Abstract
Background:
The initial empirical therapy of Ventilator Associated Pneumonia (VAP) modified based on the knowledge of local microbiological data is associated with decreased morbidity and mortality. The objective was to find the incidence and risk factors associated with VAP, the implicated pathogens and their susceptibility pattern as well as to assess the final clinical outcome in VAP.
Materials and Methods:
This was a prospective cohort study of 107 patients taken on ventilatory support for two or more days and those not suffering from pneumonia prior were to be taken on ventilator. The study was done over a period of one year. VAP was diagnosed using clinical pulmonary infection score of >6. The mortality, incidence of VAP, frequency of different pathogens isolated, their antibiotic sensitivity pattern, duration of mechanical ventilation and duration of hospital stay were assessed.
Statistical Analysis:
Univariate analysis, χ2 test and paired t-test.
Results:
The incidence of VAP was 28.04%. Mortality in VAP group was 46.67%, while in the non-VAP group was 27.28%. High APACHE II score was associated with a high mortality rate as well as increased incidence of VAP. The most common organisms isolated from endotracheal aspirate of patients who developed VAP were Pseudomonas aeruginosa, Methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae and Acinetobacter baumannii. Most strains of Pseudomonas (55.56%) were resistant to commonly used beta-lactam antibiotics known to be effective against Pseudomonas. All strains of Staphylococcus aureus were MRSA and most isolates of K. pneumoniae (85.71%) were extended-spectrum beta-lactamase producing. About 50% isolates of Acinetobacter were resistant to carbapenems. Mortality was highest for infections caused by A. baumannii (83.33%) and K. pneumoniae (71.42%).
Conclusions:
APACHE II score can be used to stratify the risk of development of VAP and overall risk of mortality. Drug-resistant strains of various organisms are an important cause of VAP in our setting.
Keywords: APACHE II score, clinical pulmonary infection score, mechanical ventilation, ventilator associated pneumonia
Introduction
Ventilator Associated Pneumonia (VAP) refers to a type of pneumonia that occurs more than 48–72 hours after endotracheal intubation, and is one of the most common nosocomial infections in patients receiving mechanical ventilation.[1,2] VAP occurs in 9–27% of all intubated patients.[3,4] Delay in initiating appropriate antibiotic therapy can increase the mortality associated with VAP, and thus therapy should not be postponed for the purpose of performing diagnostic studies.[5,6] This initial empirical therapy can be modified based on the knowledge of local microbiological data, patient characteristics, and sensitivity pattern of expected pathogens at the institution. One of the consequences of increasing antimicrobial resistance is an increased probability of inappropriate initial empiric antimicrobial treatment of infections.[7] The aim of this study was to find the incidence of VAP and mortality associated with VAP at our institution. We also aimed to find the proportion of various bacterial pathogens isolated from tracheal aspirate of patients with VAP, their antibiotic sensitivity pattern and patient outcome in terms of duration of mechanical ventilation and hospital stay. This information will help us in formulating an institutional antimicrobial policy. Such an approach of guided empirical antibiotic therapy has shown to decrease morbidity and mortality, duration of treatment and hospital stay, and has also helped in cost reduction and prevention of development of multi-drug resistant strains.
Materials and Methods
This was a prospective cohort study done over a period of 1 year. APACHE II score of all critically ill patients admitted to medical intensive care unit likely to be taken on ventilator was evaluated. If the patients were taken on ventilatory support for two or more days and were not suffering from pneumonia prior to taking on ventilator, they were included in the study. A total of 107 patients met the required criteria over 1 year duration of study period and were included in the study.
