Abstract
N-Phenethyl-substituted ortho-a and para-a oxide-bridged phenylmorphans have been obtained through an improved synthesis and their binding affinity examined at the various opioid receptors. Although the N-phenethyl substituent showed much greater affinity for μ- and κ-opioid receptors than their N-methyl relatives (e.g., Ki = 167 nM and 171 nM at μ- and κ-receptors vs >2800 and 7500 nM for the N-methyl ortho-a oxide-bridged phenylmorphan), the a-isomers were not examined further because of their relatively low affinity. The N-phenethyl substituted ortho-b and para-b oxide-bridged phenylmorphans were also synthesized and their enantiomers were obtained using supercritical fluid chromatography. Of the four enantiomers, only the (+)-ortho-b isomer had moderate affinity for μ- and κ-receptors (Ki = 49 and 42 nM, respectively, and it was found to also have moderate μ- and κ-opioid antagonist activity in the [35S]GTP-γ-S assay (Ke = 31 and 26 nM).
Keywords: ortho-a and ortho-b oxide-bridged phenylmorphans, para-a and para-b oxide-bridged phenylmorphans, synthesis, optical resolution
1.Introduction
We have synthesized the structurally rigid oxide-bridged phenylmorphans in order to gain further insight into the receptor-active conformation of the various opioid receptor subtypes, and have been correlating this information with their agonist, antagonist, or inverse-agonist activity. The synthesis of the racemic ortho-a and ortho-b, and para-a and para–b, N-methyl substituted oxide-bridged phenylmorphans (rac-(4R,6aR,11bR)-2,3,4,5,6,6a-hexahydro-3-methyl-1H-4,11b-methanobenzofuro[3,2-d]azocin-8-ol and 10-ol and rac-(4R,6aS,11bR)-2,3,4,5,6,6a-hexahydro-3-methyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-8-ol and 10-ol, Figure 1)1–4 was previously accomplished and provided four of the twelve possible racemic ortho- and para-hydroxyphenyl substituted a through f oxide-bridged phenylmorphans (Figure 1). The a-isomers were among the earliest compounds prepared2, 3 and, at that time, we did not realize that an N-methyl substituted compound was unlikely to interact well with opioid receptors. An insufficient amount of the compound was initially prepared and this did not allow us to pursue conversion to other N-substituents. The b-isomers were prepared last4 because of the difficulty of synthesizing the strained 5,6-trans-fused ring junction that must be formed to obtain them. We have now obtained the N-phenethyl-substituted ortho-a and para-a-isomers, have improved the synthesis of the N-phenethyl substituted ortho and para-b-isomers, optically resolved the b-isomers, and examined the binding affinities as well as the efficacies of the compounds with higher affinity. An N-phenethyl substituent usually, but not always,5 increases the affinity of benzomorphan or phenylmorphan-like compounds for opioid receptors,4, 6 as well as their activity as either agonists or antagonists.4 We have previously reported that the racemic N-phenethyl substituted ortho-b isomer exhibited moderate affinity for κ-opioid receptors (Ki = 26 nM),4 and less affinity for μ-opioid receptors. Since it is theoretically possible for one enantiomer to retain all of the κ-opioid receptor affinity and the other the μ-affinity, we examined the enantiomers of both the ortho-b and para-b isomers to see if there was enhanced receptor-selectivity.
Figure 1.
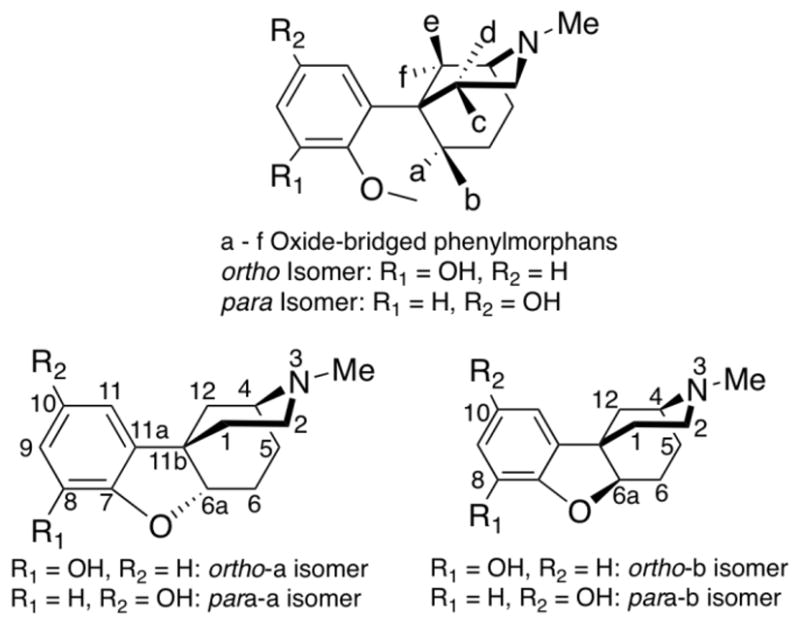
General structure of the a through f oxide-bridged phenylmorphans, and the structures of the ortho- and para-a and ortho- and para-b oxide-bridged phenylmorphans
Oxide-bridged phenylmorphans are structurally rigid molecules and those that interact with opioid receptors probably do so by presenting a specific spatial pattern in the binding pocket of the receptor. The receptor binding pocket is likely to allow only a limited number of orientations for these molecules and the structural rigidity of the oxide-bridged phenylmorphans severely restricts that number. The oxide-bridged phenylmorphans cannot change their conformation to enable or facilitate receptor interaction. In our work on the synthesis of all of the a through f-racemates1–4, 7–13 we began with the view that if an oxide-bridged phenylmorphan had high affinity for an opioid receptor and acted as an opioid agonist or antagonist we would separate the enantiomers, determine absolute configuration through X-ray crystallographic structure analysis and, via quantum chemical studies,13 examine the spatial characteristics of a molecule needed for their activity. In order to explore the effects of the ortho-a and para-a compounds on opioid receptors, the two racemic N-methyl analogues were re-synthesized and a new N-substituent, the N-phenethyl, was introduced and evaluated in both. Similarly, to examine the enantiomeric b-isomers, we resynthesized the racemate, improving its synthesis, and separated the enantiomers using supercritical fluid chromatography.14
2. Chemistry
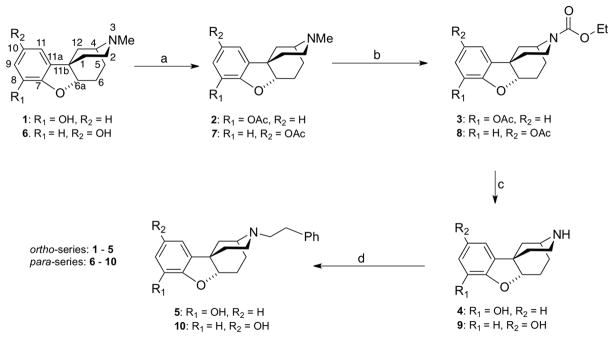
The synthesis of the racemic N-methyl substituted ortho-a and para-a isomers was carried out essentially as previously reported.2, 3 The spectral properties of intermediates were found to be similar to those in the literature and those of the N-methyl substituted ortho-a and para-a isomers were identical to the previously prepared compounds.2, 3 To prepare the N-phenethyl analogues, rac-(4R,6aR,11bR)-3-methyl-2,3,4,5,6,6a-hexahydro-1H-4,11b-methanobenzofuro[3,2-d]azocin-8-ol (1) was heated in acetic anhydride to give acetate (2, Scheme 1). The acetate was reacted with ethylchloroformate under basic conditions to give the ethylcarbamate (3), and the secondary amine 4 was obtained from 3 by refluxing in H2SO4 overnight. Reaction with phenethylbromide in DMF under basic conditions gave the desired ortho-a compound, rac-(4R,6aR,11bR)-2,3,4,5,6,6a-hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocin-8-ol (5). A similar series of reactions in the para-a series (Scheme 1) gave rac-(4R,6aR,11bR)-2,3,4,5,6,6a-hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocin-10-ol (10).
