Abstract
With the epidemic of childhood obesity, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease in pediatrics. NAFLD is strongly associated with insulin resistance, and increased level of serum free fatty acids (FFA)s. FFAs have direct hepatotoxicity through induction of an endoplasmic reticulum (ER) stress response and subsequently activation of the mitochondrial pathway of cell death. FFAs may also result in lysosomal dysfunction and alter death receptor gene expression. Lipoapoptosis is a key pathogenic process in NAFLD, and correlates with progressive inflammation, and fibrosis. Accumulation of triglyceride in the liver results from uptake and esterification of FFAs by the hepatocyte, and is less likely to be hepatotoxic per-se. To date, there are no proven effective therapies that halt NAFLD progression, or unfortunately improve prognosis in children. The cellular mechanisms of lipotoxicity are complex but provide potential therapeutic targets for NAFLD. In this review we discuss several potential therapeutic opportunities in detail including inhibition of apoptosis, c-Jun-N-terminal kinase (JNK), and endoplasmic reticulum (ER) stress pathways.
Keywords: endoplasmic reticulum (ER) stress, lipoapoptosis, nonalcoholic steatohepatitis (NASH), nonalcoholic fatty liver disease (NAFLD), free fatty acids (FFA)s, C/EBP-homologous protein (CHOP), c-Jun-N-terminal kinase (JNK), Bcl-2 homology 3 (BH3)-only protein.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has emerged as a growing public health problem linked to the increased incidence of childhood obesity. It is currently the most common cause of chronic liver disease in pediatrics. A population based autopsy study reported that 13% of children and adolescents are affected with NAFLD; 23% of the subjects with NAFLD had evidence for steatohepatitis, whereas bridging fibrosis or cirrhosis was observed in 9% of the children with NASH. Overweight and obese children accounted for 81% of all of the cases of NAFLD [1]. A cross-sectional study of liver biopsies obtained during gastric bypass surgery performed in morbidly obese adolescents, also reported an increased prevalence of NAFLD in obese children; while 83% of the subjects had NAFLD, histopathological evidence of NASH was present in 20% of subjects, and portal inflammation and fibrosis were prevalent in some patients despite not meeting the diagnostic criteria for NASH [2]. Long-term follow-up studies in adults with NASH suggest that the disease is usually slowly progressive but can ultimately lead to cirrhosis in a subset of patients [3-5] with its sequelae of portal hypertension, end-stage liver disease, hepatocellular carcinoma and an increase in overall mortality [5, 6] . Many patients with “cryptogenic” cirrhosis occurring in adulthood have, in fact, had NASH since childhood [7].
The impact of NAFLD and it is associated metabolic derangement during the active period of growth in childhood is substantial. In a series from Toronto, liver biopsies from pediatric patients with NAFLD displayed steatohepatitis in 88% and fibrosis in 71% of patients; a 10 year old patient had already progressed to cirrhosis [8]. Recent multicentric pediatric studies of patients with biopsy proven NAFLD revealed the presence of fibrosis in 87% of subjects [9, 10]. Worsening of hepatic fibrosis was reported within a relatively short period in pediatric patients with NASH, raising the concern of a more aggressive natural history of NASH in childhood [11]. Children with NAFLD may develop end-stage liver disease with the consequent need for liver transplantation in adulthood. These children with serious liver disease may have a significantly shorter survival as compared to the general population [12] . Thus, hepato-lipotoxicity in children is a genuine public health concern.
In the context of the metabolic syndrome and obesity, insulin resistance plays an important role in the pathogenesis of NAFLD [13]. Insulin resistance results in impaired suppression of lipolysis in adipose tissue and increased levels of circulating non-esterified or free fatty acids (FFA)s [14]. FFAs are taken up by the liver where they are esterified into neutral triglycerides (TG)s. However, an excess of saturated FFAs overwhelms the capacity of the liver to esterify FFA, and induces lipotoxicity. Although the amount of fat in the liver is often considered as an index of disease severity, it is unlikely the neutral triglycerides are lipotoxic. Rather the magnitude of neutral triglycerides in the liver likely serves as a biomarker for the flux of FFAs delivered to the liver. This is in accordance with the finding that the majority of obese children with NAFLD have simple steatosis without features of NASH such as inflammation and/or fibrosis [2]. Indeed, disease severity is directly proportional to the level of circulating FFA [15].
Lipotoxicity is known to promote hepatocyte death, which in the context of NAFLD is termed lipoapoptosis [16, 17]. The severity of NAFLD correlates with the degree of hepatocyte lipoapoptosis [18], and is reflected by an increase of serum caspase-cleaved cytokeratin–fragments [19]. Recent studies have elucidated the cellular and molecular mechanisms of FFA-mediated liver injury in NASH; these studies will be discussed in detail, together with potential mechanism-based therapeutic interventions for NASH.
HEPATIC LIPID METABOLISM IN NAFLD
Free Fatty Acids
Circulating FFAs derived from adipose tissue lipolysis play a critical role in the development of steatosis in NAFLD, contributing to 60% of hepatic fat content [20, 21]. De novo lipogenesis is the second most important mechanism, responsible for up to 30% of stored hepatic fat [20, 22] while dietary lipid contributes only up to 10% of the hepatic lipid content [20]. Decrease in the export of triglycerides (TG)s in the form of very low-density lipoproteins (VLDL)s, and decreased oxidation of fatty acids, mainly at the level of the mitochondria, may play a role in selected cases with genetic susceptibility [23]. FFAs levels are increased in patients with NASH and correlate with disease severity [15]. There is an increased body of evidence indicating that saturated FFAs are more hepatotoxic than unsaturated FFAs [24-26]. In vitro studies indicate that the mono-unsaturated FFAs palmitoleate (PO) (a 16 carbon FFA with 1 carbon-carbon double bonds, indicated C16:1), and oleate (C18:1) are less toxic than saturated FFAs such as palmitate (PA) (C16:0) and stearate (C18:0) [25-27]. Moreover, PO was recently identified as an adipose tissue-derived lipid hormone (lipokine) that enhances muscle insulin sensitivity and suppresses hepatic steatosis [28]. This difference in toxicity between saturated and unsaturated FFA is believed to be related to the ability of unsaturated FFA to more readily be esterified into neutral triglycerides (TG)s than saturated FFAs [27, 29, 30]. Thus these data together suggest that hepatic esterification maybe a detoxification process for FFA, and not a toxic event itself.
Human observations further suggest that impaired cellular capacity to incorporate toxic FFAs into neutral TG maybe a potential mechanism of liver injury in NASH [31]. For example, total hepatic lipid content is greater in patients with simple steatosis when compared to patients with more advanced NAFLD, indicating that the presence of fat in the liver is either an innocent bystander or decreases as the disease progresses [32]. A recent multicentric pediatric study exploring the relation between characteristics of hepatic steatosis and other histological features of NAFLD did not associate the severity of steatosis with any other histological feature of disease severity such as lobular inflammation, ballooning, or fibrosis [9]. The lack of association between hepatic TG accumulation and insulin resistance has been previously demonstrated in human subjects with familial hypobetalipoproteinemia. Subjects with this disorder have high levels of hepatic TG because of their genetic defect in hepatic TG export. In these patients, hepatic and muscle insulin sensitivity was similar to controls matched for body mass index with normal hepatic TG levels [33].