The baseline evaluation of all cases included the patient-related factors such as age, concomitant diseases, immune suppression, indication for mechanical ventilation, and the severity of illness based on APACHE II score[8] during first 24 hours of admission. The diagnosis of VAP was established using clinical pulmonary infection score (CPIS),[9] which was evaluated on a daily basis until the patient was on ventilator support. CPIS of greater than six was used as diagnostic criteria for VAP. Early-onset VAP was defined as VAP occurring within the first 4 days of hospitalisation and late-onset VAP was defined as VAP occurring after 4 days of hospitalisation. Endotracheal aspirate was preferred over protected specimen brush (PSB) sampling and broncho-alveolar lavage (BAL), as these techniques are more invasive and studies have shown no mortality benefit of using these over endotracheal aspirate.[10–12]
Collection of endotracheal aspirate
Endotracheal aspirate was obtained under aseptic precautions using a 22-inch Ramson's 12F suction catheter with a mucus extractor, which was gently introduced through the endotracheal tube for a distance of approximately 25 cm. Gentle aspiration was then performed without instilling saline, and the catheter was withdrawn from the endotracheal tube. After this, 2 ml of 0.9% saline was injected in the endotracheal tube with a sterile syringe to flush the exudates into a sterile container for collection. The samples were immediately taken to the laboratory for processing.
Microbiological processing
Gram stain preparations were made from all aspirate samples within the first hour. Samples were inoculated onto 5% blood agar, MacConkey agar, which were reconstituted according to the manufacturer's specifications, and sterilised at 121°C for 15 minutes. The plates were incubated at 37°C for 18-24 hours. The plates were read the following day, but extended to 48 hours if there was no bacterial growth within 24 hours. Isolated colonies were subjected to Gram staining and biochemical tests for identification. Identification was carried out according to standard biochemical tests. Antimicrobial susceptibility test was performed using Mueller-Hinton agar plates by Kirby-Bauer disc diffusion method, according to the Clinical Laboratory Standards Institute (CLSI) guidelines. The patients diagnosed with VAP were started on initial empirical antibiotic therapy, which was guided by the fact whether a multi-drug resistant pathogen was expected. Later on, based on culture sensitivity reports the treatment was modified.
The primary outcome measure assessed was mortality. Other measures assessed included the incidence of VAP, frequency of different pathogens isolated, their antibiotic sensitivity pattern, duration of mechanical ventilation and duration of hospital stay. The results were also analysed to find out any association between patient characteristics, severity of underlying illness as assessed by APACHE II score, factors related to course of care like re-intubation with the incidence and rate of mortality in VAP.
Statistical analysis
The study cohort was classified into four groups: VAP, non-VAP, survivors, and non-survivors. The data obtained were subjected to univariate analysis using the Chi-square test. The level of significance was set at P < 0.05.
Results
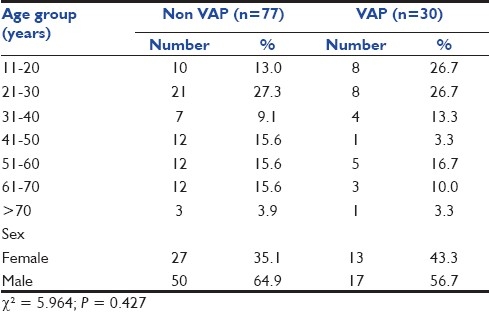
Out of 107 patients included in study cohort, 68 (63.54%) were males and 39 (36.44%) were females. The mean age was 39.9 years. The incidence of VAP in our study was 28.04%, with 30 of 107 patients developing VAP. Of these 30 patients, 4 (13.3%) developed early-onset VAP and 26 (86.7%) developed late-onset VAP. A majority of VAP cases were observed in patients aged up to 30 years (53.4%). However, age-wise, no significant statistical association with VAP could be seen (P = 0.427). There were 27 (35.1%) females in the non-VAP group, while in VAP group, this proportion was slightly higher (n = 13; 43.3%). However, the proportion of males was higher in the non-VAP group (64.9%) as compared to VAP group (56.7%). On statistical evaluation, this difference between two genders was not found to be statistically significant (P = 0.427) [Table 1].
Table 1.
