Abstract
The observation of a micellar cubic phase is reported for a mixture of an antimicrobial peptide from the Lactoferrin family, LFampin 265-284, and a model membrane system of dimyristoylphosphatidylcholine/dimyristoylphosphatidylglycerol (3:1), as derived from small-angle x-ray diffraction (SAXD) measurements. The system shows remarkable thermotropic polymorphism: the peptide disrupts the lipid bilayer, forming a cubic phase of the space group Pm3n (t < 28°C), and as the temperature increases it shows a complex phase behavior (not fully clarified by SAXD). The onset, volume fraction of each phase, and phase parameters are seen to vary with peptide/lipid ratio and temperature. The obtained SAXD data represent the first experimental evidence, to our knowledge, of a micellar cubic phase in the context of antimicrobial peptide/membrane interaction. We propose that the micellization of the membrane according to the carpet model, for long proposed as a possible mechanism of action, can go through the formation of a cubic micellar phase.
The biological relevance of lipid polymorphism has long been a subject of interest, stemming mainly from the seminal work of Luzzati (1). The subject has been taken up again, in particular as it regards the role of cubic phases, related initially to the crystallization of proteins but later also to the activity of proteins and peptides (2). Much more recently, the formation of cubic phases has also been reported in relation to antimicrobial peptides (3–6) (AMPs). AMPs are regarded as potential therapeutic agents to overcome the growing resistance of bacterial strains to conventional antibiotics (7). Furthermore, they are key components of the innate and acquired immunity. Although their antimicrobial action is established, the detailed mechanism of action is still prone to discussion. In particular, although it is known that AMPs can alter the bilayer structure, and the formation of cubic phases has been reported, a detailed discussion of this change as an actual possible mechanism of action is only now being addressed (6) and is by no means fully understood. Most work reported so far with induction of cubic phases by AMPs uses phosphatidylethanolamine, and the results show that some AMPs can induce cubic-phase formation of the bicontinuous type before the hexagonal phase transition.
In this work we report the occurrence of a different cubic phase involving two lipids, dimyristoylphosphatidylcholine (DMPC) and dimyristoylphosphatidylglycerol (DMPG), induced by a peptide of the Lactoferrin family, recently discovered by Bolscher et al., namely, LFampin 265-284, which is particularly active against Candida albicans (8). We have been studying peptides of this new antimicrobial domain in Lactoferrin (9) by a variety of biophysical techniques, in an attempt to characterize their mechanism of action. We used a mixture of DMPC/DMPG at a molar ratio of 3:1 in HEPES buffer, pH 7.4 (10 mM, NaCl 100 mM) as a simplified model for the lipidic cell membrane. Peptide solution in the same buffer was added to the liposome suspension, and the mixtures were incubated for 30 min at 40°C and transferred into glass capillaries or sandwiched between Kapton foils. Small angle x-ray diffraction (SAXD) experiments were performed at beamline A2 in HASYLAB at Deutsches Elektronen-Synchrotron, Hamburg, Germany. The sample was equilibrated at each selected temperature for 1–5 min.
