gr)* | Mean (SD) | 3406 (484) | 2603 (425) | 3086 (606) |
| Smaller Birth Weight between twin pair(gr)* | Mean (SD) | 3406 (484) | 2426 (417) | 3016 (664) |
| Maternal education (years)* | Mean (SD) | 16 (3) | 14 (4) | 15 (3) |
| Total household income (USD) | Mean (SD) | 83000 (135000) | 58000 (44000) 73000 (110000) | |
p < 0.0001
Image Acquisition
Magnetic Resonance Imaging was carried out at the UNC MRI Research Center on a Siemens head-only 3T scanner (Allegra, Siemens Medical System Inc., Erlangen, Germany). All subjects were studied without sedation. Once a child was asleep, they were fitted with earplugs and placed in the MRI scanner with head in a Vac-Fix immobilization device, and additional foam padding to diminish the sounds of the scanner. Scans were carried out with a neonatal nurse present and a pulse oximeter was used to monitor heart rate and oxygen saturation.
For the majority of the scans, T1-weighted images were obtained using a 3D spoiled gradient (FLASH TR/TE/Flip Angle 15/7msec/25°). The first 20 singletons and the first 2 twin pairs to attend were scanned using a 3D magnetization prepared rapid gradient echo (MP-RAGE TR/TI/TE/Flip Angle 1820/400/4.38ms/7°) T1 sequence. Proton density and T2 weighted images were obtained with a turbo spin echo sequence (TSE TR/TE1/TE2/Flip Angle 6200/20/119ms/150°). Spatial resolution was 1 × 1 × 1mm3 voxel size for T1 weighted images and 1.25 × 1.25 × 1.5mm3 voxel size with 0.5 mm interslice gap for proton density/T2 weighted images. The Siemens head-only 3T scanner is FDA approved for use in all age groups. Specific absorption rates are kept within safe levels for body weight by both hardware and software features of the scanner. We have previously confirmed that the scan sequences did not cause significant temperature increases with a phantom (Gilmore et al., 2005).
Tissue Segmentation Neonates
Brain tissue was classified as gray matter (GM), unmyelinated white matter (uWM), myelinated white matter (mWM) and cerebrospinal fluid (CSF) using an automatic, atlas-moderated expectation maximization segmentation tool as previously described (Gilmore et al., 2007; Prastawa et al., 2005). Note that the major separation of WM/GM at neonate is driven by the T2w image, which has a stronger WM/GM contrast than the T1w image in this age group. As a result, the difference between using the MP-RAGE T1 or the FLASH T1 is relatively minor. In addition, the use of a joint T1w & T2w segmentation reduces the influence of the T1 protocol change. Parcellation of each subject’s brain into regions was achieved in the neonate by non-linear warping of a parcellation atlas template as previously described (Gilmore et al., 2007; Knickmeyer et al., 2008). Left and right hemispheres were subdivided into four regions along the anterior-posterior axis (roughly corresponding to pre-frontal, frontal, parietal, and occipital regions). The cerebellum, brainstem and combined sets of subcortical structures are represented separately. After deformation, the parcellation template is combined with the tissue classification maps and results in estimates of GM, uWM, mWM, and CSF for each region. The volume of mWM in the cortex was very small and likely represented partial-volume effects; therefore we did not perform statistical tests on cortical mWM.
Ventricle Volumes
The neonatal lateral ventricles are segmented using InsightSnap (SNAP), a semi-automated 3D segmentation tool, which uses a level-set evolution method (Yushkevich et al., 2006). SNAP is controlled by both a user-defined initialization and by data-specific segmentation protocols with region-growing parameters that operate in conjunction with the probabilistic CSF map generated during tissue segmentation.
Statistical Analysis
For cross-sectional analyses of demographic characteristics between MZ and DZ twins and singletons, we used two sample t-tests (for two group comparisons) or ANOVA (Analysis of Variance) F-tests (for three group comparisons) for continuous variables and Fisher Exact tests for categorical variables. Tests of differences in the occurrence of birth complications between twin pregnancies and singleton pregnancies was conducted based on chi-squared tests and Fisher’s exact tests.
To test for differences in variance of brain volumes between MZ and DZ twins and singletons we used all of the singletons and randomly selected 1 of the twins in each pair. We used GLM to predict brain volumes using only the grouping variable as a predictor; then the HOVTEST option was used to calculate Levene’s homogeneity of variances.