Scheme 1.
Synthesis of racemic N-phenethyl ortho- and para-a isomers. Reagents and conditions: (a) Ac2O, 60 °C, 1 h, 92–94%; (b) EtOCOCl, K2CO3, ClCH2CH2Cl, overnight, 92–93%; (c) H2SO4, overnight, 80–81%; (d) phenethyl bromide, DMF, 90 °C, 3h, 68–70%.
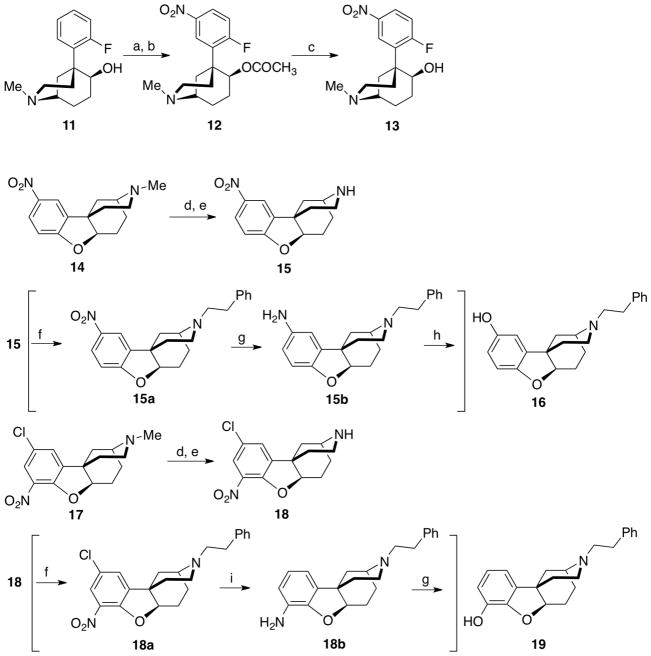
The synthesis of the racemic ortho and para-b series essentially followed the reported route4 except for the modification of a few procedures to improve the yield. rac-(1R,5R,6S)-5-(2-Fluoro-5-nitrophenyl)-2-methyl-2-azabicyclo[3.3.1]nonan-6-ol (13) was originally prepared from rac-(1R,5R,6S)-(5-(2-fluorophenyl)-2-methyl-2-azabicyclo[3.3.1]nonan-6-ol (11) in 62% yield on a 0.8 mmol scale without isolation of the intermediate acetate (12).4 However, when the reaction was carried out on a larger scale (~25 mmol) needed for optical resolution, the yields were lower (<50%). TLC indicated that all of the starting material had been used and a significant amount of a lower Rf compound was obtained. This procedure was modified to provide 13 in two steps (Scheme 2). Using the path shown in Scheme 2, compound 11, prepared in eight steps from rac-(S)-2-(2-(dimethylamino)ethyl)-2-(2-fluorophenyl)cyclohexanone,11 was acetylated using Ac2O and AcOH and nitrated with fuming nitric acid to give rac-(1R,5S,6R)-5-(2-fluorophenyl)-2-methyl-2-azabicyclo[3.3.1]nonan-6-yl acetate (12) in 92 to 96% yields over several runs. The acetyl moiety was removed with 2N HCl to give the azabicyclo[3.3.1]nonan-6-ol (13) in 93 to 96% yields.
Scheme 2.
Improved steps in synthesis of ortho and para-b isomers. Reagents and conditions: (a) Ac2O, AcOH, 90 °C; (b) fuming HNO3, 92–96% from 11; (c) 2N HCl, reflux 3 h, 5N NaOH, 93–96%; (d) BrCN, K2CO3, acetonitrile, reflux 2 H; (e) HCl, AcOH, reflux 18 h; (f) Ph(CH2)2Br, NaI, CH3CN, reflux; (g) 10% Pd-C, EtOH; (h) NaNO2, Cu(NO3)2• 2.5H2O, Cu2O, 35% H2SO4; (i) 10% Pd-C, HCO2NH4, EtOH. For experimental procedures used to prepare 15a, 15b, 18a, and 18b, see Kurimura et al.4
Conversion to the desired N-phenethyl substituent required an N-demethylation step. In the reported route,4 14 was converted to 15 using 1-chloroethyl chlorformate (56% yield and 17% recovery of starting material). Similarly, 1-chloroethyl chlorformate was used to N-demethylate 17 to 18 in 52% yield with a 42% recovery of starting material after chromatography. To improve the procedure, the N-methyl compound 14 was first reacted with BrCN in acetonitrile, then refluxed in glacial acetic acid and 2N HCl to give 15 quantitatively (Scheme 2). Compound 15 was used to obtain the N-phenethyl substituted para-b compound 16 in three additional steps.4 The BrCN procedure was also used to convert 17 to 18 in 95% yield (Scheme 2), and 18 was used to obtain the N-phenethyl substituted ortho-b compound 19 in three further well-known steps.4
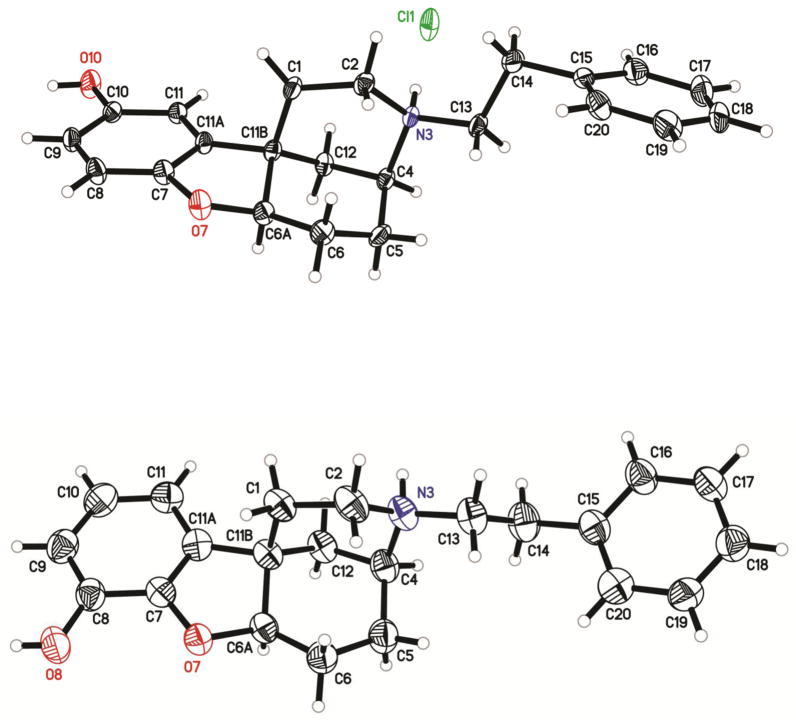
The racemic compounds 16 and 19 (Scheme 3) were optically resolved by supercritical fluid chromatography14 to give the (+)- and (−)-enantiomers of the N-phenethyl substituted para-b racemate (16a and 16b, respectively) and the (+)- and (−)-enantiomers of the N-phenethyl substituted ortho-b racemate (19a and 19b, respectively). X-ray crystallographic analysis determined the stereochemistry of 16b, and 19b the levo enantiomers ((4S,6aR,11bS)-2,3,4,5,6,6a-hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-10-ol and ((4S,6aR,11bS)-2,3,4,5,6,6a-hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-8-ol, respectively (Fig. 2)).