Age and sex distribution of non-VAP and VAP group of patients
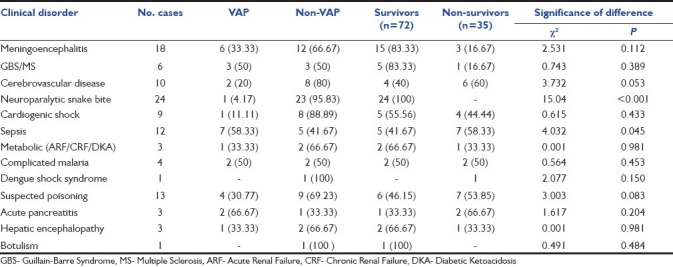
The clinical spectrum of cases indicates that the maximum number of cases enrolled in the study were of neuroparalytic snake bite (24 cases) followed by central nervous system infections (18 cases), suspected poisoning (13 cases), sepsis (12 cases), and cerebrovascular disease (10 cases). No significant association between VAP and diagnosis was seen (P = 0.071) [Table 2].
Table 2.
Comparison of clinical conditions in VAP group, non-VAP group, survivors, and non-survivors
Re-intubation was done in 8 patients, of which 7 (87.5%) developed VAP, clearly indicating that re-intubation is an important predisposing factor in the development of VAP (P < 0.001). In fact, 23.33% of patients who developed VAP had undergone re-intubation. Early tracheostomy was done in 11 patients who required prolonged ventilatory support, of which 4 (36.36%) developed VAP and 7 (63.36%) did not.
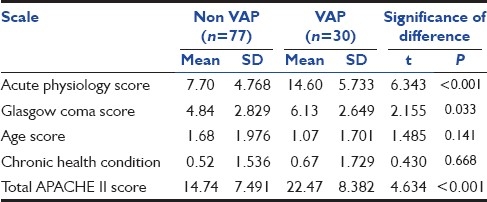
The mean APACHE II score (during first 24 hours of admission) of the patients who developed VAP was 22.47 ± 8.382, while that of the patients who did not develop VAP was 14.74 ± 7.491. The difference between the two groups was statistically significant (P < 0.001) [Table 3]. The mean of Acute Physiology Score (APS) component of APACHE II score in VAP group was 14.6 ± 5.73 and in non-VAP group, it was 7.7 ± 4.768. The difference was statistically significant (P < 0.001). The mean of Glasgow coma score (GCS) in VAP group was 6.13 ± 2.649, while in non-VAP group, it was 4.84 ± 2.829; the difference was found to be statistically significant (P = 0.033). However, no significant difference was found in the age score and chronic health condition score in the two groups.
Table 3.
Comparison of individual scores and total APACHE II Score
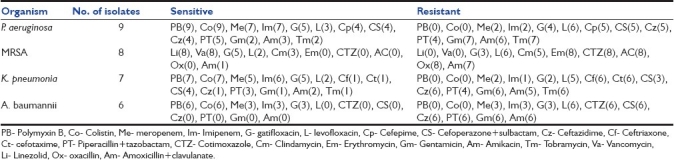
Of 30 patients diagnosed as VAP based on a CPIS score of more than six, 26 (86.67%) patients had monomicrobial infection and 4 (13.33%) had polymicrobial infection. The most common organism isolated was Pseudomonas aeruginosa followed by Methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae, and Acinetobacter baumannii [Table 4]. Most strains of P. aeruginosa were resistant to the commonly used beta-lactam antibiotics known to be effective against P. aeruginosa, with 5 (55.56%) isolates being resistant to ceftazidime, cefepime, cefoperazone+sulbactam. All 9 (100%) isolates were sensitive to polymyxin B and colistin, while 7 (87.88%) isolates were sensitive to carbapenems. All 8 (100%) isolates of S. aureus were resistant to oxacillin, indicating the high prevalence of MRSA as a cause of VAP in our setting. All 8 isolates (100%) were sensitive to vancomycin and linezolid. Six isolates of K. pneumoniae (85.71%) out of 7 were resistant to ceftriaxone, cefotaxime and ceftazidime, suggesting a high prevalence of extended-spectrum beta-lactamase (ESBL) producing organism as a cause for VAP in our setting. Five isolates (71.43%) were sensitive to gatifloxacin and meropenem, while 6 isolates (85.71%) were sensitive to imipenem. All 7 isolates (100%) were sensitive to polymyxin B and colistin. Only 3 isolates (50%) of A. baumannii were sensitive to carbapenems. However, all 6 isolates (100%) were sensitive to polymyxin B and colistin [Table 5].