Considerable experimental data in animals also suggests that saturated FFAs are hepatotoxic, while unsaturated FFAs are not injurious to the liver. In an experimental animal study where leptin receptor-deficient mice (a genetic model of obesity and steatosis) were fed methionine and choline-deficient (MCD) diet, hepatic steatosis, apoptosis, reactive oxygen species (ROS) production, and fibrosis increased inversely to TG accumulation. Genetic deletion of diacylglycerol acyltransferase (DGAT)2, an enzyme responsible for intracellular free fatty acid esterification, prevents cellular steatosis, but accentuates FFA cytotoxicity through increased oxidative stress, hepatocellular apoptosis, and resultant fibrosis [34, 35]. Mice with hepatic overexpression of DGAT2 developed marked hepatic steatosis, without liver injury, hepatic or systemic insulin resistance [35]. Likewise, mice overexpressing DGAT1 in both macrophages and adipocytes were prone to obesity, but were protected against systemic inflammation and insulin resistance [36]. In addition, genetic or pharmacological inhibition of stearoyl-CoA desaturase-1 (SCD1), the enzyme that converts saturated FFA to monounsaturated FFA, sensitizes hepatocytes to apoptosis induced by saturated FFA. SCD1 knock-out mice fed a MCD diet accumulate less TG compared to wild-type mice, but have increased serum saturated FFA, hepatocellular apoptosis and liver injury [30]. In contrast, high-oleate MCD-diet fed animals have less severe apoptosis and liver injury in comparison with the animals fed a regular MCD diet, supporting the fact that monounsaturated fatty acids are preferentially incorporated into TG [30]. Also, incorporation of palmitic acid into TG by co-treatment with oleic acid or by overexpression of SCD1 attenuates saturated FFA toxicity [29, 34]. Collectively these data strongly implicate an increase in serum saturated FFA as a hepatotoxic stimulus, while suggesting their esterification into TG as a detoxification process.
FFA transporters
Fatty acids in the circulation cross the plasma membrane by diffusion or with the assistance of fatty acid transport proteins (FATP) [37] or fatty acid uptake transporters (e.g., CD36) [38]. Modification of the expression of these fatty acid transporters influences TG accumulation in the hepatocyte. FATP5 is exclusively expressed by hepatocytes and aids in the uptake of long chain FFAs. FATP5 knockout animals are less likely to accumulate dietary lipid in the hepatocyte [39]. Mice with liver-specific FATP2 knockdown have significantly reduced hepatic steatosis despite continued high-fat feeding, and improved fasting glucose, and insulin levels [40]. Likewise, liver-fatty acid binding protein (L-Fabp) (an abundant cytosolic lipid-binding protein expressed in mammalian hepatocytes) plays an important role in fat deposition in the liver. Indeed genetic deletion of L-Fabp in mice results in decreased VLDL secretion and TG deposition in the liver [41]. FATP5, CD36 and L-Fabp hepatic expression is increased in the livers of patients with early stage NAFLD, and correlates with liver fat content [42, 43]. These data emphasize the concept that hepatic TG accumulation is not due to de novo hepatic lipogenesis, but rather FFAs flux to the liver.
Palmitoleate (PO) as a lipokine
A study by Caoe et al. suggested PO as a lipokine (adipocyte-derived lipid hormones). They utilized quantitative lipidemic analyses in mice deficient in adipose tissue lipid chaperones. PO is virtually the only fatty acid derived from adipocytes that could significantly alter serum FFA composition. Also, PO has a low basal level that fluctuates rapidly under normal physiological conditions reflecting de novo lipogenesis. In parallel with a variety of adipokines (e.g., leptin and adiponectin), PO improves peripheral insulin resistance and suppresses hepatic steatosis, supporting its role as a systemic metabolic regulator [28]. These animal data are observational and the role of PO in human NASH requires further verification and study.
Role of genes in steatosis
There is an increased body of literature demonstrating the influence of genes in the development and severity of hepatic steatosis in NAFLD. The first genome-wide association study in NAFLD patients identified adiponutrin/patatin-like phospholipase domain containing 3 gene (PNPLA3) as a key inherited determinant of liver triglyceride accumulation. A single variant in PNPLA3 (rs738409 C/G) was strongly associated with hepatic fat content [44]. A recent metaanalysis showed that PNPLA3 (rs738409) act as a strong modifier of the natural history of NAFLD in different populations. NASH and higher serum ALT were more frequently observed in GG homozygous compared to CC homozygous. GG homozygous exerted a strong influence not only on liver fat accumulation but also on the susceptibility for a more aggressive disease [45]. In pediatric patients, the high-risk PNPLA3 (rs738409) G allele is associated with an earlier presentation of disease [46]. Moreover, PNPLA3 (rs738409) G allele was shown to confer susceptibility to hepatic steatosis in obese youths without increasing the level of hepatic and peripheral insulin resistance [47]. In addition to PNPLA3, a recently published study revealed that single-nucleotide polymorphisms (SNPs) in the apolipoprotein C3 gene (APOC3), resulting in high expression of APOC3, is also an important mechanism related to increase susceptibility to triglyceride accumulation and subsequently steatosis in NAFLD by impairing the clearance of diet-derived triglyceride-rich particles [48].
Adipose tissue as a key player in hepatic steatosis
There is an increased body of evidence suggesting that the interaction between peripheral tissues, mainly adipose tissue, and the liver play an important role in the development of NAFLD. During the development of obesity, expansion of adipose tissue results in activation of the death receptor and mitochondrial pathways of apoptosis in adipose tissue. Increase in adipocytes death results in recruitment of macrophages to adipose tissue, with subsequent development of insulin resistance, and hepatic steatosis [23]. Sabio and colleagues demonstrated that, selective knockdown of adipose tissue c-Jun N-terminal kinase (JNK)-1 in mice fed a high fat diet was protective against the development of hepatic insulin resistance and steatosis [49]. Adipose tissues of obese mice and human display a proapoptotic phenotype as evidenced by a marked pro-apoptotic gene expression, such as Fas (a key death receptor belonging to the tumor necrosis factor (TNF) - receptor family), and its specific ligand (FasL) [50]. Several publications have linked Fas activation in adipose tissue to the metabolic dysregulation of obesity [50, 51]. Thus, blocking adipocyte apoptosis by inhibiting Fas-mediated pathways may be a potential therapeutic option for the treatment of obesity-associated metabolic complications including NAFLD.
LIPOAPOTOSIS
Apoptosis or programmed cell death is a morphologic and pathogenic hallmark of NASH [18, 52], which in the context of NAFLD, and secondary to its association with excess lipid deposition, is referred to as lipoapoptosis. In patients with NAFLD, the magnitude of hepatocyte apoptosis correlates with liver injury [18, 52]. For example, lipoapoptosis correlates with aspartate aminotransferase/alanine aminotransferase ratio higher than one and hepatic fibrosis [18].