Scheme 3.
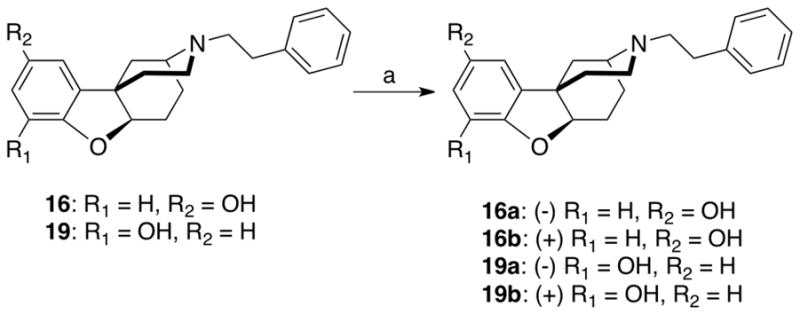
Optical resolution of the N-phenethyl substituted ortho- and para-b racemates. (a) Supercritical fluid chromatography, CO2-MeOH:2-propanol (1:1) with 1% propan-2-amine.
Figure 2.
X-ray crystallographic structures of (−)-(4S,6aR,11bS)-2,3,4,5,6,6a-hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-10-ol hydrochloride (top) and -8-ol hydrobromide (bottom) (N-phenethyl para-b isomer, 16b, and N-phenethyl ortho-b isomer 19a)
3. Results and Discussion
The racemic N-methyl analogues in the ortho- and para-a oxide-bridged phenylmorphan series (1 and 6, respectively) did not have much affinity at any of the opioid receptors (Ki > 2800 nM at μ, Table 1). The racemic N-phenethyl-substituted ortho-a analogue 5 (Table 1) had the same low affinity at both μ- and κ-receptors (Ki = 170 nM), and the N-phenethyl-substituted para-a analogue 10 had higher affinity for κ-receptors (Ki = ~100 nM) than for μ-receptors (Ki = ~500 nM). The N-phenethyl-substituted ortho-a and para-a isomers were weak or moderately potent κ-antagonists (Ke = 163 and 46 nM, respectively), and weak μ-antagonists (Table 2, Ke = 274, and 123 nM, respectively, Table 2). Although the N-phenethyl analogues had markedly higher affinity than the comparable N-methyl compounds (Table 1), the racemic a-isomers did not have sufficient affinity at any opioid receptor to warrant separation of their enantiomers or further pharmacological evaluation.
Table 1.
[3H] Opioid receptor binding dataa for ortho-a and para-a isomers 1, 5, 6, and 10 and for ortho-b and para-b enantiomers 16a, 16b, 19a, and 19b
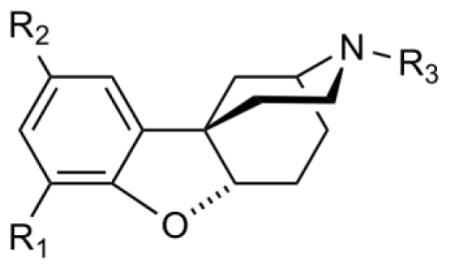 Ki (nM) | ||||||
|---|---|---|---|---|---|---|
| Cmpd | R1 | R2 | R3 | μb | δc | κd |
| rac 1 | OH | H | Me | > 2800 | > 4900 | 7500 ± 1029 |
| rac 5 | OH | H | PhEt | 167 ± 14 | > 4900 | 171 ± 14 |
| rac 6 | H | OH | Me | > 2800 | > 4900 | > 8600 |
| rac 10 | H | OH | PhEt | 486 ± 39 | > 4900 | 98 ± 5 |
| 16a: (+)-4R,6aS,11bR | H | OH | PhEt | 250 ± 28 | > 5,000 | 199 ± 10 |
| 16b: (−)-4S,6aR,11bS | H | OH | PhEt | 352 ± 28 | > 5,000 | 102 ± 3 |
| 19a: (−)-4S,6aR,11bS | OH | H | PhEt | 384 ± 38 | > 5,000 | 367 ± 19 |
| 19b: (+)-4R,6aS,11bR | OH | H | PhEt | 49 ± 3 | > 5,000 | 42 ± 1.8 |
| Morphine | 2.55 ± 0.01 | |||||
Assays were conducted15 using CHO cells, which were stably transfected and express the μ-, δ- or κ-opiate receptors respectively. All results n=3.
Table 2.
