Abstract
Objective
To determine long-term tumor recurrence rates after treatment of primary nonmelanoma skin cancer (NMSC). Data are currently insufficient to permit evidence-based choices among treatments for NMSC.
Design
Prospective study of an inception cohort observed for a median of 6.6 years after treatment.
Setting
Dermatology clinic at a Veterans Affairs hospital. Care was provided by dermatology resident or attending physicians.
Patients
Consecutive sample of all 495 patients with 616 primary NMSCs diagnosed in 1999 and 2000 and treated with electrodessication and curettage (ED&C), excision, or Mohs surgery. Follow-up was available for 608 tumors (99%).
Main Outcome Measure
Tumor recurrence, determined by medical record review, with validation by clinical examination.
Results
The mean age at diagnosis was 71 years; 97% were men. Overall, 127 tumors were treated with ED&C (20.9%); 309 with excision (50.8%); and 172 with Mohs surgery (28.3%). Over the course of the study, 21 tumors recurred (3.5% [95% confidence interval (CI), 2.2%–5.2%]): 2 after ED&C (1.6% [95% CI, 0.2%–5.6%]), 13 after excision (4.2% [95% CI, 2.2%–7.1%]), and 6 after Mohs surgery (3.5% [95% CI, 1.3%–7.4%])
Conclusions
Recurrence of primary NMSC after treatment occurred in less than 5% of tumors. The recurrence rate after ED&C was lower than expected, and the recurrence rate after Mohs surgery was higher than expected. These findings may be related to the risk for recurrence in the treatment groups.
In the United States, the most common treatments for primary basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) of the skin (nonmelanoma skin cancer [NMSC]) are tumor destruction with electrodessication and curettage (ED&C), excision, and Mohs surgery. For most tumors, existing data about tumor recurrence after treatment are insufficient to permit evidence-based choices among therapies.1 Previous studies have been limited by retrospective designs, select samples, imprecise determination of recurrence, or incomplete follow-up. To measure tumor recurrence rates after treatment, we prospectively enrolled and observed for over 6 years a consecutive sample of patients with primary NMSC diagnosed at a Veterans Affairs (VA) clinic.
METHODS
Details of the study and early data collection have been described.2 In brief, through daily review of pathology reports and clinical records we identified all patients with primary NMSCs treated with ED&C, excision, or Mohs surgery in 1999 and 2000 at a large, academically affiliated VA medical center. Most care, except Mohs surgery, was provided by dermatology residents supervised by attending physicians. Except in rare cases, Mohs surgery was performed by a Mohs surgeon who was a member of the American College of Mohs Micrographic Surgery and Cutaneous Oncology, and who was within five years after fellowship training. Nurse practitioners provided a minority of care but could perform ED&C or, rarely, excisions.
Electrodessication and curettage was performed in general dermatology clinics. Excision and Mohs surgery were most often performed in clinics specifically designated for surgical procedures. The vast majority of excisions were simple excisions (ie, margins of excised specimens were examined histologically in fixed specimens after closure)3; for a small number of excised tumors (4 tumors, 1.3%) frozen sections were used to assess the presence of tumor at the margins before closure.
The primary source of follow-up data was the medical record. A mean (SD) of 8.3 (0.6) years after therapy, trained dermatologic nurse practitioners reviewed the medical record using structured dataforms; as much as possible, these chart abstracters were blinded to treatment type. Most records were in electronic form; for 10% of tumors, early clinical encounters had been recorded on paper records, which were also reviewed. For 15% of tumors, the records indicated that the patient had also received care at another VA facility during the follow-up period; the records from these facilities were reviewed. For each tumor, the follow-up period ended at the last date on which the patient received care in the hospital system. Because patients who visited the dermatology clinic more often may have been more likely to have had recurrence detected, for each patient, we calculated the average annual number of visits to the dermatology clinic throughout the follow-up period.
To validate the determination of recurrence, the treatment sites of patients who consented were examined by a dermatologist (M.-M.C.) who was blinded to treatment type. These examinations occurred a mean (SD) of 8.6 (0.6) years after therapy. A recurrence was judged to be possible if there was evidence of scale, papule, erythema, erosion, ulceration, crust, or induration on or near the treatment site. The examiner also estimated the likelihood of recurrence as less than 20%, 21% to 40%, 41% to 60%, 61% to 80%, or greater than 80%. Patients with possible recurrences were instructed to be evaluated by a dermatologist, and all subsequent dermatologic records were reviewed.