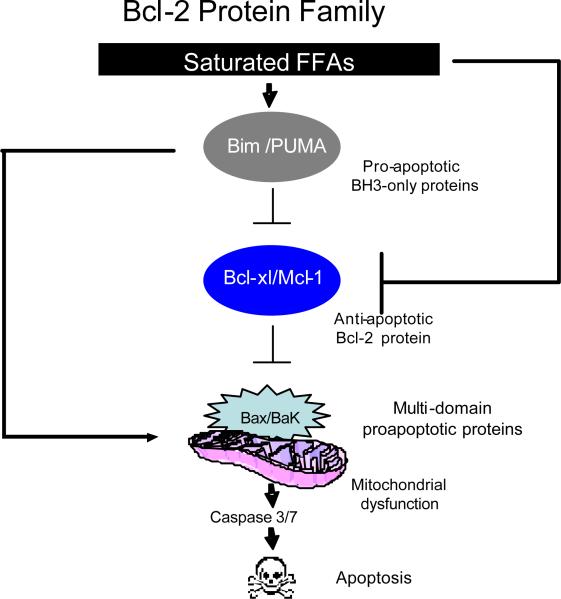
Apoptosis is regulated by members of the Bcl-2 protein family at the level of the mitochondria [53]. These proteins can be classified into three categories as follows: the guardians or anti-apoptotic members of this family, which include Mcl-1, and Bcl-xL; the multi-domain executioners or proapoptotic members of this family, that include Bax and Bak; and the messengers or biosensors of cell death, referred to as BH3-only proteins that include Bim and Puma (Figure 1). Hepatocytes can undergo apoptosis via either an extrinsic or intrinsic pathway of cell death. The extrinsic pathway is activated by death ligands, Fas and TNF related apoptosis inducing ligand (TRAIL). The intrinsic pathway is the apoptotic pathway activated by intracellular stress of membrane-bound organelles, such as ER, lysosomes, and mitochondria [54]. In hepatocytes, both the intrinsic and extrinsic pathways converge onto the mitochondria, hence, the critical role of Bcl-2 protein in hepatic injury.
Figure 1. Bcl-2 Protein Family.
Saturated FFAs activate the BH3-only proteins (BIM and PUMA), resulting in inactivation of the antiapoptotic Bcl-2 family members (Mcl-1 and Bcl-xL), releasing Bax and Bak from the inhibitory effect of the antiapoptotic Bcl-2 proteins, and causing their activation. Bim and PUMA can also activate Bax and/or Bak directly. Once activated, Bax and Bak promote mitochondrial dysfunction, leading to the activation of the caspase cascade and apoptosis.
Endoplasmic reticulum (ER) stress in obesity
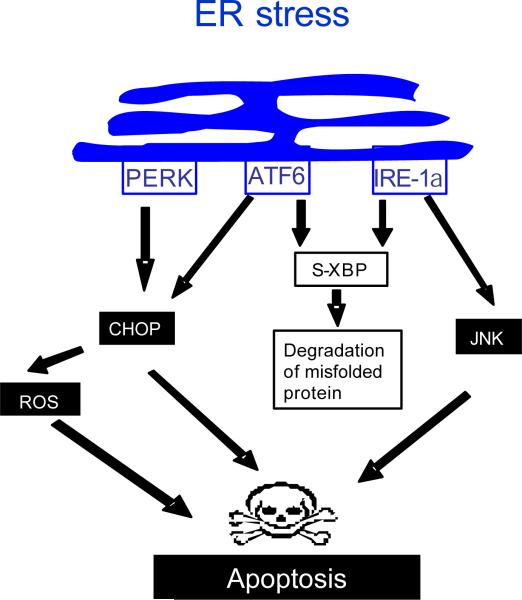
The ER is an intracellular membranous organelle that is responsible for many vital cellular functions including protein synthesis, and glycosylation, lipid synthesis, carbohydrate metabolism, calcium homeostasis, and drug detoxification. In conditions of ER stress, adaptive functions are activated by membrane sensors, which collectively initiate the unfolded protein response [55, 56]. The unfolded protein response serves to overcome the stress stimulus; however, with prolonged ER stress, apoptotic pathways are activated, causing cell demise [57]. Biosensors of ER stress include: protein kinase RNA-like ER kinase (PERK), inositol-requiring protein-1a (IRE-1a), and activating transcription factor 6 (ATF6). PERK dimerization drives the expression of the proapoptotic transcription factor C/EBP-homologous protein (CHOP) [58, 59]. IRE-1a activation leads to JNK activation and creates a spliced form of XBP-1 mRNA which promotes degradation of misfolded ER glycoproteins [60, 61]. ATF6 optimizes protein folding during ER stress and ultimately facilitates recovery from acute stress [62]. ATF6 also heterodimerizes with XBP1 and induces the ER-associated degradation components [63]. ATF6 can also transcriptionally induce CHOP [57] (Figure 2).
Figure 2. ER stress.
In the setting of ER stress the three trans-membrane biosensors PERK, ATF6, and IRE1-a are activated. PERK activation induces the expression of the proapoptotic transcription factor CHOP. CHOP in turn, mediates apoptosis through several pathways including generation of ROS. IRE1-a activates JNK a key player in apoptosis. IRE1-a also generates a spliced form of XBP (s-XBP) that promotes degradation of misfolded proteins. ATF6 contributes in CHOP induction, and heterodimerizes with XBP, enhancing protein degradation.
Ozcan et al have shown that ER stress is increased in obesity. They investigated the expression of several markers of ER stress in dietary and genetic models of murine obesity. In these models, ER stress-induced JNK activation mediates insulin resistance through phosphorylation of insulin receptor substrate 1 (IRS-1) [64]. In a nutritional murine model of steatohepatitis, ER stress-induced XBP-1 mRNA splicing and CHOP expression, correlates with the severity of apoptosis [65]. In liver samples from patients with NAFLD, PERK is strongly activated. JNK activity is also increased, particularly in patients with NASH and correlates with the degree of apoptosis [66]. ER stress markers are elevated in the obese state when assessed on liver biopsies of patients undergoing bariatric surgery and decline following weight loss [67]. Thus ER stress appears to be a major injurious pathway in NAFLD.
There is increased evidence that the composition of fatty acids in the steatotic liver, rather than the steatosis, is the important factor in inducing ER stress. Mice with impaired ability to synthesize mono-unsaturated fatty acids due to a deletion of SCD1 exhibited marked induction of ER stress by enhanced splicing of XBP1, and increased expression of the stress-induced transcription factors CHOP in their livers [68]. Rats fed a diet enriched with saturated fatty acids exhibited stronger activation of ER stress markers and liver injury in comparison with rat fed normal or polyunsaturated fatty acid (PUFA) enriched diet [65]. Several in vitro studies demonstrate that ER stress is the mediator of saturated FFA-induced apoptosis [25-27]. Unsaturated fatty acids do not induce ER stress or apoptosis, and co-incubation with monounsaturated fatty acid rescues hepatocyte from saturated free fatty acid-induced ER stress and apoptosis [25-27]. Saturated FFAs also contribute to ER stress through depleting ER-calcium stores in liver cells [26].
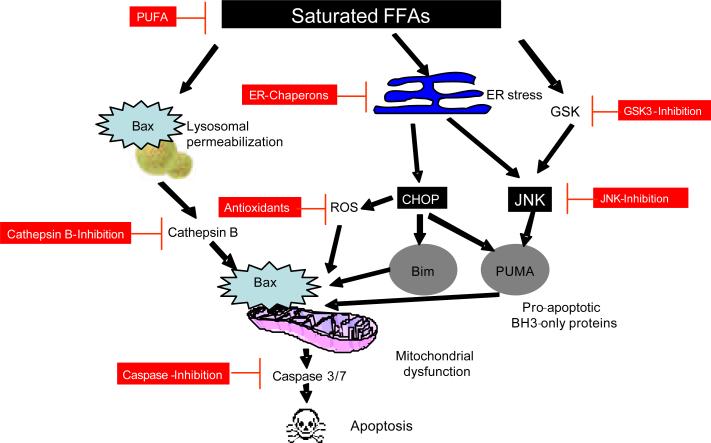
As will be discussed in greater detail later, ER stress-associated JNK and CHOP activation promotes apoptosis by enhancing expression and function of pro-apoptotic members of the Bcl-2 family, PUMA [69] and Bim [70, 71]. JNK activation stimulates formation of the transcription factor complex, activator protein (AP)-1 via phosphorylation of c-Jun. AP-1 facilitates PUMA expression by saturated FFA, in cooperation with the ER stress related transcrip tion factor CHOP [27]. FFA mediated BIM, and PUMA induction results in activation of the multi-domain proapoptotic member of Bcl-2 family Bax [24, 69], causing mitochondrial dysfunction, activation of the caspase cascade and subsequent cell death [72] (Figure 3).
