Summary
The migration of polymorphonuclear leukocytes (PMN) across the intestinal epithelium is a histopathological hallmark of many mucosal inflammatory diseases including inflammatory bowel disease (IBD). The terminal transmigration step is the detachment of PMN from the apical surface of the epithelium and their subsequent release into the intestinal lumen. The current study sought to identify epithelial proteins involved in the regulation of PMN migration across intestinal epithelium at the stage where PMN reach the apical epithelial surface. A panel of antibodies reactive with IFNγ-stimulated T84 intestinal epithelial cells was generated. Screening efforts identified one mAb, GM35, which prevented PMN detachment from the apical epithelial surface. Microsequencing studies identified the GM35 antigen as human CD44. Transfection studies confirmed this result by demonstrating the loss of the functional activity of the GM35 mAb following attenuation of epithelial CD44 protein expression. Immunoblotting and immunofluoresence revealed the GM35 antigen to be an apically expressed v6 variant-exon containing form of CD44 (CD44v6). ELISA analysis demonstrated the release of soluble CD44v6 by T84 cells during PMN transepithelial migration (TEM). In addition, the observed release of CD44v6 was blocked by GM35 treatment supporting a connection between CD44v6 release and PMN detachment. Increased expression of CD44v6 and the GM35 antigen was detected in inflamed ulcerative colitis tissue. This study demonstrates for the first time that epithelial expressed CD44v6 plays a role in PMN clearance during inflammatory episodes through regulation of the terminal detachment of PMNs from the apical epithelial surface into the lumen of the intestine.
Keywords: neutrophil, CD44v6, inflammation, clearance, glycoprotein
Introduction
Polymorphonuclear leukocytes (PMNs) play a key role in host defense through phagocytosis and destruction of invading microorganisms and as such are important effectors of the acute inflammatory response (1). A key event in these processes is the migration of PMNs out of the circulation and across both endothelial and epithelial tissue barriers in response to chemotactic stimuli. While the steps involved in PMN migration across vascular endothelium have been extensively characterized (2–4), much less is known about the sequential cell-cell interactions that define PMN transepithelial migration. Previous studies have demonstrated stimulus-specific PMN epithelial migratory interactions, with fMLP mediating its effects through binding of leukocyte-specific β2-integrins to epithelial counter-ligands (5), but with other chemoattractants such as IL-8 and C5a acting independently of β2-integrins (6). Subsequent migration by PMN into the intraepithelial space is reported to be regulated through epithelial glycoprotein CD47 interaction with PMN-expressed signal regulatory protein α (Sirpα) (7–9). In addition, neutrophil expressed junctional-adhesion-like protein (JAML) binding to epithelial coxsackie and adenovirus receptor (CAR) protein has been shown to regulate the passage of PMN through epithelial tight junctions (10).
As well as expressing ligands facilitating initial PMN binding to the basal surface and subsequent intracellular passage, the intestinal epithelium also expresses proteins mediating PMN detachment and clearance from the luminal surface. Although these epithelial ligands have not been extensively characterized, it has been previously reported that epithelial intracellular adhesion molecule 1 (ICAM-1) is expressed apically and acts as a PMN retention ligand under inflammatory conditions(11). In addition PMN Fc receptor interactions with apical epithelial proteins have also been implicated in PMN-epithelial retention (12). More recently, it has been reported that decay-accelerating factor (DAF) functions as an anti-adhesive epithelial glycoprotein that regulates PMN detachment from the epithelium (13). An increased understanding of the processes governing the terminal release of PMN into the intestinal lumen has important clinical implications, as inflammatory infiltrates characterized by accumulations of PMNs within mucosal tissues are pathognomonic of both acute and chronic inflammatory conditions. Specifically, PMN accumulation and abscess formation within intestinal crypts at the apical epithelial surface are pathological features of multiple inflammatory disease processes of the intestine including ulcerative colitis (14), infectious colitis (15), and necrotizing enterocolitis (16).
In the current study we aimed to identify novel epithelial ligands important in the terminal stages of PMN transmigration including detachment into the intestinal lumen. To this end a panel of monoclonal antibodies was generated against IFNγ-treated T84 epithelial plasma membranes. This screening process identified one mAb, designated GM35, that inhibited the detachment/release of PMN from the apical epithelial surface of polarized monolayers of epithelial cells following N-formylpeptide (fMLF)-stimulated PMN migration. Extensions of these observations revealed the GM35 antigen to be an apically expressed, v6-containing variant of human CD44 (CD44v6). The current study also demonstrated that T84 epithelial cells release soluble CD44v6 during PMN transmigration. The release of epithelial CD44v6 during transmigration was blocked by apical treatment of epithelia with GM35 suggesting a connection between CD44v6 protein shedding and PMN detachment. Our results indicate for the first time that CD44v6 acts as a mediator in PMN clearance from the apical surface of the intestinal epithelium. Increased understanding of the role of CD44v6 in neutrophil epithelial clearance has the potential to allow for the design of specific targeting mechanisms to better facilitate intestinal inflammatory resolution.
Materials and Methods
Cell Culture
Cultures of T84 cells (passage 68–72) (17), HT29 cells (passage 128–138) (18), Caco2 cells (passage 28–38) (5), SK-CO15 cells (passage 8–11) (19) and HeLa cells (passage 63–68) (20) were grown as described previously. Human dermal microvascular endothelial cells (HDMEC) (passage 4–9) were purchased from Promocell (Heidelberg, Germany) and grown according to manufacturer’s instructions.
Antibodies and Reagents
Monoclonal anti-CD44v3, anti-CD44v4,5 and anti-CD44v6 antibodies were purchased from R&D Systems (Minneapolis, MN). Monoclonal anti-CD44v7 and anti-CD44v10 antibodies were purchased from Abcam (Cambridge, MA). Monoclonal anti-CD16 F(ab′)2 and anti-CD32 F(ab′)2 antibody fragments were purchased from Fitzgerald Industries International, Inc (Concord, MA). Monoclonal anti-desmoglein mAb was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). J10.4 an isotype matched binding control IgG1 for GM35, the anti-CD11b/CD18 mAb CBRM1/29 and OE-1 the anti-CD55 mAb have been described elsewhere (13, 21, 22). Protein G Sepharose beads were purchased from GE healthcare. 3′,6′-bis(Acetyloxy)-5(or 6)-(acetyloxy)methoxy]carbonyl-3-oxo-spiroisobenzofuran-1(3H),9′-9Hxanthene]-2′,7′-dipropanoic acid 2′,7′-bis(acetyloxy)methyl ester (BCECF-AM) was purchased from Merck (Damstadt, Germany). Zenon ® Alexa Fluor ® 488 Mouse IgG1, 568 Mouse IgG1 and 568 Mouse IgG2b labeling kits were purchased from Invitrogen Corporation (Carlsbad, CA). Chemotactic peptide N-Formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLF) and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO). Full length CD44 in a pCMv6 entry vector plasmid, CD44 specific short hairpin RNA (shRNA) constructs and a Scr construct plasmid in pGFP-V-RS vectors were purchased from Origene (Rockville, MD). Recombinant human IFNγ was provided by Genentech. Human stdCD44 (CD44s) and human CD44var6 (CD44v6) instant ELISA kits were purchased from Bender Medsystems (Vienna, Austria).
Antibody Isolation
IFNγ responsive T84 cell membranes were isolated as previously described (23), and used as antigens to generate a panel of antibodies as described previously (12). Briefly, BALB/c mice were injected intraperitoneally with a 50:50 mix of complete Freund’s adjuvant and 1 × 107 cell equivalent of T84 membrane proteins. All procedures for the immunization of mice and production of monoclonal antibodies were approved by the Emory Institutional Animal Care and Use Committee (IACUC). Mice were inoculated every 2 weeks for a total of 6 weeks before harvesting of splenocytes three days after a terminal boost. Harvested splenocytes were washed in PBS, mixed 2:1 with hybridoma cells in the presence of 50% polyethylene glycol and plated in DMEM supplemented with 1% HAT for selection. 920 single clones were obtained by limiting dilution. T84 cells grown in 96-well plates were used in an ELISA to select for clones producing antibodies to epithelial surface proteins. Supernatants from these clones were used in PMN transmigration assays to select for antibodies that significantly inhibited PMN migration in the physiologically relevant basolateral to apical direction. Of these antibodies, one IgG1 subclone designated GM35 was chosen for further characterization.
Generation of F(ab′)2 fragments from mAb GM35
F(ab′)2 fragments of the IgG1 GM35 antibody were generated by digestion with Ficin, using a commercial kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s instructions. The purity of GM35 F(ab′)2 fragments was assessed by polyacrylamide gel–electrophoretic analysis and subsequent densitometry.
