Summary
Mutations in both RAS and the PTEN/PIK3CA/AKT signaling module are found in the same human tumors. PIK3CA and AKT are downstream effectors of RAS, and the selective advantage conferred by mutation of two genes in the same pathway is unclear. Based on a comparative molecular analysis, we show that activated PIK3CA/AKT is a weaker inducer of senescence than is activated RAS. Moreover, concurrent activation of RAS and PIK3CA/AKT impairs RAS-induced senescence. In vivo, bypass of RAS-induced senescence by activated PIK3CA/AKT correlates with accelerated tumorigenesis. Thus, not all oncogenes are equally potent inducers of senescence and, paradoxically, a weak inducer of senescence (PIK3CA/AKT) can be dominant over a strong inducer of senescence (RAS). For tumor growth, one selective advantage of concurrent mutation of RAS and PTEN/PIK3CA/AKT is suppression of RAS-induced senescence. Evidence is presented that this new understanding can be exploited in rational development and targeted application of pro-senescence cancer therapies.
Introduction
Different human cancers frequently arise due to genetic and epigenetic alterations in the same relatively small number of cancer pathways. Commonly mutated pathways include the Receptor Tyrosine Kinase (RTK)-RAS-BRAF growth factor signaling pathway, and the ARF-MDM2-p53 and p16-cyclin D1-pRB tumor suppressor pathways (Yeang et al., 2008). Although these same pathways are commonly deregulated in different tumor types, the specific gene that is altered often varies between tumors. For example, approximately 70% of melanomas harbor mutations in BRAF, with most of the remainder containing mutations in N-RAS (Brose et al., 2002; Davies et al., 2002; Pollock and Meltzer, 2002). In most cases, mutations in N-RAS and BRAF are mutually exclusive, presumably because there is no selective advantage for a tumor cell to alter both genes, since they act in the same linear signaling pathway.
However, the genetics of human cancers is not always this simple. An important effector of RAS is the lipid kinase, PIK3CA, and its downstream effector, protein kinase AKT (hereafter referred to as the PIK3CA/AKT signaling module) (Shaw and Cantley, 2006). PIK3CA/AKT is also negatively regulated by the lipid phosphatase PTEN, which is itself frequently mutated in human cancers. Surprisingly, mutations in both RAS and the PTEN/PIK3CA/AKT signaling axis can be found in the same tumors. For example, Vogelstein and coworkers recently reported that approximately 24% of human colon cancers harbor mutations in both K-RAS and PIK3CA (Parsons et al., 2005). Mutations in RAS genes and PIK3CA also co-occur in endometrial and thyroid cancer and Acute Lymphoblastic Leukemia (ALL) (Yeang et al., 2008). Some pancreatic cancers contain K-RAS mutations and amplification of AKT2 (Tuveson and Hingorani, 2005). Since PIK3CA/AKT is an effector of RAS, the specific selective advantage conferred by simultaneous mutation of two genes in the same pathway is unclear. In this manuscript, we set out to understand the molecular basis of the selective advantage conferred by concurrent mutation of RAS and PIK3CA/AKT in human tumors.
Oncogene-induced cellular senescence (OIS) is a permanent cell growth arrest caused by an activated oncogene within a primary untransformed cell (Adams, 2009). Although oncogenes are best known for their ability to drive transformation, a single oncogene in a primary cell often activates senescence as a tumor suppression mechanism. Activation of senescence depends on the pRB and p53 tumor suppressor pathways. Many studies have demonstrated the role of OIS as an in vivo tumor suppression mechanism. For example, many benign neoplasms harboring activated oncogenes contain senescent cells (Braig et al., 2005; Chen et al., 2005; Collado et al., 2005; Courtois-Cox et al., 2006; Michaloglou et al., 2005). In a number of mouse models, inactivation of the senescence program allows progression of such benign precursor lesions to full-blown malignant cancers (Braig et al., 2005; Chen et al., 2005; Dankort et al., 2007; Ha et al., 2007; Sarkisian et al., 2007; Sun et al., 2007). Underscoring the ability of senescence to block tumor growth, its reactivation in murine tumors is associated with tumor regression (Ventura et al., 2007; Xue et al., 2007).
