Abstract
Background and Aims:
An association of high lactate levels with mortality has been found in adult patients with septic shock. However, there is controversial literature regarding the same in children. The aim of this study was to find the correlation of serum lactate levels in pediatric septic shock with survival.
Settings and Design:
This was a prospective observational study at PICU of a tertiary care center of North India.
Materials and Methods:
A total of 30 children admitted to PICU with diagnosis of septic shock were included in the study. PRISM III score and demographic characteristics of all children were recorded. Serum lactate levels were measured in arterial blood at 0-3, 12, and 24 h of PICU admission. The outcome (survival or death) was correlated with serum lactate levels.
Results:
Septic shock was the most common (79.3%) type of shock and had 50% mortality. Initial as well as subsequent lactate levels were significantly higher in nonsurvivors. A lactate value of more than 45 mg/dl (5 mmol/l) at 0–3, 12, and 24 h of PICU admission had an odds ratio for death of 6.7, 12.5, and 8.6 (95% CI: 1.044–42.431, 1.850–84.442, 1.241–61.683) with a positive predictive value (PPV) of 38%, 71%, 64% and a negative predictive value (NPV) of 80%, 83%, and 83%, respectively.
Conclusions:
Nonsurvivors had higher blood lactate levels at admission as well as at 12 and 24 h. A lactate value of more than 45 mg/dl (5 mmol/l) was a good predictor of death.
Keywords: Lactate level, pediatric, PRISM III score, septic shock
Introduction
Septic shock is one of the most frequent life-threatening conditions encountered in pediatric hospitals.[1] The optimal management of pediatric septic shock patients includes early recognition of inadequate tissue perfusion and its timely correction in an effort to prevent anaerobic metabolism, acidosis, and cellular death.[2–4] The knowledge of factors which affect the outcome in septic shock will help in the early recognition of children who are at the highest risk of death and may allow timely changes in therapy, which may improve the outcome.[5] Multiple conditions resulting in inadequate oxygen delivery, disproportionate oxygen demand, and diminished oxygen use may lead to elevated lactate levels. Hyperlactatemia is a cardinal finding of sepsis and septic shock. It is said that the mechanism for hyperlactatemia under both the conditions is different. While in sepsis, an increased lactate level represents the increased glycolytic flux due to hypermetabolism; in septic shock, an increased glycolytic flux is due to tissue hypoxia. This suggests that there are two varieties of lactate, namely, the “stress lactate” and the “shock lactate.”[6] Blood lactate levels of up to 18 mg/dl (2 mmol/l) are usually defined to be normal for critically ill patients. Hyperlactatemia is defined as lactate levels between 18 and 45 mg/dl without metabolic acidosis whereas lactic acidosis is defined as lactate levels greater than 45 mg/dl and pH below 7.35.[7] Increased lactate levels may be considered an early marker of a potentially reversible state, e.g., early septic shock, possibly indicating that “there is still room” to boost fast intervention.[8] In adults with sepsis/septic shock, the lactate level is an important and well-studied prognostic marker of mortality.[9,10] There are not many studies on lactate levels in the pediatric age group with sepsis/septic shock. In neonates, studies have shown a poor correlation between pH or base excess and blood lactate concentration, and independent measurements of the latter are needed.[11] In preterm newborns, hyperlactatemia has been described as an indicator of the presence of sepsis, but the predictive value for the outcome is uncertain.[12] In pediatric sepsis/septic shock, results of lactate are conflicting. Duke et al. reported lactate as a good predictor of mortality whereas in a study by Hatherill et al. there was no significant difference in lactate levels between survivors and nonsurvivors.[13,14] The present study was conducted with an objective to measure serial lactate levels in children with septic shock, and correlates these levels with the outcome.