Due to the negatively charged DMPG, the mixture DMPC/DMPG (3:1) does not show a well organized lamellar phase (typical for DMPC), and in SAXD we observed a broad scattering with intensity at the level of background (not shown), compatible with unilamellar and/or oligolamellar vesicles. Our dynamic light scattering experiments confirmed the presence of DMPC/DMPG vesicles with a size of ∼250 nm. LFampin 265-284 was added to DMPC/DMPG vesicles at molar ratios (peptide/lipid (P/L)) 1:5, 1:8, and 1:14. We did not observe any changes in the lipid diffraction pattern at the lowest ratio, 1:14 (not shown), upon addition of the peptide. Fig. 1 shows SAXD of the 1:5 mixture at selected temperatures. The diffraction peaks were fitted by Lorentzians above a linear background. At low temperature, we observed a cubic phase whose diffraction maxima can be assigned to positions √2, √4, √5, √6, √8, √13 and √18. They fit extremely well a cubic phase of Pm3n space group symmetry. The lattice parameter a = 129.0 ± 0.1 Å (at 22°C) was determined as the slope of the line s (Å–1) = f(√(h2 + k2 + l2) (where h, k, and l are Miller indices) passing through the origin (0,0), with R2 = 0.99996. Upon further sample heating, we observed a broad scattering underneath one to three sharp maxima in the range 30–50°C. The broad scattering can represent the superposition of more cubic phases, or structures without long-range order. A small peak at s ∼ 0.013 Å–1 at 50°C indicates the onset of the next structural changes in the mixture. Two sharp peaks (L1 and L2) in diffractograms taken above 70°C fit well with a lamellar phase. The detailed inspection of diffractograms has revealed marked asymmetry of both peaks, and their deconvolution (not shown) proposes the coexistence of two phases: lamellar, with periodicity ∼66 Å, and hexagonal (including peaks marked by arrows in Fig. 1), with lattice parameter ∼77 Å. However, the phase transition is not completed, as indicated by the small peak at s ∼ 0.018 Å–1, confirming that the phase composition of the mixture is complex and the that full understanding of the system requires study at additional P/L ratios.
Figure 1.
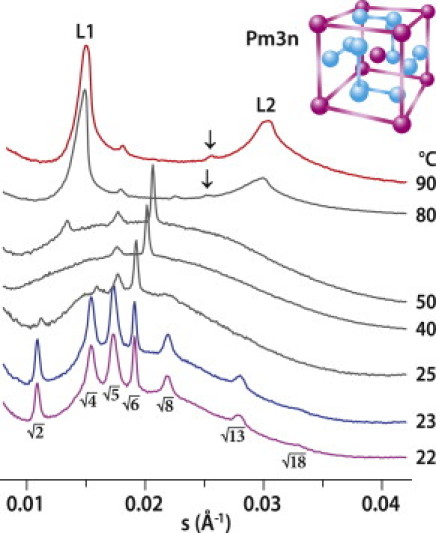
SAXD patterns for P/L mixture 1:5 (mol/mol) at selected temperatures. (Inset) Pm3n unit cell.
The micellar cubic phase Pm3n was identified in aqueous solutions of surfactants (10,11), lysophosphatidylcholines (10), and gangliosides (12), but not in connection with the AMP effect on membranes. The Pm3n phase usually observed at high water content is supposed to consist of discrete micelles of amphiphile arranged on the cubic lattice (Fig. 1, inset) and separated by a continuous film of water. Two types of micelles, one spherical and one somewhat flattened (12), or eight prolate micelles (13) divided into two groups corresponding to two types of sites with different properties, have been discussed in the literature. The positively charged AMP (nominal charge +4) has a preferential interaction with the negatively charged DMPG, and our other biophysical experiments confirm that this peptide does not partition to pure zwitterionic membranes. We believe that it induces charge segregation, as also recently suggested (14), and therefore, we hypothesize that we have P/L mixed micelles and vesicles, with the flattened form representing elongated forms of the bilayer (Fig. 1 inset, blue), less rich in DMPG, with AMP helices at the rims (15). The observation of a lamellar phase at high temperatures is surprising, as micellar-phase Pm3n is known to change either to micellar solution or to hexagonal phase. The diffractograms of the 1:8 mixture (Fig. 2) shed more light on the appearance of the lamellar phase. At temperatures below 28°C, the mixture also forms a cubic phase, Pm3n. We found the