Table 4.
Causative organisms in VAP- correlation of frequency, type of VAP, and associated mortality
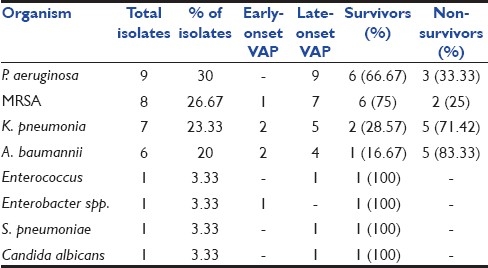
Table 5.
Antibiotic sensitivity pattern of the common isolates
The primary outcome measure assessed in the present study was mortality. The overall mortality in our study was 32.71%, with the death of 35 patients during the course of their illness. Mortality in VAP group was 46.67%, with the death of 14 patients out of 30 who developed VAP during the course of their illness. Mortality in non-VAP group was significantly low at 27.28%, with the death of 21 patients out of 77 during the course of their illness. The difference between the two groups was statistically significant (P < 0.001). Mortality in late-onset VAP was significantly high at 50%, with the death of 13 patients out of 26 during the course of their illness. However, mortality was relatively low at 25% in early-onset VAP, with the death of 1 patient out of 4 during the course of their illness, but the difference between two groups was not significant statistically (P = 0.351).
The overall risk of mortality was stratified by the APACHE II score during the first 24 hours of admission. The mean APACHE II score among survivors was 13.64 ± 6.79 and among non-survivors was 23.63 ± 7.62. This difference was found to be highly significant statistically (P < 0.001). Mortality rate was high in patients with cerebrovascular disease (60%), sepsis and septic shock (58.33%), and in cases of suspected poisonings (53.85%). All patients with neuroparalytic snake bite survived [Table 2]. Mortality rate was maximum in infections caused by A. baumannii. Five (83.33%) of 6 patients died during the course of their illness. The next most lethal organism was K. pneumoniae, with a mortality rate of 71.42% [Table 4].
Secondary outcome measures assessed were duration of mechanical ventilation and duration of hospital stay. The mean duration of ventilation in the VAP group was 12.03 ± 6.531 days and in non-VAP group, 5.13 ± 3.945 days. The mean duration of hospital stay in VAP group was 16.13 ± 10.02 days and in the non-VAP group, 8.81 ± 6.262 days. Both these parameters were observed to be significantly lower in non-VAP group as compared to VAP group (P < 0.001).
Discussion
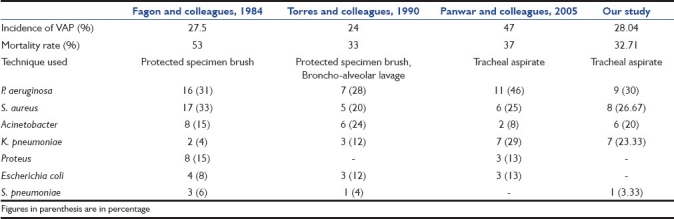
The incidence of VAP in our study was 28.04%. This correlates with other similar studies in which the incidence of VAP was 15.5-47%, depending on the diagnostic criteria used.[13–16] There was no statistically significant association between the age of the patient and development of VAP (P = 0.427), indicating that the age of the patient has no predisposition and no protective value for the development of VAP. Also, no statistically significant difference was found between the two genders with respect to the development of VAP, indicating that both males and females are equally predisposed to develop VAP.[17] Distribution of various diseases in VAP and non-VAP groups shows that the incidence of VAP was high in infectious conditions like septic shock (58.33%), meningoencephalitis (33.33%), complicated malaria (50%) and also in conditions requiring prolonged mechanical ventilation like Guillain-Barre Syndrome (GBS) (50%). The incidence was significantly low in conditions requiring mechanical ventilation for short duration like neuroparalytic snake bite (4.17%) and in conditions with no underlying lung disease or infection like cerebrovascular disease (20%), cardiogenic shock (11.11%). However, no significant association between VAP and diagnosis was seen (P = 0.071). This indicates that the primary disease of the patient is not correlated with the probability of developing VAP.