For all comparisons of mean brain volumes between MZ and DZ twins and singletons, we performed analysis of covariance (ANCOVA) controlling for gender and gestational age at MRI using PROC MIXED of SAS system (ver 9.12). Previous studies demonstrate the controlling for age at MRI and gender are critical (Gilmore et al., 2005). We included both twin members, and we fit a mixed model with twin pair as a random effect to capture correlation between subjects within twin pairs. In other words, the individual twins within a pair are treated as replications, while singleton neonates had no replicates (singletons are considered random block with size 1). This enables twins and singletons to be used in the same analysis without violating independence assumptions or discarding information by using only one of two twins. There were 126 twin neonates with scans available for both members of the pair (63 pairs) and 28 twin neonates where scans were only available for 1 member of a pair. We fitted a heterogeneous covariance model to estimate covariance between twin members differently by MZ twin and DZ twin since we expected the correlation between MZ twin members would be higher than the correlation between DZ twin members (Munoz et al., 1986). ANCOVA with gestational age at MRI as a continuous variable was used to examine differences in slopes between singleton and twin groups. Graphical exploration of the data, including plots of “Leverage” and “Cook’s distance” suggested that one twin subject with an extremely high gestational age at MRI could be a potential leverage point or influential observation that would affect the estimate of regression coefficients. This subject was excluded from the analysis of brain volumes, demographic data and comparison of birth complications. All statistical hypothesis tests are two-tailed and conducted at a significance level of 0.05.
RESULTS
Comparison of demographic characteristics revealed that the twin and singleton groups did not differ in the reported ancestry of the mother, the ratio of males to females, maternal age at birth, or total household income. Twins had a significantly lower gestational age at birth (p < 0.0001) and at MRI (p < 0.0001). The interval between birth and MRI was greater for twins than it was for singletons (p < 0.0001). Regardless of whether one compared singletons to the heaviest twin in each pair, the lightest twin in each pair, or the average weight of the twin pair; twins had significantly lower birth weights than singletons with (p ≤ 0.0005) or without (p < 0.0001) correcting for gestational age at birth. Mothers of twins had fewer years of education than mothers of singletons (p < 0.0001) (See Table 1). Review of participants’ medical records revealed that singletons had significantly greater head circumference (HC) at birth (p < 0.0001, 4% greater in singletons compared to twins), but this difference was eliminated when correcting for gestational age at birth. Singletons had significantly greater HC than both MZ (p = < 0.0001, 6% difference) and DZ twins (p = 0.001, 4% difference) at birth. DZ twins had slightly greater HC at birth than MZ twins (p = 0.006, 2% difference) at birth. When correcting for gestational age at birth, there was a statistical trend for group differences in HC at birth (p = 0.08). Post-hoc comparisons showed that DZ twins continued to have slightly greater HC at birth than MZ twins after correction for gestational age at birth (p = 0.02, 1.5%). However, there were no significant differences in head circumference at the MRI visit.
Comparison of birth complications indicated that twin and singleton pregnancies did not differ in the occurrence of maternal diabetes, pregnancy hypertension, bleeding, placental problems (brevia or abruption), rubella immunity, RH incompatibility, or maternal medical problems. Being a twin was significantly associated with occurrence of preterm labor (p < 0.0001), preterm premature rupture of membranes (p < 0.0001), and other pregnancy problems (p = 0.0033).
For our comparison of group differences in the variance of intracranial volume, total tissue volumes, regional tissue volumes, and lateral ventricle volume we were unable to rule out the null hypothesis (i.e. there was no convincing evidence that the groups differed in variance).
Comparison of mean intracranial volume, total tissue volumes, regional tissue volumes, and lateral ventricle volume between singletons and twins indicated that neonatal brain structure is very similar in the two groups when controlling for gestational age at MRI and gender (see Table 2). The only areas which showed significant differences between groups were total volume of CSF (p = 0.001; 8% greater in twins compared to singletons) and volume of frontal uWM (p = 0.014; 4% greater in twins compared to singletons). Dividing the twin group based on zygosity revealed additional information (See Table 3). First, while total volume of CSF was significantly larger in both MZ and DZ twins when compared to singletons (p = 0.002 for the overall comparison), the size of the effect was larger for MZ twins (p < 0.001, 12% difference) than for DZ twins (p = 0.045, 6% difference). CSF volume was not significantly different when comparing MZ twins and DZ twins to each other. These differences do not appear to reflect differences in lateral ventricle volume, which did not differ significantly between groups. There were also significant group differences for frontal and parietal uWM (p = 0.035 and p = 0.041). Post-hoc comparisons showed that this effect was driven by DZ twins who had significantly greater frontal uWM volumes than singletons (p = 0.012, 4% difference) and significantly greater parietal uWM volumes than both singletons and MZ twins (p = 0.037, 3% difference and p = 0.026, 4% difference). Intriguingly, group comparisons of total GM volume, total cortical GM, and parietal GM could not be performed as the p value for the homogeneity of slope (volume versus gestational age at MRI) was less than 0.05 for these variables, suggesting that there were group differences in the relationship between gestational age and gray matter volumes. In other words, there may be group differences in the developmental trajectory of GM; this was examined next.