lattice parameter a = 117.8 ± 0.1 Å at 24°C (Fig. 2 inset; R2 = 0.999995). This value is smaller than that obtained for the 1:5 mixture, showing that the lattice parameters depend on the P/L ratio. In this mixture, SAXD recognizes the lamellar-phase onset already at 29°C, as indicated by two peaks whose ratio between their s values is L2/L1 = 2, superimposed over a broad background, similar to that observed for P/L = 1:5. With increasing temperature, the intensity of the broad background decreased. The diffractogram at 50°C (Fig. 2) shows a well ordered lamellar phase with the repeat distance d = 65.9 ± 0.1 Å, similar to the value retrieved at higher temperature for the ratio 1:5. At 50°C, DMPC multilamellar vesicles show a repeat distance of 60.7 Å. The higher spacing observed compared to pure DMPC can be accounted for either by the presence of peptide (positively charged) between the negatively charged patches of the membranes (formed by DMPG) or by a residual charge imbalance of the bilayer itself. The volume fraction of the lamellar phase in the mixture is indeed related to the molar ratio, P/L, and that would be the reason why the lamellar phase is less well defined for P/L = 1:5. As the temperature is raised, the cubic phase vanishes and a redistribution of charged AMP induces massive structural changes. Most probably, part of the mixture forms mixed lipid/peptide aggregates, and some fraction of the peptide is bound with lipid in the lamellar phase. The quality of the order of the Pm3n cubic phase was found to depend on the P/L ratio in the process of the sample heating/cooling.
Figure 2.
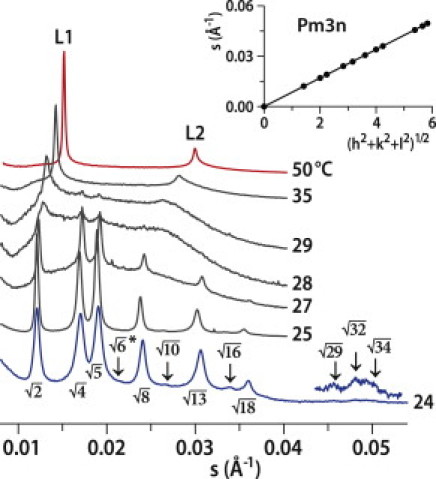
SAXD patterns for P/L mixture 1:8 at selected temperatures. The peak at position √6 is systematically absent. (Inset) A plot of s = f(sqrt(h2 + k2 + l2)) for all observed reflections at 24°C.
Altogether, these observations suggest a complex phase behavior, with temperature-dependent phases of different chemical composition. It should be mentioned that the data presented do not give complete information about the phase diagram of the system. By its nature, SAXD identifies only structures with long-range order, and temperature-induced structural changes represent a dynamic process with the experimental protocol used here. In all cases, it should be stressed that the studied system shows remarkable polymorphic behavior.
To the best of our knowledge, the Pm3n cubic phase has never been reported in the context of AMP action. As the P/L ratios tested are now known to be biologically relevant (16), these results can thus provide experimental proof of one of the mechanisms of antimicrobial action that has long been proposed, namely, the micellization mechanism that would follow an initial carpet model (7,17,18). In this model, after an initial covering of the membrane with peptide, when the peptide reaches a threshold value, the membrane eventually disintegrates, being solubilized upon formation of mixed micelles. This peptide is particularly active against C. albicans, which is richer in phosphatidylcholine than phosphatidylethanolamine, and we thus hypothezise that this is a possible mechanism of action against this pathogen.
Acknowledgments
This research received funding from the European Commission's 7th Framework Program (FP7/2007-2013) under grant agreement No. 226716 (HASYLAB project II-20090024 EC), and financial support from a Fundação para a Ciência e a Tecnologia/Centro de Investigaçãos em Química (Universidade do Porto) to M.B., Dutch Digestive Foundation (grant WS 01-42) to J.G.M.B., and Ministerstva školstva Slovenskej republiky grants VEGA 1/0292/09 (D.U.) and SK-PT-0015-10 (D.U. and M.B.).
Contributor Information
Margarida Bastos, Email: mbastos@fc.up.pt.
Daniela Uhríková, Email: uhrikova@fpharm.uniba.sk.
