Abstract
Aspirin is effective for the prevention of cardiovascular events in patients with a history of vascular disease, as so-called secondary prevention. In general populations with no history of previous myocardial infarction or stroke, aspirin also seems useful for primary prevention of cardiovascular events, although the absolute benefits are smaller than those seen in patients with previous cardiovascular disease. Patients with diabetes mellitus are at an increased risk of cardiovascular events, but new trials have raised questions about the benefit of aspirin for primary prevention in patients with this disorder. This Review comprehensively examines the basic pharmacology of aspirin and provides an overview of the randomized, controlled trials of aspirin therapy that have included patients with diabetes mellitus. On the basis of currently available evidence from primary prevention trials, aspirin is estimated to reduce the relative risk of myocardial infarction and stroke by about 10% in patients with diabetes mellitus; however, aspirin also increases the risk of gastrointestinal bleeding. As such, low-dose aspirin therapy (75–162 mg) is reasonable for patients with diabetes mellitus and a 10-year risk of cardiovascular events >10%. Results from upcoming large trials will help clarify the effects of aspirin with greater precision, including whether the benefits differ between men and women.
Introduction
Aspirin is well-proven to reduce cardiovascular events in patients with a history of cardiovascular disease.1 In addition, meta-analyses of multiple randomized trials conducted over the past 25 years suggest that aspirin can reduce nonfatal myocardial infarction in men with no previous history of cardiovascular disease.2–4 Few trials are available in women, but the current evidence (mainly obtained from one large trial) suggests that aspirin may reduce stroke in women.4,5 However, aspirin also increases the risk of gastrointestinal bleeding; therefore, the net benefit of aspirin depends on the absolute cardiovascular risk of the patient who is being considered for treatment. If one assumes that the reduction in relative risk of cardiovascular events with the use of aspirin is approximately constant across different patient populations, those at high cardiovascular risk might potentially derive net benefit from aspirin, while those at very low risk of cardiovascular disease could experience deleterious rather than beneficial effects.
Patients with diabetes mellitus are at an increased relative risk of cardiovascular events.6 Cardiovascular risk in middle-aged and older adults is frequently as high as that in adults without diabetes mellitus who have had a previous cardiovascular event. The absolute excess risk increases with age and the presence of other risk factors for cardiovascular diseases including smoking, dyslipidemia, hypertension, albuminuria and a family history of premature atherosclerosis. As such, patients with diabetes mellitus would seem to be a good target population for aspirin therapy, and practice guidelines have generally recommended aspirin use in patients with this disorder. However, two new trials conducted specifically in patients with diabetes mellitus have raised questions about the benefits of aspirin in this patient population.7,8 The results have prompted guideline-issuing organizations to re-examine their recommendations for aspirin use for primary prevention.9
This Review will first address the pharmacology and physiology of aspirin use and how it may differ in patients with diabetes mellitus. The clinical trial evidence for the primary prevention of cardiovascular disease with aspirin therapy in patients with diabetes mellitus will then be examined in light of these potential mechanisms. Finally, suggestions for clinical practice are provided, and the limitations in current evidence as well as the upcoming trials that may help resolve these important clinical issues are highlighted.
Pharmacology of aspirin
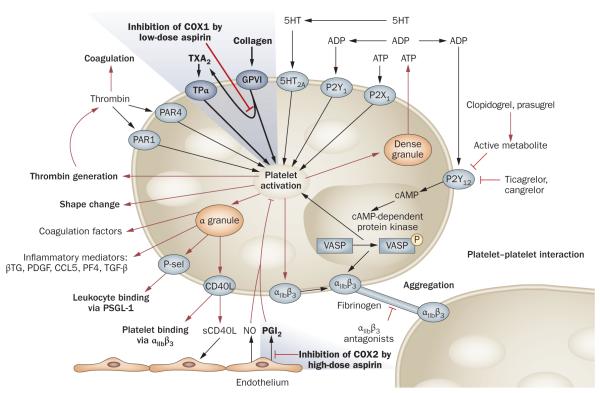
Upon damage to the endothelium of a blood vessel, activated platelets form a plug at the site of injury, in conjunction with the coagulation protein fibrin, to halt bleeding. Disorders of coagulation can increase the risk of hemorrhage (bleeding) or result in thrombosis (the formation of an obstructing blood clot). Obstruction of >70% of the lumenal area of an artery can cause significant organ hypoxia and symptoms. Obstruction of >90% can result in cell necrosis and death. Pharmacological agents that reduce blood clotting include antiplatelet drugs, which prevent thrombosis by decreasing platelet aggregation and thus inhibiting formation of a platelet plug (Box 1). The antiplatelet drug aspirin inhibits platelet activation via inhibition of the enzyme cyclooxygenase (COX; also known as prostaglandin G/H synthase), which reduces the production of thromboxane A2 (TXA2; Figure 1).10,11 TXA2 normally exerts an autocrine effect on the platelet it activates, as well as a paracrine effect on surrounding platelets. Activation of platelets by TXA2 occurs via its binding to one of two variant TXA2 receptors on the platelet surface. This binding causes the activation of a series of guanine nucleotide-binding proteins (G proteins) that, in turn, activate phospholipase C and ultimately lead to the upregulation of the surface receptors integrin αIIbβ3 (also known as platelet membrane glycoprotein IIB/IIIA). Surface integrin αIIbβ3 receptors crosslink activated platelets with fibrinogen to help stabilize the platelet plug produced by aggregation of adjacent platelets.
Box 1. Antiplatelet drugs.
Cyclooxygenase inhibitors
▪ Aspirin
P2Y12 inhibitors
▪ Clopidogrel
▪ Prasugrel
▪ Ticlopidine
▪ Ticagrelor (under FDA review at time of publishing)
▪ Cangrelor (clinical trials ongoing at time of publishing)
Phosphodiesterase inhibitors
▪ Cilostazol
▪ Pentoxifylline
Adenosine reuptake inhibitors
▪ Dipyridamole
Integrin αIIbβ3 (also known as platelet membrane glycoprotein IIB/IIIA) inhibitors
▪ Abciximab
▪ Eptifibatide
▪ Tirofiban
Figure 1.