PMN isolation
PMN were isolated from whole blood obtained from normal human volunteers, with approval from the Emory University Institutional Review Board on human subjects, by using a previously described density gradient centrifugation technique (24). PMN were re-suspended in HBSS with 10mM Hepes, pH7.4, and without Ca2+ or Mg2+ at a concentration of 5 × 107 cells/ml. Neutrophils isolated in this way were 97% pure and >95% viable and were used for transmigration within 2 hours of isolation.
PMN Transmigration and Cell Adhesion Assays
For transmigration experiments, cells were grown on collagen-coated, permeable 0.33-cm2 polycarbonate filters (5μm pore size; Costar Corp.) as described previously (7, 17, 25). All epithelial migration experiments were performed in the presence of a chemotactic gradient of 100nM fMLF and in the physiologically relevant basolateral-to-apical direction (i.e. inverted monolayers) unless otherwise indicated, and all HDMEC transmigration studies were performed in the apical-to-basolateral direction with a 10nM fMLF gradient as described previously (26). For migration experiments, 1×106 PMN were added to the upper chambers of transwell inserts and migration levels assessed at 37oC following indicated intervals. Transmigrated PMN were quantified by colorimetric enzyme activity assay specific for the PMN azurophilic marker myeloperoxidase (MPO) as described previously (27). Briefly, migrated neutrophils were lysed by the addition of Triton X-100 to a final concentration of 0.5% and acidified by the addition of citrate buffer (100mM, pH 4.2). Neutrophil standards in the range of 0.05×106 to 1.0×106 neutrophils were prepared and similarly lysed. Standard and experimental sample aliquots (in triplicate) were added to equal volumes of ABTS solution (1 mM ABTS, 0.03% H2O2, 100 mM sodium citrate buffer, pH 4.2) in a 96-well plate and resulting color was quantitated on a plate reader at 405 nm. A standard curve with numbers of neutrophils vs MPO activity was constructed and used to determine the number of migrated neutrophils in experimental samples. PMNs remaining adherent to T84 monolayers after basolateral-to-apical migration were quantified using a previously described monolayer washing procedure (12). Briefly, after completion of transmigration, T84 monolayers were removed and transferred to new tissue culture plates containing 1ml HBSS+/well. Plates were spun for 5 min. (50g, 4°C) and detached PMN quantified by MPO assay as above.
For IFNγ studies T84 epithelial monolayers grown to 70–80% confluency in 24 well tissue culture plates were treated for 24 hours with 100U/ml IFNγ immediately prior to assessment of PMN adhesion. PMN adhesion to confluent T84 and HT29 epithelial cells was measured directly using modifications of previous protocols (13, 28, 29).
Briefly, epithelial cells were washed free of media and treated with 10μg/ml GM35 or isotype control binding antibody in the presence of 100nM fMLF for 10 minutes at 37°C. BCECF-labeled PMN (5×105) pre-incubated with relevant antibodies were added to epithelial monolayers and plates were centrifuged at 50g for 5 min to uniformly settle PMN, before adhesion was allowed to proceed for 10 minutes at 37°C. Monolayers were gently washed with HBSS+, and fluorescence intensity (excitation, 485nm; emission, 530nm) was measured on a fluorescent plate reader. Adherent PMN numbers were determined from standard curves generated by serial dilution of known numbers of BCECF-AM-labeled cells.
Immunoblotting and Immunofluoresence
Cell lysates for Western blotting were prepared with the following lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% TX-100, 1 mM Na3VO4, and 1 mM PMSF) supplemented with 10% mammalian tissue protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). For immunoprecipitation experiments, pre-cleared cell lysates were incubated with 2μg of relevant mAb for 4h at 4°C followed by incubation with protein G-Sepharose beads overnight at 4°C. Washed immunoprecipitates and regular cell lysates were boiled in SDS-PAGE sample buffer under reducing conditions and subjected to SDS-PAGE followed by transfer to PVDF under standard conditions. Membranes were blocked with 0.5% milk, incubated with 1μg/ml GM35 or anti-CD44 variant antibodies. Primary antibodies were detected using HRP-linked secondary antibodies (Jackson Immunoresearch laboratories West Grove, PA). All blocking, antibody incubations and intervening washes with TBS-Tween20 were carried out using the SNAP i.d. protein detection system (Millipore, Billerica, MA).
Immunfluoresecent labeling of T84 epithelial cells was achieved as follows. Non-permeabilized T84 monolayers were fixed using 10% Formalin (20°C, 20 min) and subsequently blocked with 2% BSA in PBS. Monolayers were then incubated with 10μg/ml GM35 labeled with Zenon ® Alexa Fluor ® 488 Mouse IgG1, 5μg/ml anti-CD44 antibody labeled with Zenon ® Alexa Fluor ® 568 Mouse IgG1 or Alexa Fluor ® 568 Mouse lgG2b or 10μg/ml anti-CD55 antibody labeled with Zenon ® Alexa Fluor ® 568 Mouse IgG2a for 1hr at room temperature. After three washes with PBS, monolayers were mounted in ProLong anti-fade embedding solution (Invitrogen corp, Carlsbad CA). Images shown were representative of at least three experiments with multiple images taken per monolayer.
For human tissue staining, frozen sections (6 μm) of discarded resection specimen colonic mucosa from patients with ulcerative colitis were obtained. Inflamed and non-inflamed sections of discarded tissue were characterized based on observed disease extent and activity. Tissue was fixed in absolute ethanol, non-specific protein binding was blocked with 3% bovine serum albumin and tissue sections were incubated with primary antibodies, washed in HBSS+, and subsequently labeled with appropriate secondary antibodies. All procedures on discarded human tissue were carried out under Emory IRB approval. All images were captured using an LSM 510 confocal microscope (Carl Zeiss Microimaging, Thornwood NY) with pan-Neofluar 40x/1.3 oil objective using software supplied by the vendor.
shRNA and DNA transfections
For CD44 knockdown studies, one of four HuSh 29mer shRNA constructs against CD44 (p313, p314, p315, p316) or a Scramble construct (Scr) was transfected into HT29 cells. Transfection complexes consisting of 1μg plasmid DNA, 3μl lipofectamine 2000 and 100μl opti-MEM I (Invitrogen Corp, Carlsbad CA) were incubated for 30 minutes at room temperature before addition to HT29 cells grown in 6 well culture plates. Extent of protein knockdown was assessed after 72 hrs by immunoblotting for CD44 as described above. The functional effect of CD44 knockdown on mAb GM35 activity was measured by transfecting HT29 cells with the shRNA constructs 72 hours before examining the effect of GM35 on PMN-HT29 adhesion as described above.
Enzyme Linked Immunoadsorbent Assay (ELISA) detection of soluble CD44
PMN were isolated and prompted to migrate across confluent T84 monolayers in the physiologically relevant basolateral to apical direction in the presence or absence of apically applied GM35 (10μg/ml) as described above. Samples from the apical reservoir were removed at 0,5,15,30,45 and 60 minutes and assessed for levels of soluble CD44 standard (sCD44std) and soluble CD44v6 (sCD44v6) using CD44std and CD44v6 ELISA kits respectively according to manufacturer’s instructions. A standard curve was prepared from six standard dilutions of sCD44std or sCD44v6 and levels of sCD44std and sCD44v6 in experimental samples and standards were measured at 450nm.
Data analysis
Data were analyzed by 2-factor ANOVA using PRISM 5 for Mac OSX version 5.0a 1992–1998, Graphpad software, Inc. Values are expressed as the mean ± SE from a minimum of at least three independent experiments.
Results
In order to identify epithelial mediators of PMN clearance up-regulated by inflammatory stimuli, T84 cells were treated with the pro-inflammatory cytokine IFNγ, a key mediator of intestinal inflammation (30) and barrier function (31). Cytokine-treated T84 epithelial cell membrane preparations were then used to generate mouse monoclonal antibodies (mAbs) against epithelial epitopes. The resulting antibodies were tested using T84 monolayers, as a model intestinal epithelial cell line, to determine their influence on PMN transepithelial migration (TEM) in response to fMLF. Upon initial investigation, one subclone IgG1 antibody, designated GM35, was found to significantly reduce the level of PMN transmigration when applied to the apical surface of T84 monolayers and was chosen for further investigation.