Table 2.
Twin-Singleton Comparison of Global and Regional Tissue Volumes (Values Corrected for Gestational Age at MRI and Gender)
| Brain Volumes (all in mm3) | Singleton LSmeans(SE) |
Twin LSmeans(SE) |
|---|---|---|
| Intracranial Volume | 467874 (3591) | 477474 (3809) |
| Total Brain Volume | 412730 (3053) | 417483 (3255) |
| Total Brain Gray Matter | 241901 (1814) | 243253 (1820) |
| Total Brain Unmyelinated White Matter | 160841 (1323) | 163877 (1475) |
| Total Brain Myelinated White Matter | 10009 (327) | 10374 (355) |
| Total Brain Cerebrospinal Fluid* | 55248 (889) | 59835 (1051) |
| Total cortical volume | 360258 (2680) | 364905 (2946) |
| Cortical Gray Matter | 201050 (1543) | 202416 (1592) |
| Cortical Unmyelinated White Matter | 152561 (1243) | 155212 (1414) |
| Cerebellum | 25157 (252) | 24891 (262) |
| Subcortical Area and Brainstem | 27319 (363) | 27697 (339) |
| Prefrontal Gray Matter | 28429 (382) | 27330 (421) |
| Prefrontal Unmyelinated White Matter | 24351 (282) | 24898 (329) |
| Frontal Gray Matter | 43937 (385) | 44588 (405) |
| Frontal White Unmyelinated Matter+ | 38186 (361) | 39576 (405) |
| Parietal Gray Matter | 60073 (553) | 60930 (555) |
| Parietal Unmyelinated White Matter | 48375 (486) | 49206 (499) |
| Occipital Gray Matter | 68629 (643) | 69754 (707) |
| Occipital Unmyelinated White Matter | 41652 (427) | 41826 (481) |
| Lateral Ventricles | 4398 (146) | 4261 (196) |
p < 0.01,
p < 0.05; LSmeans = Least-squares means
Table 3.
Singleton-MZ-DZ Comparison of Global and Regional Tissue Volumes (Values Corrected for Gestational Age at MRI and Gender)
| Brain Volumes (all in mm3) | Singleton LSmeans(SE) |
MZ LSmeans(SE) |
DZ LSmeans(SE) |
|---|---|---|---|
| Intracranial Volume | 467925 (3593) | 475535 (5548) | 478996 (4934) |
| Total Brain Volume | 412824 (3054) | 413419 (4787) | 420556 (4131) |
| Total Brain Gray Matter# | 242117 (1936) | 244130 (2799) | 243894 (2256) |
| Total Brain Unmyelinated White Matter | 160883 (1324) | 161945 (2099) | 165517 (1916) |
| Total Brain Myelinated White Matter | 10013 (327) | 10151 (559) | 10511 (439) |
| Total Brain Cerebrospinal Fluid* | 55208 (890) | 61964 (1599) | 58473 (1300) |
| Total cortical volume | 360339 (2681) | 361431 (4255) | 367716 (3798) |
| Cortical Gray Matter# | 201186 (1644) | 203445 (2436) | 202769 (1988) |
| Cortical Unmyelinated White Matter | 152603 (1243) | 153273 (1977) | 156968 (1860) |
| Cerebellum | 25162 (252) | 24529 (462) | 25045 (303) |
| Subcortical Area and Brainstem | 27324 (363) | 27450 (551) | 27831 (408) |