In addition to proliferation arrest, cell senescence is associated with many other phenotypes, and depends on activation of various signaling and effector pathways. In the nucleus of senescent cells, activated DNA damage signaling pathways, reflected in a focal distribution of DNA damage sensing proteins, γH2AX and 53BP1, are instrumental in driving senescence (d’Adda di Fagagna, 2008). Also, formation of specialized domains of facultative heterochromatin, called Senescence Associated Heterochromatin Foci (SAHF), is thought to silence proliferation promoting genes such as cyclin A2, thereby contributing to a more permanent cell cycle arrest (Narita et al., 2003). Formation of SAHF depends on a complex of histone chaperones, HIRA/UBN1/ASF1a (Banumathy et al., 2009; Zhang et al., 2005). In turn, function of this chaperone complex in senescent cells depends on phosphorylation of HIRA by GSK3β and recruitment of HIRA to a subnuclear organelle, the PML body (Ye et al., 2007). Notably, GSK3β has also been shown to be an important inducer of senescence in other contexts (Kortlever et al., 2006; Liu et al., 2008; Zmijewski and Jope, 2004).
Senescent cells also upregulate autophagy (Gamerdinger et al., 2009; Young et al., 2009), an organelle recycling process, and this might contribute to remodeling of senescent cells and provide the raw materials for altered biosynthetic processes. Prominently, senescent cells show a marked change in their secretory program (Coppe et al., 2008). Upregulated genes whose products are secreted from senescent cells include cytokines and chemokines, such as IL6 and IL8, as well as extracellular proteases, such as Matrix MetalloProteinases (MMPs) (Acosta et al., 2008; Kuilman et al., 2008; Xue et al., 2007). Secretion of these extracellular signaling molecules, collectively referred to as the senescence secretome, may facilitate clearance of senescent cells by the immune system, and so limit tumor growth.
Given the apparent potency of OIS in tumor suppression, it is not surprising that many oncogenes have been reported to induce OIS. However, previous studies do not present a clear picture regarding the ability of activated PIK3CA/AKT to induce senescence (see Discussion). In this study, by profiling the full spectrum of phenotypes that constitute the senescent state, we show that activation of the PIK3CA/AKT pathway is a poor inducer of senescence, compared to activated RAS. This manifests as an inefficient proliferation arrest, a deficient senescence secretome, weak DNA damage signaling and autophagy and no detectable SAHF. Remarkably, we find that, when both pathways are activated, the senescence-impaired PIK3CA/AKT phenotype is in some respects dominant over RAS-induced senescence. The dominance of PIK3CA/AKT depends on the ability of this pathway to intersect and counteract downstream effectors of RAS-induced senescence, such as GSK3β and likely mTOR. The significance of GSK3β in human cancer is underscored by the demonstration that a high level of phosphorylated GSK3β is a predictor of poor survival in human pancreatic cancer. In a mouse model of pancreatic carcinogenesis, genetic inactivation of PTEN, an inhibitor of PIK3CA/AKT, leads to bypass of RAS-induced proliferation arrest (with features of senescence) and accelerated formation of pancreatic ductal adenocarcinoma (PDAC). Together, these results indicate that activation of the PIK3CA/AKT pathway cooperates with activation of RAS in tumorigenesis through its ability to suppress RAS-induced senescence.