Overview of platelet activation and its inhibition by antiplatelet drugs. The platelet has many routes of activation and multiple sites of pharmacologic inhibition. Platelet activation is initiated by soluble agonists, such as thrombin, thromboxane A2, 5HT, ADP and ATP, and by adhesive ligands, such as collagen. Consequently, granule secretion of platelet agonists and secretion of thromboxane A2 leads to amplification of platelet activation and its associated responses. Low-dose aspirin inhibits platelet activation via inhibition of the enzyme cyclooxygenase 1 (COX1), which reduces the production of thromboxane A2, whereas high-dose aspirin inhibits COX2 in endothelial cells, thereby suppressing prostacyclin production. By contrast, ADP receptor inhibitors, such as clopidogrel, prasugrel, ticagrelor and cangrelor, mediate their actions by inhibiting guanine nucleotide-binding protein-coupled purinergic receptor P2Y12. Abbreviations: αIIbβ3, integrin αIIbβ3 (also known as platelet membrane glycoprotein IIB/IIIA); βTG, platelet basic protein (also known as β-thromboglobulin); CCL5, CC-chemokine ligand 5 (also known as RANTES); CD40L, CD40 ligand; COX, cyclooxygenase (also known as prostaglandin G/H synthase); 5HT, 5-hydroxytryptamine (also known as serotonin); NO, nitric oxide; PDGF, platelet-derived growth factor; PF4, platelet factor 4; PGI2, prostacyclin; P-sel, P-selectin; PSGL1, P-selectin glycoprotein ligand 1; P2Y12, P2Y12 platelet ADP receptor; TGFβ, transforming growth factor β; TXA2, thromboxane A2; VASP, vasodilator-stimulated phosphoprotein. Adapted with permission from Bentham Science Publishers © Storey, R. F. Curr. Pharm. Des. 12, 1255–1259 (2006).
COX is expressed in two isoforms, COX1 and COX2, although mature platelets express almost exclusively COX1, which is more sensitive to inhibition by aspirin.12,13 This effect can be measured by determining levels of a stable, inactive metabolite of TXA2, TXB2, in urine or in serum. Serum TXB2 is converted in the liver into two major metabolites, 2,3-dinor-TXB2 and 11-dehydro-TXB2 that are excreted unchanged in the urine along with unconverted TXB2.11,14 Whereas measurement of serum TXB2 can be performed at any single time point, the variable conversion and elimination of TXB2 metabolites in urine requires the collection of a 24-h sample if COX1 inhibition is quantified via measurement of urine metabolites of TXB2.12,14
The efficacy of low-dose aspirin (75–162 mg) as an antiplatelet agent results primarily from two features of the drug’s effect. First, the inhibition of the COX enzyme in the platelet is irreversible. This irreversibility is due to the acetylation of the enzyme and accounts for the difference in antiplatelet effects between acetylsalicylic acid (aspirin) and salicylic acid,15 which is similar to other nonsteroidal anti-inflammatory agents with its transient platelet-inhibiting effect. The circulating lifespan of a platelet is about 10 days and, therefore, this permanent inhibition by aspirin allows for an accumulation effect over the first 2 to 3 days of aspirin therapy. After the first dose of aspirin, 50–80% of TXA2 production is suppressed and after a second dose this suppression increases >90%.10,16 Once all the platelets have been affected, COX1 inhibition can be maintained with a low, once-daily aspirin dose.10,15
Second, the effect of aspirin on platelets is presystemic and begins in the portal circulation. Unlike other antiplatelet drugs, such as clopidogrel and prasugrel, aspirin is active in its original form, and inhibition of platelets begins in gut capillaries as aspirin encounters platelets and passively diffuses across the phospholipid cell membrane to the intracellular COX1 target.10,15 Reductions in TXB2 levels are observed before aspirin is detectable in the serum.10 Given that the portal blood volume is low, the concentration of aspirin that platelets are exposed to in the portal circulation is relatively high, even at low doses. Although the serum half-life of aspirin is short (~20 min), gastrointestinal absorption occurs over about 2 h, which ensures adequate exposure of platelets in the portal circulation to inhibitory concentrations of aspirin.
The result of these pharmacokinetic and pharmacodynamic aspects of aspirin therapy is that low doses exhibit complete inhibition of COX1 in platelets as measured by serum or urine TXB2 levels. In a study of 10 patients published in 1991,17 75 mg of immediate-release aspirin showed 78% inhibition of TXB2 production after a single dose and 99% inhibition by day three.16 The same finding of 99% inhibition of TXB2 production has been replicated in a study in 48 patients who received 100 mg aspirin.
Systemic effects of aspirin
Notably, the bioavailability of aspirin is approximately 50% across a wide range of dosing.10 Higher doses can therefore produce higher systemic aspirin concentrations than low doses. Exposure of the endothelial COX2 enzyme to aspirin reduces the production of vascular prostacyclin—a lipid molecule that inhibits platelet aggregation (Figure 1). This exposure can clearly have prothrombotic, localized effects in endothelium, the clinical importance of which is still debated.12 Regardless, one reason to keep doses low (<500 mg daily) in clinical practice is to avoid systemic effects of aspirin.
Aspirin resistance
Aspirin resistance in the general population
Variability in the measured bleeding time response to aspirin therapy was noted several years before the mechanism of the antiplatelet effect was known.18,19 The source of this variability is only somewhat better understood today. Despite complete suppression of TXA2 production in the platelet by low-dose aspirin, numerous studies document a range of residual platelet activation on aspirin doses of 75–325 mg per day.17,20–22 Some of these studies show apparent improvement in reducing this residual platelet reactivity with daily aspirin doses >325 mg.21,22 This apparent heterogeneity in the response to aspirin has led to the classification of some patients as ‘aspirin resistant’.
Resistance to the effects of aspirin continues to be a source of confusion for clinicians.23,24 Defining resistance as the occurrence of vascular events in patients who take aspirin is too broad, as it implies that vascular events could be eliminated with optimal dosing of aspirin. In the absence of a clear clinical definition, the results of several ex vivo and in vitro testing methods (for example, platelet aggregometry, measurement of levels of phosphorylation of vasodilator-stimulated phosphoprotein or serum TXB2) have been used to measure platelet function and thus potentially predict the risk of thrombosis or bleeding.25 As surrogates for clinical endpoints, however, these methods are flawed for several reasons.17,26
First, the platelet has numerous pathways of activation (Figure 1), and complete blockade of any one biochemical pathway (for example, TXA2 inhibition by aspirin) should not be expected to lead to complete suppression of platelet activity. Second, much of what is known about surrogate platelet testing with aspirin comes from post-hoc analyses of prospective trials.27–29 In such analyses, however, patients with a high residual platelet reactivity invariably have a greater predominance of other risk factors for vascular events, such as smoking, hypertension and diabetes mellitus, than those with low residual platelet reactivity.27,29 Thus, although these data are adequate for the generation of a hypothesis, they do not prove cause and effect.