| Prefrontal Gray Matter | 28427 (382) | 27425.39 (642) | 27263 (531) |
| Prefrontal Unmyelinated White Matter | 24350 (282) | 24921 (432) | 24867 (478) |
| Frontal Gray Matter | 43946 (385) | 44104 (638) | 44881 (496) |
| Frontal White Unmyelinated Matter+ | 38193 (361) | 39226 (582) | 39869 (528) |
| Parietal Gray Matter# | 60272 (589) | 60640 (878) | 61511 (667) |
| Parietal Unmyelinated White Matter+ | 48398 (486) | 47921 (744) | 50061 (603) |
| Occipital Gray Matter | 68628 (644) | 69846 (1069) | 69685 (897) |
| Occipital Unmyelinated White Matter | 41666 (427) | 41220 (692) | 42323 (621) |
| Lateral Ventricles | 4400 (147) | 4193 (294) | 4307.09 (244) |
p for homogeneity of slopes is < 0.05, test for overall group differences not appropriate;
p < 0.01;
p < 0.05; LSmeans = Least-squares means
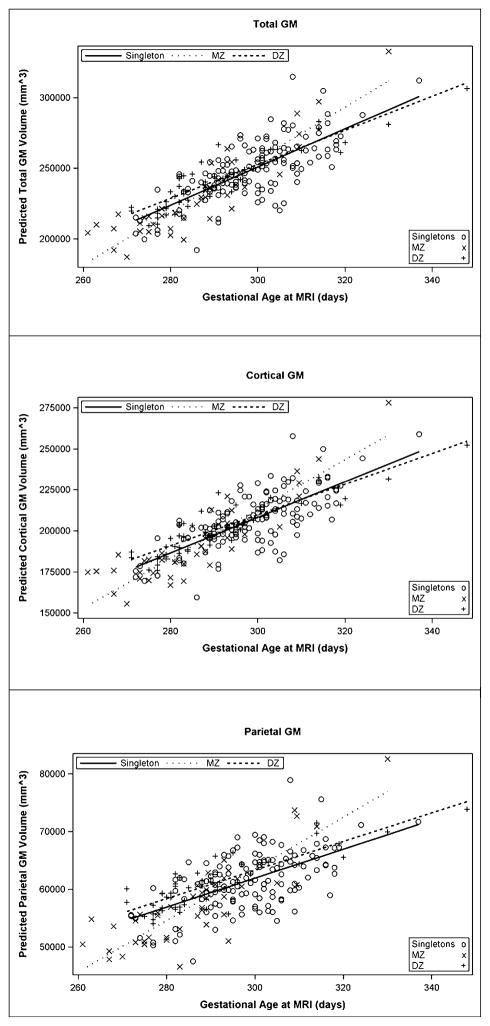
When comparing the combined twin group to singletons, there were no significant differences in the trajectory of brain development for any variable. However, when the twin group was divided based on zygosity, group differences were seen for the developmental trajectories of total GM volume (p = 0.024), total cortical GM (p = 0.024), and parietal GM (p = 0.021). Post-hoc comparisons showed that the slope of the relationship between total GM volume and gestational age at MRI was steeper in MZ twins compared to DZ twins (p = 0.009) – that is total GM was increasing at a greater rate in MZ twins than DZ twins. The same relationship was observed for total cortical GM (p = 0.007). For parietal GM, the slope of the relationship between parietal GM volume and gestational age at MRI was higher in MZ twins compared to both DZ twins (p = 0.009) and singletons (p = 0.017). Though significant, these differences are not very large in absolute terms (see Table 4). No group differences were seen for the other brain volume variables examined.
Table 4.
Singleton-MZ-DZ comparison of growth trajectory in first 10 weeks of life
| Brain Volumes | Group | Increase per week | Percent increase over 10 weeks |
|---|---|---|---|
| Total Gray Matter | Singleton | 9653 mm3 | 16% |
| MZ | 12887 mm3 | 17% | |
| DZ | 8449 mm3 | 14% | |
| Cortical Gray Matter | Singleton | 7735 mm3 | 15% |
| MZ | 10458 mm3 | 17% | |
| DZ | 6559 mm3 | 14% | |
| Parietal Gray Matter | Singleton | 1813 mm3 | 14% |
| MZ | 3073 mm3 | 17% | |
| DZ | 1757 mm3 | 14% |
Calculations based on regression equations applied to the period from 280 days (40 weeks) gestational age to 350 days (50 weeks) gestational age.