Results
Activation of PIK3CA/AKT fails to induce a robust senescence program
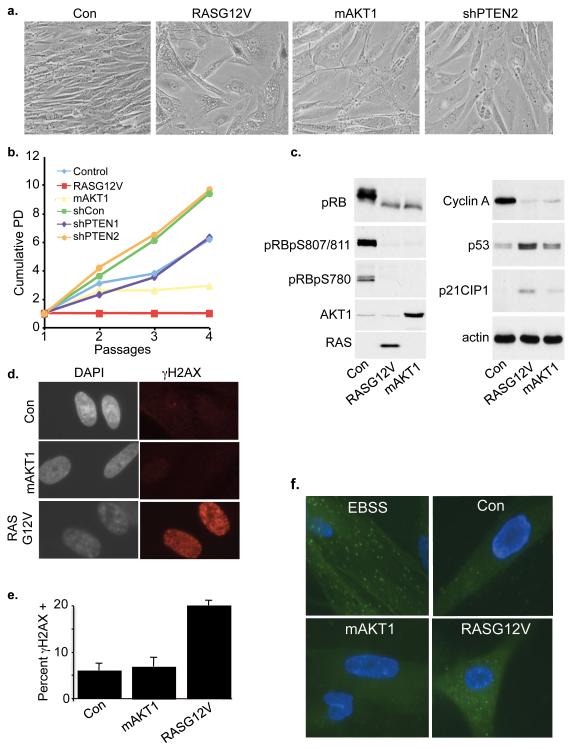
We set out to compare the spectrum of senescence phenotypes induced by activated RAS and PIK3CA/AKT. Human BJ fibroblasts immortalized with hTERT (BJ-hTERT) were infected with a control retrovirus or viruses encoding activated H-RAS (RASG12V) or activated myristoylated AKT1 (mAKT1), or an shRNA (shPTEN) to knock down the PIK3CA pathway inhibitor, PTEN. As expected, cells infected with activated RAS assumed a flattened vacuolated morphology, characteristic of senescence induced by this oncogene (Figure 1a). Compared to RASG12V-infected cells, mAKT1 and shPTEN-transduced fibroblasts were less vacuolated, but did become larger and flatter. However, activated AKT1 and shPTEN were both weaker inducers of proliferation arrest (Figure 1b and Supplementary Figure 1a). Consistent with this, cells expressing mAKT1 expressed reduced amounts of cyclin A, and exhibited some biochemical changes consistent with senescence, such as dephosphorylation of pRB and upregulation of p53 and p21CIP1 (Figure 1c and Supplementary Figure 1b). But, mAKT1 tended to be less efficient in these respects than RASG12V (Figure 1c and Supplementary Figure 1b), and after passaging at least a proportion of mAKT1-expressing cells did resume growth (data not shown). Similarly, shPTEN failed to arrest colony outgrowth after infection and drug selection (Supplementary Figure 1c). In line with these observations, only activated RAS upregulated expression of p16INK4a, an activator of the p16-cyclin D1-pRB tumor suppressor pathway and key effector of senescence-associated proliferation arrest (Supplementary Figure 1d). Our results suggest that perturbation of this pathway can induce some features of senescence, but is markedly less potent in this regard than is activated RAS.
Figure 1. Inactivation of PTEN and activation of AKT1 fails to induce robust growth arrest.
(a) BJ-hTERT fibroblasts were transduced with either control, mAKT1, RasG12V retroviruses or a lentivirus encoding a short hairpin to PTEN. Cells were drug selected and bright field images taken 7 days later. (b) Growth curves of cells from (a). (c) IMR90 fibroblasts were transduced with control, mAKT1 or RASG12V. Cells were drug selected for 7 days, lysates prepared and western blotted. (d) Cells from (c) were fixed and stained for γH2AX. (e) Percent cells from (d) containing at least 20 foci of γH2AX. Mean of 3 experiments with standard deviation. (f) IMR90 cells were co-transduced with EGFP-LC3 and control, RASG12V or mAKT1. As a positive control for autophagosome formation, EGFP-LC3 expressing cells were treated with Earle’s Balanced Salt Solution (EBSS) for 1 hour.