Third, patient intravariability of various surrogate platelet testing modalities is large—a finding that casts further doubt on the clinical utility of surrogate testing for aspirin resistance at this time. In one trial, 48 study participants were randomly allocated to 100 mg of aspirin daily over 8 weeks and four different methods of platelet reactivity testing were performed weekly on each participant.17 A wide intrapatient as well as inter patient variation was found for all testing modalities used. This variation occurred despite uniform suppression of serum TXB2.17 Finally, as drug assays for antiplatelet agents become more sophisticated, some of the resistance reported is simply noncompliance with antiplatelet therapy.30 To account for this variable in clinical practice is problematic, given the short half-life of aspirin and absence of a stable metabolite that could be measured as a surrogate for compliance with therapy.
Dose response
Surrogate tests for platelet reactivity sometimes show increased effects when high doses of aspirin (>500 mg) are administered, although this finding is in contrast to currently available indirect data from trials measuring clinical outcomes.1,31 In the 2002 Antithrombotics Trialists’ Collaboration (ATC) analysis,1 doses of aspirin >500 mg per day were no more effective for the prevention of vascular events than doses of 75–325 mg per day. Similarly, in the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study,31 aspirin doses >100 mg daily, whether used in combination with clopidogrel or as monotherapy, were not more effective in reducing vascular endpoints than doses of 75–100 mg daily.
This discordance—an apparent dose-dependent effect of aspirin observed with surrogate measures of platelet function and no such effect found for vascular endpoints in clinical trials—casts further doubt on the clinical utility of surrogate platelet testing to identify aspirin resistance and the prescription of high-dose aspirin. Evidence that promotes the use of high-dose aspirin would need to come from studies in which platelet response is determined first and then patients with apparent resistance are randomly allocated to regimens of either standard antiplatelet therapy or more intensive regimens. Such trials would avoid the confounding of post-hoc analyses with nonrandomized patient populations, and a prospective determination could be made whether or not patients with platelet resistance (defined by surrogate measures) benefit from more intensive antiplatelet regimens. As described below, such studies are ongoing, although they may potentially not offer definitive data for the patient with diabetes mellitus.
Aspirin resistance in diabetes mellitus
Controversy regarding the relative benefit of aspirin in patients with diabetes mellitus has included concerns about aspirin resistance in this patient population. How the presence of diabetes mellitus affects platelet responsiveness to aspirin has yet to be fully elucidated. Increased platelet aggregation in response to ADP and epinephrine was noted in patients with diabetes mellitus over 30 years ago.32 New evidence obtained by surrogate platelet testing also notes about 50% increased rates of aspirin resistance in patients with diabetes mellitus compared with individuals without this disorder.27,33 Indeed, independent from diabetes mellitus, obesity itself is associated with a reduced response to aspirin.34 The mechanism of increased residual platelet reactivity in patients with diabetes mellitus is probably multifactorial. Increased systemic production of isoprostanes, including thromboxane, likely plays a role.35
In patients with diabetes mellitus and cardiovascular disease, a higher rate of TXA2 production has been found compared with that in healthy control indivi duals.36 Tight control of blood glucose with insulin reduces some of the excess production of TXA2.36 Diabetes mellitus is also associated with increased levels of platelet adhesion molecules that could increase platelet aggregation.37 Reconstituted HDL cholesterol therapy has been found to reduce platelet reactivity in patients with type 2 diabetes mellitus.38
Whereas many of these pathophysiologic changes probably result from the metabolic consequences of insulin resistance, increased platelet reactivity has also been found in patients with type 1 diabetes mellitus without insulin resistance.39 Thus, hyperglycemia alone potentially accounts for at least part of the altered platelet response in patients with diabetes mellitus. This hypothesis is consistent with the observation that patients with type 1 diabetes mellitus have an increased risk of cardiovascular disease without concomitant dysipidemia and hypertension.40 Despite findings of increased platelet reactivity in patients with diabetes mellitus, the therapeutic implications remain undetermined.
No clear evidence from clinical trials exists to suggest that a different dosing strategy should be used for aspirin in patients with diabetes mellitus compared with individuals without this disease. In the ATC meta-analysis,1 the reduction in relative risk of cardiovascular events with aspirin was similar in patients with diabetes mellitus and those without the disorder, although this analysis did not include three trials performed exclusively in patients with diabetes mellitus.2,7,8,41 This finding argues against a greater or lesser pharmacologic effect of aspirin in this patient population. Similarly, although some evidence suggests that newer antiplatelet agents are more effective than aspirin in patients with diabetes mellitus, the observed benefits are very similar to those found in patients without diabetes mellitus.42–44 These observations, therefore, probably reflect differences in antiplatelet agents themselves rather than the studied patient populations. To date, no large, randomized trials have directly compared different antiplatelet regimens for the primary prevention of cardiovascular events in patients with diabetes mellitus. Thus, the clinical value of currently available surrogate platelet studies and retrospective analyses in these patients remains unknown until the findings of comparative outcome studies are available.
Primary prevention in diabetes mellitus
Multiple randomized trials of aspirin have been performed in patients without cardiovascular disease (Table 1) and have included patients with or without diabetes mellitus. This review only addresses trials that included patients with diabetes mellitus and that involved a randomized comparison of aspirin with placebo rather than other antiplatelet therapies. Trials also needed to report at least 2 years of follow-up and state individual vascular endpoints, so that drug effects could be estimated specifically for patients with diabetes mellitus. Nine trials met these criteria. Three of these trials exclusively studied patients with diabetes mellitus; the other six trials enrolled general populations but included some patients with diabetes mellitus.
Table 1.