Since being a twin was significantly associated with occurrence of preterm labor and preterm premature rupture of membranes in this sample, we also reran our analyses with gestational age at birth as a covariate. Twin-singleton differences in total CSF and frontal WM were not present when correcting for gestational age at birth. However, DZ twins continued to have larger parietal WM volumes than both singletons and MZ twins after correction (p = 0.03 and 0.02 respectively). Also, the observed group differences in the developmental trajectory of GM volumes remained significant and were of a similar magnitude even after correcting for gestational age at birth (See Figure 1). This could indicate that gray matter development was delayed in utero in MZ twins and that MZ twins are experiencing a period of “catch-up” growth post-birth. To test this hypothesis we performed a post-hoc analysis in which we calculated the least squares means for each of these regions at birth and examined differences between the three groups, adjusting for gestational age at birth. Singletons had significantly larger estimated GM volumes (total, cortical, and parietal) at birth than DZ twins (p < 0.001, 7–9% difference) who had significantly larger GM volumes than MZ twins (p < 0.001, 20–25% difference).
Figure 1.
Linear regression plot between predicted gray matter volumes (total, cortical, and parietal) and gestational age at MRI in singletons, DZ twins, and MZ twins. Data is cross-sectional and has been corrected for gestational age at birth and gender.
The pattern of results was similar when we reran analyses excluding those individuals scanned with the MP-RAGE T1 sequence.
Discussion
This study compared neonatal brain morphology between twins and singletons. We examined intracranial volume, total tissue volumes, lobar tissue volumes, and lateral ventricle volume and the developmental trajectory of these variables. Intracranial volume, total white matter, and ventricle volumes did not differ between twins and singletons or between MZ and DZ twins. These results indicate that heritability estimates for these variables made in neonatal twin samples are generalizable to the singleton population. In addition, it suggests that for some aspects of structural brain development, twins and singletons can be combined in statistical analyses without controlling for group membership. This will allow more powerful studies addressing the relationship between neonatal brain structure and cognitive development and the impact of specific genetic polymorphisms and environmental factors on early brain development.
However, we also observed potentially important differences between twins and singletons. First, we observed that twins had significantly greater total CSF volume and that the size of this effect was larger for MZ twins than for DZ twins. This does not appear to reflect ventricle enlargement, as no significant differences were observed when CSF within the lateral ventricles was measured on its own. There were also significant group differences for frontal and parietal uWM, driven by greater WM in DZ twins. While we did not observe any differences in gray matter volume when comparing the combined twin group to singletons, we did observe some intriguing differences when we divided the samples based on zygosity. Total GM and cortical GM increased at a greater rate in MZ twins than DZ twins and parietal GM increased at a greater rate in MZ twins than it did in either DZ twins or singletons. We hypothesize that gray matter development is delayed in utero in MZ twins and that MZ twins are experiencing a period of “catch-up” growth post-birth. Graphical exploration of the data is compatible with this interpretation (see Figure 1) as was a post-hoc analysis we performed to compare predicted brain volumes at birth, corrected for gestational age at birth. Twins appear to have caught up to singletons by 20 days post-term. While MRI is not currently capable of identifying the neurodevelopmental processes which underlie this growth, post-mortem studies suggest that dendritic and axonal arborization and spine growth are likely candidates (Mrzljak et al., 1990).
Longitudinal follow-up will be necessary to confirm group differences in developmental trajectory and to ascertain if and when overall volume differences disappear. Ordaz et al. (2010) carried out a similar study in children and adolescents (mean age 11.0, SD 3.6, youngest participants were 4 years old) and observed no differences between twins and singletons. They also tested group by age interactions in a regression framework and reported no interactions – suggesting that the twin-singleton differences we observed will resolve by early childhood. However, they did not perform comparisons with the twin group separated by zygosity. In a study of adults (mean age of 30.7 years, standard deviation 9.6 years), no effects of zygosity on adult brain volumes were observed (Pol et al., 2002).