In light of these provocative differences between activated RAS and PIK3CA/AKT, we investigated the status of other molecular markers of senescence in mAKT1 and RASG12V-transduced cells. Induction of senescence by activated RAS has been shown previously to depend on RAS-induced hyper-replication or unscheduled DNA synthesis, and subsequent DNA damage (d’Adda di Fagagna, 2008). We monitored oncogene-induced DNA damage in mAKT1 and RASG12V-transduced cells by examining two commonly used markers of DNA damage, γH2AX and 53BP1. Cells transduced with RASG12V, as expected, had an increase in DNA damage over control cells. However, transduction of activated AKT1 did not lead to an increase in DNA damage, as judged by either γH2AX or 53BP1 (Figure 1d, e and Supplementary Figure 1e, f). When we examined levels of γH2AX by western blotting, we observed consistent results (Supplementary Figure 1g). Thus, analysis of DNA damage signals support the notion that activated AKT1, compared to RASG12V, does not induce the full senescence program.
In RASG12V-infected cells, induction of autophagy is also important for onset of senescence (Gamerdinger et al., 2009; Young et al., 2009). To compare autophagy in RASG12V and mAKT1- infected cells, we introduced either oncogene together with GFP-LC3, a fluorescent fusion protein that is incorporated into autophagosomes (Klionsky et al., 2008). As shown previously, activated RAS induced formation of autophagosomes, reflected in a punctate distribution of GFP-LC3 in the cytoplasm (Figure 1f). However, by this measure, activated AKT1 failed to induce autophagy. These results also support the notion that, compared to activated RAS, activated AKT1 does not induce a robust senescence program.
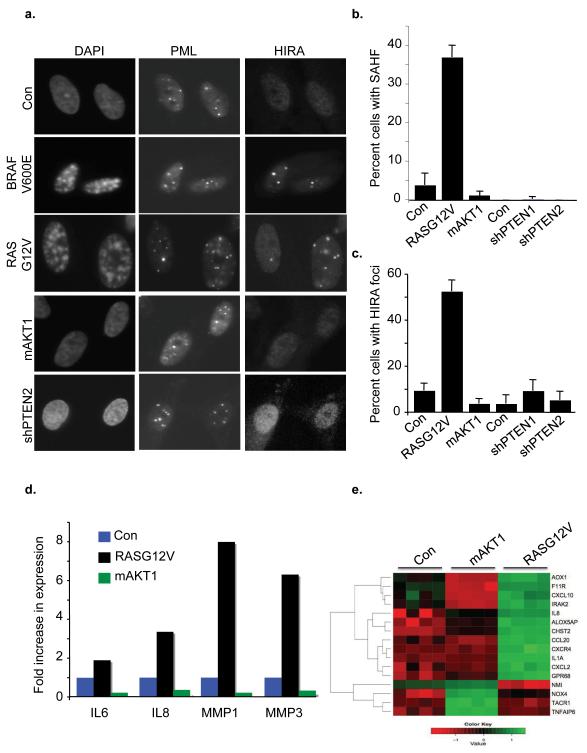
Next, we compared the ability of activated RAS, AKT and shPTEN to induce senescence-associated chromatin changes, manifest as SAHF and recruitment of the HIRA histone chaperone to PML bodies (Narita et al., 2003; Zhang et al., 2005). SAHF can be visualized by conventional epifluorescence microscopy as punctate domains of DAPI-stained chromatin that stain with specific heterochromatin proteins, such as histone variant macroH2A. We observed characteristic macroH2A-containing SAHF in cells transduced with activated RAS (and an activated mutant of one of its effectors, BRAF (BRAFV600E)), but not in activated AKT1- or shPTEN-transduced cells (Figure 2a-b and Supplementary Figure 2). Consistent with this, activated RAS and BRAF also triggered HIRA’s relocalization to PML bodies, whereas activated AKT1 did not (Figure 2a, c). Rather, activated AKT1-infected cells were much like control, lacking both HIRA foci and SAHF. Finally, we compared induction of the senescence secretome by activated RAS and AKT1, by quantitative PCR. Activated RAS robustly increased expression of IL6, IL8, MMP1 and MMP8, as expected. However, activated AKT1 was unable to achieve this (Figure 2d). To confirm and extend these findings, we performed a gene expression microarray of cells infected with activated RAS, activated AKT1 or control. Gene Ontology (GO) classification of genes induced by RASG12V compared to control showed that the top-ranked GO term was “Inflammation”. Specific genes in this group upregulated by RASG12V included IL8, CXCL2 and IL1α. This GO group as a whole was not significantly altered by mAKT1, and, typically, individual genes in this group were not upregulated by this oncogene (Figure 2e). In sum, by several measures, namely proliferation arrest, DNA damage signaling, autophagy, activation of HIRA and formation of SAHF and upregulation of the secretome, activated AKT1 fails to induce a senescence program as robust as that induced by activated RAS.