GM35 inhibits detachment of transmigrated PMN from the apical epithelial surface
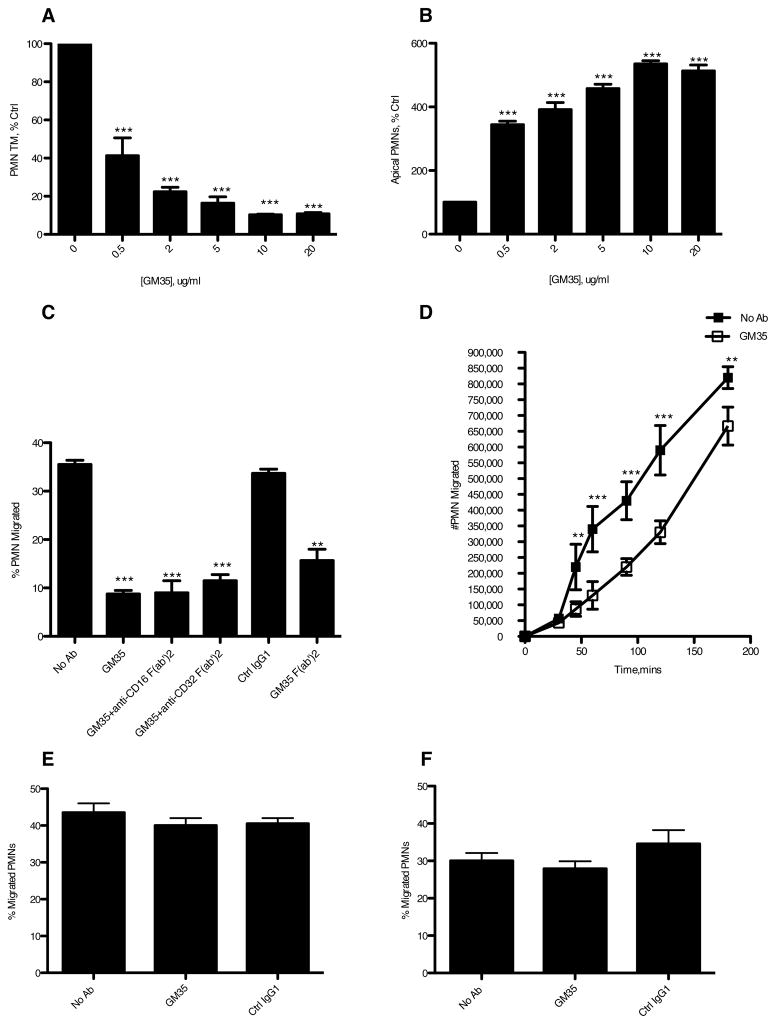
Initial observations revealed that GM35 reduced the levels of PMN migration across T84 epithelial monolayers in a dose-dependent manner in the physiologically relevant basolateral-to-apical direction. At a concentration of 10μg/ml GM35 reduced detectable PMN numbers in the apical chamber by ≥ 80% (P<0.0001) compared to either a no mAb control (Fig. 1A), or an isotype matched non-inhibitory binding control mAb (Fig. 1C). GM35 had no effect on apical to basolateral TEM of PMNs (Fig. 1E), suggesting that GM35 influences PMN transmigration in a polarized fashion. Further, GM35 had no effect on transendothelial migration (Fig. 1F), implying that the effects of GM35 on PMN transmigration are restricted to interactions between PMN and the epithelium. This epithelial specificity of GM35 is further supported by flow cytometric analyses, which, indicate that GM35 does not bind PMN (data not shown).
Figure 1. GM35 inhibits detachment of transmigrated PMNs from the apical epithelial surface.
Confluent T84 monolayers were pre-treated apically with indicated concentrations of GM35 mAb (GM35) before 1×106 PMNs were added to the basolateral surface. PMNs were allowed to migrate in the physiologically relevant basolateral to apical direction for 1 hour in response to a 100nM gradient of n-formyl-methionyl-leucyl-phenylalanine (fMLF). The number of migrated PMNs (A) and the number of PMNs which were adherent to the apical epithelial surface (B) were quantified by myeloperoxidase assay. Data are means +/− SE (n=5). Transmigration assays were also performed in the presence of apically applied GM35, GM35 F(ab′)2, or isotype matched binding control IgG1, with PMN exposed to 5μg/ml functionally inhibitory F(ab′)2 anti-FCR CD32 and CD16 mAbs (C). (D) Confluent T84 monolayers were treated apically with 10μg/ml GM35 (open square) or vehicle (closed square) before the addition of 1×106 PMN. PMN transmigration was then measured over a three-hour time course. (E) 1×106 PMN were added to confluent T84 monolayers pre-treated apically with 10μg/ml GM35 or isotype matched binding control IgG1 before migration in the apical to basolateral direction was measured by MPO assay. (F) Confluent HDMEC monolayers were pre-treated apically with 10μg/ml GM35 or isotype control IgG1 before the apical addition of 1×106 PMN. PMNs were allowed to migrate for 1hr in response to a 10nM gradient of fMLF. The number of migrated PMN was quantified by MPO assay. Data are mean +/− SE (n=3). Significance was defined at p<0.05 (*, p<0.05; **, p<0.01; ***, p<0.001).
In order to determine if the GM35-mediated reduction in neutrophil TEM was due to interference with the process of neutrophil detachment from the apical epithelial surface, we measured the numbers of neutrophils that had reached but not detached from, the apical epithelial surface. Fig. 1B demonstrates that GM35 increases the level of PMN adherence to the apical surface of the epithelium with 10μg/ml increasing the number of adherent neutrophils by ≥ 80% (P<0.0001) in a dose dependent manner. Taken together these data demonstrate that mAb GM35 exerts its effects on PMN transmigration through promoting PMN adherence to the apical surface of the epithelium.
Previous studies have demonstrated that interactions between epithelium-bound antibody and PMN Fc receptors influence PMN transmigration. Specifically, Reaves et al. report an Fc receptor CD32A epitope-mediated decrease in PMN detachment from the epithelium during PMN transmigration (12). To rule out the involvement of PMN Fc receptors in GM35 activity, transmigration assays in the presence of anti-Fc receptor antibodies were carried out. Fig. 1C demonstrates that the GM35-induced attenuation of PMN transmigration is independent of PMN Fc receptors in that anti-CD16 F(ab′)2 and anti-CD32 F(ab′)2 antibodies, that bound PMN by FACS (data not shown), had no effect on the GM35-mediated increase in PMN adhesiveness. This result was further supported by demonstration that GM35 F(ab′)2 fragments, which were determined to be 98% pure by gel electrophoresis and densitometry (data not shown), resulted in the same increase in PMN adherence/decrease in PMN detachment as intact GM35 mAb (Fig. 1C). Kinetic analysis of the GM35 effect on PMN TEM revealed a maximal effect detectable at 60 minutes (P<0.0001) with significant effects on detachment of transmigrated PMN also detectable at 2hrs (p<0.001) and 3hrs (p<0.001) (Fig. 1D).
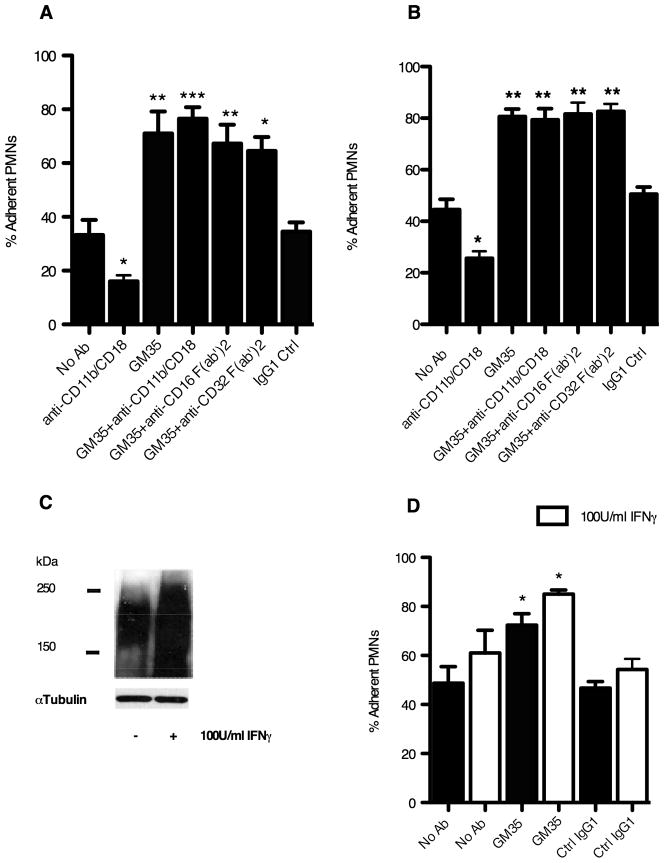
In order to confirm the specific effect of GM35 on PMN-epithelial adhesion/attachment interactions, the effect of this mAb on neutrophil adhesion to epithelial cells was measured directly. GM35 (10μg/ml) significantly increased the level of PMN adhesion to T84 cells compared to either a no Ab control (p<0.001) or an isotype-matched non-inhibitory binding control antibody (p<0.001) (Fig. 2A). Further, as was observed during transmigration, GM35 exerted its effects independently of PMN Fc receptors in that anti-CD16 and anti-CD32 F(ab′)2 antibodies had no effect on the GM35-mediated increase in PMN adhesion. In addition while the anti-CD11b/CD18 antibody CBRM1/29 effectively blocked PMN epithelial adhesive interactions, it had no effect on the ability of GM35 to alter PMN adhesion suggesting that GM35 acts independently of the β2-integrin Mac-1. A similarly significant increase (p<0.001) in PMN adhesion induced by 10μg/ml GM35 was also observed for HT29 cells (Fig. 2B).