Figure 3. Integrated model of lipoapotosis by saturated FFAs and potential antiapoptotic agents.
Saturated FFAs induce ER stress which in turn activates JNK and CHOP. JNK leads to the upregulation of the pro-apoptotic BH3-only proteins PUMA. CHOP enhances the expression of the proapoptotic BH3-only protein Bim, contributes to PUMA upregulation and mediates the generation of ROS. Bim in cooperation with PUMA induces the activation of the multi-domain executioner proapoptotic protein Bax. Bax activation results in mitochondrial dysfunction, activation of the caspase cascade, and cell death. Saturated FFAs also cause Bax dependent lysosomal permeabilization and release of cathepsin B into the cytosol, cathepsin B mediates down stream mitochondrial permeabilization and apoptosis. Therapeutic strategies to prevent cell death in the setting of saturated FFA-induced apoptosis include as outlined: PUFA, ER Chaperons, GSK3-inhibition, JNK-inhibition, antioxidants, cathepsin B-inhibition, and caspase-inhibition.
Controversies regarding CHOP in NASH
CHOP is an inducible leucine zipper transcription factor that is present at low levels under normal conditions but is strongly expressed in response to ER stress [73]. CHOP plays an important role in ER stress-induced apoptosis through activating both the intrinsic and the extrinsic pathways of apoptosis. Several in vitro studies have demonstrated CHOP induction in response to FFA-induced ER stress in liver cells [27, 65], although the mechanism by which CHOP promotes apoptosis remains incompletely understood. CHOP was found to induce apoptosis, in part, through enhancing expression of TRAIL receptor 2 or death receptor 5 (DR5), a member of the TNF-receptor gene superfamily [74]. Upregulation of DR5 promotes apoptosis by the extrinsic cellular pathway of cell death [75]. FFAs upregulate DR5 expression in hepatocyte in vitro and silencing DR5 expression protects against FFAs-mediated apoptosis (unpublished observation). DR5 expression is also increased in human NASH [76], as well as animal models of NASH [77]. In addition, CHOP-induced apoptosis involves generation of ROS [57].
Despite all the data implicating CHOP in ER stress associated lipotoxicity, the role of CHOP in NAFLD remains complex. Genetic ablation of CHOP only reduces hepatocyte toxicity in vitro at high doses of FFA [27, 78, 79]. More importantly, genetic deletion of CHOP does not reduce, and even accentuates NAFLD in nutritional models of this disease in mice [78, 79]. The mechanism responsible for this paradoxical observation is unknown. However, perhaps CHOP deletion accentuates responses in cells other than hepatocyte (e.g., Kupffer cells, adipose tissue macrophages, etc.), which aggravates liver injury. A hepatocyte conditional CHOP knockout will need to be developed to better understand and perhaps resolve this controversy.
C-Jun N-terminal kinase-1 (JNK)
C-Jun N-terminal kinase-1 (JNK) belongs to a family of intracellular mitogen activated protein (MAP) kinases; of three known JNK genes, JNK1 and JNK2 are expressed in the liver [80]. JNK activation is pivotal in both the metabolic syndrome accompanying NAFLD and cellular apoptosis. JNK is activated in experimental murine dietary and genetic models of NASH [81-83], and also in human NASH [66, 69]. In mice models of genetic and dietary obesity ER stress-induced JNK activation phosphorylates IRS-1, suppressing insulin receptor signaling, and inducing insulin resistance [64]. Both JNK1 and JNK2 have been implicated in insulin resistance, although JNK1 is more strongly associated with steatohepatitis [82, 83]. In a mouse model of obesity, absence of JNK1 results in decreased adiposity, and significant improvement of insulin sensitivity [81]. Liver specific knockdown of JNK1 in obese mice lowers blood glucose and insulin levels, but interestingly, increases TG level [84]. JNK1 but not JNK2, phosphorylates c-Jun, a member of the AP-1transcription factor complex, which induces expression of the BH3-only protein PUMA [69, 85]. Genetic deletion of JNK1 prevents FFA-mediated c-Jun activation and PUMA induction by FFA.[69]
JNK phosphorylates and activates the BH3-only proteins Bim and BAD and the proapoptotic Bcl-2 proteins Bax, which directly triggers the mitochondrial apoptotic pathway [24, 85, 86]. Finally it has been shown that JNK induces Fas and DR5 expression, sensitizing steatotic hepatocyte to circulating Fas or TRAIL mediated toxicity [54, 76]. Thus, JNK activation appears to play a major role in the metabolic syndrome and lipotoxicity.
BH3 only proapoptotic proteins
Proapoptotic BH3 only proteins, especially Bim and PUMA, are key regulators of lipoapoptosis. Saturated FFA treated hepatocytes have increased Bim and PUMA expression [69, 70]. Saturated FFAs induce protein phosphatase 2A (PP2A) activation by the transcription factor FoxO3a, a member of the forkhead box- containing proteins, class O. This transcription factor drives expression of the intracellular death mediator Bim [70]. PP2A also increases Bim protein levels through rendering it refractory to proteasomal degradation [71]. Interestingly, PP2A is known to be activated through ER stress [71, 87]. This observation links ER stress to Bim induction and potent apoptosis inducing proteins.
PUMA induction is JNK1/AP-1-dependent [69]. Genetic deletion of PUMA conveys resistance against PA induced apoptosis in vitro. PUMA expression is increased in liver biopsies from patients with NASH when compared to patients with simple steatosis or controls [69]. FFA mediated BIM, and PUMA induction results in activation of the multi-domain proapoptotic member of Bcl-2 family, Bax, initiating a mitochondrial pathway of cell death, with activation of caspase 3, 6, 7, resulting ultimately in apoptosis [24, 69] . Moreover, Bax expression is also increased in a nutritional murine model of steatohepatitis [88], and structural and functional mitochondrial abnormality are observed in human NASH [10, 14, 18].
Antiapoptotic Bcl-2 proteins
Mcl-1 is an anti-apoptotic member of the Bcl-2 family. Saturated FFA induces a rapid degradation of Mcl-1 in hepatocytes by a proteasome-dependent pathway involving protein kinase C; inhibition of Mcl-1 degradation attenuates saturated FFA-induced apoptosis [89]. Bcl-xL is another member of the anti-apoptotic Bcl-2 family. Bcl-xL expression is reduced in a murine nutritional model of NASH [77]. Overexpression of Bcl-xL blocks Bax-induced lysosomal permeabilization and attenuates saturated FFA induced-apoptosis in vitro [90]. Furthermore, PUMA co-immuno-precipitates with Bcl-xL and Mcl-1, causing their inactivation, enhancing indirectly Bax activity [91].