Figure 2. AKT activation fails to induce SAHF or the senescence-secretome.
(a) IMR90 fibroblasts were transduced with BRAFV600E, RASG12V, mAKT1 or a short hairpin that targets PTEN. Cells were drug selected, fixed and stained for SAHF, PML or HIRA foci. (b) and (c) One hundred cells from (a) were scored for HIRA foci or SAHF. Mean of 3 experiments with standard deviation. (d) RNA was harvested from mAKT1, RASG12V or control cells and assayed for expression of IL-6, IL-8, MMP-1 and MMP-3 by quantitative RT-PCR. (e) Expression profiling was performed on control, RASG12V or mAKT1 transduced IMR90. Heatmap of significantly upregulated (green) and downregulated (red) genes with GO classification “Inflammation” is shown.
Activated AKT antagonizes RAS-induced senescence
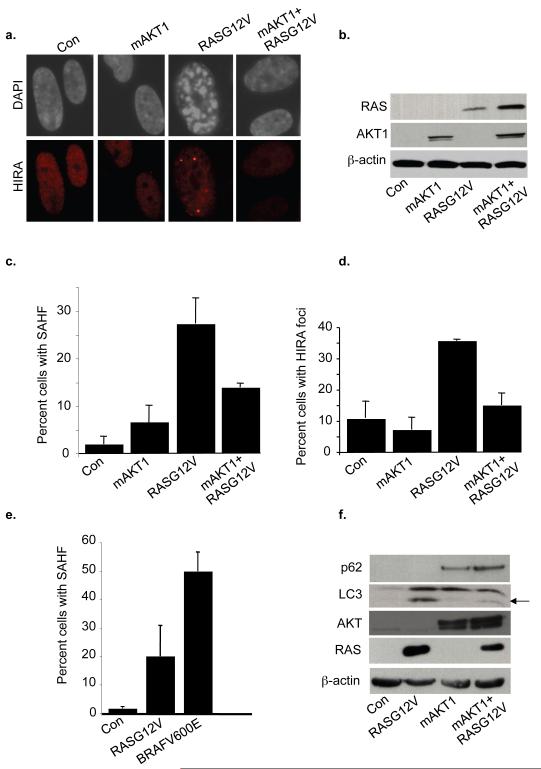
Knowing that some human tumors contain mutations in both RAS and the PTEN/PIK3CA/AKT axis (Parsons et al., 2005; Tuveson and Hingorani, 2005; Yeang et al., 2008), we wanted to know whether the senescence program of cells containing activated RAS and AKT was more or less robust than cells containing activated RAS alone. To do this, we transduced IMR90 fibroblasts with each oncogene alone, or both activated AKT and RAS together, and scored markers of senescence. First, we asked whether activated AKT1 is able to suppress RASG12V-induced upregulation of p16INK4a. As shown previously (Supplementary Figure 1d), activated RAS caused upregulation of p16INK4a, whereas activated mAKT1 did not. Coinfection of RASG12V and mAKT1 showed that activated AKT1 suppressed RASG12V-induced upregulation of p16INK4a (Supplementary Figure 3a). Next, we looked at recruitment of HIRA to PML bodies and formation of SAHF. Compared to RASG12V alone, co-expression of activated AKT and RAS decreased both SAHF formation and HIRA foci (Figure 3a-d). Activated RAS and AKT were both efficiently expressed in all infections (Figure 3b). Significantly, we also observed that activated BRAF is a more potent inducer of SAHF than is activated RAS (Figure 3e). This is consistent with the ability of RAS, but not BRAF, to activate AKT1 (Supplementary Figure 3b) (Shaw and Cantley, 2006), which in turn is able to antagonize SAHF formation. Finally, we examined