Figure 2. GM35 binding increases PMN adhesion to T84 and HT29 cells.
(A) T84 or (B) HT29 monolayers were treated with 10μg/ml GM35 or isotype control IgG1 before the addition of 5 × 105 BCECF labeled PMNs pretreated with 5μg/ml of indicated antibodies. Data shown are the % of total PMN remaining adherent following washing (n=3). (C) T84 cells were treated for 24h with 100U/ml IFNγ before cell lysis and western blotting with GM35. Data depicts representative results from n=3 blots. (D) T84 cells were incubated with 100U/ml IFNγ for 24 hours before treatment with 10μg/ml GM35 or isotype control antibody followed by the addition of 5 × 105 BCECF labeled PMNs. Data shown are % of total PMN remaining adherent following washing (n=3). Significance was defined at p<0.05 (*, p<0.05; **, p<0.01; ***, p<0.001).
IFNγ increases GM35 antigen expression and functional effect
As a membrane preparation from IFNγ-treated T84 cells was the immunogen used to generate mAb GM35, we next sought to verify inducibility of the GM35 antigen in T84 cells upon treatment with IFNγ. Immunoblotting to confirm the up-regulation of the GM35 antigen in T84 cells upon IFNγ treatment revealed the expected increase in protein expression (Fig. 2C), as well as robust baseline expression, of the heavily glycosylated ~ 195kDa GM35 ligand. In addition pre-treatment of T84 cells with IFNγ resulted in a significant increase in the functional effect of GM35 (Fig. 2D). These data show that IFNγ pre-treatment leads to a significant increase in the GM35-mediated increase in PMN adhesion to T84 cells (p<0.05). Further, in keeping with previously reported findings, these data also show enhanced retention of PMN by T84 cells following treatment with IFNγ even in the absence of GM35 (27).
Cell-Specific expression of the GM35 ligand
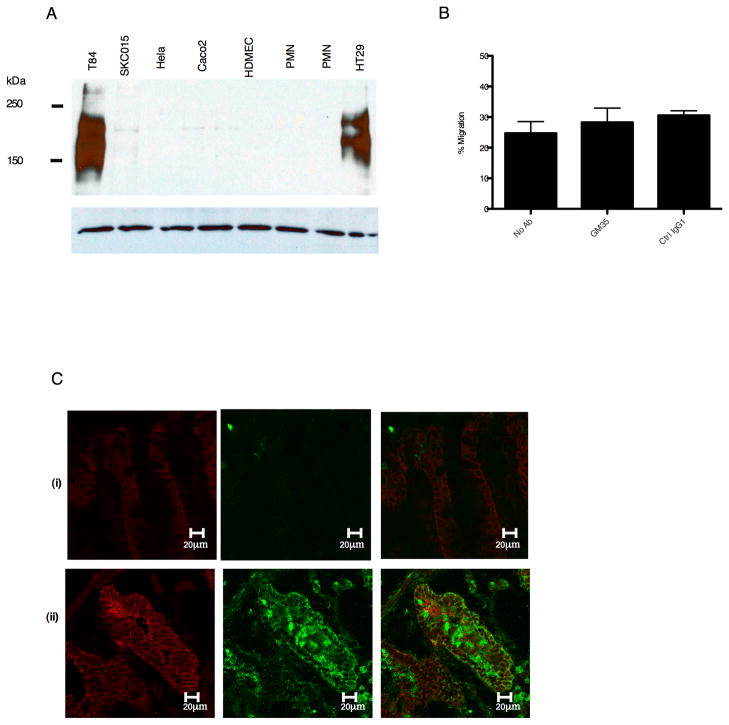
Examination of the expression of the GM35 antigen in a panel of different cell types revealed a restricted pattern of expression for the GM35 ligand. Expression of the protein recognized by GM35 was observed in T84 and HT29 cells but not in Caco2, SK-CO15, HELA, PMNs or human dermal microvascular endothelial cells (HDMEC) (Fig. 3A). Also in contrast to observed functional effects on T84 and HT29 cells, GM35 had no effect on PMN transmigration across Caco2 monolayers (Fig. 3B).
Figure 3. GM35 recognizes an IFNγ responsive glycoprotein.
(A) Following cell lysis, equal amounts of proteins from indicated cells were subjected to SDS-PAGE under reducing conditions and Western blotted with GM35. Data depicts representative results from n=3 Western Blots. (B) Confluent Caco2 monolayers were pre-treated apically with 10 μg/ml GM35 mAb (GM35) before 1×106 PMNs were added to the basolateral surface. PMNs were allowed to migrate in the physiologically relevant basolateral to apical direction for 1 hour in response to a 100nM gradient of n-formyl-methionyl-leucyl-phenylalanine (fMLF). The number of migrated PMNs was quantified by myeloperoxidase assay. Data are means +/− SE (n=3). (C) Cryosections of non-inflamed sections of colonic mucosa (i) and inflamed sections of colonic mucosa (ii) from patients with active ulcerative colitis were examined for localization of the GM35 antigen (green) or the epithelial protein desmoglein (red) as described in methods.
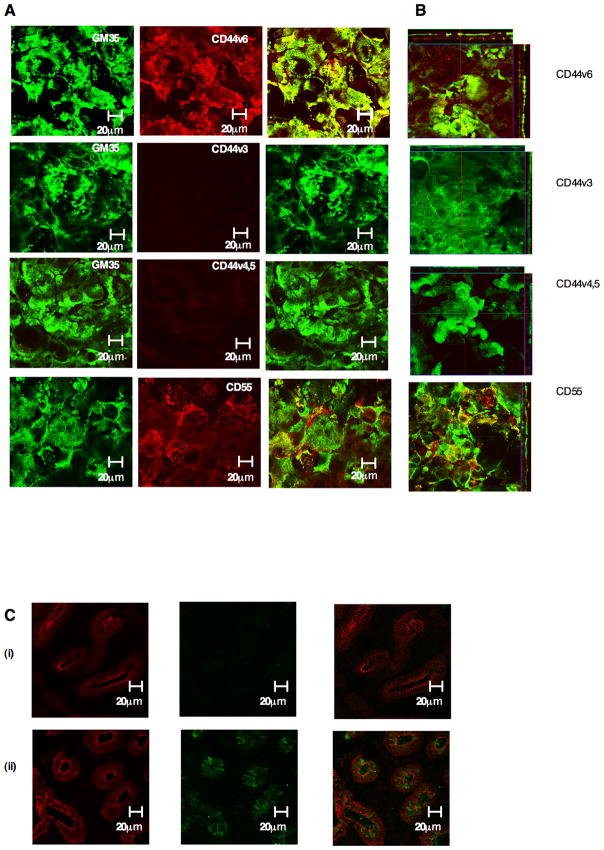
In order to determine if the GM35 antigen was expressed in human colonic epithelia, non-inflamed and inflamed sections of discarded human colonic epithelium were examined by confocal microscopy. Staining of non-inflamed tissue from a patient with ulcerative colitis demonstrated minimal expression of the GM35 ligand (Fig. 3Ci). In stark contrast, there was significant expression of the GM35 antigen in the intestinal epithelium of patients with active ulcerative colitis (Fig. 3Cii). Further, the GM35 antigen in inflamed tissue was localized in the apical and lateral membranes of the epithelial cells. Some increase in the expression of desmoglein (the desmosomal protein used to define the cellular architecture of the intestinal epithelium) was also observed in the inflamed tissue. This finding is consistent with a previously observed up-regulation of this protein upon exposure to inflammatory stimulus (unpublished observations).
Together, these results suggest that the GM35 antigen is an epithelial glycoprotein with a cell specific expression profile, inducible under inflammatory conditions and expressed in an apically-directed fashion. These observations are also consistent with our observation of GM35-mediated disruption of PMN detachment from the apical epithelial surface.