Materials and Methods
It was a prospective observational study conducted in PICU of a tertiary care center of North India. Thirty cases of septic shock between the ages of 1 month and 12 years were enrolled prospectively in the study over a period of 1 year. Septic shock was defined as sepsis with either hypotension, i.e., systolic BP < 2 SD adjusted for age or at least one manifestation of inadequate organ perfusion, i.e., (1) altered mentation (defined as irritability, lethargy, semicoma/coma), (2) hypoxia (PaO2 < 45 mmHg while breathing room air or PaO2/FiO2 < 350), (3) metabolic acidosis (arterial pH < 7.35 or base deficit > 5), or (4) oliguria (i.e., urine output < 1 ml/kg/h for >2 h measured with a urinary catheter), along with signs of poor peripheral perfusion, i.e., poor capillary refill (CFT >3 s), cyanosis, or diminished peripheral circulation. Sepsis was defined as clinical or laboratory evidence of infection in the presence of more than two of the following findings: (1) temperature > 38°C or < 36°C, (2) WBC count abnormalities (i.e., >15,000 cells/mm3, <4000 cells/mm3, or 10% immature neutrophils), and (3) increased acute phase reactants (i.e., ESR >20 mm/h or CRP >20 mg/l). A positive blood culture for a likely pathogen or bacterial culture from an otherwise sterile site was not necessary for diagnosis of sepsis.
Following patients were excluded from the study: (1) patients with other causes of shock, not due to sepsis itself, e.g., cardiogenic, oligemic, anaphylactic, neurogenic, endocrinological, and dengue shock; (2) patients with known malignancies and immunosuppressive treatment; (3) patients with serious underlying neurological disease, chronic illness, and major congenital malformations, and (4) postoperative cases. Following clinical data were recorded for all patients: age/sex, underlying infection, PRISM III score, duration of the illness before the onset of septic shock, duration of shock, fluid boluses and inotropes received before the admission to PICU, and need and indication of mechanical ventilation.[15] Patients were monitored for vital parameters, Glasgow Coma Scale (GCS), urine output, and central venous pressure.
Arterial blood gas (ABG) analysis was done at 0–3, 12, 24, 48 h, and then as and when required. The serum lactate level was measured in arterial blood at 0–3 h (lactate 1), 12 h (lactate 2) and 24 hs (lactate 3) by the NADH-dependent kinetic method using the reagent manufactured by Randex Laboratory Ltd., UK. Standard treatment for septic shock was used for all patients as per guidelines.[2,16] The outcome of patients was recorded as “survived” or “expired.” Serial serum lactate levels were compared between survivors and nonsurvivors. Secondly, the outcome was also correlated with the PRISM III score at admission to PICU. The correlation between the PRISM III score and lactate level at admission (lactate 1) was measured. The study was approved by the institution's ethics committee.
Statistical methods
Statistical methods were performed using the Windows SPSS software version 16. The continuous variables with normal distribution were expressed as means ± 2 SD and were compared using Student's t-test, whereas continuous variables with an asymmetric distribution were expressed as median and the respective range interval (minimum and maximum) and were compared using the nonparametric Mann-Whitney test, for independent samples. In the case of categorical variables, Pearson's chi-square test, Yates’ correction in 2 × 2 contingency tables, and Fisher's exact test were used to analyze differences in proportions. Lactate levels and PRISM III score were further analyzed using the receiver operating characteristic (ROC) and optimal cut-off points were chosen for the calculation of positive and negative predictive values and odds ratios. A test that predicts an outcome no better than chance has an area under the ROC curve of 0.5. An area under the ROC curve above 0.8 indicated fairly good prediction.[17] The relationship of lactate at admission (lactate 1) with the PRISM III score (at admission) was determined by calculating the Spearman correlation coefficient and two-tailed significance.
Results
A total of 250 children were admitted in PICU during the study period. Out of these, 58 (23.2%) cases were admitted with shock. Forty-six of these (79.4% of all patients with shock and 18.4% of total PICU admissions) were diagnosed clinically as septic shock and 12 (4.8% of total PICU cases) with other types of shock. Other types of shock were enteric fever with shock, nine cases, cardiogenic shock, two cases, and dengue shock, one case. Sixteen cases of septic shock were excluded from the study: eight postoperative cases and eight having associated chronic disease.