A tumor was defined as recurrent if it was in the same body location as, and had histopathologic findings consistent with, the primary tumor and was described by the dermatologic clinician as recurrent or previously treated. For recurrent tumors, the medical records were reviewed by 2 chart abstracters to ensure agreement in assignment of the primary outcome.
Before therapy, patients were surveyed about their skin cancer history and health status. Health status was measured with the Short Form 12 instrument of the Medical Outcomes Study (SF-12).4 The SF-12 scores are reported as a Physical Component Summary Score and a Mental Component Summary Score; on these scales, higher is healthier and norm-based standardized scores have means of 50 in the general US population. Co-morbidity was measured by a version of the Charlson instrument adapted for self-report.5,6
Characteristics of patients, tumors, and care were compared across the 3 treatment groups using χ2 tests for binary characteristics, and either parametric or nonparametric analysis of variance for continuous characteristics. We calculated recurrence rates through the end of follow-up and displayed tumor recurrence over time in Kaplan-Meier plots; data were right-censored at the last date on which a patient received any care at the hospital. Statistical analyses were performed using Stata statistical software, version 11.0 (StataCorp LP, College Station, Texas).
RESULTS
CHARACTERISTICS OF THE SAMPLE
From January 1999 through December 2000, a total of 495 patients had 616 primary NMSCs (Table 1). Patients, tumors, and care differed in the 3 treatment groups. For example, tumors in the H-zone of the face8 were much more common in the group treated with Mohs surgery. The treating clinician was a resident for almost all tumors treated with ED&C or excision, and almost no tumors treated with Mohs surgery, which is typical at this institution.
Table 1.
Baseline Characteristics of 487 Patients With 608 Tumors Treated With ED&C, Excision, or Mohs Surgerya
| Treatment Groupb | ||||
|---|---|---|---|---|
| Characteristic | ED&C | Excision | Mohs Surgery | P Value |
| Patient Characteristicsc | ||||
| Patients, No. | 93 | 256 | 138 | NA |
| Age, mean (SD), y | 71.3 (12.0) | 72.3 (11.4) | 69.6 (10.9) | .09 |
| Male sex | 100 | 95.7 | 96.4 | .11 |
| Physical SF-12 score, mean (SD) | 41.6 (12.7) | 42.3 (11.9) | 41.4 (11.2) | .84 |
| Mental SF-12 score, mean (SD) | 47.8 (11.8) | 48.0 (12.0) | 48.8 (10.7) | .85 |
| Charlson comorbidity index, mean (SD) | 2.5 (2.7) | 2.4 (2.8) | 3.1 (3.6) | .76 |
| History of NMSC | 57.0 | 58.6 | 46.4 | .06 |
| NMSCs at enrollment, mean (SD), No. | 1.4 (1.0) | 1.3 (0.6) | 1.3 (0.7) | .83 |
| Annual visits to dermatology clinic, mean (SD), No. | 1.7 (1.3) | 1.9 (1.5) | 1.8 (1.8) | .13 |
| Immunocompromisedd | 2.2 | 2.3 | 5.1 | .32 |
| Tumor Characteristicsc | ||||
| Tumors, No. | 127 | 309 | 172 | NA |
| Histologic type | <.001 | |||
| Basal cell carcinoma | 86.6 | 66.0 | 75.0 | |
| Squamous cell carcinoma | 13.4 | 34.0 | 25.0 | |
| Tumor diameter, mean (SD), mm | 11.5 (10.3) | 13.3 (14.1) | 9.1 (6.9) | .95 |
| Tumor location | <.001 | |||
| Head or neck | 34.7 | 66.7 | 99.4 | |
| H-zone of the face | 17.3 | 35.6 | 79.1 | |
| Trunk | 43.3 | 18.8 | 0 | |
| Extremities | 20.5 | 13.6 | 0.6 | |
| Training Level of Cliniciansc | ||||
| Attending physician | 11.3 | 19.2 | 97.6 | <.001 |
| Resident physician | 67.0 | 79.1 | 2.4 | |
| Nurse practitioner | 21.7 | 1.7 | 0 | |
Abbreviations: ED&C, electrodessication and curettage; NMSC, nonmelanoma skin cancer; SF-12, Short Form 12 instrument of the Medical Outcomes Study.4
Unless otherwise indicated, all data are reported as percentages.