References and Footnotes
- 1.Luzzati V. Biological significance of lipid polymorphism: the cubic phases. Curr. Opin. Struct. Biol. 1997;7:661–668. doi: 10.1016/s0959-440x(97)80075-9. [DOI] [PubMed] [Google Scholar]
- 2.Killian J.A., Nyholm T.K.M. Peptides in lipid bilayers: the power of simple models. Curr. Opin. Struct. Biol. 2006;16:473–479. doi: 10.1016/j.sbi.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Zweytick D., Tumer S., Lohner K. Membrane curvature stress and antibacterial activity of lactoferricin derivatives. Biochem. Biophys. Res. Commun. 2008;369:395–400. doi: 10.1016/j.bbrc.2008.01.176. [DOI] [PubMed] [Google Scholar]
- 4.Fuhrmans M., Knecht V., Marrink S.J. A single bicontinuous cubic phase induced by fusion peptides. J. Am. Chem. Soc. 2009;131:9166–9167. doi: 10.1021/ja903224q. [DOI] [PubMed] [Google Scholar]
- 5.Staudegger E., Prenner E.J., Lohner K. X-ray studies on the interaction of the antimicrobial peptide gramicidin S with microbial lipid extracts: evidence for cubic phase formation. Biochim. Biophys. Acta. 2000;1468:213–230. doi: 10.1016/s0005-2736(00)00260-1. [DOI] [PubMed] [Google Scholar]
- 6.Haney E.F., Nathoo S., Prenner E.J. Induction of non-lamellar lipid phases by antimicrobial peptides: a potential link to mode of action. Chem. Phys. Lipids. 2010;163:82–93. doi: 10.1016/j.chemphyslip.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 8.van der Kraan M.I.A., Nazmi K., Bolscher J.G. Distinct bactericidal activities of bovine lactoferrin peptides LFampin 268-284 and LFampin 265-284: Asp-Leu-Ile makes a difference. Biochem. Cell Biol. 2006;84:358–362. doi: 10.1139/o06-042. [DOI] [PubMed] [Google Scholar]
- 9.Bolscher J.G.M., Adão R., Veerman E.C. Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie. 2009;91:123–132. doi: 10.1016/j.biochi.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Vargas R., Mariani P., Luzzati V. Cubic phases of lipid-containing systems. The structure of phase Q223 (space group Pm3n). An x-ray scattering study. J. Mol. Biol. 1992;225:137–145. doi: 10.1016/0022-2836(92)91031-j. [DOI] [PubMed] [Google Scholar]
- 11.Sakya P., Seddon J.M., Tiddy G.J.T. Micellar cubic phases and their structural relationships: the nonionic surfactant system C12EO12/water. Langmuir. 1997;13:3706–3714. [Google Scholar]
- 12.Gulik A., Delacroix H., Luzzati V. Polymorphism of ganglioside-water systems: a new class of micellar cubic phases. Freeze-fracture electron microscopy and x-ray scattering studies. J. Phys. II France. 1995;5:445–464. [Google Scholar]
- 13.Fontell K., Fox K.K., Hansson E. On the structure of the cubic phase I1 in some lipid-water systems. Mol. Cryst. Liq. Cryst. 1985;1:9–17. [Google Scholar]
- 14.Epand R.M., Epand R.F. Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim. Biophys. Acta. 2009;1788:289–294. doi: 10.1016/j.bbamem.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Bechinger B. Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Curr. Opin. Colloid. Interface Sci. 2009;14:349–355. [Google Scholar]
- 16.Melo M.N., Ferre R., Castanho M.A.R.B. Antimicrobial peptides: linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009;7:245–250. doi: 10.1038/nrmicro2095. [DOI] [PubMed] [Google Scholar]
- 17.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 18.Oren Z., Shai Y. Mode of action of linear amphipatic α-helical antimicrobial peptides. Biopolymers. 1998;47:413–491. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]