Comparison of trials of primary prevention for coronary heart disease in patients with diabetes mellitus
| Study | Aspirin dose (mg)* |
Follow-up (years) |
Patients with DM |
Mean age |
CHD endpoint |
CHD endpoint event rate (control % vs aspirin %) |
10-year extrapolated CHD event rates (%)‡ |
CHD events RR (95% CI)§ |
Stroke events (control vs aspirin) with RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| TPT9 | 75 | 6.7 | 68 | 58 | MCE | 15.4 vs 13.8 | 23.0 vs 20.6 | 0.90 (0.28–2.89) |
2 vs 1 0.67 (0.06–7.06) |
| HOT9 | 75 | 3.8 | 1,501 | 62 | MCE | 3.6 vs 2.8 | 9.5 vs 7.3 | 0.77 (0.44–1.36) |
24 vs 22 0.91 (0.52–1.61) |
| JPAD7 | 81–100 | 4.4 | 2,539 | 65 | Fatal and nonfatal MI |
1.1 vs 1.0 | 2.5 vs 2.3 | 0.87 (0.40–1.87) |
32 vs 28 0.89 (0.54–1.46) |
| POPADAD8 | 100 | 6.7 | 1,276 | 60 | CHD death and nonfatal MI |
12.9 vs 13.9 | 19.3 vs 20.7 | 1.09 (0.82–1.44) |
50 vs 37 0.74 (0.49–1.12) |
| PPP50∥ | 100 | 3.7 | 1,031 | 64 | Fatal and nonfatal MI |
2.0 vs 1.0 | 5.4 vs 2.7 | 0.49 (0.17–1.43) |
11 vs 10 0.90 (0.38–2.09) |
| WHS5∥ | 100 mg every other day |
10.1 | 1,027 | 55 | Fatal and nonfatal MI |
5.9 vs 7.9 | 5.9 vs 7.9 | 1.34 (0.85–2.12) |
31 vs 15 0.45 (0.25–0.82) |
| PHS46∥ | 325 mg every other day |
5.0 | 533 | NA | Fatal and nonfatal MI |
10.5 vs 6.2 | 21 vs 12.4 | 0.59 (0.33–1.06) |
10 vs 16 1.50 (0.69–3.25) |
| BMD9 | 500 | 5.6 | 101 | NA | MCE | 18.8 vs 18.8 | 33.48 vs 33.6 | 1.00 (0.42–2.40) |
1 vs 0 1.39 (0.15–12.86) |
| ETDRS41 | 650 | 5.0 | 3,711 | NA | Fatal and nonfatal MI |
15.3 vs 13.0 | 30.6 vs 26.0 | 0.85 (0.73–1.00) |
78 vs 92 1.18 (0.88–1.58) |
Daily dose, unless specified otherwise.
The 10-year extrapolated coronary heart disease event rate was calculated by (10 ÷ study duration) × event rate.
Based on event counts.
Values slightly different from original reports based on updated information.9
Abbreviations: BMD, British Medical Doctors study; CHD, coronary heart disease; DM, diabetes mellitus; ETDRS, Early Treatment of Diabetic Retinopathy trial; HOT, Hypertension Optimal Treatment; JPAD, Japanese Prevention of Atherosclerosis with Aspirin in Diabetes trial; MCE, major coronary event (CHD death or nonfatal MI or sudden death); MI, myocardial infarction; NA, not applicable; PHS, Physicians Health Study; POPADAD, Prevention of Progression of Arterial Disease and Diabetes trial; PPP, Primary Prevention Project; RR, relative risk; TPT, Thrombosis Prevention Trial; vs, versus; WHS, Womens Health Study. Adapted with permission from the American Diabetes Association © Pignone, M. et al. Diabetes Care 33, 1395–1402 (2010).
The trials that exclusively studied patients with diabetes mellitus were the Early Treatment of Diabetic Retinopathy (ETDRS),41 the Prevention of Progression of Arterial Disease and Diabetes (POPADAD)8 and the Japanese Prevention of Atherosclerosis with Aspirin in Diabetes (JPAD).7 These trials included a total of 7,526 patients with 38,275 patient–years of follow-up between them.
The ETDRS trial41 was notably larger than POPADAD8 or JPAD,7 enrolled patients with type 1 and type 2 diabetes mellitus and was conducted before the era of statins. Although the trial was designed to exclude patients with a history of major cardiovascular events, 1.7% of patients who took part in the trial had a history of stroke and 7.7% had a history of coronary heart disease.41 These characteristics contributed to the ETDRS study participants being at a higher risk of cardiovascular disease than the participants of POPADAD and JPAD. In the ETDRS control group,41 the 10-year adjusted incidence rate of coronary heart disease was 30.6% compared with 19.3% and 2.5% in the control groups of POPADAD and JPAD, respectively (Table 1).
Aspirin therapy resulted in a 15% relative risk (RR) reduction in fatal plus nonfatal myocardial infarction in patients in the ETDRS (RR 0.85, 95% CI 0.73–1.00).41 In the JPAD study,7 a similar reduction was observed for fatal plus nonfatal coronary heart disease events (RR 0.81, 95% CI 0.49–1.33) but the number of events was small (28 in the treatment group versus 35 in the control group) and the findings were not statistically significant. Neither trial reduced the risk of stroke, although not many strokes occurred (Table 1).
In the POPADAD trial,8 which enrolled patients with an ankle brachial pressure index <0.99 (the ratio of blood pressure in the lower legs to the blood pressure in the arms as a measure of peripheral vascular disease), no benefit from aspirin was found for the endpoint of death from coronary heart disease plus nonfatal myocardial infarction, despite the relatively high risk of the population studied. A nonsignificant reduction in the risk of stroke was determined. The use of statins was not reported. The finding that aspirin provided no benefit in the POPADAD trial should be considered in the context of the very high rate of drug discontinuation in this trial. Although POPADAD averaged a mean 6.7 years in duration of follow-up, the rate of discontinuation of assigned therapy was reported as 50% at 5 years. A beneficial trend for the composite vascular endpoint with aspirin therapy could be detected after 2 years but was lost after 4 years.8 What part the high rate of aspirin discontinuation played in this finding is unknown. In addition, the rate of adverse events did not differ between groups, which again raises the possibility that low adherence may have led to the few observed differences.
In the six studies that analyzed aspirin therapy in general populations, a variable number of patients with diabetes mellitus were enrolled, with a wide range of baseline risk levels (Table 1). These trials were the British Medical Doctors (BMD) study, the Physicians Health Study (PHS), the Thrombosis Prevention Trial (TPT), the Hypertension Optimal Treatment (HOT) study, the Primary Prevention Project (PPP) and the Women’s Health Study (WHS).5,45–50 Three of these trials included 1,000 or more patients with diabetes mellitus (PPP, WHS and HOT), whereas the PHS included about 500 patients with diabetes mellitus, and the BMD study and TPT enrolled about 100 each. The 10-year extrapolated risk of coronary heart disease in these patients ranged from 5.4% to 33.5%. Given that the trials did not specifically randomize on the basis of diabetes mellitus status, post-hoc analyses of their results do not preserve all the benefits of randomization and should be interpreted with caution.