Functional data ([35S]GTP-γ-S) for N-phenethyl-substituted ortho-a and para-a racemates 5 and 10 and for ortho-b and para-b enantiomers 16a, 16b, 19a, and 19b
 Ke (nM)a | ||||
|---|---|---|---|---|
| Cmpd | R1 | R2 | μ-Antagonism | κ-Antagonism |
| rac 5 | OH | H | 274 ± 49 | 163 ± 29 |
| rac 10 | H | OH | 23 ± 10 | 46 ± 9 |
| 16a: (+)-4R,6aS,11bR | H | OH | 47 ± 9 | 100 ± 3 |
| 16b: (−)-4S,6aR,11bS | H | OH | 227 ± 33 | 103 ± 17 |
| 19a: (−)-4S,6aR,11bS | OH | H | 328 ± 67 | 761 ± 191 |
| 19b: (+)-4R,6aS,11bR | OH | H | 31 ± 6 | 22 ± 6 |
| Naloxone | 2.3 ± 0.3 | - | ||
| norBNI | - | 0.11 ± 0.02 | ||
[35S]GTP-γ-S binding was performed using CHO hMOR cells which express the human μ-opiate receptor, and were conducted as described in section 4.2.1. All values n=3.
Interestingly, the N-phenethyl substituted chiral ortho-b enantiomer 19b lost the racemate’s selectivity for κ-opioid receptors (Table 1). Kurimura et al.,4 reported that the N-phenethyl substituted ortho-b racemate was seven-fold selective for κ over μ-receptors. This chiral ortho-b enantiomer 19b had considerably higher affinity at μ-receptors than the racemate (Ki = 49 nM (Table 1) vs 190 nM4 for the racemate) and about the same, or perhaps a little less affinity than the racemate at κ-receptors (Ki = 42 nM (Table 1) vs. 26 nM4 for the racemate). The enantiomer 19b was found to be both a moderately potent κ- and μ-antagonist (Ke = 31 and 26 nM, respectively, Table 2) in the [35S]GTP-γ-S assay.
The opioid receptor affinity and the agonist and antagonist activity of the rigid N-phenethyl substituted ortho- and para-a through -f oxide-bridged phenylmorphans is likely to be related to their specific 3-dimensional molecular shape and the different interatomic distances between their heteroatoms, and their aromatic ring, with sets of amino acids in a receptor binding pocket. In our work with the phenylmorphans, we have found that only the (−)-para-e isomer had morphine-like agonist activity in vivo.13 The (−)-ortho-f isomer was four times as potent as naloxone as a μ-antagonist,13 and the rac ortho-c isomer had much higher affinity for the μ-receptor than the (−)-ortho-f isomer, and was a very potent μ-antagonist.16 The (+)-ortho-b isomer was a considerably weaker antagonist (Table 2). Future research on the oxide-bridged phenylmorphans will focus on the optical resolution of the rac ortho-c isomer, as well as the use of different N-substituents that could provide further data for our probe of the opioid receptor from the viewpoint of the ligand.
4. Experimental Section
4.1 Chemistry
All melting points were determined on a Thomas-Hoover melting-point apparatus and are uncorrected. Proton nuclear magnetic resonance (1H NMR, 300 or 500 MHz) and carbon nuclear magnetic resonance (13C NMR, 75 or 125 MHz) spectra were recorded on a Varian Gemini-300 or a Bruker DMX500 wide-bore spectrometer ((proton frequency 500.13 MHz) running XWINNMR v3.1, carbon 125.757) in CDCl3 (unless otherwise noted) with the values given in ppm (TMS as internal standard) and J (Hz) assignments of 1H resonance coupling. The high resolution electrospray ionization (ESI) mass spectra were obtained on a Waters LCT Premier time-of-flight (TOF) mass spectrometer. Thin-layer chromatography (TLC) was performed on 0.25 mm Analtech GHLF silica gel. Flash column chromatography was performed with Bodman silica gel LC 60 A. Elemental analyses were performed by Atlantic Microlabs, Inc., Norcross, GA, or Micro-Analytics, Inc, Wilmington, DE. Optical resolution of 16 and 19 was carried out by Avery Discovery Services, Worcester, MA, using supercritical fluid chromatography.
4.1.1 rac-(4R,6aR,11bR)-3-Methyl-2,3,4,5,6,6a-hexahydro-1H-4,11b-methanobenzofuro[3,2-d]azocine-8-acetate (2)
A mixture of acetic anhydride (1.0 mL) and the phenol 1 (60 mg, 0.25 mmol) was heated at 80 °C for 1 h. After removal of the solvent, the residue was diluted with CHCl3 (15 mL) and washed with NH4OH, dried over MgSO4. The solvent was evaporated to provide 2 (66 mg, 92%) as a light yellow oil. This was used directly in the next step. HRMS [M+H]+ calcd for C17H22NO3: 288.1600. Found, 288.1602
4.1.2 rac-(4R,6aR,11bR)-3-Ethylcarboxylate-2,3,4,5,6,6a-hexahydro-1H-4,11b-methanobenzofuro[3,2-d]azocine-8-acetate (3)
To a solution of 2 (66 mg, 0.23 mmol) in ClCH2CH2Cl (3.0 mL) were added K2CO3 (159 mg, 1.15 mmol) followed by ethylchloroformate (124 mg. 1.15 mmol). The reaction mixture was refluxed overnight. After filtration and removal of the solvent, the residue was diluted with H2O, extracted with CHCl3 (3 × 15 mL), and the organic layer dried over MgSO4. The solvent was removed in vacuo to afford carbamate 3 (73 mg, 92%) as light yellow oil. It was used directly in the next step without further purification. HRMS [M+H]+ calcd for C19H24NO5: 346.1654. Found, 346.1662.
4.1.3 rac-(4R,6aR,11bR)-2,3,4,5,6,6a-Hexahydro-1H-4,11b-methanobenzofuro[3,2-d]azocin-8-ol (4)
H2SO4 (3.0 mL, 40% w/w) was added to compound 3 (73 mg, 0.21 mmol) and the mixture was heated to reflux and stirred overnight. After cooling to 0 °C, the reaction mixture was made basic with aqueous NaOH (pH 8). The basicity was raised to pH 9 with NH4OH and the mixture was extracted with CHCl3/MeOH (10:1). The organic solution was dried over MgSO4 and the solvent was removed in vacuo to give the secondary amine 4 (38 mg, 80%) as a white powder that was used directly for the next step without further purification.