Lysosomal pathway
Lysosomes are membrane bound organelles; with an acidic intravesicular pH. Lysosomes contain hydrolytic enzymes that are active at an acid to neutral pH. Cathepsin B is a cysteine protease whose endoproteolytic activity is maintained at neutral pH. Cathepsin B can be released into the cytosol with lysosomal permeabilization, where it mediates down stream mitochondrial permeabilization and caspase activation [92]. Treatment of hepatocytes with FFA may activate the lysosomal pathway of apoptosis through Bax induced lysosomal permeabilization, and release of cathepsin B into the cytosol. Cytosolic cathepsin B precipitates mitochondrial dysfunction and cell death [90, 93]. Likewise, release of cathepsin B into the cytoplasm is observed in human liver specimens with NAFLD and correlates with disease severity [93]. In a dietary murine model of NAFLD, inactivation of cathepsin B protects against development of hepatic steatosis, liver injury, and insulin resistance [93]. Furthermore lysosomal permeabilization results in nuclear factor ?B (NF-?B) activation, and TNF-a release; TNF-a in turn promotes lysosomal destabilization leading to a feed forward mechanism for lipotoxicity [93].
Ceramides
Ceramide is the main lipid in the metabolism of sphingolipids; ceramides have been recognized as an important factor in cell stress and death ligand-induced hepatocellular death [94, 95], and also in insulin resistance and obesity [95, 96]. Ceramides are synthesized in the endoplasmic reticulum (ER) from sphingosine and a fatty acid moiety, usually palmitoyl CoA. Long chain saturated FFA availability is the rate-limiting step in ceramide synthesis; therefore, obesity can be associated with excess ceramide synthesis. In addition ceramide can also be rapidly generated from sphingomyelin by sphingomyelinase. The death ligands, tumor necrosis factor-alpha (TNF-a) and Fas can both activate sphingomyelinase leading to rapid accumulation of ceramide [97, 98]. Thus, ceramide is a recognized intermediate linking both excess nutrients (i.e. saturated FFA) and inflammatory cytokines (e.g. TNF-a) to insulin resistance. Ceramides also play a role in insulin resistance by inhibiting insulin-induced glucose uptake [95]. Despite the association of genes involved in ceramide signaling with liver fat content in patients with NAFLD [42], ER stress and apoptosis are independent of ceramide synthesis in an animal model of nutritional NASH [25]. Moreover, PA induced lysosomal permeabilization is ceramide independent [93], as well as, the induction of the proapoptotic Bcl-2 protein, Bim by FoxO3a [70]. Although, it is believed that ceramides may contribute to lipopaoptosis, perhaps via a Fas-induced signaling mechanism, liver ceramide content in patients with NAFLD is similar to normal controls [86]. Thus the potential role of ceramide in the pathogenesis of human NAFLD remains unclear and for the most part speculative.
Cholesterol
Free cholesterol (FC) has been recognized as a hepatotoxic agent, while cholesterol esters are either stored in lipid droplets in macrophages, or incorporated in apolipoprotein B-containing lipoproteins, such as chylomicrons in enterocytes and very-low-density lipoprotein (VLDL) in hepatocytes [99]. In an animal study designed to evaluate the role of dietary FC loading, rats fed a high cholesterol diet developed microvesicular steatosis and were sensitized to the apoptotic effect of TNF-a. Sensitization to TNF-a was secondary to reduced mitochondrial glutathione stores; repletion of mitochondrial glutathione rescued free cholesterol loaded liver from TNF-a induced steatohepatitis [100]. Although atherosclerotic macrophage-free cholesterol accumulation leads to calcium depletio n and ER stress induced apoptosis [101], free cholesterol loading of the ER in nutritional and genetic models of hepatic steatosis does not cause ER stress [100]. A progressive increase in hepatic free cholesterol from controls with normal histology to subjects with simple steatosis and NASH has been reported, together with an increase of serum total cholesterol; in contrast to FC, liver cholesterol ester contents in subjects and controls were comparable [32]. FC increases in human NASH, and correlates with SREBP-2 (Sterol regulatory element-binding protein-2) and StAR (steroidogenic acute regulatory protein) expression. SREBP-2 a transcription factor that plays an important role in cholesterol synthesis through hydroxymethylglutaryl-CoA (HMG-CoA) reductase, and StAR a mitochondrial-cholesterol transporting polypeptide, were overexpressed in patients with NASH compared to those with simple steatosis [102]. These findings suggest a role for mitochondrial FC in disease progression from steatosis to steatohepatitis; although the role of FC in FFA induced ER stress in hepatocyte requires further investigation. We note that there is an association between atherosclerotic cardiovascular disease and NAFLD in adults. Animal models of atherosclerosis may also manifest NAFLD [103, 104]. Given the mechanistic link between cholesterol and atherosclerosis, the role for FC in NAFLD remains an important area of investigation.
POTENTIAL THERAPEUTIC INTERVENTIONS
Weight loss, thiazolidinedione, and antioxidants are the most extensively evaluated therapeutic options for NAFLD. According to a recent meta-analysis of randomized controlled trial for treatment of NAFLD, none can be considered markedly effective. Weight loss improves histological activity in NASH but more than 50% of patients fail to achieve the target weight. Thiazolidinedione improves steatohepatitis markers, but leads to significant weight gain [105]. In the PIVENS trial, vitamin E improved histological and serum biomarkers of liver injury in adult patients with NASH. However, fibrosis (the ultimate goal of hepato-protective therapy) was not reduced, although the 2 year timeline of the study may not have been long enough to detect reversible fibrosis [106]. The recently published treatment of NAFLD in children (TONIC) trial revealed that neither vitamin E nor metformin were effective in maintaining a sustained reduction in ALT level in pediatric patients with NAFLD, although the resolution of NASH was significantly greater in children treated with vitamin E, fibrosis was not improved [107]. Clearly, there is unmet need for other therapies. As apoptosis is a key pathogenic mechanism in NAFLD [18], several antiapoptotic agents may serve as potential therapeutic targets (Figure 3).
Chemical Chaperones
If ER stress and protein misfolding are implicated in NAFLD pathogenesis, it is likely that improvement of protein folding may serve as a therapeutic strategy in NAFLD [108]. Chemical chaperones, such as glycerol and 4-phenyl butyric acid (PBA) represent a group of low molecular weight compounds that can stabilize protein conformation, improve ER folding capacity, and facilitate the appropriate trafficking of mutant proteins [108]. PBA is an orally administered agent that was developed for treatment of urea-cycle disorders [109]. It was also used in patients with homozygous ß-thalassemia without problematic side effects [110]. Bile acid derivatives, such as taurine-conjugated ursodeoxycholic acid (TUDCA), are effective in abolishing ER stress, and preventing apoptosis by blocking calcium-mediated apoptotic pathway and caspase-12 activation [111]. Treatment of obese and diabetic mice with PBA and TUCA compounds alleviates ER stress resulting in normalization of blood glucose level, restoration of systemic insulin sensitivity, and resolution of fatty liver disease [112]. Clinical trials in human NASH, however, did not show a benefit of ursodeoxycholic acid over placebo based on post treatment histological criteria [79, 105], although a recent study has shown that ursodeoxycholic acid is effective in improving hepatic and muscle insulin sensitivity in obese subjects [113]. PBA has not been evaluated in human NASH.
Poly-unsaturated fatty acids (PUFA)s
Unsaturated FFAs attenuate saturated FFA-induced ER stress and cell death in liver cells [25-27, 29]. Unsaturated FFAs protect against lipotoxicity by reducing CHOP induction, JNK activation, and upregulation of the proapoptotic BH3-only proteins (PUMA and Bim) [27]. Quantification of hepatic lipids by capillary gas chromatography showed decrease levels of n-3 PUFA eicosapentaenoic and docosahexaenoic acids in patients with NASH [32]; this deficiency may contribute to the progression of steatosis to steatohepatitis [114]. In a small pilot trial of NAFLD patients receiving eicosapentaenoic acid, post treatment liver biopsy showed improvement of hepatic steatosis, fibrosis, hepatocyte ballooning, and lobular inflammation [115]. Larger scale clinical trials are needed to confirm a potential therapeutic benefit of dietary supplementation with n-3 PUFAs in patients with NAFLD.