Identification of the GM35 Protein Antigen as CD44
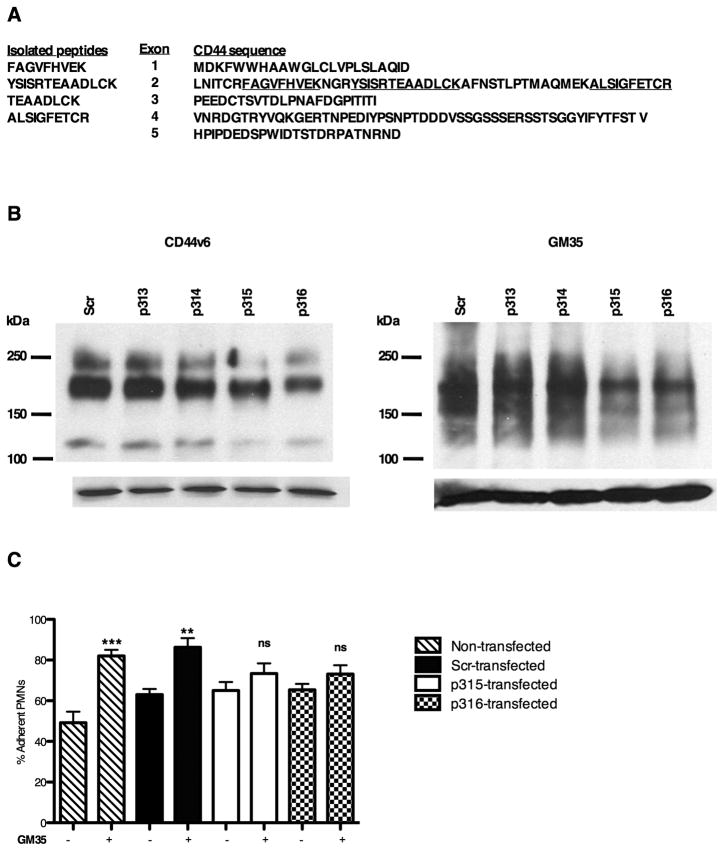
Further experiments were performed to identify the antigen recognized by mAb GM35. 500cm2 of confluent T84 cells were used to generate sufficient GM35 antigen as detailed in methods. While protein identification was limited by the highly glycosylated nature of the GM35 antigen, four tryptic peptides resulting from mass spectrometry showed direct sequence homology with human CD44 (Fig. 4A). The polymorphic, though monogenic, CD44 family of proteins is composed of 19 exons, 10 of which (exons 1–5 and 15–19) are non-variant and are included in the ubiquitously expressed 80–95kDa standard form of CD44, termed CD44s(32). The remaining variant exons can be differentially inserted into the mature mRNA via alternative splicing giving rise to tissue-specific variant CD44 isoforms, the expression of which has been reported in endothelial cells, epithelial cells, activated lymphocytes and some tumor cells (33–36).
Figure 4. Identification of the GM35 antigen as CD44.
(A) 500cm2 of T84 cells were lysed, immunoprecipitated with GM35 and subjected to tryptic digestion and microsequence analysis. Isolated tryptic peptides mapped to CD44 are underlined. (B) Representative immunoblots demonstrating that transfection of HT29 cells with CD44 gene silencing plasmids (p315, p316) decreased the expression of CD44v6 and the GM35 antigen as compared to scramble control (Scr). (C) HT29 cells were transfected with the same plasmids before measurement of neutrophil adhesion levels. Data are presented as mean ± SE (n=4). Significance was defined at p<0.05 (*, p<0.05; **, p<0.01; ***, p<0.001).
In order to demonstrate a direct requirement for CD44 binding in GM35 activity, HT29 cells were transfected with one of four gene silencing shRNA plasmids specific for human CD44 (p313, p314, p315, p316) or with a control scramble plasmid (Scr). HT29 cells were chosen for transfection studies over T84 cells due to the difficulties of transfecting T84 epithelial cells by conventional methods. Knockdown of CD44 protein expression was verified by Western blot (Fig. 4B). Data show that transfection of HT29 cells with either of the CD44 gene silencing plasmids p315 or p316 markedly reduced the expression of CD44v6 (a large glycosylated isoform of CD44 previously reported to be expressed in both T84 and HT29 cells (37)). In contrast, the Scr plasmid and two of the shRNA plasmids p313 and p314 failed to reduce the level of CD44v6 protein expression. Importantly, the same two shRNA plasmids that reduced expression levels of CD44v6 also resulted in a significant reduction in the expression of the GM35 antigen.
Having demonstrated a decrease in GM35 antigen expression upon CD44 knockdown, we next determined the functional effect of CD44 knockdown on the GM35-mediated increase in neutrophil-HT29 adhesion (Fig 2B). GM35 at 10μg/ml significantly increased PMN adhesion compared to no Ab control in non-transfected cells (p<0.001), and in HT29 cells transfected with a Scr plasmid (p<0.01) (Fig. 4C). Transfection of HT29 cells with plasmids p313 or p314 (which had failed to significantly alter the expression of CD44 or the GM35 antigen, Fig. 4B) had no effect on the GM35 induced increase in PMN adhesion (data not shown). In contrast, transfection of HT29 cells with plasmids that successfully knocked down expression of CD44 (p315 or p316) resulted in loss of the GM35 mediated increase in PMN adhesion. This loss of the functional activity of the GM35 mAb following attenuation of epithelial CD44 expression is consistent with our observation that GM35 mediates its effects on PMN transmigration through interaction with epithelial expressed CD44.
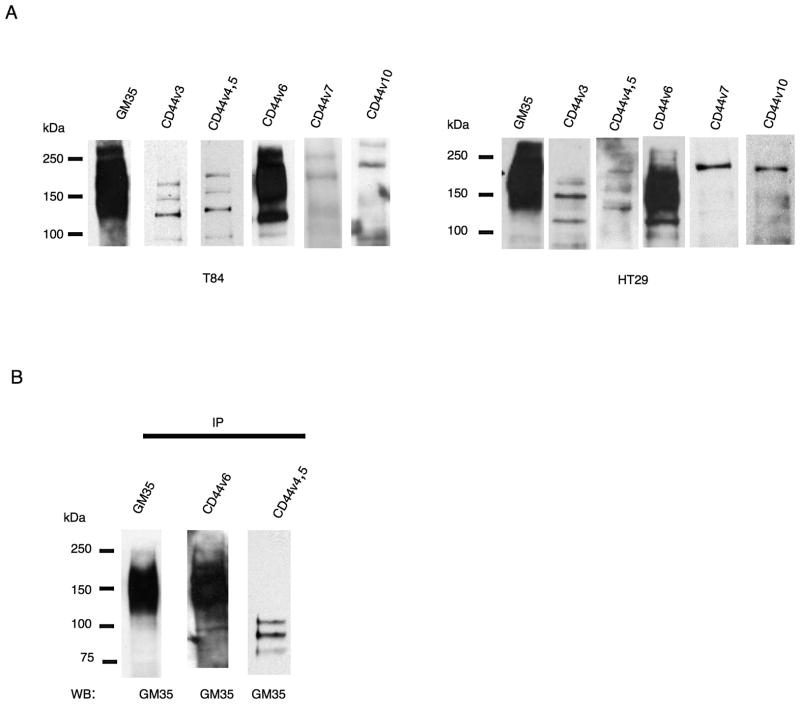
Identification of the GM35 antigen as CD44v6
To determine the specific CD44 splice variant recognized by GM35, T84 and HT29 cell lysates were probed with antibodies specifically raised against different variants of CD44. Immunoblotting with a panel of variant-specific CD44 antibodies revealed that only an anti-CD44v6 antibody bound to a glycoprotein with a molecular weight similar to that recognized by mAb GM35 (Fig. 5A). Conversely, anti-CD44v3, anti-CD44v4,5, anti-CD44v7 and anti-CD44v10 antibodies recognized smaller non-glycosylated proteins, suggesting that the GM35 antigen represents an epithelial CD44 variant containing the v6 domain (CD44v6). The binding of GM35 to CD44v6 was further explored through co-immunprecipitation experiments. CD44v6 immunoprecipitates contained readily detectable amounts of the heavily glycosylated ~ 195kDa GM35 ligand (Fig. 5B). By contrast immunoprecipitates of CD44v4,5 (an isotype matched variant CD44 mAb) did not contain the large glycosylated protein recognized by GM35. However several smaller non-glycosylated proteins (between 75kDa and 100kDa) recognized by anti-CD44v4,5 mAb were also recognized by GM35. These bands may represent non-specific binding or may represent smaller proteins, different than the large 195kDa CD44v6 containing protein that are also recognized by GM35.
Figure 5. GM35 binds to CD44v6.
(A) T84 or HT29 whole cell lysates were analyzed by Western blot and probed with GM35 or variant specific anti-CD44 mAbs. (B) T84 cell lysates were immunoprecipitated with GM35, anti-CD44v6 or anti-CD44v4,5 mAbs and subsequently analyzed by Western blot with GM35. Data depicts representative results from n=3 Western Blots.