The BMD, TPT and PHS trials only enrolled men. The BMD trial showed no benefit of aspirin for the reduction of coronary heart disease events or stroke in the subset of patients with diabetes mellitus. Similarly, little effect was observed in the TPT. The number of events in participants with diabetes mellitus was quite small in both trials, however, and the CIs were wide. The PHS showed a large, but not statistically significant, reduction in myocardial infarction but also a nonstatistically significant increase in stroke among study participants with diabetes mellitus treated with aspirin.
The PPP contained a mixed population of men and women and showed a nonstatistically significant reduction in coronary heart disease events among aspirin-treated patients with diabetes mellitus. Researchers of the HOT study also enrolled a mixed population of men and women and had similar findings. The WHS, the only trial to enroll exclusively women and the trial that used the lowest aspirin dosage (100 mg every other day), showed a nonsignificant trend for increased coronary heart disease events (RR 1.34, 95% CI 0.85–2.12) but also found a reduction in stroke (RR 0.45, 95% CI 0.25–0.82) with aspirin.
Given the limited power and mixed results of the available individual trials, several analysts have attempted to combine the data on the effectiveness of aspirin in patients with diabetes mellitus by meta-analysis (Table 2).2,9,51–53 The meta-analyses differed somewhat in the specific trials included, how outcomes were defined and the data available from each trial. However, they consistently identified a nonstatistically significant 10–15% relative risk reduction in coronary heart disease events. Pooled estimates for reduction in stroke were similar in magnitude (10–17%) across analyses that included the majority of trials and were also not statistically significant. Given the limited duration (<10 years) of the available trials, the effects of aspirin on all-cause mortality are currently undetermined. In addition, none of the meta-analyses had sufficient data at the individual patient level to stratify their estimates of effect of aspirin in diabetes mellitus for men and women, despite some evidence suggesting that the effects may differ between the sexes.51
Table 2.
Meta-analyses of trials examining the effect of aspirin in diabetes mellitus
| Reference | Trials included | Effect of aspirin on coronary heart disease events* |
Effect of aspirin on stroke* |
Comments |
|---|---|---|---|---|
| Baigent et al.2 |
BMD, PHS, PPP, WHS, TPT, HOT |
NA | NA | Effect of aspirin on cardiovascular events: 0.88 (0.82–0.94) |
| Calvin et al.52 |
PHS, PPP, WHS, HOT, ETDRS, JPAD, POPADAD‡ |
0.86 (0.67–1.11) | 0.62 (0.31–1.24) | NA |
| DeBerardis et al.51 |
PHS, PPP, WHS, ETDRS, JPAD, POPADAD§ |
0.86 (0.61–1.21) | 0.83 (0.60–1.14) | NA |
| Pignone et al.9 |
BMD, PHS, PPP, WHS, TPT, HOT, ETDRS, JPAD, POPADAD |
0.91 (0.79–1.05) | 0.90 (0.71–1.13) | Used additional data provided by ATT investigators |
| Zhang et al.53 |
PHS, PPP, WHS, HOT, ETDRS, JPAD, POPADAD§ |
0.85 (0.65–1.11) | 0.83 (0.63–1.10) | Important differences in effect for men and women identified with meta-regression No evidence of publication bias |
Values state the relative risk and CIs are listed in brackets.
Only included JPAD and WHS for stroke.
Did not include PHS for stroke.
Abbreviations: ATT, Anti-Thrombotic Trialists; BMD, British Medical Doctors study; ETDRS, Early Treatment of Diabetic Retinopathy trial; HOT, Hypertension Optimal Treatment; JPAD, Japanese Prevention of Atherosclerosis with Aspirin in Diabetes trial; NA, not applicable; POPADAD, Prevention of Progression of Arterial Disease and Diabetes trial; PHS, Physicians Health Study; PPP, Primary Prevention Project; RR, relative risk; TPT, Thrombosis Prevention Trial; WHS, Womens Health Study.
Several meta-analyses have examined the adverse effects of aspirin on gastrointestinal bleeding in general populations that included individuals with and without diabetes mellitus. These analyses have consistently found that aspirin increases the risk of bleeding, with relative risks of 1.5–1.7.54 The absolute rates of gastrointestinal bleeding in randomized trials have been low; the absolute excess risk was approximately three per 10,000 in the six general population primary prevention trials. Nevertheless, a 2009 ATT meta-analysis,2 an update from the same group that published the ATC meta-analysis1 in 2002, also suggested that patients with diabetes mellitus may be at somewhat higher risk of gastrointestinal bleeding than those in the general population. In addition, data from real-world settings in general populations (outside the context of a research study) have also suggested higher rates of bleeding with aspirin in patients with diabetes mellitus than those without this disorder.55 On the basis of these data, the actual excess risk of gastrointestinal bleeding may be 1–2 in 1,000 individuals per year in middle-aged adults with diabetes mellitus. This risk also increases substantially with age and may be >5 in 1,000 per year in those aged >70 years.
Benefit versus harm of aspirin use
Given the potential beneficial and deleterious effects of aspirin, clinicians and policy makers must decide which patients, if any, should receive aspirin for the primary prevention of cardiovascular disease (Box 2). This question is difficult for patients both with and without diabetes mellitus. Many different analysts have attempted to weigh the relative benefits (reductions in myocardial infarction and/or stroke) against the adverse effects of gastrointestinal bleeding and the added burden of taking a preventive medication every day.56–58 These calculations must be made in the presence of uncertainty about the true long-term effects of aspirin and whether these effects vary across different patient populations, including those with diabetes mellitus.
Box 2. Pros and cons of aspirin for primary prevention in diabetes mellitus.
Arguments in favor of aspirin therapy
▪ Meta-analyses show trend for modest benefit
▪ Patients with diabetes mellitus are at high risk of cardiovascular events
▪ Patients with diabetes mellitus in clinical practice often lack optimal cardiovascular disease risk control (control of LDL cholesterol levels, hypertension control, smoking cessation) and would derive more benefit than trials suggest
▪ Avoiding a major cardiovascular event is generally of more value to a patient than experiencing a gastrointestinal bleed
Arguments against aspirin therapy
▪ Trend for benefit in meta-analyses not statistically significant
▪ Patients with diabetes mellitus may be more ‘resistant’ to effects of antiplatelet therapies
▪ ATT meta-analysis2 suggests a higher risk of adverse effects related to gastrointestinal bleeding from aspirin in patients with diabetes mellitus than in the general population
Some analysts have counted the number of potential cardiovascular events prevented and directly compared them with the number of gastrointestinal bleeds caused.56 Such comparisons are misleading, as each cardiovascular event, on average, has a greater effect on health than each gastrointestinal bleed. In order to accurately judge net health effect, immediate and delayed effects of preventing or failing to prevent myocardial infarctions, strokes, and gastrointestinal bleeds must be considered, including their effects on quality of life, costs of care and mortality.