The differences we observed could be the result of a suboptimal intrauterine environment in twins compared to singletons, particularly in the case of MZ twins. Twin pregnancies have a higher rate of maternal and perinatal complications (Rao et al., 2004) and higher extended perinatal mortality (Glinianaia et al., 2000) then singleton pregnancies, which is partially the result of the higher proportion of preterm and low birthweight twins. Preterm birth in singletons may be associated with alterations in brain structure and development. The most common neuropathology reported in association with preterm birth is periventricular white matter damage which may also produce ventricular system enlargement as a downstream effect (Hart et al., 2008; Mathur & Inder, 2009; Rutherford et al., 2010). Preterm birth has been associated with decreased volume of cortical gray matter at term-equivalent age (Inder et al., 2005; Peterson et al., 2003) and poor postnatal head growth (Cheong et al., 2008). Volume reductions associated with prematurity may persist into late childhood (Kesler et al., 2008). This pattern seems quite distinct from the pattern we observed in monozygotic twins. In addition, these studies primarily focused on very preterm children (born < 30 weeks gestational age) with very low birth weights and often with observable white matter damage. Such children would have been excluded from the current study. However, since being a twin was significantly associated with occurrence of preterm labor and preterm premature rupture of membranes in this sample, we also reran our analyses with gestational age at birth as a covariate. Twin-singleton differences in total CSF and frontal WM were not present when correcting for gestational age at birth. However, DZ twins continued to have larger parietal WM volumes than both singletons and MZ twins and the observed group differences in the developmental trajectory of total, cortical, and parietal GM volumes remained significant and were of a similar magnitude even after correcting for gestational age at birth.
In addition to the increased incidence of preterm birth, two-thirds of MZ twin pregnancies are monochorionic; that is the two offspring share the same placenta (Machin, 1995). The presence of vascular anastomoses in monochorionic placentas can produce an unequal blood supply to the twins, in extreme cases producing twin-twin transfusion syndrome (TTS) (Rao et al., 2004). While we excluded infants with major perinatal or postnatal complications from this analysis and twins and singletons did not differ on many common pregnancy complications, being a twin was significantly associated with “other pregnancy problems” in this sample. However, MZ and DZ twins did not differ from each other in the frequency of other pregnancy problems. In addition, we did not observe significant differences between MZ and DZ pairs in discordance of head circumference at birth or discordance of weight at birth (Mukherjee et al., 2009), which suggests that growth competition between the twins was not a major factor.
It is unclear whether the early differences in brain development we observed between twins and non-twins have any long-term functional relevance. Several sizeable studies of twins born during the mid-20th century showed that twins scored 4–5 points lower on IQ tests than singletons (Deary et al., 2005; Record et al., 1970; Ronalds et al., 2005), but a recent large cohort of ninth grade children (age 15 or 16) who were born in the late 1980s showed no effect of twin status on a general test of academic achievement (Christensen et al., 2006). The authors suggest that improvements in obstetric and pediatric practices may be responsible for the differences between these studies. However, methodological differences may also be involved. Posthuma et al. (2000) found no twin-singleton differences in IQ in a sample of adults born mid-century. Unlike most other studies, this study compared twins to their own non-twin siblings, thereby matching for genetic background and familial environment. Age of testing may also be a factor with significant twin-singleton differences reported more frequently in young twins (Alin-Akerman, 1995; Dezoete & MacArthur, 1996; Koeppen-Schomerus et al., 2003; Myrianthopoulos et al., 1976; Wilson, 1979). We will continue to follow this cohort with magnetic resonance imaging at 1, 2, 4, and 6 years of age and will also carry out developmental testing to help resolve this issue. It should also be kept in mind that delayed gray matter development might reflect a physiological adaptation to the limited uterine environment rather than a pathological process. A similar argument has been made regarding birth weight in twins (Blickstein, 2004).
In conclusion, we observed significant differences in gray matter development in MZ twins compared to DZ twins and singletons which was not related to gestational age at birth. We hypothesize that gray matter development is delayed in utero in twins (particularly MZ twins), followed by a period of “catch-up” growth post-birth. We also observed that twins had significantly greater total CSF and frontal and parietal uWM. These findings were, in part, related to gestational age at birth. In contrast, we observed that intracranial volume, total white matter volume, and lateral ventricle volume did not differ between twins and singletons in the first month of life and neither did the developmental trajectory of these variables. Our findings require replication, but should be taken into account when interpreting and designing studies in this age-range. Even if the differences we observed were to persist, this does not mean that MZ twins cannot be included with DZ twins and singletons in studies of typical brain development, but researchers should consider controlling for zygosity especially if predictor variables of interest could vary with zygosity.
Acknowledgments
This work was supported by NIMH Conte Center Grant MH064065 (J.H.G.), MH070890 (J.H.G), HD03110 (G.G.), and MH083045 (R.C.K). We thank Joe Blocher, Meghan Casey, Brooke Taylor and the staff of the UNC MRI Research Center for technical assistance and valuable discussions. We thank Dianne Evans and Mary Norton, study coordinators, and their research team. We also thank our participating families who have made this project possible.