APACHE II score was an important parameter to stratify risk of developing VAP. A significantly higher value was observed in VAP group (P < 0.001). In a similar study done by Panwar and colleagues,[16] it was concluded that patients who developed VAP had a significantly higher APACHE III score within the first 24 hours of admission. Also, statistically significant difference was seen between VAP and non-VAP group with respect to the mean of Acute Physiology Score (APS) component (P < 0.001) and Glasgow Coma Score (GCS) (P = 0.033) of APACHE II score. However, no significant difference was found in the age score and chronic health condition score in the two groups. The risk of VAP was greatly increased in patients who underwent re-intubation (P < 0.001). A similar high incidence of VAP was found to be associated with re-intubation in other studies.[16,18] This might be because this invasive procedure of intubation was repeated and also duration of ventilation was increased. Another hypothesis for this is that the patient who required re-intubation would have been vulnerable to aspiration in the interval between extubation and re-intubation. This underlies the importance of proper weaning protocols in the prevention of VAP and the associated mortality. Although the incidence of VAP was found to be lower in patients who underwent early tracheostomy, but this was not found to be statistically significant (P = 0.516). Thus, from the analysis of various factors used to stratify risk for development of VAP, we conclude that a high total APACHE II score along with a high APS and GCS during the first 24 hours and re-intubation during the course of patient care significantly predispose them to development of VAP. Other factors like age and sex of the patient and underlying chronic health condition do not predispose them to the development of VAP.
The most common organism isolated was P. aeruginosa, with nine isolates. All nine isolates were from patients with late-onset VAP. The next most common organism isolated was MRSA, with a total of eight isolates, of which seven were isolated from patients with late onset VAP. Other common organisms isolated were K. Pneumoniae (7 isolates) and A. baumannii (6 isolates). In four cases of polymicrobial infection, two cases had MRSA and K. pneumoniae in combination, and in one case, K. pneumoniae in combination with P. aeruginosa, and in the remaining one case, Enterococcus in combination with P. aeruginosa was seen. The organisms implicated in VAP were similar in other such studies[13–16] [Table 6].
Table 6.
Comparison of incidence, mortality rate, and pathogens causing VAP in various studies
Analysis of antibiotic sensitivity pattern of these organisms suggests that a large number of these are highly resistant to the commonly used drugs. Most strains of P. aeruginosa were resistant to the commonly used beta-lactam antibiotics known to be effective against P. aeruginosa, with 5 (55.56%) isolates being resistant to ceftazidime, cefepime, cefoperazone+sulbactam. All isolated strains of S. aureus were MRSA and 85.71% isolates of K. pneumoniae were ESBL producing. One isolate of K. pneumoniae was resistant to both the carbapenems used. This might be due to a carbapenemase producing strain. Carbapenem resistance was noted still higher with A. baumannii, with 50% isolates resistant to carbapenems. The overall picture suggests that number of drug-resistant strains of various organisms is rising and is an important cause of VAP in our setting.
Mortality in VAP group was 46.67%, while in non-VAP group, it was 27.28% and the difference was statistically significant (P < 0.001). Although VAP was not independently associated with mortality, mortality rate was higher in patients with VAP. The rate of mortality was less in early-onset VAP group as compared to late-onset VAP, but the difference was found to be statistically insignificant (P = 0.351). A similar high mortality rate associated with VAP was observed in other similar studies[13–16] [Table 6]. The mean APACHE II score was significantly higher (P < 0.001) in non-survivors than survivors. It was further concluded that derangements in acute physiology and severity of underlying disease of the patient at the time of admission are more responsible for mortality than the underlying chronic health condition. A similar association of mortality with a high mean APACHE III score was observed in a study by Panwar and colleagues.[16] Mortality was also influenced by the type of organism isolated being highest for infections caused by A. baumannii (83.33%) and K. pneumoniae (71.42%).