The effects of aspirin for primary prevention in patients without diabetes mellitus at different levels of cardiovascular risk have previously been modeled.59–61 In general, these analyses have found that the benefits of aspirin appear to clearly exceed the adverse effects in men with 10-year coronary heart disease risk over 10%.60 In women, the net benefit is less evident because of limited data, but may be present for older women (>65 years) at increased risk of stroke.59
A cost-effectiveness model examined the role of aspirin specifically in patients with diabetes mellitus. The investigators assumed an 18% reduction in coronary heart disease events and 5% reduction in stroke and found aspirin use to be efficacious and cost-effective ($5,428 per life year gained) compared with no aspirin use. Men derived more benefit than women, but aspirin was cost-effective for both sexes.62
Although the estimate of overall cardiovascular disease risk reduction in the ATT meta-analysis2 did not differ with the presence or absence of diabetes mellitus, aspirin might be somewhat less effective (that is, a smaller relative risk reduction) for patients with diabetes mellitus compared with the general population.2,9 In other words, the cardiovascular disease risk threshold for prescribing aspirin would be higher for patients with diabetes mellitus than those without this disorder. Further data on the effects of aspirin in adults (particularly women) with diabetes mellitus is needed to clarify this question. In addition, aspirin must be considered in the context of other effective preventive therapies in diabetes mellitus, including statins, smoking cessation, blood pressure control and possibly glycemic control.
Guidelines
In January 2009, the American Diabetes Association (ADA) revised the strength of its recommendation for the use of aspirin for primary prevention of cardiovascular events in patients with diabetes mellitus, going from evidence level A (clear evidence from well-conducted, randomized trials) to level C (conflicting evidence with weight supporting recommendation).63 Canadian guidelines were similarly revised.64 Others have suggested that aspirin should not be used for primary prevention in patients with diabetes mellitus because they consider the benefits to be unproven in the face of known deleterious effects.2,65,66
On the basis of new trial evidence, as well as conflicting expert opinion regarding the use of aspirin in patients with diabetes mellitus, the ADA, American Heart Association and American College of Cardiology Foundation convened a panel of experts to review all the available evidence and provide updated practice recommendations. A total of nine trials were analyzed (including additional data from three trials that were provided by the ATC) with meta-analyses generated for both cardiovascular events and stroke. New summary recommendations were published by the ADA in January 2010 and a complete document was published in June, 2010.67 The panel concluded that, on the basis of currently available evidence, aspirin (75–162 mg per day) for primary prevention is reasonable for adults with diabetes mellitus and moderate or higher cardiovascular disease risk (10-year risk of cardiovascular disease events >10%), assuming that they are not at high risk of gastrointestinal bleeding. By contrast, those with low cardiovascular disease risk (10-year cardiovascular disease risk <5%) should not use aspirin as a preventive measure. Men aged >50 years and women aged >60 years with an additional risk factor were identified as those most likely to benefit from aspirin therapy.67
Future research
Surrogate platelet testing
Given the debate regarding the potential for diabetes mellitus to induce a relative aspirin resistance, the availability of a reliable test to measure platelet response to aspirin therapy would be an important step forward. Prospective trials are needed to determine whether or not higher doses of aspirin (>325 mg daily) or the use of other antiplatelet therapies in addition to aspirin are effective in patients with apparent aspirin resistance. Two ongoing trials, both of which include patients with diabetes mellitus, may offer some insight into the latter question.
The trial GRAVITAS (Gauging Responsiveness with a Verifynow® assay, Impact on Thrombosis and Safety) has a target enrollment of nearly 3,000 patients and will test whether higher doses of clopidogrel (150 mg daily versus standard 75 mg) improve clinical outcomes in patients with apparent antiplatelet resistance despite background therapy with aspirin and standard doses of clopidogrel. Researchers of GRAVITAS completed recruiting in 2009, but the trial is ongoing. The smaller ASCET trial (Aspirin Non-responsiveness and Clopidogrel Endpoint Trial) will enroll about 1,000 patients and will also include those with diabetes mellitus. ASCET is using a different surrogate platelet testing modality to test platelet response to 160 mg aspirin daily and is randomizing patients on the basis of their response to either continued aspirin or a switch to clopidogrel. Although neither trial will include enough patients with diabetes mellitus to provide definitive answers, both should offer some insight into the clinical implications of prospectively determined anti-platelet resistance and its impact on clinical outcomes.
Ongoing trials of primary prevention
The findings of two ongoing studies of the use of aspirin for primary prevention in diabetes mellitus are eagerly awaited, especially given the lingering question about the role of aspirin in patients already on statin therapy. The Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D) is an open-label study that compares 100 mg aspirin to placebo in patients with diabetes mellitus (>50 years of age) without coronary heart disease.68 A second trial conducted in the UK will also compare 100 mg of aspirin with placebo.69 Researchers of the ASCEND (A Study of Cardiovascular Events in Diabetes) trial will examine patients with diabetes mellitus (aged >40 years) without coronary heart disease.69 Given that ASCEND is still recruiting, a large number of patients in this trial are expected to also be on statin therapy. ACCEPT-D and ASCEND will include 5,000 and 10,000 men and women with no prespecified number for either sex, respectively. A third trial, the Japanese Primary Prevention Project (JPPP) has completed enrollment and includes about 5,000 patients with diabetes mellitus among the nearly 15,000 patients enrolled.70 Investigators of the JPPP trial recruited individuals >60 years of age to further delineate the risk of bleeding in the elderly population with diabetes mellitus.70 Similar to the ACCEPT-D68 and ASCEND69 trials, JPPP70 is investigating the effect of 100 mg of aspirin daily versus placebo.
Conclusions
Patients with diabetes mellitus are at an increased risk of cardiovascular events. Aspirin, through platelet inhibi tion, is effective in reducing thrombosis, although this benefit comes at the expense of an increased risk of gastrointestinal bleeding. Evidence from trials in patients with diabetes mellitus and subgroup analyses of participants with diabetes mellitus in trials performed in general populations suggest that aspirin may reduce coronary heart disease events and stroke in diabetes mellitus, although definitive conclusions about aspirin’s effects are limited by the low number of events in the trials to date and paucity of data from trials conducted solely in patients with diabetes mellitus. Ongoing trials such as ASCEND69 and ACCEPT-D68 should help clarify these questions in the near future. Until then, it seems reasonable to consider aspirin as one of the potential therapies for cardiovascular disease risk reduction in patients with diabetes mellitus and elevated cardiovascular disease risk.