Patients with more than 1 tumor were assigned to the treatment group of the tumor they reported bothered them the most.7
Numbers of patients and/or tumors for which data on the following characteristics were missing: 178, health status; 143, comorbidity; 102, diameter; 4, H-zone; and 22, treating clinician type.
Described in the medical record as “immunocompromised” or as having received an organ transplant or as having human immunodeficiency virus infection/AIDS.
TUMOR RECURRENCE
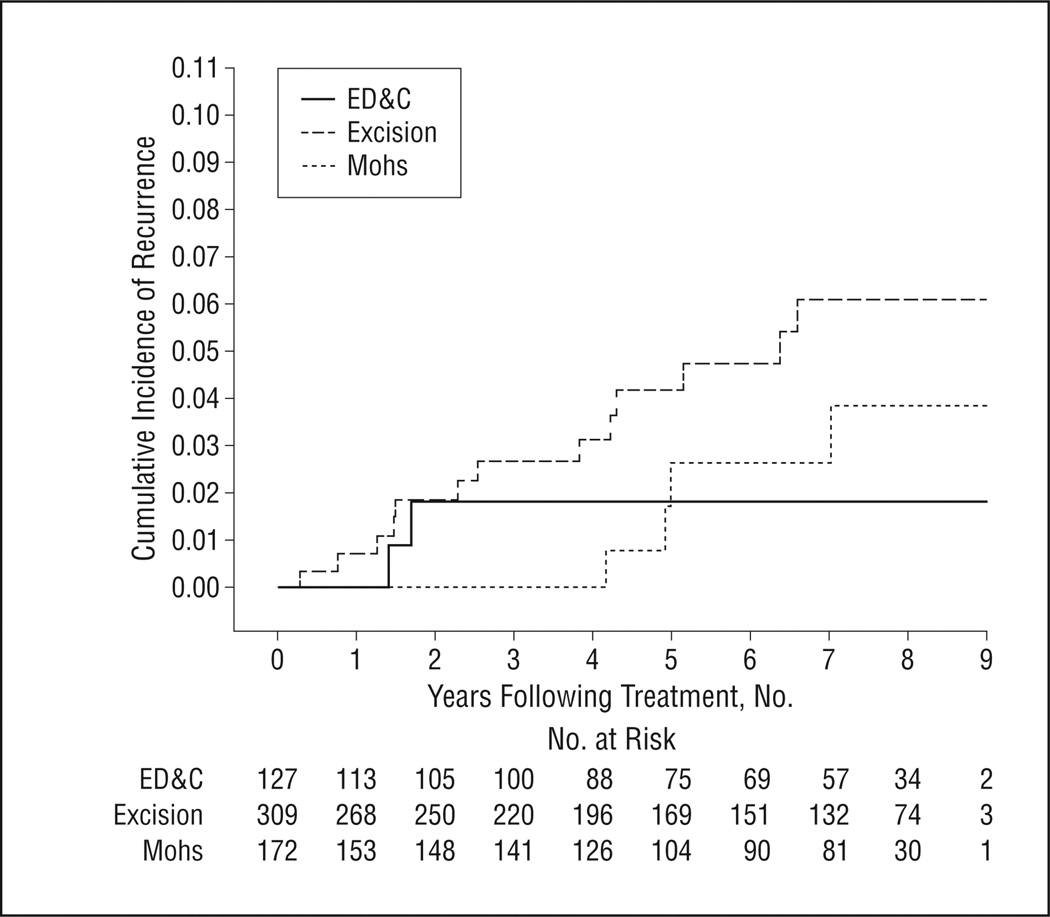
Follow-up information was available for 487 patients (98%) with 608 tumors (99%). The median time after treatment for which follow-up information was available was 6.6 years (interquartile range [IQR], 4.5 years) (ED&C, 6.7 years; excision, 6.2 years; and Mohs surgery, 7.0 years) (P = .60). Overall, 21 of 608 tumors recurred (3.5% [95% confidence interval (CI), 2.2%–5.2%]): 2 after ED&C (1.6% [95% CI, 0.2%–5.6%]), 13 after excision (4.2% [95% CI, 2.2%–7.1%]), and 6 after Mohs surgery (3.5% [95% CI, 1.3%–7.4%]). Five-year recurrence rates calculated using survival analysis techniques to account for differential follow-up were 1.8% (95% CI, 0.4%–6.9%) after ED&C, 4.0% (95% CI, 2.2%–7.4%) after excision, and 2.6% (95% CI, 0.8%–7.7%) after Mohs surgery. Details about the individual tumors that recurred are listed in Tables 2, 3, and 4.