References
- Alin AB. Eight-year follow-up of cognitive development in 33 twin pairs. Acta Geneticae Medicae et Gemellologiae (Roma) 1995;44:179–188. [PubMed] [Google Scholar]
- Baaré WFC, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJC, Schnack HG, van Haren NEM, van Oel CJ, Kahn RS. Quantitative genetic modeling of variation in human brain morphology. Cerebral Cortex. 2001;11(9):816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Blickstein I. Is it normal for multiples to be smaller than singletons? Best Practice & Research in Clinical Obstetrics & Gynaecology. 2004;18(4):613–623. doi: 10.1016/j.bpobgyn.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews Genetics. 2002;3(11):872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Cheong JLY, Hunt RW, Anderson PJ, Howard K, Thompson DK, Wang HX, Bear MJ, Inder TE, Doyle LW. Head growth in preterm infants: Correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics. 2008;121(6):E1534–E1540. doi: 10.1542/peds.2007-2671. [DOI] [PubMed] [Google Scholar]
- Christensen K, Petersen I, Skytthe A, Herskind AM, Mcgue M, Bingley P. Comparison of academic performance of twins and singletons in adolescence: follow-up study. British Medical Journal. 2006;333(7578):1095–1097. doi: 10.1136/bmj.38959.650903.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Pattie A, Wilson V, Whalley LJ. The cognitive cost of being a twin: Two whole-population surveys. Twin Research and Human Genetics. 2005;8(4):376–383. doi: 10.1375/1832427054936709. [DOI] [PubMed] [Google Scholar]
- Dezoete JA, MacArthur BA. Cognitive development and behavior in very low birthweight twins at four years. Acta Geneticae Medicae et Gemellologiae (Roma) 1996;45:325–332. doi: 10.1017/s0001566000000921. [DOI] [PubMed] [Google Scholar]
- Gilmore J, Looney C, Vesta Y, Smith JK, Lin W, Lieberman JA, Gerig G. Early postnatal brain structure and development in humans: Sexual dimorphism and cerebral asymmetry are present at birth. Neuropsychopharmacology. 2005;30:S154–S154. [Google Scholar]
- Gilmore J, Schmitt J, Knickmeyer R, Smith JK, Lin W, Styner M, Gerig G, Neale MC. Genetic and environmental contributions to neonatal brain structure: A twin study. Human Brain Mapping. 2010;31:1174–1182. doi: 10.1002/hbm.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. Journal of Neuroscience. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinianaia SV, Pharoah P, Sturgiss SN. Comparative trends in cause-specific fetal and neonatal mortality in twin and singleton births in the North of England, 1982–1994. British Journal of Obstetrics and Gynaecology. 2000;107(4):452–460. doi: 10.1111/j.1471-0528.2000.tb13261.x. [DOI] [PubMed] [Google Scholar]
- Hart AR, Whitby EW, Griffiths PD, Smith MF. Magnetic resonance imaging and developmental outcome following preterm birth: review of current evidence. Developmental Medicine and Child Neurology. 2008;50(9):655–663. doi: 10.1111/j.1469-8749.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Hüppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Reiss AL, Vohr B, Watson C, Schneider KC, Katz KH, Maller-Kesselman J, Silbereis J, Constable RT, Makuch RW, Ment LR. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. Journal of Pediatrics. 2008;152(4):513–520. doi: 10.1016/j.jpeds.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knickmeyer R, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen-Schomerus G, Spinath FM, Plomin R. Twins and non-twin siblings: Different estimates of shared environmental influence in early childhood. Twin Research. 2003;6(2):97–105. doi: 10.1375/136905203321536227. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: Initial findings from a neuroimaging study of pediatric twins. Development and Psychopathology. 2008;20(4):1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney CB, Smith JK, Merck LH, Wolfe HM, Chescheir NC, Hamer RM, Gilmore JH. Intracranial hemorrhage in asymptomatic neonates: Prevalence on MR images and relationship to obstetric and neonatal risk factors. Radiology. 2007;242(2):535–541. doi: 10.1148/radiol.2422060133. [DOI] [PubMed] [Google Scholar]
- Machin G. Twins and their disorders. In: Reed G, Claireaux A, Cockburn F, editors. Diseases of the fetus and newborn. London: Chapman & Hall; 1995. pp. 201–225. [Google Scholar]