GM35 co-localizes apically with CD44v6
Examination of the sub-cellular co-localization of specific CD44 variant proteins with the GM35 antigen in non-permeabilized T84 monolayers revealed a pattern of apical staining. In addition, the only variant form of CD44 found to co-localize apically with GM35 was CD44v6 (Fig. 6B). Analysis of protein localization in permeabilized T84 cells also revealed apical co-localization of CD44v6 and the GM35 antigen as well as basolateral co-localization of GM35 with CD44v6, CD44v3 and CD44v4,5 (data not shown). In addition analysis of co-localization of GM35 and CD55 (an apically expressed protein previously implicated in neutrophil release from the apical surface of the epithelium) revealed only intermittent, patchy areas of co-localization, distinct from the almost complete co-localization observed between the GM35 antigen and CD44v6.
Figure 6. GM35 co-localizes apically with CD44v6.
(A) Non-permeabilized T84 monolayers were co-stained with 10μg/ml Zenon-labeled GM35 (green) and 5μg/ml anti-CD44 antibodies specific for CD44v3, CD44v4,5, CD44v6 or 5μg/ml anti-CD55 mAb. (red). Apical protein localization was determined by confocal microscopy. Images shown are en face (A) or in the x–z plane of section (B). (C) Cryosections of non-inflamed sections of colonic mucosa (i) and inflamed sections of colonic mucosa (ii) from patients with active ulcerative colitis were examined for localization of CD44v6 (Green) or the epithelial marker Desmoglein (red) as described in methods.
This apical co-localization of CD44v6 and the GM35 antigen is consistent with the interaction of GM35 and CD44v6, disrupting PMN detachment from the apical epithelial surface. Examination of the expression of CD44v6 in un-inflamed versus inflamed ulcerative colitis tissue demonstrated an increase in the expression of this variant form of CD44 in the inflamed tissue (Fig. 6C). This is in keeping both with the findings of others (38) and with the recognition of CD44v6 by GM35, as a similar pattern of tissue staining was also observed with mAb GM35 (Fig. 3C). Taken together these data suggest that GM35 binding to apically expressed epithelial CD44v6 interferes with the process of PMN detachment from the luminal surface of the epithelium.
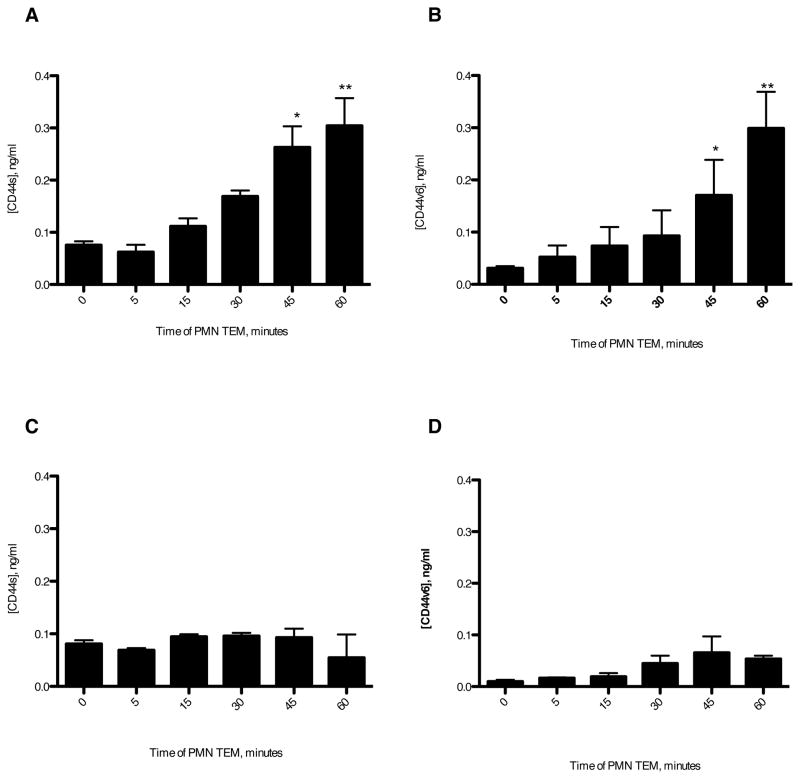
GM35 binding blocks PMN-dependent release of soluble CD44v6
Recent studies have identified a role for extracellular domain shedding in the functioning of CD44 variant proteins (39). Therefore we sought to examine whether the release of soluble CD44v6 fragments occurs during neutrophil migration across epithelium. Analysis of the presence of CD44 proteins released by T84 epithelial cells during PMN transmigration (Fig. 7A,B), revealed significant levels of both CD44std and CD44v6 released between 30 and 60 minutes of PMN migration. Furthermore, levels of detectable protein were similar for both CD44s and CD44v6, suggesting recognition of a single v6 domain containing CD44 protein recognized by both capture antibodies. In order to ascertain if apical exposure of T84 cells to GM35 affected the release of soluble CD44 protein fragments, assays in the presence of GM35 (10μg/ml) were carried out. These data revealed that apical treatment of T84 cells with GM35 prevents the release of soluble CD44std and soluble CD44v6 (Fig. 7C,D). In contrast to the effects seen when GM35 is present for the duration of the migration assay, addition of mAb GM35 following migration of PMNs did not prevent the detection of soluble CD44s or CD44v6 (data not shown). These data therefore demonstrated that GM35 did not prevent binding of released CD44 proteins to the capture or detection antibodies of the ELISA, but rather that GM35 interfered with the release of soluble CD44 proteins. Taken together these data suggest that binding of GM35 to CD44v6 prevents PMN-TEM-dependent release of CD44v6 and, that this failure to release CD44v6 potentially contributes to the retention of PMN at the apical epithelial surface.
Figure 7. GM35 blocks release of soluble CD44v6.
1×106 PMNs were added to confluent T84 monolayers treated apically with 10μg/ml binding control IgG1 (A,B) or 10 μg/ml GM35(C,D). PMNs were allowed to migrate in the basolateral to apical direction in response to a 100nM gradient of fMLF. At the time- points indicated the T84 coated filters were moved into a fresh fMLF-containing well of a 24-well tissue culture plate. For each indicated time-point the solution from the apical migration reservoir was tested for the presence of soluble CD44s (A,C) or soluble CD44v6 (B,D) by ELISA as described in Methods. Data depicts the mean ± SEM from 3 independent experiments. Significance was defined at p<0.05 (*, p<0.05; **, p<0.01; ***, p<0.001).
Discussion
Mucosal recruitment of PMNs involves sequential migration across endothelial, lamina propria and epithelial barriers (40). Subsequent adhesion of PMNs to apical epithelial membranes can result in activated PMNs persisting in crypt abscesses, an event that has implications for disease states including inflammatory bowel disease, periodontitis, cystitis, and infectious enterocolitis (41). As epithelial proteins involved in the retention/release of PMN have not been comprehensively characterized, the current study devised a monoclonal strategy to screen for apically expressed epithelial ligands that modulate the late stages of PMN migration. This strategy identified one mAb GM35 that was found to regulate PMN detachment from the apical surface of epithelial cell monolayers. Specifically, treatment of confluent epithelial monolayers with GM35 significantly inhibited the terminal release of PMN from the apical epithelial surface.
Expansion of these observations identified the GM35 antigen as a member of the highly polymorphic, though monogenic, CD44 protein family. CD44 class I transmembrane glycoproteins have previously been implicated in cell-cell adhesion and cytoskeletal rearrangements, as well as in cell signaling, cell survival and malignant transformation processes (42–44). This multifunctional capacity is facilitated by the expression of different CD44 isoforms generated both from alternative splicing and from post-translational modifications of the extracellular domain of CD44 proteins.
The large molecular weight and highly glycosylated nature of the GM35 antigen in the current study was highly suggestive of binding to a variant exon containing form of CD44 rather than binding to the ubiquitously expressed 80–95kDa non-variant form of CD44 (CD44s). Further analysis revealed binding of GM35 to a v6-containing variant of CD44 (CD44v6). The expression of CD44v6 in epithelial cells reported in the current work is supported by previous studies which report that alternative splicing of CD44 is mediated by tissue-specific factors with certain variant exon products being differentially expressed (or at least predominantly found) on defined tissues such as epithelium (45–48). Further, the expression of the GM35 ligand in T84 and HT29 cells but not in other epithelial cell lines is in keeping with its binding to CD44v6 as it has been reported that this large, heavily glycosylated protein is expressed in these epithelial cell lines but not in Caco2 colon cells (37). This cell-specific expression pattern of the glycoprotein recognized by GM35 in the current study is supported by other previously reported cell-specific differences in protein glycosylation (49, 50). Further, glycosylation and more specifically the oligosaccharides that decorate cell surface O and N-linked glycoproteins have previously been shown to play an important role in the regulation of leukocyte trafficking (51).