Protease inhibitors
Caspase activation and apoptosis are key pathogenic features of NAFLD [18]. In vitro studies have shown that the pan-caspase inhibitor Z-VAD-fmk is affective in attenuating saturated FFA induced apoptosis [24, 116]. Also, the pancaspase inhibitor (VX-166) reduces progression to fibrosis in a mouse model of nutritional NASH; treatment with VX-166 decreases active caspase-3, TUNEL-positive cells, triglyceride content, and fibrosis. However, ALT levels are similar in VX-166-treated mice and vehicle-treated controls [117]. Although, a phase 2 clinical trial was recently reported, accessing tolerability and efficacy of a new caspase inhibitor (GS9450) in adults with NASH [118], a longer trial was stopped due to drug toxicity. Other caspase inhibitors, however, should be tried in this disease.
Mitochondrial dysfunction is a key factor in the pathogenesis of NASH [14, 119, 120]. Saturated FFAs induce mitochondrial dysfunction through Bax translocation to the lysosome, lysosomal disruption and release of cathepsin B into the cytosol [93]. This process was attenuated in vitro by either pharmacological cathepsin B inhibition (by cathepsin inhibitors CA07 and E-64) or genetic inhibition by shRNA technology [121]. Likewise, in a dietary murine model of NAFLD, both genetic and pharmacological inactivation of cathepsin B, preserved mitochondrial function, decreased oxidative stress, and protected against development of hepatic steatosis, liver injury, and insulin resistance [93, 121]. Thus, inhibition of cathepsin B activity may present another potential therapeutic target for treating NAFLD.
Kinase inhibitors
Glycogen synthase kinase (GSK) a and ß are serine/threonine kinases [122, 123]. In an acute model of liver injury by acetaminophen, GSK-3ß mediates JNK activation [124]. In the context of saturated FFA-mediated lipoapotosis in vitro either pharmacological or genetic inhibition of GSK-3ß attenuates apoptosis, through inhibition of JNK activation, and subsequent PUMA induction [116]. Further preclinical data are encouraged regarding a role of GSK inhibition in this disease. Such agents are being developed for cancer therapy, and merit investigation in NASH.
JNK is a central mediator of the metabolic syndrome, through it is involvement in insulin resistance [82, 83]. It is also an important mediator of steatohepatitis [24, 69], as JNK1 promotes liver injury in a murine model of steatohepatitis, while JNK2 inhibits hepatocyte cell death by blocking the mitochondrial death pathway [82, 83]. Although no selective JNK inhibitors are available, nonselective JNK inhibition pharmacologically by SP600125 decreased lipoapotosis in vitro [24, 69, 125] by attenuating saturated FFA induced PUMA induction [69]. The role of JNK in the metabolic syndrome is complex, as it has effects in the hypothalamus, ß cells of the pancreas, adipocytes, inflammatory cells, and liver. The role of JNK in obesity has recently been reviewed [126]. Since, the tissue distribution and target function of pharmacological inhibitors are not uniform; it is still likely that a JNK inhibitor with efficient hepatocyte uptake could be beneficial in decreasing lipoapoptosis and NASH.
Oxidative stress and anti-oxidants
Oxidative stress, due to increased production of ROS and decreased antioxidant defense is observed in human and experimental models of steatohepatitis [14, 127, 128]. Yu et all investigated the role of heme oxygenase-1 (HO-1), an antioxidant defense enzyme in nutritional steatohepatitis, and showed that HO-1 can interrupt progression of steatohepatitis by up-regulation of antioxidant chaperones, enzymes, and anti-inflammatory interleukin-22 and down-regulation of proinflammatory cytokines in vitro and in vivo [129]. Induction of haem oxygenase with haemin, in a nutritional murine model of steatohepatitis down regulates fibrosing genes, ameliorates fibrosing hepatitis [130], and suppresses hepatocyte apoptosis [131]. In the PIVENS trial Vitamin E, a well known antioxidant, induced histological improvement in adult patients with NASH, but did not reverse fibrosis during the 2 year period of the study [106]. In a pediatric study, histological improvement of NASH provided by a combination of vitamin E, C, diet and exercise was not superior to diet and exercise alone [132]. The NASH Clinical Research Network (CRN) has recently completed the treatment of NAFLD in children (TONIC) randomized clinical trial using Vitamin E and metformin. The results of which revealed neither vitamin E nor metformin were superior to placebo in maintaining a sustained reduction of ALT over the 96 week period of the study. The resolution of NASH was significantly greater in children treated with vitamin E than with placebo; this was mainly attributable to significant improvement in hepatocellular ballooning by vitamin E treatment. Vitamin E treatment also significantly improved NAFLD activity score; however, vitamin E did not affect significantly steatosis, inflammation, or fibrosis. Patients treated with metformin had also significant reduction in ballooning but there was no significant improvement in NAFLD activity score and NASH resolution compared with placebo [107]. Clearly, the role of vitamin E as therapy for this disease requires further investigation.
CONCLUSION
NAFLD is today the most common pediatric liver disease in developed countries. To date, there is no therapy proven to be effective in reversing disease progression. NAFLD is associated with insulin resistance and increased level of circulating FFA which leads to deposition of fat mainly in the form of TG in the liver, causing steatosis. Excess FFA delivery to the liver, exceeds the coping mechanisms of the liver and induces ER stress. ER stress activates numerous signaling pathways, including CHOP and JNK dependent upregulation of proapoptotic BH3 only proteins leading to Bax activation lysosomal permeabilization, mitochondrial dysfunction, caspase activation and subsequent lipoapoptosis. Lipoapoptosis is a key player in the progression of steatosis into steatohepatitis. Understanding the molecular mechanism of lipotoxicity is of biomedical and public health importance for the development of new diagnostic markers, and targeted therapies to halt disease progression.
Acknowledgments
Grants: This work was supported by NIH Grants DK41876 to GJG, DK084310-01 to RK, the Mayo Foundation, and Cincinnati Children's Hospital Medical Center.
Footnotes
Disclosure: The authors have no potential conflict of interest in regards to this manuscript.
Abbreviations: activator protein-1 (AP-1), apolipoprotein C3 gene (APOC3), Bcl-2 homology 3 (BH3), Bcl-2-interacting mediator of cell death (Bim), C/EBP-homologous protein (CHOP), diacylglycerol acyltransferase (DGAT), death receptor 5 (DR5), elongation initiation factor 2a (eIF2a), endoplasmic reticulum (ER), free cholesterol (FC), free fatty acids (FFA), fatty acid transport proteins (FATP), glycogen synthase kinase (GSK), heme oxygenase-1 (HO-1), inositol-requiring protein-1a (IRE-1a), insulin receptor substrate 1 (IRS-1), c-Jun-N-terminal kinase (JNK), liver-fatty acid binding protein (L-Fabp), methionine and choline-deficient (MCD), nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), nuclear factor ?B (NF-?B), patatin-like phospholipase domain containing 3 gene (PNPLA3), palmitate (PA), 4-phenyl butyric acid (PBA), protein kinase RNA-like ER kinase (PERK), palmitoleate (PO), protein phosphatase 2A (PP2A), poly-unsaturated fatty acids (PUFA), p53-upregulated modulator of apoptosis (PUMA), reactive oxygen species (ROS), stearoyl CoA desaturase-1 (SCD1), short hairpin (sh), sterol regulatory element-binding protein-2 (SREBP-2), StAR (steroidogenic acute regulatory protein), triglyceride (TG), tumor necrosis factor-alpha (TNF-a), tumor necrosis factor related apoptosis inducing ligand (TRAIL), taurine-conjugated ursodeoxycholic acid (TUDCA), very low-density lipoproteins (VLDL)s.