Table 2.
Tumors Treated With ED&C
| Tumor Characteristics | Care Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient Age, y |
Clinical Subtype or Descriptor |
Type | Additional Histologic Features |
Body Location |
Diameter, mm |
Training Level of Treating Clinician |
Cycles of ED&C, No. |
Size of Defect, mm |
Annual Visits to Dermatology Clinic, Mean No. |
Time After Treatment to Detection of Recurrence, mo |
| 76 | None specified | BCC | None described | Back | Not specified | Nurse practitioner | 3 | Not specified | 2.8 | 20 |
| 71 | Nodular | BCC | Superficial and nodular | Shoulder | 8 | Resident | 3 | 9 | 1.8 | 17 |
Abbreviations: BCC, basal cell carcinoma; ED&C, electrodessication and curettage; NMSC, nonmelanoma skin cancer.
Table 3.
Tumors Treated With Excision
| Tumor Characteristics | Care Characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient Age, y |
Clinical Subtype or Descriptor |
Type | Additional Histologic Features |
Body Location |
Diameter, mm |
Training Level of Treating Clinician |
Curettage Before Excision |
Size of Margins, mm |
Margins Clear of Tumor? |
Annual Visits to Dermatology Clinic, Mean No. |
Time After Treatment to Detection of Recurrence, mo |
| 77 | None specified | BCC | Infiltrative, keratinizing | Cheek, not in H-zone | 4 | Resident | Yes | Not specified | Yes | 1.9 | 30 |
| 82 | None specified | BCC | Nodular | Anterior mid-chest | 10 | Resident | Yes | 3 | Yes | 2.9 | 79 |
| 58 | Nodular | BCC | Superficial, nodular | Temple, H-zone | 4 | Resident | Not described | Not specified | Yes | 1.5 | 76 |
| 66a | Nodular | BCC | Superficial, nodular | Shoulder | 15 | Resident | Yes | 3 | Yes | 3.1 | 3 |
| 63 | Nodular | BCC | Nodular | Mid-back | 13 | Resident | Not described | Not specified | Yes | 2.6 | 51 |
| 77 | Nodular | BCC | Infiltrative, nodular | Temple, H-zone | Not specified | Resident | Yes | 3 | Yes | 2.4 | 17 |
| 72 | None specified | BCC | Nodular | Temple, H-zone | 6 | Resident | Yes | Not specified | Yes | 1.3 | 46 |
| 82 | None specified | BCC | Nodular, infiltrative | Back | 13 | Resident | Yes | Not specified | Yes | 3 | 9 |
| 72 | None specified | BCC | Superficial | Forehead, H-zone | 2 | Resident | Yes | Not specified | Yes | 1.7 | 51 |
| 83 | Papule | SCC | In situ | Ear helix, H-zone | 3 | Resident | Yes | 3 | Yes | 1.5 | 15 |
| 80 | None specifie | SCC | In situ | Forehead, H-zone | 14 | Resident | Yes | 3 | Yes | 3.3 | 62 |
| 54 | Indistinct margins | SCC | Invasive | Pubic area | 25 | Resident | Yes | 3 | Yes | 0.1 | 90 |
| 50 | Papule | SCC | In situ, acantholytic | Temple, H-zone | 8 | Resident | Yes | Not specified | Yes | 2.3 | 27 |
| Tumor That Possibly Recurred After Excision (No Histologic Confirmation) | |||||||||||
| 50 | None specified | SCC | Invasive | Anterior chest | 12 | Resident | Not described | Not specified | Yes | 2.5 | Examined at 104 mo |
Abbreviations: BCC, basal cell carcinoma; ED&C, electrodessication and curettage; NMSC, nonmelanoma skin cancer; SCC, squamous cell carcinoma.