Key points.
▪ Patients with diabetes mellitus exhibit increased platelet reactivity
▪ Randomized trials of aspirin for the primary prevention of cardiovascular events in patients with diabetes mellitus have studied heterogeneous populations and exhibit heterogeneous results
▪ Aspirin (75–162 mg daily) for primary prevention is reasonable for patients with diabetes mellitus and moderate or high cardiovascular disease risk (10-year risk >10%) that are not at high risk of gastrointestinal bleeding
▪ Those with low cardiovascular disease risk (10-year risk <5%) should not use aspirin as a preventive measure
▪ Ongoing trials of aspirin therapy in patients with diabetes mellitus will help to provide more definitive conclusions
Review criteria.
A search for original studies published since 1966 and focusing on aspirin therapy was conducted in MEDLINE. Search terms included “aspirin”, “therapeutic use” and “placebo controlled”. All articles were English language, full-text papers. Trials had to report at least 2 years of follow-up and include patients with diabetes mellitus. Publications needed to include a listing of specific vascular endpoints so that drug effects could be estimated specifically for patients with diabetes mellitus. We also searched the reference lists of identified articles for further papers. Nine trials met the search criteria and were included in Table 1. The author’s expertise in this field was used to determine other articles.
Footnotes
Competing interests The authors declare no competing interests.
Author contributions M. Pignone and C. D. Williams researched the data for the article and provided a substantial contribution to discussions of the content.
References
- 1.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2002;136:161–172. doi: 10.7326/0003-4819-136-2-200201150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Berger JS, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N. Engl. J. Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 6.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch. Intern. Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa H, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 8.Belch J, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pignone M, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Diabetes Care. 2010;33:1395–1402. doi: 10.2337/dc10-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N. Engl. J. Med. 1984;311:1206–1211. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 11.FitzGerald GA, Pedersen AK, Patrono C. Analysis of prostacyclin and thromboxane biosynthesis in cardiovascular disease. Circulation. 1983;67:1174–1177. doi: 10.1161/01.cir.67.6.1174. [DOI] [PubMed] [Google Scholar]
- 12.Patrono C, Rodríguez L. A. García, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 13.Rocca B, et al. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and characterizes newly formed platelets. Proc. Natl Acad. Sci. USA. 2002;99:7634–7639. doi: 10.1073/pnas.112202999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tohgi H, Konno S, Tamura K, Kimura B, Kawano K. Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin. Stroke. 1992;23:1400–1403. doi: 10.1161/01.str.23.10.1400. [DOI] [PubMed] [Google Scholar]
- 15.Patrono C. Aspirin as an antiplatelet drug. N. Engl. J. Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- 16.Clarke RJ, Mayo G, Price P, FitzGerald GA. Suppression of thromboxane A2 but not of systemic prostacyclin by controlled-release aspirin. N. Engl. J. Med. 1991;325:1137–1141. doi: 10.1056/NEJM199110173251605. [DOI] [PubMed] [Google Scholar]
- 17.Santilli F, et al. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin “resistance”. J. Am. Coll. Cardiol. 2009;53:667–677. doi: 10.1016/j.jacc.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 18.Smith JB, Willis AL. Aspirin selectively inhibits prostaglandin production in human platelets. Nat. New Biol. 1971;231:235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- 19.Quick AJ. Salicylates and bleeding: the aspirin tolerance test. Am. J. Med. Sci. 1966;252:265–269. doi: 10.1097/00000441-196609000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hovens MM, et al. Prevalence of persistent platelet reactivity despite use of aspirin: a systematic review. Am. Heart J. 2007;153:175–181. doi: 10.1016/j.ahj.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 21.Abaci A, et al. Effect of increasing doses of aspirin on platelet function as measured by PFA-100 in patients with diabetes. Thromb. Res. 2005;116:465–470. doi: 10.1016/j.thromres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Mirkhel A, et al. Frequency of aspirin resistance in a community hospital. Am. J. Cardiol. 2006;98:577–579. doi: 10.1016/j.amjcard.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 23.Eikelboom JW, Hankey GJ. Aspirin resistance: a new independent predictor of vascular events? J. Am. Coll. Cardiol. 2003;41:966–968. doi: 10.1016/s0735-1097(02)03013-9. [DOI] [PubMed] [Google Scholar]
- 24.Schneider DJ. On defining aspirin resistance. J. Am. Coll. Cardiol. 2005;46:1710–1711. doi: 10.1016/j.jacc.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Michelson AD. Methods for the measurement of platelet function. Am. J. Cardiol. 2009;103(Suppl. 3):20A–26A. doi: 10.1016/j.amjcard.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Williams CD, Cherala G, Serebruany V. Application of platelet function testing to the bedside. Thromb. Haemost. 2010;103:29–33. doi: 10.1160/TH09-06-0375. [DOI] [PubMed] [Google Scholar]
- 27.Eikelboom JW, et al. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 28.Snoep JD, Hovens MM, Eikenboom JC, van der Bom JG, Huisman MV. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: a systematic review and meta-analysis. Arch. Intern. Med. 2007;167:1593–1599. doi: 10.1001/archinte.167.15.1593. [DOI] [PubMed] [Google Scholar]
- 29.Geisler T, et al. The Residual Platelet Aggregation after Deployment of Intracoronary Stent (PREDICT) score. J. Thromb. Haemost. 2008;6:54–61. doi: 10.1111/j.1538-7836.2007.02812.x. [DOI] [PubMed] [Google Scholar]
- 30.Serebruany V, et al. Association of platelet responsiveness with clopidogrel metabolism: role of compliance in the assessment of “resistance”. Am. Heart J. 2009;158:925–932. doi: 10.1016/j.ahj.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Peters RJ, et al. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation. 2003;108:1682–1687. doi: 10.1161/01.CIR.0000091201.39590.CB. [DOI] [PubMed] [Google Scholar]
- 32.Colwell JA, et al. Altered platelet function in diabetes mellitus. Diabetes. 1976;25(Suppl. 2):826–831. [PubMed] [Google Scholar]