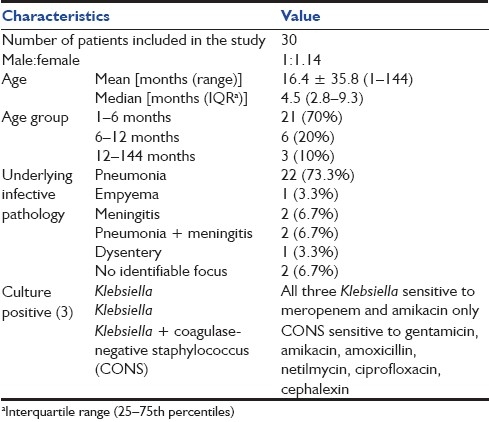
Characteristics of patients admitted in PICU with septic shock are shown in Table 1. Pneumonia (73.3%) was the most common underlying infection associated with septic shock. There were only 3 (10%) patients who had culture-positive sepsis; all of these survived.
Table 1.
Characteristics of patients admitted in PICU with septic shock
The median PICU stay of our cohort was 8.5 (IQR 2–14; range 0.2–35) days. The mean duration of the PICU stay among survivors and nonsurvivors was 12.2 ± 7.4 and 6.0 ± 9.0 days, respectively. The overall mortality of PICU admissions was 34.8% (87 out of 250) and 15 out of 30 cases of septic shock died (50% mortality).
Twenty (66.7%) patients of septic shock required mechanical ventilation. The median duration of ventilation was 72 (IQR 13.4–258; range 2–840) h. Among the survivors and nonsurvivors, the median duration of ventilation was 264 (IQR 132–408; range 96–456) and 32 (IQR 9–192; range 2–840) h, respectively. In our study, 20 (66.7%) cases of septic shock had one or more organ dysfunction other than cardiovascular system. Among these, most common organ dysfunction was respiratory in 20 (66.7%) followed by central nervous system in 16 (53.3%), renal failure in 5 (16.7%), hematologic (DIC) in 2 (6.7%) patients, and hepatic failure in 1 (3.3%) patient.
In four patients, lactate 2 and 3 levels could not be measured as patients died within 12 h of PICU admission. Mean and median lactate 1, 2, and 3 levels were 60.1 ± 49.0, 51.7 (range 7.1–252.7, IQR 31.9–73.9); 56.4 ± 38.6, 47.8 (range 7.4–138.3, IQR 23.6–85.9); and 49.2 ± 44.7, 43.8 (range 5.9-–70.0, IQR 11.0–61.0) mg/dl, respectively. The distribution of lactate levels were as follows: lactate 1: less than 18 mg/dl, 3 (survived: 2, death:- ); 18–45 mg/dl, 7 (survived: 6, death: 1); more than 45 mg/dl, 16 (survived: 6, death: 10); lactate 2: less than 18 mg/dl, 5 (survived: 4, death: 1); 18–45 mg/dl, 7 (survived: 6, death: 1); more than 45 mg/dl, 14 (survived: 4, death: 10); and lactate 3: less than 18 mg/dl, 6 (survived: 5, death: 1); 18–45 mg/dl- 6 (survived: 5, death: 1); more than 45 mg/dl, 11 (survived:4, death, 7).
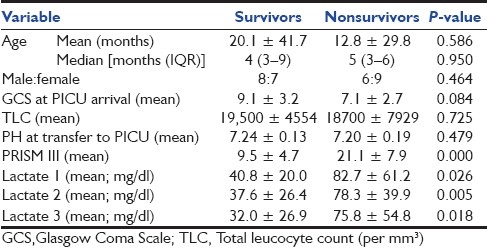
Table 2 shows that age, sex, total leucocyte counts, GCS, and pH at the time of transfer to PICU were not significantly different between survivors and nonsurvivors. All three lactate levels and PRISM III score were significantly higher in nonsurvivors as compared to survivors.
Table 2.