Patient was described in the chart has having human immunodeficiency virus/AIDS.
Table 4.
Tumors Treated With Mohs Surgery
| Patient Age, y |
Clinical Subtype or Descriptor |
Type | Additional Histologic Features |
Body Location | Diameter, mm |
Training Level of Treating Clinician |
Stages, No. |
Defect Size Before Repairs, mm |
Annual Visits to Dermatology Clinic, Mean No. |
Time After Treatment to Detection of Recurrence, mo |
|---|---|---|---|---|---|---|---|---|---|---|
| 62 | Nodular | BCC | Nodular | Lower eyelid, H-zone | 8 | Attending | 2 | 13 | 2.0 | 55 |
| 70 | Indistinct margins | BCC | Superficial, nodular | Medial canthus, H-zone | 7 | Attending | 3 | 24 | 2.6 | 84 |
| 73 | Nodular | BCC | None specified | Nasal ala, H-zone | 10 | Attending | 2 | 18 | 0.5 | 50 |
| 63 | Nodular | BCC | Superficial, nodular | Lower eyelid, H-zone | 16 | Attending | 2 | 34 | 0.9 | 92 |
| 65 | None specified | SCC | None specified | Nasal sidewall, H-zone | 8 | Attending | 3 | 10 | 1.4 | 105 |
| 64a | None specified | SCC | in situ | Frontal scalp, H-zone | Not specified | Attending | 1 | 11 | 1.3 | 59 |
Abbreviations: BCC, basal cell carcinoma; ED&C, electrodessication and curettage; NMSC, nonmelanoma skin cancer; SCC, squamous cell carcinoma.
Patient was described in the chart has having human immunodeficiency virus/AIDS.
Visits to the Dermatology Clinic
The median number of annual visits to the dermatology clinic for all patients was 1.7 (IQR, 2.2) (ED&C, 1.7; excision, 1.8; and Mohs surgery, 1.4). Within each treatment group, there was no significant difference in annual visit rates for patients whose tumors recurred and those whose tumors did not recur.
Validation by Patient Examinations
A total of 249 patients with 301 tumors were alive at the time of recruitment for examination of the tumor treatment site; 127 patients (51%) with 152 tumors (50%) consented to have examinations. Patients who consented were similar at baseline to those who did not consent in all patient and tumor variables except that those who consented had somewhat better mental health status at baseline (Mental Component Scores of the SF-12 were 50.3 and 47.1, respectively) (P = .06). Evidence of possible tumor recurrence was found in 21 tumor sites (14%). Based on subsequent chart review, 4 of the possibly recurrent tumors were determined to be recurrent; 16 were determined not to be recurrent; and for 1 tumor, insufficient records existed to make a determination of recurrence. For this tumor, the likelihood of recurrence was judged by the examiner to be 41% to 60%. The tumor with possible recurrence but for which insufficient records existed to make a determination of recurrence is also included in Table 3.
Time Course of Recurrence
The median time of detection of recurrence was 4.2 years (IQR, 4.7 years). Recurrent tumors were detected earliest after ED&C and latest after Mohs surgery (median times of detection of recurrence after ED&C, excision, and Mohs surgery were 1.5, 3.8, and 6.0 years, respectively) (P = .04). The Figure contains Kaplan-Meier curves depicting the cumulative incidence of recurrence in the years following the 3 therapies.
Figure.
Time to detection of recurrence of nonmelanoma skin cancer according to treatment. ED&C indicates electrodessication and curettage.
Recurrence in Clinical Subgroups
Most tumor recurrences (71%) were BCCs (3.4% of all BCCs recurred; 3.6% of all SCCs recurred) (P = .53). The mean size of tumors that did or did not recur was similar (11.5 mm vs 11.7 mm, P = .96). Tumors in the H-zone of the face were somewhat more likely to recur than those not in the H-zone (4.9% vs 2.4%) (P = .08). Although overall only 7 patients had human immunodeficiency virus (HIV)/AIDS, 2 of the recurrent tumors occurred in these patients (1 BCC and 1 SCC). Table 5 summarizes recurrence rates within each treatment group in clinical subgroups that did or did not have conventionally accepted risk factors for recurrence.9
Table 5.