- 33.Watala C, et al. Reduced sensitivity of platelets from type 2 diabetic patients to acetylsalicylic acid (aspirin)-its relation to metabolic control. Thromb. Res. 2004;113:101–113. doi: 10.1016/j.thromres.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Anfossi G, Russo I, Trovati M. Platelet dysfunction in central obesity. Nutr. Metab. Cardiovasc. Dis. 2009;19:440–449. doi: 10.1016/j.numecd.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Davì G, et al. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288:2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- 36.Davì G, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N. Engl. J. Med. 1990;322:1769–1774. doi: 10.1056/NEJM199006213222503. [DOI] [PubMed] [Google Scholar]
- 37.Tschoepe D, et al. Evidence for abnormal platelet glycoprotein expression in diabetes mellitus. Eur. J. Clin. Invest. 1990;20:166–170. doi: 10.1111/j.1365-2362.1990.tb02264.x. [DOI] [PubMed] [Google Scholar]
- 38.Calkin AC, et al. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation. 2009;120:2095–2104. doi: 10.1161/CIRCULATIONAHA.109.870709. [DOI] [PubMed] [Google Scholar]
- 39.Tschoepe D, et al. Elevated platelet activation in type I diabetics with chronic complications under long-term near-normoglycemic control. Haemostasis. 1990;20:93–98. doi: 10.1159/000216113. [DOI] [PubMed] [Google Scholar]
- 40.Retnakaran R, Zinman B. Type 1 diabetes, hyperglycaemia, and the heart. Lancet. 2008;371:1790–1799. doi: 10.1016/S0140-6736(08)60767-9. [DOI] [PubMed] [Google Scholar]
- 41.Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. ETDRS Investigators. JAMA. 1992;268:1292–1300. doi: 10.1001/jama.1992.03490100090033. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 42.Wiviott SD, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel—Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626–1636. doi: 10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
- 43.King SB, 3rd, Mahmud E. Will blocking the platelet save the diabetic? Circulation. 1999;100:2466–2468. doi: 10.1161/01.cir.100.25.2466. [DOI] [PubMed] [Google Scholar]
- 44.Bhatt DL, et al. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am. J. Cardiol. 2002;90:625–628. doi: 10.1016/s0002-9149(02)02567-5. [DOI] [PubMed] [Google Scholar]
- 45.Peto R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br. Med. J. (Clin. Res. Ed.) 1988;296:313–316. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N. Engl. J. Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 47.Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet. 1998;351:233–241. [No authors listed] [PubMed] [Google Scholar]
- 48.Hansson L, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 49.de Gaetano G. Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative Group of the Primary Prevention Project. Lancet. 2001;357:89–95. doi: 10.1016/s0140-6736(00)03539-x. [DOI] [PubMed] [Google Scholar]
- 50.Sacco M, et al. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care. 2003;26:3264–3272. doi: 10.2337/diacare.26.12.3264. [DOI] [PubMed] [Google Scholar]
- 51.De Berardis G, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: meta-analysis of randomised controlled trials. BMJ. 2009;339:b4531. doi: 10.1136/bmj.b4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calvin AD, et al. Aspirin for the primary prevention of cardiovascular events: a systematic review and meta-analysis comparing patients with and without diabetes. Diabetes Care. 2009;32:2300–2306. doi: 10.2337/dc09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, et al. Aspirin for primary prevention of cardiovascular events in patients with diabetes: A meta-analysis. Diabetes Res. Clin. Pract. 2010;87:211–218. doi: 10.1016/j.diabres.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 54.Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ. 2000;321:1183–1187. doi: 10.1136/bmj.321.7270.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernández-Díaz S, Rodríguez L. A. García. Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 2006;4:22. doi: 10.1186/1741-7015-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart. 2001;85:265–271. doi: 10.1136/heart.85.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mulrow C, Pignone M. An editorial update: should she take aspirin? Ann. Intern. Med. 2005;142:942–943. doi: 10.7326/0003-4819-142-11-200506070-00015. [DOI] [PubMed] [Google Scholar]
- 58.Aspirin for the prevention of cardiovascular disease: U. S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 59.Pignone M, Earnshaw S, Pletcher MJ, Tice JA. Aspirin for the primary prevention of cardiovascular disease in women: a cost-utility analysis. Arch. Intern. Med. 2007;167:290–295. doi: 10.1001/archinte.167.3.290. [DOI] [PubMed] [Google Scholar]
- 60.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann. Intern. Med. 2006;144:326–336. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 61.Greving JP, Buskens E, Koffijberg H, Algra A. Cost-effectiveness of aspirin treatment in the primary prevention of cardiovascular disease events in subgroups based on age, gender, and varying cardiovascular risk. Circulation. 2008;117:2875–2883. doi: 10.1161/CIRCULATIONAHA.107.735340. [DOI] [PubMed] [Google Scholar]
- 62.Li R, Zhang P, Barker LE, Hoerger TJ. Cost-effectiveness of aspirin use among persons with newly diagnosed type 2 diabetes. Diabetes Care. 2010;33:1193–1199. doi: 10.2337/dc09-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(Suppl. 1):S13–61. doi: 10.2337/dc09-S013. [No authors listed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can. J. Diabetes. 2008;32(Suppl. 1):S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Barnett H, Burrill P, Iheanacho I. Don’t use aspirin for primary prevention of cardiovascular disease. BMJ. 2010;340:c1805. doi: 10.1136/bmj.c1805. [DOI] [PubMed] [Google Scholar]
- 66.Sirois C, Poirier P, Moisan J, Grégoire JP. The benefit of aspirin therapy in type 2 diabetes: what is the evidence? Int. J. Cardiol. 2008;129:172–179. doi: 10.1016/j.ijcard.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 67.Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(Suppl. 1):S11–S61. doi: 10.2337/dc10-S011. [No authors listed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Berardis G, et al. Aspirin and Simvastatin Combination for Cardiovascular Events Prevention Trial in Diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials. 2007;8:21. doi: 10.1186/1745-6215-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.British Heart Foundation A Study of Cardiovascular Events In Diabetes (ASCEND) Trial. 2010 [online], http://www.ctsu.ox.ac.uk/ascend/
- 70.Teramoto T, et al. Rationale, design, and baseline data of the Japanese Primary Prevention Project (JPPP)-a randomized, open-label, controlled trial of aspirin versus no aspirin in patients with multiple risk factors for vascular events. Am. Heart J. 2010;159:361–369. doi: 10.1016/j.ahj.2009.11.030. [DOI] [PubMed] [Google Scholar]