Various clinical parameters, PRISM III scores, and lactate levels among survivors and nonsurvivors
PRISM III score and lactate levels were further analyzed using the ROC curve. Results are shown in Table 3 and Figure 1. The area under the ROC curve for the PRISM III score (0.909) suggests that it was a strong predictor of mortality in septic shock patients. For all three lactate levels, the area under the ROC curve was almost 0.8 (P-value < 0.05) which indicates a fair correlation between the lactate level and death [Table 3].
Table 3.
Area under the receiver operating characteristic curve
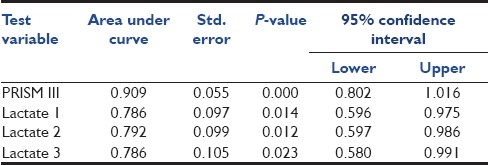
Figure 1.
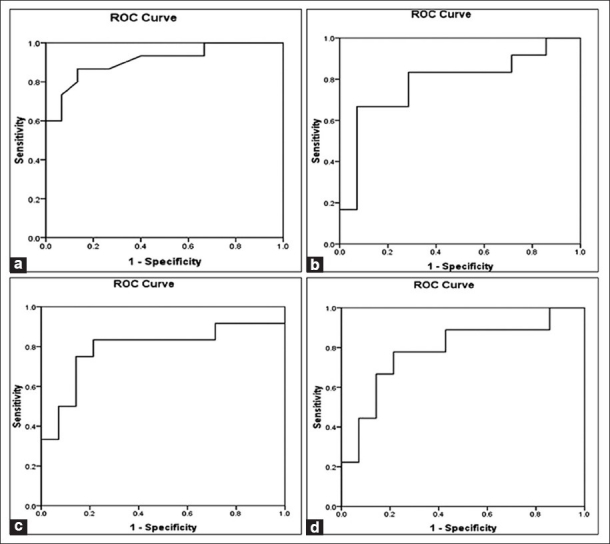
Receiver operating characteristic (ROC) curves. (a) PRISM III score, (b) lactate 1 levels, (c) lactate 2 levels, and (d) lactate 3 levels
The relationship of lactate at admission (lactate 1) with the PRISM III score (done at admission) was determined by calculating the Spearman correlation coefficient and two-tailed significance. A highly significant positive correlation existed between the PRISM III score and lactate level (lactate 1) at admission (r = 561; P = 0.003).
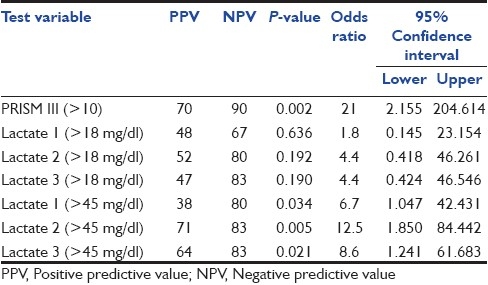
Cut-off points for the PRISM III score and lactate levels along with the positive predictive value (PPV), negative predictive value (NPV), and odds ratio for the prediction of death are shown in Table 4. A PRISM score more than 10 and a lactate level more than 45 mg/dl (5 mmol/l) at all three time periods (0–3, 12, and 24 h) significantly discriminated nonsurvivors from survivors [Table 4]. When we analyzed serial values of lactate, it was observed that the lactate level remained high in nonsurvivors even at 24 h (third lactate value) of PICU admission [Table 2].
Table 4.