indicators of autophagy in single or double oncogene-infected cells. Consistent with activated RAS-induced upregulation of autophagy described previously and demonstrated in Figure 1f, activated RAS caused accumulation of LC3-II, the lipidated form of the protein that is incorporated into autophagosomes and which characteristically migrates faster in SDS-PAGE (Klionsky et al., 2008) (Figure 3f). In contrast, cells transduced with both RASG12V and mAKT1 showed decreased LC3-II and an increased level of p62, a protein whose accumulation is indicative of decreased autophagy (Klionsky et al., 2008). These experiments indicate that the combination of activated AKT and RAS in cells results in a less complete senescence program than does activated RAS alone.
Figure 3. Activation of AKT antagonizes RASG12V-induced SAHF formation and Autophagy.
IMR90 fibroblasts were transduced with either control, mAKT1, RASG12V or both mAKT1 and RASG12V and double drug selected for 7 days. Cells were then fixed and stained for HIRA foci and SAHF. (b) Expression of transduced proteins and phosphorylation of AKT (AKTpS473) was assayed by western blotting. (c) and (d) One hundred cells from (a) were scored for HIRA foci or SAHF. Mean of 3 experiments with standard deviation. (e) IMR90 cells were transduced with RASG12V or BRAFV600E and scored for SAHF formation at 5 days post drug selection. Mean of 3 experiments with standard deviation. (f) Western blotting of cell lysates from (a) with indicated antibodies. The arrow marks the cleaved lipidated form of LC3, LC3-II.
Mechanism of antagonism of senescence by activated AKT
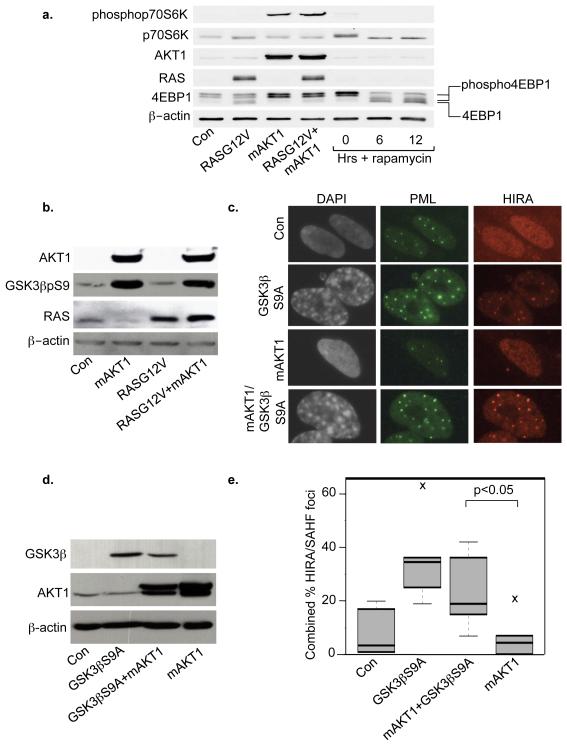
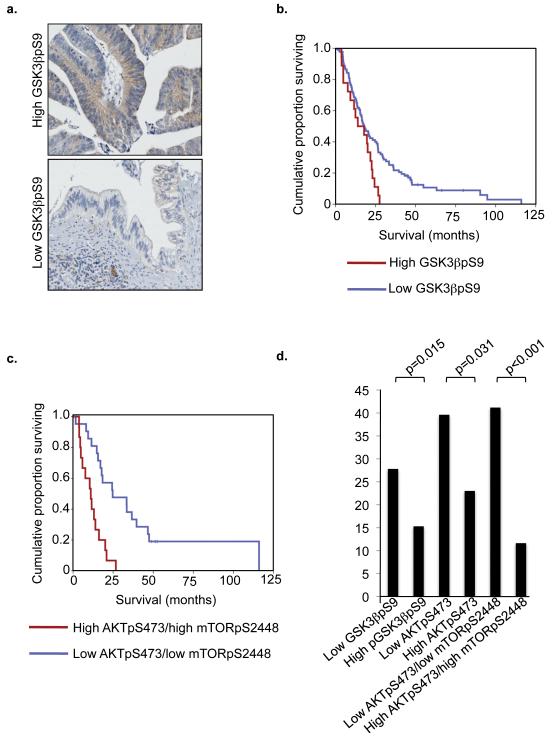
We next wanted to know the mechanism by which activated AKT1 antagonizes aspects of RASG12V-induced senescence. Since AKT1 activates mTOR and mTOR is a potent inhibitor of autophagy (He and Klionsky, 2009), we hypothesized that activated AKT1 suppresses RASG12V-induced autophagy by activation of mTOR. Consistent with this idea, in the presence of activated RAS, activated AKT1 activated mTOR, as judged by phosphorylation of mTOR substrates, 4EBP1 and p70S6K (Figure 4a). With respect to SAHF, we previously showed that activated RAS induces