REFERENCES
- 1.Schwimmer JB, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Xanthakos S, et al. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006;4(2):226–32. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 3.Powell EE, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 4.Propst A, et al. Prognosis in nonalcoholic steatohepatitis. Gastroenterology. 1995;108(5):1607. doi: 10.1016/0016-5085(95)90724-6. [DOI] [PubMed] [Google Scholar]
- 5.Adams LA, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Ekstedt M, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 7.Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA : the journal of the American Medical Association. 2003;289(22):3000–4. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 8.Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30(1):48–53. doi: 10.1097/00005176-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Carter-Kent C, et al. Relations of steatosis type, grade, and zonality to histological features in pediatric nonalcoholic Fatty liver disease. Journal of pediatric gastroenterology and nutrition. 2011;52(2):190–7. doi: 10.1097/MPG.0b013e3181fb47d3. [DOI] [PubMed] [Google Scholar]
- 10.Carter-Kent C, et al. Nonalcoholic Steatohepatitis in Children: A Multicenter Clinicopathological Study. Hepatology. 2009;50(4):1113–1120. doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohli R, et al. Rapid progression of NASH in childhood. Journal of pediatric gastroenterology and nutrition. 2010;50(4):453–6. doi: 10.1097/MPG.0b013e3181a9387b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldstein AE, et al. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58(11):1538–44. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. Journal of gastroenterology and hepatology. 2002;17(Suppl):S186–90. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 14.Sanyal AJ, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 15.Nehra V, et al. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Digestive diseases and sciences. 2001;46(11):2347–52. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- 16.Kusminski CM, et al. Diabetes and apoptosis: lipotoxicity. Apoptosis : an international journal on programmed cell death. 2009;14(12):1484–95. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 17.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144(12):5159–65. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 18.Feldstein AE, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–43. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 19.Feldstein AE, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–8. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnelly KL, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115(5):1343–51. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. The Journal of clinical investigation. 2004;114(2):147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. The Journal of clinical investigation. 2008;118(3):829–38. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldstein AE. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis. 2010;30(4):391–401. doi: 10.1055/s-0030-1267539. [DOI] [PubMed] [Google Scholar]
- 24.Malhi H, et al. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, et al. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. American journal of physiology. Endocrinology and metabolism. 2006;291(2):E275–81. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 26.Wei Y, et al. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Molecular and cellular biochemistry. 2009;331(1-2):31–40. doi: 10.1007/s11010-009-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akazawa Y, et al. Palmitoleate attenuates palmitate-induced Bim and PUMA up-regulation and hepatocyte lipoapoptosis. J Hepatol. 2010;52(4):586–93. doi: 10.1016/j.jhep.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao H, et al. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134(6):933–44. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Listenberger LL, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(6):3077–82. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li ZZ, et al. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284(9):5637–44. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta. 2010;1801(3):299–310. doi: 10.1016/j.bbalip.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Puri P, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 33.Amaro A, et al. Dissociation Between Intrahepatic Triglyceride Content and Insulin Resistance in Familial Hypobetalipoproteinemia. Gastroenterology. 2010;139(1):149–153. doi: 10.1053/j.gastro.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi K, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–74. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 35.Monetti M, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6(1):69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Koliwad SK, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. The Journal of clinical investigation. 2010;120(3):756–67. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pohl J, et al. Role of FATP in parenchymal cell fatty acid uptake. Biochimica et biophysica acta. 2004;1686(1-2):1–6. doi: 10.1016/j.bbalip.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 2008;134(2):556–67. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Doege H, et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130(4):1245–58. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Falcon A, et al. FATP2 is a hepatic fatty acid transporter and peroxisomal very long-chain acyl-CoA synthetase. American Journal of Physiology-Endocrinology and Metabolism. 2010;299(3):E384–E393. doi: 10.1152/ajpendo.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Newberry EP, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. The Journal of biological chemistry. 2003;278(51):51664–72. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 42.Greco D, et al. Gene expression in human NAFLD. American journal of physiology. Gastrointestinal and liver physiology. 2008;294(5):G1281–7. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 43.Charlton M, et al. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49(4):1375–84. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romeo S, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011 doi: 10.1002/hep.24283. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Rotman Y, et al. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52(3):894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santoro N, et al. A Common Variant in the Patatin-Like Phospholipase 3 Gene (PNPLA3) Is Associated with Fatty Liver Disease in Obese Children and Adolescents. Hepatology. 2010;52(4):1281–1290. doi: 10.1002/hep.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petersen KF, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. The New England journal of medicine. 2010;362(12):1082–9. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322(5907):1539–43. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alkhouri N, et al. Adipocyte Apoptosis, a Link between Obesity, Insulin Resistance, and Hepatic Steatosis. Journal of Biological Chemistry. 2010;285(5):3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wueest S, et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. Journal of Clinical Investigation. 2010;120(1):191–202. doi: 10.1172/JCI38388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ribeiro PS, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99(9):1708–17. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 53.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 54.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28(4):360–9. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature reviews. Molecular cell biology. 2007;8(7):519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 56.Ji C, Kaplowitz N. ER stress: Can the liver cope? Journal of hepatology. 2006;45(2):321–333. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nature cell biology. 2011;13(3):184–90. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplowitz N, et al. Endoplasmic reticulum stress and liver injury. Seminars in liver disease. 2007;27(4):367–77. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 59.Rahman SM, et al. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45(5):1108–17. doi: 10.1002/hep.21614. [DOI] [PubMed] [Google Scholar]
- 60.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends in cell biology. 2004;14(1):20–8. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. The Journal of clinical investigation. 2002;110(10):1389–98. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu J, et al. ATF6 alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Developmental Cell. 2007;13(3):351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto K, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Developmental cell. 2007;13(3):365–76. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 64.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 65.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–51. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 66.Puri P, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):568–76. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 67.Gregor MF, et al. Endoplasmic Reticulum Stress Is Reduced in Tissues of Obese Subjects After Weight Loss. Diabetes. 2009;58(3):693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flowers MT, et al. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiological Genomics. 2008;33(3):361–372. doi: 10.1152/physiolgenomics.00139.2007. [DOI] [PubMed] [Google Scholar]