PPV, NPV, and odds ratio for PRISM III score >10 and lactate levels >18 mg/dl (2 mmol/l) and > 45 mg/dl (5 mmol/l)
Discussion
Septic shock was the most common type of shock in patients admitted to PICU, comprising 79.3% of all patients with shock and 18.4% of all PICU admissions although it does not mean that the occurrence of septic shock is commonest. Hypovolemic shock is easily managed by fluid boluses and often does not need admission to PICU. This figure is comparable to various studies where sepsis and septic shock comprised 12.3–26.7% of PICU admissions.[1,4,18–20]
Pneumonia was the most common underlying infection with septic shock, accounting for 73.3% of total cases. Pneumonia was most common in other studies from abroad as well as India[1,18] whereas in a study by Jacobs et al., meningitis was the most frequent localized site of infection occurring in 49.7% of cases.[19] Two cases (6.7%) had no identifiable underlying infective pathology in our study. Llorens et al. observed 24% cases of septic shock with no identifiable focus of infection.[1] Our patients of septic shock had 50% mortality. In various studies, the mortality in pediatric septic shock varied from 9.8% to 50%.[1,14,18,21–23] Higher mortality in our study may be due to the fact that the majority of septic shock patients admitted to PICU were fluid refractory and also refractory to one inotrope. With presently available bed strength, it is not possible to admit all cases with septic shock to PICU and those who respond to fluid boluses or small doses of inotropes were managed in the wards and survived. They have not been included in our study. Therefore, patients coming to our PICU were sicker and had a higher mortality. In infants, the incidence of sepsis and associated mortality is higher.[23] Most of patients in our study were infants thus contributing to high mortality.
Trials have demonstrated the prognostic value of lactate levels in postcardiac surgery patients, surgical patients, in infections/sepsis and septic shock.[24–30] Marecaux et al. showed that when correlated with other markers, lactate has a better prognostic value than the tumor necrosis factor and IL-6.[31] Lactate clearance can be used to risk stratify patients and determine their response to therapy.[32] Vincent et al. described that shock patients with the best prognosis were those in whom lactate levels had considerably decreased within 1 h after resuscitation.[33] Besides these studies with adults, observations in pediatric patients for the establishment of laboratory parameters as predictors of death have presented controversial results. When compared with other parameters, blood lactate levels were not correlated with mortality.[34] Hatherill et al. found that the initial lactate concentration in children submitted to cardiac surgery cannot predict death.[35] In another study by Hatherill et al., mortality correlated with gastric intramucosal pH but not with the lactate levels.[14] Siegel et al. observed that in children admitted to the ICU after a cardiac surgery, high levels of lactate had a PPV of 100% and a NPV of 97% for death.[36] By using univariate logistic regression, Duke et al. found that lactate allowed distinguishing survivors from nonsurvivors among children with sepsis at 12 and 24 h of admission.[13] Hatherill et al. suggested that hyperlactatemia can indicate death on admission and if it persists after 24 h of treatment.[37] In another study, as a predictor of death, the blood lactate level at 24 h of PICU admission presented the best sensitivity and specificity.[38]
In our study, all three different lactate levels were significantly higher among nonsurvivors as compared to survivors [Table 2] and the area under the ROC curve for all three lactate levels was just at the significance level [Table 3]. Further, a lactate value more than 45 mg/dl (5 mmol/l) predicted death at a significant level [Table 4]. In previous studies by Duke et al.[13] and Koliski et al.,[38] a lactate level of >3 mmol/l significantly predicted mortality. This value was high (5 mmol/l) in our study. The reason for this difference may be patient selection: Duke et al. included patients with sepsis with or without shock; Koliski et al. included all patients admitted to PICU whereas in our study only patients with septic shock were included where a high lactate level was expected. A small number of patients is the limitation of our study.