- Mathur A, Inder T. Magnetic resonance imaging-Insights into brain injury and outcomes in premature infants. Journal of Communication Disorders. 2009;42(4):248–255. doi: 10.1016/j.jcomdis.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HBM, Van Eden GG, Judáš M. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Progress in Brain Research. 1990;85:185–222. doi: 10.1016/s0079-6123(08)62681-3. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Kang C, Wolfe HM, Hertzberg BS, Smith JK, Lin WL, Gerig G, Hamer RM, Gilmore JH. Discordance of prenatal and neonatal brain development in twins. Early Human Development. 2009;85(3):171–175. doi: 10.1016/j.earlhumdev.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz A, Rosner B, Carey V. Regression-analysis in the presence of heterogeneous intraclass correlations. Biometrics. 1986;42(3):653–658. [PubMed] [Google Scholar]
- Myrianthopoulos N, Nichols P, Broman S. Intellectual development of twins comparison with singletons. Acta Geneticae Medicae et Gemellologiae (Roma) 1976;25:376–380. doi: 10.1017/s0001566000014458. [DOI] [PubMed] [Google Scholar]
- Ordaz S, Lenroot R, Wallace GL, Clasen L, Blumenthal J, Schmitt J, Giedd JN. Are there differences in brain morphometry between twins and unrelated singletons? A pediatric MRI study. Genes, Brain and Behavior. 2010;9(3):288–295. doi: 10.1111/j.1601-183X.2009.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper J, Schnack H, Brouwer R, van Baal C, van Leeuwen M, Collins L, Evans A, Boomsma D, Kahn R, Hulshoff-Poll H. Heritability of brain structure at the onset of puberty: An MRI study in 9-year old twin-pairs. Psychophysiology. 2008;45:S17–S17. [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff-Poll HE. Genetic influences on human brain structure: A review of brain imaging studies in twins. Human Brain Mapping. 2007;28(6):464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, Gore JC, Duncan CC, Makuch R, Ment LR. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111(5):939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- Pol HEH, Posthuma D, Baare WFC, De Geus EJC, Schnack HG, van Haren NEM, van Oel CJ, Kahn RS, Boomsma DI. Twin-singleton differences in brain structure using structural equation modelling. Brain. 2002;125:384–390. doi: 10.1093/brain/awf035. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJC, Bleichrodt N, Boomsma DI. Twin-singleton differences in intelligence. Twin Research. 2000;3:83–87. doi: 10.1375/136905200320565535. [DOI] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin WL, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Medical Image Analysis. 2005;9(5):457–466. doi: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Rao A, Sairam S, Shehta H. Obstetric complications of twin pregnancies. Best Practice & Research in Clinical Obstetrics & Gynaecology. 2004;18(4):557–576. doi: 10.1016/j.bpobgyn.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Record RG, McKeown T, Edwards JH. An investigation of difference in measured intelligence between twins and single births. Annals of Human Genetics. 1970;34:11. doi: 10.1111/j.1469-1809.1970.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Ronalds GA, De Stavola BL, Leon DA. The cognitive cost of being a twin: evidence from comparisons within families in the Aberdeen children of 1950s cohort study. British Medical Journal. 2005;331(7528):1306. doi: 10.1136/bmj.38633.594387.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MA, Supramaniam V, Ederies A, Chew A, Bassi L, Groppo M, Anjari M, Counsell S, Ramenghi LA. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology. 2010;52:505–521. doi: 10.1007/s00234-010-0700-y. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Wallace GL, Rosenthal MA, Molloy EA, Ordaz S, Lenroot R, Clasen LS, Blumenthal JD, Kendler KS, Neale MC, Giedd JN. A multivariate analysis of neuroanatomic relationships in a genetically informative pediatric sample. Neuroimage. 2007;35(1):70–82. doi: 10.1016/j.neuroimage.2006.04.232. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Schmitt JE, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN. A pediatric twin study of brain morphometry. Journal of Child Psychology and Psychiatry. 2006;47(10):987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Wilson RS. Twin Growth - Initial deficit, recovery, and trends in concordance from birth to 9 years. Annals of Human Biology. 1979;6(3):205–220. doi: 10.1080/03014467900007212. [DOI] [PubMed] [Google Scholar]
- Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET. Genetic contributions to regional variability in human brain structure: Methods and preliminary results. Neuroimage. 2002;17(1):256–271. doi: 10.1006/nimg.2002.1163. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]