4.1.4 rac-(4R,6aR,11bR)-2,3,4,5,6,6a-Hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro [3,2-d]azocin-8-ol (5)
The secondary amine 4 (32 mg, 0.14 mmol) was dissolved in DMF (1.0 mL), followed by the addition of NaHCO3 (13 mg, 0.154 mmol) and (2-bromoethyl)benzene (29 mg, 0.154 mmol). The reaction mixture was stirred at 90 °C for 3 h. After removal of the solvent in vacuo, the residue was diluted with CHCl3 (10 mL), and the organic material was washed with H2O (2 × 4 mL). The organic phase was dried over MgSO4, evaporated and the resulting residue was purified by flash column chromatography on silica gel (CHCl3/MeOH 40:1) to afford compound 5 (32 mg, 70%) as a white powder, mp 82–83 °C. 1H NMR (300 MHz, CDCl3) δ 1.56–1.73 (m, 4H), 1.78–1.89 (m, 2H), 2.11–2.24 (m, 2H), 2.50 (td, J = 12.9 Hz, 3.3 Hz, 1H), 2.58–2.73 (m, 2H), 2.79–2.89 (m, 3H), 3.34 (br, 1H), 4.58 (t, J = 6.6 Hz, 1H), 6.64–6.79 (m, 3H), 7.18–7.33 (m, 5H); 13C NMR (75 MHz, CHCl3) δ 16.1, 29.9, 34.5, 35.2, 36.6, 43.4, 44.2, 50.7, 56.9, 87.9, 115.0, 115.2, 121.5, 126.4, 128.7, 128.9, 140.4, 145.8, 146.0; HRMS [M+H]+ calcd for C22H26NO2: 336.1964. Found, 336.1957. Anal. Calcd for C22H25NO2.0.85CHCl3: C, 62.81, H, 5.96, N, 3.21. Found: C, 62.84, H, 5.89, N, 3.28.
4.1.5 rac-(4R,6aR,11bR)-3-Methyl-2,3,4,5,6,6a-hexahydro-1H-4,11b-methanobenzofuro[3,2-d]azocine-10-acetate (7)
Acetic anhydride (1.0 mL) and the phenol 6 (64 mg, 0.26 mmol) were heated at 80 °C for 1 h. After removal of the solvent, the residue was diluted with CHCl3 (16 mL) and the organic solution was washed with NH4OH and dried over MgSO4. The solvent was evaporated to give 7 (70 mg, 94%) as a light yellow oil. This was used directly in the next step. HRMS [M+H]+ calcd for C17H22NO3: 288.1600. Found, 288.1500.
4.1.6 rac-(4R,6aR,11bR)-3-Carboxylate-2,3,4,5,6,6a-hexahydro-1H-4,11b-methanobenzofuro [3,2-d]azocine-10-acetate (8)
To a solution of 7 (70 mg, 0.24 mmol) in ClCH2CH2Cl (3.0 mL) were added K2CO3 (168 mg, 1.21 mmol) followed by ethylchloroformate (131 mg. 1.21 mmol). The reaction mixture was refluxed overnight. After filtration and removal of solvent, the residue was diluted with H2O, extracted with CHCl3 (3 × 16 mL) and dried over MgSO4. The solvent was evaporated to afford carbamate 8 (77 mg, 93%) as light yellow powder. It was used directly in the next step without further purification. HRMS [M+H]+ calcd for C19H24NO5: 346.1654. Found, 346.1638.
4.1.7 rac-(4R,6aR,11bR)-2,3,4,5,6,6a-Hexahydro-1H-4,11b-methanobenzofuro[3,2-d]azocin-10-ol (9)
H2SO4 (3.0 mL, 40% w/w) was added to compound 8 (77 mg, 0.22 mmol) and the mixture was heated to reflux and stirred overnight. After cooling down to 0 °C, the reaction mixture was made basic with aq NaOH (pH 8). The basicity was raised to pH 9 using NH4OH and the mixture was extracted with CHCl3/MeOH (10:1). The organic solution was dried over MgSO4 and the solvent was evaporated in vacuo to give the secondary amine 9 (41 mg, 81%) as a white powder that was used directly in the next step without further purification.
4.1.8 rac-(4R,6aR,11bR)-2,3,4,5,6,6a-Hexahydro-3-phenylethyl-1H-4,11b-methanobenzofuro [3,2-d]azocin-10-ol (10)
The secondary amine 9 (40 mg, 0.18 mmol) was dissolved in DMF (1.0 mL), followed by addition NaHCO3 (13 mg, 0.19 mmol) and (2-bromoethyl)benzene (36 mg, 0.193 mmol). The reaction mixture was stirred at 90 °C for 3 h. After removal of the solvent in vacuo, the residue was diluted with CHCl3 (10 mL) and the organic material was washed with H2O (2 × 4 mL). The organic phase was dried over MgSO4 and the resulting residue was purified by flash column chromatography on silica gel (CHCl3/MeOH 40:1) to give compound 10 (40 mg, 68%) as off-white powder, mp 76–77 °C. 1H NMR (300 MHz, CDCl3) δ 1.66–1.87 (m, 6H), 2.10–2.26 (m, 3H), 2.54 (t, J = 12.6 Hz, 1H), 2.69 (m, 2H), 2.84–2.93 (m, 2H), 3.39 (br, 1H), 4.84 (t, J = 8.5 Hz, 1H), 6.61–6.63 (m, 3H), 7.25–7.38 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 16.7, 24.6, 24.7, 25.0, 29.9, 32.3,42.1, 44.9, 52.0, 56.0, 85.9, 109.8, 111.0, 115.6, 127.1, 128.9, 192.0, 129.2, 129.5, 151.9, 152.02. HRMS [M+H]+ calcd for C22H26NO2: 336.1964; found, 336.1962. Anal. Calcd for C22H25NO2 .0.85 CHCl3: C, 62.81; H, 5.96; N, 3.21. Found: C, 62.73; H, 5.90; N, 3.22.
4.1.9 rac-(1R,5S,6R)-5-(2-Fluoro-5-nitrophenyl)-2-methyl-2-azabicyclo[3.3.1]nonan-6-yl acetate (12)
A mixture of rac-(1R,5R,6S)-(5-(2-fluorophenyl)-2-methyl-2-azabicyclo[3.3.1]nonan-6-ol4 (11, 5.5 g, 22 mmol) and Ac2O (3.96 mL, 42 mmol) in 14 mL of AcOH was stirred at 90 °C for 18 h. The AcOH was removed under reduced pressure and fuming HNO3 (13 mL) was added slowly at −5 °C with stirring. The mixture was allowed to warm to room temperature over 1 h. A second portion of fuming HNO3 (15 mL) was then added. After stirring for 2 h at room temperature, the mixture was cooled (−5 to −10 °C) and carefully basified with 5N NaOH (ca. 100 mL), maintaining the temperature between 0 to −5 °C. The product was extracted with CH2Cl2 (2×) and the combined extracts were washed with H2O and brine and dried (MgSO4). Removal of solvent gave 12 (7.09 g, 96%) as light yellow crystals. A sample was recrystallized from EtOAc, mp 138.5–139 °C. 1H NMR (500 MHz; CDCl3) δ 8.21 (dd, J = 6.9, 2.7 Hz, 1H), 8.10 (td, J = 6.1, 2.7 Hz, 1H), 7.12 (dd, J = 11.8, 9.0 Hz, 1H), 5.36 (dd, J = 10.2, 7.6 Hz, 1H), 3.04 (td, J = 12.1, 4.8 Hz, 1H), 3.00-2.90 (m, 2H), 2.71-2.64 (m, 1H), 2.54-2.42 (m, 4H), 2.33-2.25 (m, 1H), 2.13-2.03 (m, 2H), 1.98 (d, J = 13.0 Hz, 1H), 1.89-1.77 (m, 4H), 1.61-1.51 (m, 1H); 13C NMR (125 MHz; CDCl3) δ 170.2, 166.6, 164.5, 144.1, 144.1, 135.3, 135.2, 124.9, 124.8, 124.6, 124.5, 118.0, 117.8, 75.5, 75.5, 52.9, 50.6, 43.1, 39.7, 39.6, 37.9, 37.9, 37.8, 30.0, 28.7, 23.6, 21.0.