Our results also indicate that expression of the GM35 ligand is increased by treatment of T84 cells with IFNγ. It has previously been reported that the expression of CD44v6 in epithelial cells increases during the inflammatory response associated with IBD (52) and more specifically during the inflammatory response associated with ulcerative colitis (38). One specific feature of CD44 variant proteins (including CD44v6) that may be related to the mechanism of GM35 described in the current study is the extracellular proteolytic cleavage of CD44 proteins in response to stimuli. Such proteolytic cleavage of CD44 has recently emerged as a key mechanism underlying its functional regulation, but the biological effects of this event and the proteases involved have not been comprehensively characterized (39). In the current study we demonstrate the release of soluble CD44v6 protein during PMN transmigration and further, that GM35 prevents this release of soluble CD44v6. It is therefore plausible that the release of the CD44v6 extracellular domain from the apical surface of the epithelium can facilitate PMN detachment and, that the GM35-mediated blockade of CD44v6 release results in the observed PMN accumulation at the apical epithelial surface.
Although it has been previously suggested that the expression of specific isoforms of CD44 plays a role in the regulation of the immune response, as well as in the development of autoimmune disorders (33, 53, 54), the mechanisms whereby CD44 variants govern leukocyte recruitment have not been fully characterized. It has been previously detailed that CD44v3 expressed basolaterally on epithelial cells regulates transmigration via binding to neutrophil CD11b/CD18 (5). However the basolateral expression and molecular weight of this CD44v3 isoform show it to be a different CD44 variant than the one recognized by GM35 in the current study. In addition this study reported expression of this CD44v3 variant in Caco2 cells and also in human microvascular endothelial cells, cells shown not to express the GM35 antigen in the current study. Further this role for CD44v3 in PMN transmigration is reported to be mediated through interaction of epithelial CD44v3 and PMN CD18/CD11b suggesting a mechanism unrelated to that described in current study where we show that GM35 regulates PMN migration independently of CD18/CD11b. In support of this it has previously been reported that different CD44 variant isoforms exert distinct and non-overlapping functional activities (55, 56).
The current study demonstrates that GM35 binding to the apical surface of human epithelial cells results in failed PMN detachment. A similar blockade of neutrophil detachment from the apical epithelial surface has been reported previously, mediated through binding of mAb OE-1 to epithelial decay accelerating factor (DAF), CD55 (13). Despite having the same end effect on PMN epithelial adhesiveness, the current study describes a mechanism of action for mAb GM35 that is independent of that previously reported for mAb OE-1. Specifically CD55 was reported to be a protein important for successful migration across both endothelial and epithelial cell monolayers. In contrast we report that GM35 had no effect on the migration of PMN across human dermal microvascular endothelial cells. In addition OE-1 inhibited PMN migration in both the non-physiologically relevant apical to basolateral and basolateral to apical directions whereas GM35 had no effect on PMN transmigration in the apical to basolateral direction. Further, the current study demonstrates that Caco2 cells do not express the GM35 antigen and that GM35 has no effect on PMN migration across Caco2 monolayers. In contrast, it has previously been reported that CD55 is expressed in Caco2 cells and that OE-1 treatment leads to increased PMN Caco2 adhesiveness (13). These data suggest that expression of CD55 in cells is not sufficient to facilitate the activity of GM35 and cells that express CD55 but not CD44v6 (37) have unaltered PMN transmigration in the presence of GM35. Taken together this suggests that the activity of GM35 described in the current study can be separated from that previously reported for mAb OE-1.
The current study is the first to demonstrate a role for CD44v6 in PMN transepithelial migration. Further work is required to determine whether GM35 exerts its effects on CD44v6 release and PMN detachment directly through occupying the CD44v6 extra-cellular receptor domain or whether GM35 binding delivers a transmembrane stimulus that activates downstream intracellular signaling cascades.
In summary we demonstrate that a v6 exon-containing variant of CD44, recognized by mAb GM35, mediates PMN detachment from apical epithelium during TEM. We show that CD44v6 binding by GM35 leads to disruption of PMN-epithelium detachment (the terminal step in PMN transepithelial migration). Further this novel interaction has relevance for inflammatory disorders, as while CD44v6 is not found in native non-inflamed intestine (57), the expression of CD44v6 is up-regulated in the crypt epithelium of patients with ulcerative colitis (38).
Acknowledgments
This work was supported by National Institutes of Health grants DK62007 and DK 063399 and a Seed Grant from the Emory University Research Council (N.A.L) as well as DK72564, DK079392, DK 55679, DK 59888 and DDRDC grant DK064399.
The authors would like to thank Tao Wu for his expert help and technical support in the generation and propagation of antibody hybridomas.
Abbreviations used in this paper
- BCECF
2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester
- DAF
decay accelerating factor
- ERM
ezrin radixin moesin
- fMLF
formyl-methionyl-leucyl-phenylalanine
- JAM-A
junctional adhesion molecule A
- RTK
receptor tyrosine kinase
- Sirpα
signal regulatory protein alpha
- scr
scrambled
- TEM
transepithelial migration
References
- 1.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- 2.Woodfin A, Voisin MB, Nourshargh S. Recent developments and complexities in neutrophil transmigration. Curr Opin Hematol. 2009 doi: 10.1097/MOH.0b013e3283333930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Imhof BA, Dunon D. Basic mechanism of leukocyte migration. Horm Metab Res. 1997;29:614–621. doi: 10.1055/s-2007-979112. [DOI] [PubMed] [Google Scholar]
- 5.Zen K, Liu DQ, Li LM, Chen CX, Guo YL, Ha B, Chen X, Zhang CY, Liu Y. The heparan sulfate proteoglycan form of epithelial CD44v3 serves as a CD11b/CD18 counter-receptor during polymorphonuclear leukocyte transepithelial migration. J Biol Chem. 2009;284:3768–3776. doi: 10.1074/jbc.M807805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake KM, Carrigan SO, Issekutz AC, Stadnyk AW. Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol. 2004;136:262–268. doi: 10.1111/j.1365-2249.2004.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parkos CA, Colgan SP, Liang TW, Nusrat A, Bacarra AE, Carnes DK, Madara JL. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper D, Lindberg FP, Gamble JR, Brown EJ, Vadas MA. Transendothelial migration of neutrophils involves integrin-associated protein (CD47) Proc Natl Acad Sci U S A. 1995;92:3978–3982. doi: 10.1073/pnas.92.9.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Buhring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028–10036. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 10.Zen K, Liu Y, McCall IC, Wu T, Lee W, Babbin BA, Nusrat A, Parkos CA. Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Mol Biol Cell. 2005;16:2694–2703. doi: 10.1091/mbc.E05-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkos CA, Colgan SP, Diamond MS, Nusrat A, Liang TW, Springer TA, Madara JL. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- 12.Reaves TA, Colgan SP, Selvaraj P, Pochet MM, Walsh S, Nusrat A, Liang TW, Madara JL, Parkos CA. Neutrophil transepithelial migration: regulation at the apical epithelial surface by Fc-mediated events. Am J Physiol Gastrointest Liver Physiol. 2001;280:G746–754. doi: 10.1152/ajpgi.2001.280.4.G746. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence DW, Bruyninckx WJ, Louis NA, Lublin DM, Stahl GL, Parkos CA, Colgan SP. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J Exp Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gewirtz AT, Liu Y, Sitaraman SV, Madara JL. Intestinal epithelial pathobiology: past, present and future. Best Pract Res Clin Gastroenterol. 2002;16:851–867. doi: 10.1053/bega.2002.0339. [DOI] [PubMed] [Google Scholar]
- 15.Loss RW, Jr, Mangla JC, Pereira M. Campylobacter colitis presentin as inflammatory bowel disease with segmental colonic ulcerations. Gastroenterology. 1980;79:138–140. [PubMed] [Google Scholar]