Conclusion
We conclude that most cases of VAP in our setting are those of late-onset VAP and a majority of these are caused by highly resistant strains. Local epidemiological data like this should be collected at all centres, as such information can help in guiding the initial empirical antibiotic therapy, which would be more rationale and help in decreasing mortality and morbidity. This would also help in preventing development of more resistant strains. Also, APACHE II score is a good parameter to prognosticate the risk of mortality as well as the risk of developing VAP.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Craven DE, Kunches LM, Kilinsky V, Lichtenberg DA, Make BJ, McCabe WR. Risk factors for pneumonia and fatality in patients receiving continuous mechanical ventilation. Am Rev Respir Dis. 1986;133:792–6. [PubMed] [Google Scholar]
- 2.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Healthcare Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention. Guidelines for preventing health-careassociated pneumonia, 2003: Recommendations of the CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1–36. [PubMed] [Google Scholar]
- 3.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 4.Rello J, Ollendorf DA, Oster G, Montserrat V, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 5.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–8. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Lerma F. ICU-acquired Pneumonia Study Group. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Med. 1996;22:387–94. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- 7.Kollef MH. Inadequate antimicrobial treatment: An important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31:S131–8. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]
- 8.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 9.Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–9. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Nieto JM, Torres A, Garcia-Cordoba F, El-Ebiary M, Carrillo A, Ruiz J, et al. Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator-associated pneumonia: A pilot study. Am J Respir Crit Care Med. 1998;157:371–6. doi: 10.1164/ajrccm.157.2.97-02039. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz M, Torres A, Ewig S, Marcos MA, Alcon A, Lledo R, et al. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: Evaluation of outcome. Am J Respir Crit Care Med. 2000;162:119–25. doi: 10.1164/ajrccm.162.1.9907090. [DOI] [PubMed] [Google Scholar]
- 12.Sole Violan J, Fernandez JA, Benitez AB, Cardenosa Cendrero JA, Rodriguez de Castro F. Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia. Crit Care Med. 2000;28:2737–41. doi: 10.1097/00003246-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Torres A, Puig de la Bellacasa J, Xaubet A, Gonzalez J, Rodriguez-Roisin R, Jimenez de Anta MT, et al. Diagnostic value of quantitative cultures of bronchoalveolar lavage and telescoping plugged catheters in mechanically ventilated patients with bacterial pneumonia. Am Rev Respir Dis. 1989;140:306–10. doi: 10.1164/ajrccm/140.2.306. [DOI] [PubMed] [Google Scholar]
- 14.Kollef MH. Ventilator-associated pneumonia: A multivariate analysis. JAMA. 1993;270:1965–70. [PubMed] [Google Scholar]
- 15.Fagon JY, Chastre J, Domart Y, Trouillet JL, Pierre J, Darne C, et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation: Prospective analysis of 52 episodes with use of protected specimen brush and quantitative culture techniques. Am Rev Respir Dis. 1989;139:884. doi: 10.1164/ajrccm/139.4.877. [DOI] [PubMed] [Google Scholar]
- 16.Panwar R, Vidya SN, Alka KD. Incidence, clinical outcome and risk stratification of ventilator-associated pneumonia: A prospective cohort study. Indian J Crit Care Med. 2005;9:211–6. [Google Scholar]
- 17.Apostolopoulou E, Bakakos P, Katostaras T, Gregorakos L. Incidence and risk factors for ventilator-associated pneumonia in 4 multidisciplinary intensive care units in Athens, Greece. Respir Care. 2003;48:681–8. [PubMed] [Google Scholar]
- 18.Torres A, Gatell JM, Aznar E, El-Ebiary M, Puig de la Bellacasa J, Gonzalez J, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. Am J Respir Crit Care Med. 1995;152:137–41. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]