HRMS [M+H]+ calcd for C17H22N2O4F: 337.1564. Found: 337.1560. Anal. Calcd for C17H22N2O4F: C, 60.70, H, 6.29; N, 8.33. Found: C, 60.89; H, 6.32; N, 8.32.
4.1.10 rac-(1R,5R,6S)-5-(2-Fluoro-5-nitrophenyl)-2-methyl-2-azabicyclo[3.3.1]nonan-6-ol (13)
The acetate 12 (7 g, 20 mmol) in 2N HCl (140 mL) was refluxed for 3 h. The mixture was cooled, basified with 5N NaOH (ca. 70 mL), extracted with CH2Cl2 (2×), and the combined extracts were washed with H2O and brine and dried (MgSO4). The solvent was removed to give 13 (5.95 g, 96%), identical with the known compound.4
4.1.11 rac-(4R,6aS,11bR)-2,3,4,5,6,6a-Hexahydro-10-nitro-3-N-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine (15)
To a mixture of rac-(4R,6aS,11bR)-2,3,4,5,6,6a-hexahydro-3-methyl-10-nitro-1H-4,11b-methanobenzofuro[3,2-d]azocine4 (14, 5.26 g, 19.2 mmol) and K2CO3 (2.16 g, 15.6 mmol) in 125 mL of acetonitrile was added with stirring a solution of cyanogen bromide (2.44 g, 23.0 mmol) in 5 mL of acetonitrile, and the mixture was refluxed for 2 h. After cooling, the solids were removed by filtration and the solvent evaporated. To the residue was added a mixture of 30 mL of glacial HOAc and 120 mL of 2N HCl, and the mixture was refluxed for 18 h. After cooling, the mixture was basified with 5N NaOH and extracted with CHCl3 (3×). The combined extracts were washed with a small volume of H2O, dried (MgSO4) and the solvent removed to give 15 (5 g, quantitative) as a light yellow powder sufficiently pure for the next reaction.
4.1.12 rac-(4R,6aS,11bR)-10-Chloro-2,3,4,5,6,6a-hexahydro-8-nitro-1H-4,11b-methanobenzofuro[3,2-d]azocine (18)
The secondary amine 18 was prepared from rac-(4R,6aS,11bR)-10-chloro-2,3,4,5,6,6a-hexahydro-3-methyl-8-nitro-1H-4,11b-methanobenzofuro[3,2-d]azocine4 (17) using the procedure for the preparation of compound 15 from 14, to give a yellow solid (95%) sufficiently pure for the next reaction.
4.1.13 Optical resolution of rac-(4R,6aS,11bR)-2,3,4,5,6,6a-hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-10-ol (16)
The racemic N-phenethyl substituted para-b (16, 366 mg) and ortho-b (19, 295 mg) isomers were resolved using supercritical fluid chromatography.14 Resolution of the racemic para-b isomer 16 was conducted on a preparative scale using a 3×25 cm (S,S)-Whelk-01 column (Regis Technologies, Morton Grove, IL) using 65% liquid CO2 (solvent) and 35% isopropanol with 0.1% isopropylamine (co-solvent) isocratically at 80 mL/min. The sample was applied in MeOH:CH2Cl2 (1:1) and retention times were 0.8 and 2.5 min for the para-b enantiomers 16a and 16b with recoveries of 170 mg and 168 mg, respectively, and estimated ee values of 98% for both.
4.1.13.1 rac-(4R,6aS,11bR)-2,3,4,5,6,6a-Hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-10-ol (16a)
16a (base): [α]D23 +52.1 (c 0.87, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.28 (t, J = 7.5 Hz, 2H), 7.23-7.17 (m, 3H), 6.72 (d, J = 8.4 Hz, 1H), 6.69 (d, J = 2.4 Hz, 1H), 6.62 (dd, J = 8.4, 2.4 Hz, 1H), 4.09 (dd, J = 11.6, 6.6 Hz, 1H), 3.15 (s, 1H), 2.93-2.87 (m, 1H), 2.86-2.68 (m, 5H), 2.51 (d, J = 12.4 Hz, 1H), 2.35 (dd, J = 14.8, 1.7 Hz, 1H), 2.26-2.16 (m, 1H), 1.87-1.77 (m, 2H), 1.74-1.65 (m, 1H), 1.53-1.43 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 153.2, 150.6, 140.4, 138.7, 128.9, 128.6, 126.3, 114.9, 110.9, 110.4, 91.7, 58.1, 52.8, 49.4, 44.0, 38.1, 34.5, 31.8, 27.6, 22.6; HRMS [M+H]+ calcd for C22H26NO2: 336.1964. Found, 336.1955.
4.1.13.2 rac-(4S,6aR,11bS)-2,3,4,5,6,6a-Hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-10-ol (16b)
16b (base): [α]D23 −55.7 (c 0.94, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.28 (t, J = 7.4 Hz, 2H), 7.25-7.16 (m, 3H), 6.72 (d, J = 8.4 Hz, 1H), 6.67 (s, 1H), 6.61 (d, J = 8.7 Hz, 1H), 4.09 (dd, J = 11.9, 6.3 Hz, 1H), 3.13 (s, 1H), 2.94-2.88 (m, 1H), 2.86-2.66 (m, 5H), 2.49 (d, J = 12.2 Hz, 1H), 2.35 (d, J = 14.1 Hz, 1H), 2.27-2.16 (m, 2H), 1.87-1.77 (m, 2H), 1.75-1.65 (m, 1H), 1.52-1.43 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 153.2, 150.6, 140.4, 138.7, 128.9, 128.6, 126.3, 114.9, 110.9, 110.4, 91.7, 58.1, 52.8, 49.4, 44.0, 38.1, 34.5, 31.8, 27.6, 22.6; HRMS [M+H]+ calcd for C22H26NO2: 336.1964. Found, 336.1955.