Conclusions
Septic shock is a common cause for PICU admission and high mortality. This study demonstrated that most patients who died had higher blood lactate levels than those who survived. Lactate levels at 0–3, 12, and 24 h (> 5 mmol/l) and PRISM III score (>10) were predictors of death in septic shock. There is a need for larger studies on cut-off values of lactate levels in pediatric septic shock above which mortality increases significantly. The persistence of high lactate was associated with higher mortality. This makes it useful as a prognostic marker for the risk of death. The numbers of patients were small in our study; therefore, further studies are necessary to confirm the predictive value of lactate in pediatric patients admitted to PICU.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Llorens XS, Vargas S, Guerra F, Coronado L. Application of new sepsis definitions to evaluate outcome of pediatric patients with severe systemic infections. Pediatr Infect Dis J. 1996;14:557–61. doi: 10.1097/00006454-199507000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Carcillo JA, Fields AI. Task force Committee Members.Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit care Med. 2002;30:1365–78. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 3.Jocobs BK, Carver J, Wilkinson JD. Comparison of gastric intromucosal pH and standard perfusional measurements in pediatric septic shock. Chest. 1995;108:220–5. doi: 10.1378/chest.108.1.220. [DOI] [PubMed] [Google Scholar]
- 4.Han YY, Carcillo JA, Dragotta MA, Bills DM, Watson RS, Westerman ME, et al. Early reversal of pediatric- neonatal septic shock by community physicians is associated with improved outcome. Pediatrics. 2003;112:793–9. doi: 10.1542/peds.112.4.793. [DOI] [PubMed] [Google Scholar]
- 5.Jones GR. Assessment criteria in identifying the sick sepsis patient. J Infect. 1998;37:24–9. doi: 10.1016/s0163-4453(98)92710-4. [DOI] [PubMed] [Google Scholar]
- 6.Mizock BA. The hepatosplanchnic area and hyperlacatatemia: A tale of two lactates. Crit Care Med. 2001;29:442–59. doi: 10.1097/00003246-200102000-00047. [DOI] [PubMed] [Google Scholar]
- 7.Stacpoole PW. Lactic acidosis. Endocrinol Metabol Clin North Am. 1993;22:221–45. [PubMed] [Google Scholar]
- 8.Valenza F, Aletti G, Fossali T, Chevallard G, Sacconi F, Irace M, et al. Lactate as a marker of energy failure in critically ill patients: Hypothesis. Critical Care. 2005;9:588–93. doi: 10.1186/cc3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacpoole PW, Wright EC, Baumgartner TG, Bersin RM, Buchalter S, Curry SH, et al. Natural history and course of acquired lactic acidosis in adults.DCA-Lactic Acidosis Study Group. Am J Med. 1994;97:47–54. doi: 10.1016/0002-9343(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 10.Phua J, Koay ES, Lee KH. Lactate, procalcitonin, and amino-terminal pro-b-type Natriuretic peptide versus cytokine measurements and clinical severity scores for prognostication in septic shock. Shock. 2008;3:328–33. doi: 10.1097/SHK.0b013e318150716b. [DOI] [PubMed] [Google Scholar]
- 11.Deshpande SA, Ward-Platt MP. Association between blood lactate and acid-base status and mortality in ventilated babies. Arch Dis Child Fetal Neonatal Ed. 1997;76:F15–20. doi: 10.1136/fn.76.1.f15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald MJ, Gota M, Myers TF, Zeller WP. Early metabolic effects of sepsis in the preterm infant: Lactic acidosis and increased glucose requirement. J Pediatr. 1992;121:951–5. doi: 10.1016/s0022-3476(05)80350-6. [DOI] [PubMed] [Google Scholar]
- 13.Duke TD, Butt W, South M. Predictors of mortality and multiple organ failure in children with sepsis. Intensive Care Med. 1997;23:684–92. doi: 10.1007/s001340050394. [DOI] [PubMed] [Google Scholar]
- 14.Hatherill M, Tibby SM, Evans R, Murdoch IA. Gastric tonometry in septic shock. Arch Dis Child. 1998;78:155–8. doi: 10.1136/adc.78.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated pediatric risk of mortality score. Crit Care Med. 1996;24:743–52. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 17.Bewick V, Cheek L, Ball J. Statistics review 13: Receiver operating characteristic curves. Critical Care. 2004;8:508–12. doi: 10.1186/cc3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upadhyay M, Singhi S, Murlidharan J, Kaur N, Majumdar S. Randomized evaluation of fluid resuscitation with crystalloid (saline) and colloid (polymer from degraded Gelatin in saline) in pediatric septic shock. Indian Pediatr. 2005;42:223–31. [PubMed] [Google Scholar]