Tumor Recurrence Rates in Patients and Tumors With Potential Risk Factors for Recurrencea
| Recurrence Rate, % (No. of Recurrent Tumors/ No. of Tumors) |
||
|---|---|---|
| Characteristicb | Characteristic Not Present |
Characteristic Present |
| Tumors Treated With ED&C (n=127) | ||
| Patient immunocompromisedc | 1.6 (2/124) | 0 (0/3) |
| Squamous cell carcinoma | 1.8 (2/110) | 0 (0/17) |
| Tumor diameter > 10 mmd | 2.2 (1/46) | 0 (0/21) |
| Tumor location in H-zone of the faced,e | 1.9 (2/103) | 0 (0/22) |
| Histologic risk factor for recurrencef | 1.6 (2/125) | 0 (0/2) |
| Tumors Treated With Excision (n=309) | ||
| Patient immunocompromisedc | 4.0 (12/300) | 11.1 (1/9) |
| Squamous cell carcinoma | 4.4 (9/204) | 3.8 (4/105) |
| Tumor diameter > 10 mmd | 4.8 (7/145) | 4.0 (5/126) |
| Tumor location in H-zone of the faced,e | 3.1 (6/197) | 6.4 (7/110) |
| Histologic risk factor for recurrencef | 4.5 (11/245) | 3.1 (2/64) |
| Tumors Treated With Mohs Surgery (n=172) | ||
| Patient immunocompromisedc | 3.1 (5/163) | 11.1 (1/9) |
| Squamous cell carcinoma | 3.1 (4/129) | 4.7 (2/43) |
| Tumor diameter > 10 mmd | 3.2 (4/127) | 2.4 (1/41) |
| Tumor location in H-zone of the faced,e | 0 (0/36) | 4.4 (6/136) |
| Histologic risk factor for recurrencef | 4.3 (6/141) | 0 (0/31) |
Abbreviation: ED&C, electrodessication and curettage.
Adapted from National Comprehensive Cancer Network guidelines.9
For each characteristic, P > .20.
Described in the medical record as “immunocompromised” or as having received an organ transplant or as having human immunodeficiency virus infection/AIDS.
Numbers of patients and/or tumors for which data on the following characteristics were missing, 103, diameter; 4, H-zone.
Facial areas regarded to be at higher risk for tumor recurrence.8
For basal cell carcinomas, histopathologic reading described perineural involvement or morpheaform, sclerosing, mixed infiltrative, or micronodular features. For squamous cell carcinomas, histopathologic reading described perineural involvement, poor differentiation, Clark level IV or V, or adenoid, adenosquamous, or desmoplastic features.
Recurrence After ED&C
Two BCCs recurred after ED&C (Table 2). Information about size and histologic features was available for only 1 of these tumors, which was a superficial and nodular tumor 8 mm in diameter. Both recurrences after ED&C were detected within the second year after treatment.
Recurrence After Excision
Of the 309 tumors treated with excision, data on surgical margins were available for 148 tumors (48%). The mean (SD) margin size was 3.8 (2.8) mm. The difference in margin size between tumors that recurred (3.2 [0.4] mm) and those that did not recur (3.8 [2.8] mm) was not significant (P = .58). In the entire sample of excised tumors, histopathologic examination revealed that tumor cells remained at the margins of the excised specimen for 14 tumors (4.5%). Of these 14 tumors, 2 were retreated with ED&C; 5 were retreated with additional excisions; 4 were retreated with Mohs surgery; and for 3, no information was available about subsequent treatment. None of the recurrences occurred after an incomplete excision.
Nine BCCs and 4 SCCs recurred after excision (Table 3). The original diameters of most of the recurrent tumors (12 of 13) were less than 20 mm (mean diameter, 9.3 mm; median diameter, 8.0 mm), and most (7 of 13) were in the H-zone of the face. Most recurrent BCCs (7 of 9) had nodular histopathologic features; most recurrent SCCs (3 of 4) were histopathologically in situ. The median time of detection of recurrence after excision was 2.5 years (IQR, 2.8 years).