Enantiomers 16a and 16b were converted to their HBr salts in 2-propanol-H2O. 16a HBr: [α]D23 +57.2 (c 0.51, MeOH:H2O, 1:1); mp 276–280 °C. Anal. Calcd for C22H26BrNO2: C, 63.46, H, 6.29, N, 3.36. Found: C, 63.16, H, 6.28, N, 3.32. 16b HBr: [α]D23 −55.8 (c 0.51, MeOH:H2O, 1:1); mp 276–278°C. Anal. Calcd for C22H26BrNO2: C, 63.46, H, 6.29, N, 3.36. Found: C, 63.36, H, 6.36, N, 3.33.
4.1.14 Optical resolution of rac-(4R,6aS,11bR)-2,3,4,5,6,6a-hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-8-ol (19)
Optical resolution of the racemic ortho-b isomer 19 was achieved in the same manner as with 16, above. The sample was applied in 2-propanol and retention times were 2.6 and 3.1 min for ortho-b fractions 1 and 2 (19a and 19b, respectively) with recoveries of 121 and 126 mg and estimated ee values of 99.9 and 98.5%, respectively.
4.1.14.1 rac-(4R,6aS,11bR)-2,3,4,5,6,6a-Hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-8-ol (19a)
19a (base): [α]D23 −64.1 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.28 (t, J = 7.5 Hz, 2H), 7.22-7.17 (m, 3H), 6.80 (t, J = 7.6 Hz, 1H), 6.74 (d, J = 7.9 Hz, 1H), 6.68 (d, J = 7.2 Hz, 1H), 5.60 (br s, 1H), 4.15 (dd, J = 12.6, 5.7 Hz, 1H), 3.11 (s, 1H), 2.99-2.93 (m, 1H), 2.85 (td, J = 11.7, 5.9 Hz, 1H), 2.81-2.68 (m, 4H), 2.46 (d, J = 12.4 Hz, 1H), 2.38-2.32 (m, 1H), 2.32-2.18 (m, 2H), 1.87-1.73 (m, 3H), 1.51-1.42 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 146.0, 141.0, 140.6, 138.9, 128.9, 128.5, 126.2, 122.2, 115.3, 114.0, 92.6, 58.2, 53.0, 49.3, 44.3, 38.3, 34.7, 31.9, 27.5, 23.0; HRMS [M+H]+ calcd for C22H26NO2: 336.1964. Found, 336.1967.
4.1.14.2 rac-(4S,6aR,11bS)-2,3,4,5,6,6a-hexahydro-3-phenethyl-1H-4,11b-methanobenzofuro[3,2-d]azocine-8-ol (19b)
19b (base): [α]D23 +63.4 (c 1.1, CHCl3); 1H NMR (500 MHz, CDCl3) δ 7.28 (t, J = 7.4 Hz, 2H), 7.22-7.17 (m, 3H) 6.80 (t, J = 7.6 Hz, 1H), 6.74 (d, J = 8.0 Hz, 1H), 6.68 (d, J = 7.2 Hz, 1H), 5.78, (br s, 1H), 4.15 (dd, J = 12.6, 5.7 Hz, 1H), 3.11 (s, 1H), 2.96 (dd, J = 10.9, 8.2 Hz, 1H), 2.85 (td, J = 11.7, 5.9 Hz, 1H), 2.81-2.68 (m, 4H), 2.47 (d, J = 12.2 Hz, 1H), 2.39-2.32 (m, 1H), 2.32-2.17 (m, 2H), 1.87-1.73 (m, 3H), 1.52-1.42 (m, 1H); 13C NMR (125 MHz, CDCl3) δ 146.1, 141.0, 140.5, 138.8, 128.9, 128.5, 126.2, 122.2, 115.3, 114.0, 92.6, 58.2, 53.0, 49.3, 44.3, 38.2, 34.7, 31.8, 27.5, 22.9; HRMS [M+H]+ calcd for C22H26NO2: 336.1964. Found, 336.1960.
Enantiomers 19a and 19b was converted to their HBr salts in 2-propanol. 19a HBr: [α]D23 −53.3 (c 0.59, MeOH:H2O, 1:1); mp 298–300 °C. Anal. Calcd for C22H26BrNO2: C, 63.46, H, 6.29, N, 3.36. Found: C, 63.57, H, 6.20, N, 3.32. 19b HBr: [α]D23 +52.6 (c 0.53, MeOH:H2O, 1:1); mp 298–300 °C. Anal. Calcd for C22H26BrNO2: C, 63.46, H, 6.29, N, 3.36. Found: C, 63.51, H, 6.23, N, 3.32.
4.2 Binding and Efficacy assays. Cell culture and membrane preparation
As noted previously,15 the recombinant CHO cells (hMOR-CHO, hDOR-CHO and hKOR-CHO) were produced by stable transfection with the respective human opioid receptor cDNA, and were provided by Dr. Larry Toll (SRI International, CA). The cells were grown on plastic flasks in DMEM (100%) (hDOR-CHO and hKOR-CHO) or DMEM/F-12 (50%/50%) medium (hMOR-CHO) containing 10% FBS, and G-418 (0.10–0.2 mg/mL) under 95% air/5% CO2 at 37 °C. Cell monolayers were harvested and frozen at −80 °C.
4.2.1 [35S]GTP-γ-S binding assays
The assays were conducted with minor modifications of published methods.17 In this description, buffer “A” is 50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA and buffer “B” is buffer A plus 1.67 mM DTT and 0.15% BSA. On the day of the assay, cells were thawed on ice for 15 min and homogenized using a polytron in 50 mM Tris-HCl, pH 7.4, containing 4 μg/mL leupeptin, 2 μg/mL chymostatin, 10 μg/mL bestatin and 100 μg/mL bacitracin. The homogenate was centrifuged at 30,000 × g for 10 min at 4 °C, and the supernatant discarded. The membrane pellets were resuspended in buffer B and used for [35S]GTP-γ-S binding assays. Test tubes received the following additions: 50 μL buffer A plus 0.1% BSA, 50 μL GDP in buffer A/0.1% BSA (final concentration = 40 μM), 50 μL drug in buffer A/0.1% BSA, 50 μL [35S]-GTP-γ-S in buffer A/0.1% BSA (final concentration = 50 pM), and 300 μL of cell membranes (50 μg of protein) in buffer B. The final concentrations of reagents in the [35S]GTP-γ-S binding assays were: 50 mM Tris-HCl, pH 7.4, containing 100 mM NaCl, 10 mM MgCl2, 1 mM EDTA, 1 mM DTT, 40 μM GDP and 0.1% BSA. Incubations proceeded for 3 h at 25 °C. Nonspecific binding was determined using GTP-γ-S (40 μM). Bound and free [35S]GTP-γ-S were separated by vacuum filtration (Brandel) through GF/B filters. The filters were punched into 24-well plates to which was added 0.6 mL LSC-cocktail (Cytoscint). Samples were counted, after an overnight extraction, in a Trilux liquid scintillation counter at 27% efficiency.