- 16.Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- 17.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. J Biol Chem. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 19.Mandell KJ, I, McCall C, Parkos CA. Involvement of the junctional adhesion molecule-1 (JAM1) homodimer interface in regulation of epithelial barrier function. J Biol Chem. 2004;279:16254–16262. doi: 10.1074/jbc.M309483200. [DOI] [PubMed] [Google Scholar]
- 20.Betanzos A, Schnoor M, Severson EA, Liang TW, Parkos CA. Evidence for cross-reactivity of JAM-C antibodies: implications for cellular localization studies. Biol Cell. 2009;101:441–453. doi: 10.1042/BC20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(Pt 13):2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 22.Balsam LB, Liang TW, Parkos CA. Functional mapping of CD11b/CD18 epitopes important in neutrophil-epithelial interactions: a central role of the I domain. J Immunol. 1998;160:5058–5065. [PubMed] [Google Scholar]
- 23.Kaoutzani P, Parkos CA, Delp-Archer C, Madara JL. Isolation of plasma membrane fractions from the intestinal epithelial model T84. Am J Physiol. 1993;264:C1327–1335. doi: 10.1152/ajpcell.1993.264.5.C1327. [DOI] [PubMed] [Google Scholar]
- 24.Mackarel AJ, Russell KJ, Ryan CM, Hislip SJ, Rendall JC, FitzGerald MX, O’Connor CM. CD18 dependency of transendothelial neutrophil migration differs during acute pulmonary inflammation. J Immunol. 2001;167:2839–2846. doi: 10.4049/jimmunol.167.5.2839. [DOI] [PubMed] [Google Scholar]
- 25.Colgan SP, Parkos CA, McGuirk D, Brady HR, Papayianni AA, Frendl G, Madara JL. Receptors involved in carbohydrate binding modulate intestinal epithelial-neutrophil interactions. J Biol Chem. 1995;270:10531–10539. doi: 10.1074/jbc.270.18.10531. [DOI] [PubMed] [Google Scholar]
- 26.Bruyninckx WJ, Comerford KM, Lawrence DW, Colgan SP. Phosphoinositide 3-kinase modulation of beta(3)-integrin represents an endogenous “braking” mechanism during neutrophil transmatrix migration. Blood. 2001;97:3251–3258. doi: 10.1182/blood.v97.10.3251. [DOI] [PubMed] [Google Scholar]
- 27.Colgan SP, Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. J Cell Biol. 1993;120:785–798. doi: 10.1083/jcb.120.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zund G, Uezono S, Stahl GL, Dzus AL, McGowan FX, Hickey PR, Colgan SP. Hypoxia enhances induction of endothelial ICAM-1: role for metabolic acidosis and proteasomes. Am J Physiol. 1997;273:C1571–1580. doi: 10.1152/ajpcell.1997.273.5.C1571. [DOI] [PubMed] [Google Scholar]
- 29.Louis NA, Campbell E, Colgan SP. Model systems to investigate neutrophil adhesion and chemotaxis. Methods Mol Biol. 2007;412:257–270. doi: 10.1007/978-1-59745-467-4_17. [DOI] [PubMed] [Google Scholar]
- 30.Monteleone G, Fina D, Caruso R, Pallone F. New mediators of immunity and inflammation in inflammatory bowel disease. Curr Opin Gastroenterol. 2006;22:361–364. doi: 10.1097/01.mog.0000231808.10773.8e. [DOI] [PubMed] [Google Scholar]
- 31.Utech M, Bruwer M, Nusrat A. Tight junctions and cell-cell interactions. Methods Mol Biol. 2006;341:185–195. doi: 10.1385/1-59745-113-4:185. [DOI] [PubMed] [Google Scholar]
- 32.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–579. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 33.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 34.Johnson P, Maiti A, Brown KL, Li R. A role for the cell adhesion molecule CD44 and sulfation in leukocyte-endothelial cell adhesion during an inflammatory response? Biochem Pharmacol. 2000;59:455–465. doi: 10.1016/s0006-2952(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 35.Wittig BM, Stallmach A, Zeitz M, Gunthert U. Functional involvement of CD44 variant 7 in gut immune response. Pathobiology. 2002;70:184–189. doi: 10.1159/000068152. [DOI] [PubMed] [Google Scholar]
- 36.Herrlich P, Sleeman J, Wainwright D, Konig H, Sherman L, Hilberg F, Ponta H. How tumor cells make use of CD44. Cell Adhes Commun. 1998;6:141–147. doi: 10.3109/15419069809004470. [DOI] [PubMed] [Google Scholar]
- 37.Singh R, Subramanian S, Rhodes JM, Campbell BJ. Peanut lectin stimulates proliferation of colon cancer cells by interaction with glycosylated CD44v6 isoforms and consequential activation of c-Met and MAPK: functional implications for disease-associated glycosylation changes. Glycobiology. 2006;16:594–601. doi: 10.1093/glycob/cwj108. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg WM, Prince C, Kaklamanis L, Fox SB, Jackson DG, Simmons DL, Chapman RW, Trowell JM, Jewell DP, Bell JI. Increased expression of CD44v6 and CD44v3 in ulcerative colitis but not colonic Crohn’s disease. Lancet. 1995;345:1205–1209. doi: 10.1016/s0140-6736(95)91991-0. [DOI] [PubMed] [Google Scholar]
- 39.Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–935. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madara JL. Migration of neutrophils through epithelial monolayers. Trends Cell Biol. 1994;4:4–7. doi: 10.1016/0962-8924(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 41.Jaye DL, Parkos CA. Neutrophil migration across intestinal epithelium. Ann N Y Acad Sci. 2000;915:151–161. doi: 10.1111/j.1749-6632.2000.tb05238.x. [DOI] [PubMed] [Google Scholar]
- 42.Mielgo A, van Driel M, Bloem A, Landmann L, Gunthert U. A novel antiapoptotic mechanism based on interference of Fas signaling by CD44 variant isoforms. Cell Death Differ. 2006;13:465–477. doi: 10.1038/sj.cdd.4401763. [DOI] [PubMed] [Google Scholar]
- 43.Lee JL, Wang MJ, Sudhir PR, Chen JY. CD44 engagement promotes matrix-derived survival through the CD44-SRC-integrin axis in lipid rafts. Mol Cell Biol. 2008;28:5710–5723. doi: 10.1128/MCB.00186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bajorath J. Molecular organization, structural features, and ligand binding characteristics of CD44, a highly variable cell surface glycoprotein with multiple functions. Proteins. 2000;39:103–111. [PubMed] [Google Scholar]
- 45.Camacho FI, Munoz C, Sanchez-Verde L, Saez AI, Alcantara M, Rodriguez R. CD44v6 expression in inflammatory bowel disease is associated with activity detected by endoscopy and pathological features. Histopathology. 1999;35:144–149. doi: 10.1046/j.1365-2559.1999.00712.x. [DOI] [PubMed] [Google Scholar]
- 46.Reinisch W, Heider KH, Oberhuber G, Dejaco C, Mullner M, Adolf GR, Gasche C. Poor diagnostic value of colonic CD44v6 expression and serum concentrations of its soluble form in the differentiation of ulcerative colitis from Crohn’s disease. Gut. 1998;43:375–382. doi: 10.1136/gut.43.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper DL, Dougherty G, Harn HJ, Jackson S, Baptist EW, Byers J, Datta A, Phillips G, Isola NR. The complex CD44 transcriptional unit; alternative splicing of three internal exons generates the epithelial form of CD44. Biochem Biophys Res Commun. 1992;182:569–578. doi: 10.1016/0006-291x(92)91770-q. [DOI] [PubMed] [Google Scholar]
- 48.Macdonald DC, Leir SH, Brooks C, Sanders E, Lackie P, Rosenberg W. CD44 isoform expression on colonic epithelium mediates lamina propria lymphocyte adhesion and is controlled by Th1 and Th2 cytokines. Eur J Gastroenterol Hepatol. 2003;15:1101–1110. doi: 10.1097/00042737-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Brown TA, Bouchard T, St John T, Wayner E, Carter WG. Human keratinocytes express a new CD44 core protein (CD44E) as a heparan-sulfate intrinsic membrane proteoglycan with additional exons. J Cell Biol. 1991;113:207–221. doi: 10.1083/jcb.113.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 51.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wittig B, Seiter S, Schmidt DS, Zuber M, Neurath M, Zoller M. CD44 variant isoforms on blood leukocytes in chronic inflammatory bowel disease and other systemic autoimmune diseases. Lab Invest. 1999;79:747–759. [PubMed] [Google Scholar]
- 53.Arch R, Wirth K, Hofmann M, Ponta H, Matzku S, Herrlich P, Zoller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992;257:682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- 54.Konig H, Ponta H, Herrlich P. Coupling of signal transduction to alternative pre-mRNA splicing by a composite splice regulator. EMBO J. 1998;17:2904–2913. doi: 10.1093/emboj/17.10.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farkas S, Hornung M, Sattler C, Anthuber M, Gunthert U, Herfarth H, Schlitt HJ, Geissler EK, Wittig BM. Short-term treatment with anti-CD44v7 antibody, but not CD44v4, restores the gut mucosa in established chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 2005;142:260–267. doi: 10.1111/j.1365-2249.2005.02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seiter S, Schmidt DS, Zoller M. The CD44 variant isoforms CD44v6 and CD44v7 are expressed by distinct leukocyte subpopulations and exert non-overlapping functional activities. Int Immunol. 2000;12:37–49. doi: 10.1093/intimm/12.1.37. [DOI] [PubMed] [Google Scholar]
- 57.Mackay CR, Terpe HJ, Stauder R, Marston WL, Stark H, Gunthert U. Expression and modulation of CD44 variant isoforms in humans. J Cell Biol. 1994;124:71–82. doi: 10.1083/jcb.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]