Recurrence After Mohs Surgery
Four BCCs and 2 SCCs recurred after Mohs surgery (Table 4). The diameters of the primary tumors that recurred were 16 mm or smaller (mean diameter, 9.1 mm; median diameter, 7.0 mm), and all were in the H-zone of the face. Three of the recurrent BCCs had nodular histopathologic features. The median time of detection of recurrence after Mohs surgery was 6.0 years (IQR, 4.2 years).
COMMENT
In this prospective cohort study of primary NMSCs treated with ED&C, excision, or Mohs surgery and followed up for a median of 6.6 years, the overall tumor recurrence rate was 3.5%. Rates of recurrence after ED&C, excision, and Mohs surgery were 1.6%, 4.2%, and 3.5%, respectively.
These results fill a gap in current knowledge about treatment of NMSC. Previous data on NMSC recurrence after treatment are inadequate for several reasons. First, tumor recurrence is typically a long-term outcome, requiring collection of data for at least 5 years after treatment.10 Many previous studies (over 25% of studies in a systematic review of treatments for BCC11) have reported follow-up data for less than 5 years. Second, in the United States, data on cutaneous BCCs and SCCs are not collected in standard population-based tumor registries, and collection of follow-up data requires direct review of medical records rather than tabulations from electronic databases. Third, NMSC is typically treated in the outpatient setting, so review of ambulatory care records, which can be difficult to access, is essential. Finally, automated data sets are usually inadequate for determination of recurrence of a given tumor because only brief and inadequate information is typically available about tumor location and recurrence status. Thus, precise determination of recurrence requires explicit a priori definitions of outcome and thorough medical record review by clinically trained staff who have access not only to dermatopathology records but also clinicians’ progress notes for at least 5 years after treatment. These requirements undoubtedly contribute to the widely acknowledged inadequacy of data about outcomes of NMSC treatment.1
The present study satisfies many of these requirements. We determined recurrence in a large consecutive cohort with long-term follow-up and for which a strict definition of recurrence was established before data collection by specialty trained clinicians. The follow-up rate was very high, in part because the ambulatory clinical records were accessible and complete.
The recurrence rate after ED&C, 1.6%, was lower than expected (a structured review reported recurrence rates after ED&C of 5.7%–18.8%11). This difference may be related to the small number of tumors treated with ED&C (n = 127) and the fact that this treatment was typically used in lower-risk tumors.
The recurrence rate after excision, 4.2%, was similar to what was expected.11,12 The average margin size was 3.8 mm, however, and some authors have proposed that lower recurrence rates may be attainable with larger margins.13,14
The recurrence rate after Mohs surgery, 3.5%, was higher than expected. The structured review reported rates after Mohs surgery of 0.6% to 1.7%,11 and recurrence of facial BCCs after Mohs surgery in a randomized controlled trial was 2.5%.12 Our findings may not be significantly different, or they may indicate that the treatment was used in higher-risk tumors in our sample.
Tumor recurrence rates were not significantly higher in clinical subgroups with patient or tumor characteristics that are conventionally regarded as risk factors for recurrence.9 These findings may be related to an inadequate sample size to detect any differences, particularly given the low recurrence rate overall. Tumors in the H-zone of the face, however, were somewhat more likely to recur (P = .08). Also, few patients in the sample had histories of organ transplantation or were otherwise immunocompromised, but 2 of the 7 tumors in HIV-infected patients recurred. Although the small numbers limit the conclusions we can draw from this observation, HIV infection as a risk factor for skin cancer recurrence needs further study.15,16
Some patients may have received follow-up dermatologic care outside the VA system. This possibility seems unlikely, though, since the median time between the last visit at which the patient’s skin was examined by a dermatologic clinician and his last visit to the hospital was only 6 months, indicating that most patients continued to receive follow-up dermatologic care at the VA.