4.3. X-ray crystal data on compounds 16b and 19a
Single-crystal X-ray diffraction data on compounds 16b and 19a were collected using MuKα radiation and a Bruker Platinum 135 CCD area detector. Crystals were prepared for data collection by coating with high viscosity microscope oil. The oil-coated crystal was mounted on a micro-mesh mount (Mitergen, Inc.) and transferred to the diffractometer. The structures were solved by direct methods and refined by full-matrix least squares on F2 values using the programs found in the SHELXTL suite (Bruker, SHELXTL v6.10, 2000, Bruker AXS Inc., Madison, WI).18 Corrections were applied for Lorentz, polarization, and absorption effects. Parameters refined included atomic coordinates and anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms on carbons were included using a riding model [coordinate shifts of C applied to H atoms] with C-H distance set at 0.96 Å. Complete information on data collection and refinement is available in the supplemental material.
For compound 16b a 0.643 × 0.104 × 0.026 mm3 crystal was prepared for data collection and a data set collected at room temperature. The crystal was orthorhombic in space group P 212121, with unit cell dimensions a = 7.4195(2), b = 12.5090(3), and c = 20.7761(5) Å. Data was 98.5% complete to 68.26° θ with an average redundancy of 4.98. The final anisotropic full matrix least-squares refinement on F2 with 235 variables converged at R1 = 4.56%, for the observed data and wR2 = 13.56% for all data.
For compound 19a a 0.364 × 0.085 × 0.042 mm3 crystal was prepared for data collection and a data set collected at room temperature. The crystal was monoclinic in space group P 21, with unit cell dimensions a = 9.1613(3), b = 11.7449(4), c = 18.4218(6) Å, and b = 90.721°. Data was 91.2% complete to 68.04° θ with an average redundancy of 3.09. The final anisotropic full matrix least-squares refinement on F2 with 476 variables converged at R1 = 4.99%, for the observed data and wR2 = 14.94% for all data.
Atomic coordinates for 16b and 19a have been deposited with the Cambridge Crystallographic Data Centre (deposition numbers 816329 and 816330, respectively). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK [fax: +44(0)-1223-336033 or e-mail: deposit@ccdc.cam.ac.uk.
Supplementary Material
Acknowledgments
The work of the Drug Design and Synthesis Section, CBRB, NIDA, & NIAAA, was supported by the NIH Intramural Research Programs of the National Institute on Drug Abuse (NIDA) and the National Institute of Alcohol Abuse and Alcoholism. The Clinical Psychopharmacology Section, CBRB, NIDA, was supported by the NIH Intramural Research Program of the National Institute on Drug Abuse (NIDA). We thank the Averica Discovery Services, J. P. Kiplinger, President, Worcester, MA, for carrying out the supercritical fluid chromatography used to obtain the enantiomers of 16 and 19. We also thank Dr. Klaus Gawrisch and Dr. Walter Teague (Laboratory of Membrane Biochemistry and Biophysics, NIAAA, for NMR spectral data. The authors express their thanks to Noel Whittaker and Wesley White, Laboratory of Analytical Chemistry, NIDDK, for mass spectral data and 1H NMR spectral data. The X-ray crystallographic work was supported by NIDA through an Interagency Agreement #Y1-DA1101 with the Naval Research Laboratory (NRL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burke TR, Jr, Jacobson AE, Rice KC, Weissman BA, Silverton JV. Problems of Drug Dependence 1983. In: Harris LS, editor. National Institute on Drug Abuse Research Monograph 49; DHHS ((ADM) 84–1316); Washington DC: 1984. p. 109. [Google Scholar]
- 2.Burke TR, Jr, Jacobson AE, Rice KC, Silverton JV. J Org Chem. 1984;49:1051. [PubMed] [Google Scholar]
- 3.Yamada K, Flippen-Anderson JL, Jacobson AE, Rice KC. Synthesis-Stuttgart. 2002:2359. [Google Scholar]
- 4.Kurimura M, Liu H, Sulima A, Przybyl AK, Ohshima E, Kodato S, Deschamps JR, Dersch C, Rothman RB, Lee YS, Jacobson AE, Rice KC. J Med Chem. 2008;51:7866. doi: 10.1021/jm800913d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.May EL. J Org Chem. 1956;21:899. [Google Scholar]
- 6.Zhang Y, Lee YS, Rothman RB, Dersch CM, Deschamps JR, Jacobson AE, Rice KC. J Med Chem. 2009;52:7570. doi: 10.1021/jm9004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke TR, Jr, Jacobson AE, Rice KC, Silverton JV. J Org Chem. 1984;49:2508. [PubMed] [Google Scholar]
- 8.Linders JT, Burke TR, Jacobson AE, Rice KC. Problems of Drug Dependence, 1990. In: Harris LS, editor. National Institute on Drug Abuse Research Monograph. Vol. 105. Washington, DC: 1991. p. 388. [Google Scholar]
- 9.Kodato S, Linders JTM, Gu XH, Yamada K, Flippen-Anderson JL, Deschamps JR, Jacobson AE, Rice KC. Org & Biomolec Chem. 2004;2:330. doi: 10.1039/b312633c. [DOI] [PubMed] [Google Scholar]
- 10.Tadic D, Linders JTM, Flippen-Anderson JL, Jacobson AE, Rice KC. Tetrahedron. 2003;59:4603. [Google Scholar]
- 11.Hashimoto A, Przybyl AK, Linders JTM, Kodato S, Tian XR, Deschamps JR, George C, Flippen-Anderson JL, Jacobson AE, Rice KC. J Org Chem. 2004;69:5322. doi: 10.1021/jo040159k. [DOI] [PubMed] [Google Scholar]
- 12.Zezula J, Jacobson AE, Rice KC. Heterocycles. 2007;71:881. [Google Scholar]
- 13.Zezula J, Singer LB, Przybyl AK, Hashimoto A, Dersch CM, Rothman RB, Deschamps J, Lee YS, Jacobson AE, Rice KC. Org & Biomol Chem. 2008;6:2868. doi: 10.1039/b803433h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White C, Burnett J. Journal of Chromatography A. 2005;1074:175. doi: 10.1016/j.chroma.2005.02.087. [DOI] [PubMed] [Google Scholar]
- 15.Fontana G, Savona G, Rodriguez B, Dersch CM, Rothman RB, Prisinzano TE. Tetrahedron. 2008;64:10041. doi: 10.1016/j.tet.2008.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH, Deschamps JR, Rothman RB, Dersch CM, Folk JE, Cheng K, Jacobson AE, Rice KC. Bioorg Med Chem. 2011 doi: 10.1016/j.bmc.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, Hashimoto A, Rice KC, Jacobson AE, Thomas JB, Carroll FI, Lai J, Rothman RB. Synapse. 2001;39:64. doi: 10.1002/1098-2396(20010101)39:1<64::AID-SYN9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.