- 19.Jacobs RF, Sowell MK, Moss MM. Septic shock in children: Bacterial etiologies and temporal relationship. Pediatr Infect Dis J. 1990;9:196–200. doi: 10.1097/00006454-199003000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Goh AY, Chan PW, Lum LC. Sepsis, severe sepsis and septic shock in pediatric multiple organ dysfunction syndrome. Paediatr Child Health J. 1999;35:488–92. doi: 10.1046/j.1440-1754.1999.355409.x. [DOI] [PubMed] [Google Scholar]
- 21.Dugas MA, Proulx F, Jaeger A, Lacroix J, Lambert M. Markers of tissue hypoperfusion in Pediatric Septic Shock. Intensive care Med. 2000;26:75–83. doi: 10.1007/s001340050015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242–5. [PubMed] [Google Scholar]
- 23.Jafari HS, Mccracken GH. Sepsis and septic shock: A review for clinicians. Pediatr Infect Dis J. 1992;11:739–49. doi: 10.1097/00006454-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Maillet JM, Le Besnerais P, Cantoni M, Nataf P, Ruffenach A, Lessana A, et al. Frequency, risk factors, and outcome of hyperlactatemia after cardiac surgery. Chest. 2003;123:1361–6. doi: 10.1378/chest.123.5.1361. [DOI] [PubMed] [Google Scholar]
- 25.Husain FA, Martin MJ, Mullenix PS, Steele SR, Elliott DC. Serum lactate and base deficit as predictors of mortality and morbidity. Am J Surg. 2003;185:485–91. doi: 10.1016/s0002-9610(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 26.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–7. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 27.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33:1892–9. doi: 10.1007/s00134-007-0680-5. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–8. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Bernardin G, Pradier C, Tiger F, Deloffre P, Mattei M. Blood pressure and arterial lactate level are early indicators of short term survival in human septic shock. Intensive Care Med. 1996;22:17–25. doi: 10.1007/BF01728326. [DOI] [PubMed] [Google Scholar]
- 30.Levy B, Sadoune LO, Gelot AM, Bollaert PE, Nabet P, Larcan A. Evolution of lactate/pyruvate and arterial ketone body ratios in the early course of catecholamine-treated septic shock. Crit Care Med. 2000;28:114–9. doi: 10.1097/00003246-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Marecaux G, Pinsky MR, Dupont E, Kahn RJ, Vincent JL. Blood lactate levels are better prognostic indicators than TNF and IL-6 levels in patient with septic shock. Intensive Care Med. 1996;22:404–8. doi: 10.1007/BF01712155. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32:1637–42. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 33.Vincent JL, Dufaye P, Berré J, Leeman M, Degaute JP, Kahn RJ. Serial lactate determinations during circulatory shock. Crit Care Med. 1983;11:449–51. doi: 10.1097/00003246-198306000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Balasubramanyan N, Havens PL, Hoffman GM. Unmeasured anions identified by the Fencl-Stewart method predict mortality better than base excess, anion gap, and lactate in patients in the pediatric intensive unit. Crit Care Med. 1999;27:1577–81. doi: 10.1097/00003246-199908000-00030. [DOI] [PubMed] [Google Scholar]
- 35.Hatherill M, Sajjanhar T, Tibby SM, Champion MP, Anderson D, Marsh MJ, et al. Serum lactate as a predictor of mortality after paediatric cardiac surgery. Arch Dis Child. 1997;77:235–8. doi: 10.1136/adc.77.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel LB, Dalton HJ, Hertzog JH, Hopkins RA, Hannan RL, Hauser GJ. Initial postoperative serum lactate levels predict survival in children after open heart surgery. Intensive Care Med. 1996;22:1418–23. doi: 10.1007/BF01709563. [DOI] [PubMed] [Google Scholar]
- 37.Hatherill M, McIntyre AG, Wattie M, Murdoch AI. Early hyperlactatemia in critically ill children. Intensive Care Med. 2000;26:314–8. doi: 10.1007/s001340051155. [DOI] [PubMed] [Google Scholar]
- 38.Koliski A, Cat I, Giraldi DJ, Cat ML. Blood lactate concentration as prognostic marker in critically ill children. J Pediatr (Rio J) 2005;81:287–92. [PubMed] [Google Scholar]