- 69.Cazanave SC, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284(39):26591–602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barreyro FJ, et al. Transcriptional regulation of Bim by FoxO3A mediates hepatocyte lipoapoptosis. J Biol Chem. 2007;282(37):27141–54. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 71.Puthalakath H, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–49. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 72.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305(5684):626–9. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 73.Wang XZ, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Molecular and Cellular Biology. 1996;16(8):4273–80. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279(44):45495–502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 75.Guicciardi ME, Gores GJ. Life and death by death receptors. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23(6):1625–37. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malhi H, et al. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56(8):1124–31. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farrell GC, et al. Apoptosis in experimental NASH is associated with p53 activation and TRAIL receptor expression. Journal of gastroenterology and hepatology. 2009;24(3):443–52. doi: 10.1111/j.1440-1746.2009.05785.x. [DOI] [PubMed] [Google Scholar]
- 78.Soon RK, Jr., et al. Stress signaling in the methionine-choline-deficient model of murine fatty liver disease. Gastroenterology. 2010;139(5):1730–9. 1739, e1. doi: 10.1053/j.gastro.2010.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfaffenbach KT, et al. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. American journal of physiology. Endocrinology and metabolism. 2010;298(5):E1027–35. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochimica et biophysica acta. 2007;1773(8):1341–8. doi: 10.1016/j.bbamcr.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 82.Schattenberg JM, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43(1):163–72. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 83.Singh R, et al. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49(1):87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang R, et al. Liver-specific knockdown of JNK1 up-regulates proliferator-activated receptor gamma coactivator 1 beta and increases plasma triglyceride despite reduced glucose and insulin levels in diet-induced obese mice. The Journal of biological chemistry. 2007;282(31):22765–74. doi: 10.1074/jbc.M700790200. [DOI] [PubMed] [Google Scholar]
- 85.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. The Journal of biological chemistry. 2006;281(30):21256–65. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 86.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27(48):6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Christen V, et al. Activation of endoplasmic reticulum stress response by hepatitis viruses up-regulates protein phosphatase 2A. Hepatology. 2007;46(2):558–65. doi: 10.1002/hep.21611. [DOI] [PubMed] [Google Scholar]
- 88.Wang Y, et al. Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. The Journal of nutrition. 2008;138(10):1866–71. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Masuoka HC, et al. Mcl-1 degradation during hepatocyte lipoapoptosis. J Biol Chem. 2009;284(44):30039–48. doi: 10.1074/jbc.M109.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feldstein AE, et al. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. American journal of physiology. Gastrointestinal and liver physiology. 2006;290(6):G1339–46. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jabbour AM, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell death and differentiation. 2009;16(4):555–63. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]
- 92.Guicciardi ME, Leist M, Gores GJ. Lysosomes in cell death. Oncogene. 2004;23(16):2881–90. doi: 10.1038/sj.onc.1207512. [DOI] [PubMed] [Google Scholar]
- 93.Feldstein AE, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40(1):185–94. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 94.Mari M, Fernandez-Checa JC. Sphingolipid signalling and liver diseases. Liver international : official journal of the International Association for the Study of the Liver. 2007;27(4):440–50. doi: 10.1111/j.1478-3231.2007.01475.x. [DOI] [PubMed] [Google Scholar]
- 95.Summers SA. Ceramides in insulin resistance and lipotoxicity. Progress in lipid research. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 96.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell metabolism. 2007;5(3):167–79. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 97.Morales A, et al. Sphingolipids and cell death. Apoptosis : an international journal on programmed cell death. 2007;12(5):923–39. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 98.Paris F, et al. Natural ceramide reverses Fas resistance of acid sphingomyelinase(-/-) hepatocytes. The Journal of biological chemistry. 2001;276(11):8297–305. doi: 10.1074/jbc.M008732200. [DOI] [PubMed] [Google Scholar]
- 99.Ikonen E. Mechanisms for cellular cholesterol transport: defects and human disease. Physiological reviews. 2006;86(4):1237–61. doi: 10.1152/physrev.00022.2005. [DOI] [PubMed] [Google Scholar]
- 100.Mari M, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell metabolism. 2006;4(3):185–98. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 101.Feng B, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature cell biology. 2003;5(9):781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 102.Caballero F, et al. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. Journal of hepatology. 2009;50(4):789–96. doi: 10.1016/j.jhep.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 103.Wouters K, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48(2):474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 104.Wouters K, et al. Intrahepatic cholesterol influences progression, inhibition and reversal of non-alcoholic steatohepatitis in hyperlipidemic mice. FEBS letters. 2010;584(5):1001–5. doi: 10.1016/j.febslet.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 105.Musso G, et al. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79–104. doi: 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 106.Sanyal AJ, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lavine JE, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA : the journal of the American Medical Association. 2011;305(16):1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clark JM, Diehl AM. Defining nonalcoholic fatty liver disease: implications for epidemiologic studies. Gastroenterology. 2003;124(1):248–50. doi: 10.1053/gast.2003.50032. [DOI] [PubMed] [Google Scholar]
- 109.Maestri NE, et al. Long-term treatment of girls with ornithine transcarbamylase deficiency. The New England journal of medicine. 1996;335(12):855–9. doi: 10.1056/NEJM199609193351204. [DOI] [PubMed] [Google Scholar]
- 110.Collins AF, et al. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85(1):43–9. [PubMed] [Google Scholar]
- 111.Xie Q, et al. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36(3):592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 112.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kars M, et al. Tauroursodeoxycholic Acid May Improve Liver and Muscle but Not Adipose Tissue Insulin Sensitivity in Obese Men and Women. Diabetes. 2010;59(8):1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Videla LA, et al. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free radical biology & medicine. 2004;37(9):1499–507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 115.Tanaka N, et al. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol. 2008;42(4):413–8. doi: 10.1097/MCG.0b013e31815591aa. [DOI] [PubMed] [Google Scholar]
- 116.Ibrahim SH, et al. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol. 2011;54(4):765–72. doi: 10.1016/j.jhep.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Witek RP, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50(5):1421–30. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 118.Cazanave SC, Gores GJ. Mechanisms and clinical implications of hepatocyte lipoapoptosis. Clin Lipidol. 2010;5(1):71–85. doi: 10.2217/clp.09.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pessayre D, Mansouri A, Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2002;282(2):G193–9. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- 120.Serviddio G, et al. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol Aspects Med. 2008;29(1-2):22–35. doi: 10.1016/j.mam.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 121.Li Z, et al. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47(5):1495–503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maurer U, et al. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Molecular cell. 2006;21(6):749–60. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 123.Linseman DA, et al. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(44):9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shinohara M, et al. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. The Journal of biological chemistry. 2010;285(11):8244–55. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pagliassotti MJ, Wei Y, Wang D. Insulin protects liver cells from saturated fatty acid-induced apoptosis via inhibition of c-Jun NH2 terminal kinase activity. Endocrinology. 2007;148(7):3338–45. doi: 10.1210/en.2006-1710. [DOI] [PubMed] [Google Scholar]
- 126.Vallerie SN, Hotamisligil GS. The role of JNK proteins in metabolism. Sci Transl Med. 2010;2(60):60rv5. doi: 10.1126/scitranslmed.3001007. [DOI] [PubMed] [Google Scholar]
- 127.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99(8):1497–502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 128.Kohli R, et al. Mitochondrial reactive oxygen species signal hepatocyte steatosis by regulating the phosphatidylinositol 3-kinase cell survival pathway. The Journal of biological chemistry. 2007;282(29):21327–36. doi: 10.1074/jbc.M701759200. [DOI] [PubMed] [Google Scholar]
- 129.Yu J, et al. Heme oxygenase-1 protects against steatohepatitis in both cultured hepatocytes and mice. Gastroenterology. 2010;138(2):694–704. 704, e1. doi: 10.1053/j.gastro.2009.09.058. [DOI] [PubMed] [Google Scholar]
- 130.Wang RQ, et al. Induction of heme oxygenase-1 protects against nutritional fibrosing steatohepatitis in mice. Lipids Health Dis. 2011;10(1):31. doi: 10.1186/1476-511X-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nan Y, et al. Heme oxygenase-1 prevents non-alcoholic steatohepatitis through suppressing hepatocyte apoptosis in mice. Lipids Health Dis. 2010;9:124. doi: 10.1186/1476-511X-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nobili V, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48(1):119–28. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]