This description of recurrence outcomes after treatments for NMSC must be interpreted with care. The study was conducted at a VA medical center with a limited number of providers in a single city, so the results may not be generalizable to other settings. Also, the small number of outcomes limits the precision of estimates of recurrence risk and the power of the study to detect differences in recurrence after the individual therapies. Absolute differences in recurrent rates were 2.6% (95% CI, −0.5% to 5.7%) after excision or ED&C, 1.9% (95% CI, −1.6% to 5.4%) after Mohs or ED&C, and 0.7% (95% CI, −2.8% to 4.3%) after Mohs or excision. The sample of 481 tumors undergoing Mohs or excision had 80% power to detect a difference in recurrence rates of 7%, with a 2-sided P value of .05. Comparisons in recurrence rates among the 3 groups are limited not only by this relatively low power to detect differences but also by confounding by indication because the treatment groups differed substantially in risk factors for recurrence.
The results of this study document the long-term risk of tumor recurrence in a consecutive cohort of patients with primary NMSC who were treated with the most common therapies and followed up prospectively. Overall, recurrence rates were low, particularly after ED&C.
Acknowledgments
Funding/Support: This work was supported by Investigator-Initiated Research Grant IIR 04-043-3 and the San Francisco Research Enhancement Award Program (REAP) of the Health Services Research and Development Service of the Department of Veterans Affairs, and by grants K24 AR052667 and R01 AR 054983 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr Chren had full access to all data and takes responsibility for its integrity and the accuracy of the analyses. Study concept and design: Chren. Acquisition of data: Chren, Torres, Stuart, and Labrador. Analysis and interpretation of data: Chren, Bertenthal, and Boscardin. Drafting of manuscript: Chren. Critical revision of manuscript for important intellectual content: Chren, Torres, Stuart, Bertenthal, Labrador, and Boscardin. Statistical analysis: Chren, Bertenthal, and Boscardin. Obtained funding: Chren. Administrative, technical, material support: Chren, Torres, Stuart, Bertenthal, and Boscardin. Study supervision: Chren, Torres, and Boscardin.
Financial Disclosure: None reported.
REFERENCES
- 1.Bath-Hextall F, Bong J, Perkins W, Williams H. Interventions for basal cell carcinoma of the skin: systematic review. BMJ. 2004;329(7468):705. doi: 10.1136/bmj.38219.515266.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chren MM, Sahay AP, Sands LP, et al. Variation in care for nonmelanoma skin cancer in a private practice and a veterans affairs clinic. Med Care. 2004;42(10):1019–1026. doi: 10.1097/00005650-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Do D. Mohs micrographic surgery for basal cell carcinoma of the face. Arch Dermatol. 2009;145(12):1428–1430. doi: 10.1001/archdermatol.2009.315. [DOI] [PubMed] [Google Scholar]
- 4.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei PP, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2007;127(6):1351–1357. doi: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
- 8.Swanson NA, Grekin RC, Baker SR. Mohs surgery: techniques, indications, and applications in head and neck surgery. Head Neck Surg. 1983;6(2):683–692. doi: 10.1002/hed.2890060209. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: basal cell and squamous cell skin cancers [registration required] [Accessed May 18, 2010]; http://www.nccn.org/index.asp.
- 10.Smeets NW, Kuijpers DI, Nelemans P, et al. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face—results of a retrospective study and review of the literature. Br J Dermatol. 2004;151(1):141–147. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- 11.Thissen MR, Neumann MH, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135(10):1177–1183. doi: 10.1001/archderm.135.10.1177. [DOI] [PubMed] [Google Scholar]
- 12.Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9(12):1149–1156. doi: 10.1016/S1470-2045(08)70260-2. [DOI] [PubMed] [Google Scholar]
- 13.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375(9715):673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 14.Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123(3):340–344. [PubMed] [Google Scholar]
- 15.Nguyen P, Vin-Christian K, Ming ME, Berger T. Aggressive squamous cell carcinomas in persons infected with the human immunodeficiency virus. Arch Dermatol. 2002;138(6):758–763. doi: 10.1001/archderm.138.6.758. [DOI] [PubMed] [Google Scholar]
- 16.Otley CC. Immunosuppression and skin cancer: pathogenetic insights, therapeutic challenges, and opportunities for innovation. Arch Dermatol. 2002;138(6):827–828. doi: 10.1001/archderm.138.6.827. [DOI] [PubMed] [Google Scholar]