HIRA localization to PML bodies and formation of SAHF through its ability to activate GSK3β (Ye et al., 2007). In contrast, AKT is known to directly inhibit GSK3β through inhibitory phosphorylation on serine 9 (Cross et al., 1995). Therefore, we hypothesized that mAKT1’s ability to block RASG12V-induced SAHF formation might depend on its ability to phosphorylate and inhibit GSK3β. Consistent with this idea, in cells coexpressing activated RAS and AKT, GSK3β was heavily phosphorylated on serine 9 (GSK3βpS9) (Figure 4b). This indicates that RASG12V-induced activation of GSK3β is over-ridden by mAKT1-induced inhibition of GSK3β. To test our hypothesis further, we expressed activated AKT1 with or without a non-phosphorylatable mutant of GSK3β (GSK3βS9A), and found that, even in the presence of activated AKT1, GSK3βS9A was able to induce both localization of HIRA to PML bodies and SAHF formation (Figure 4c-e). We verified appropriate expression of GSK3βS9A and activated AKT by western blotting (Figure 4d). These results are consistent with the notion that activated AKT1 suppresses HIRA activation and formation of SAHF, at least in part, through phosphorylation and inhibition of GSK3β. Underscoring the importance of AKT1-mediated GSK3β phosphorylation in human cancer, we found that in a pancreatic cancer Tissue MicroArray (TMA) the level of GSK3βpS9 correlated with poor patient survival, independent of tumor size, tumor grade, perineural invasion, resection margin involvement and lymph node status (Figure 5a, b). Phosphorylation and activation of AKT1 and its downstream effector, mTOR, and combined phosphorylation and activation of AKT1 and mTOR similarly correlated with poor disease outcome (Figure 5c, d and Supplementary Figure 4 Supplementary Tables 1-5), also emphasizing the significance of activated AKT1 in this disease.
Figure 4. mAKT1 counters effects of RASG12V on mTOR and GSK3β.
(a) IMR90 cells were transduced with control, RASG12V, mAKT1 or both RASG12V and mAKT1. Cells were double drug selected, lysates prepared and western blotted. Uninfected cells were treated with 1nM rapamycin to define unphospho and phospho-4EBP1. (b) Western blotting of cells from (a). (c) IMR90 cells were transduced with control, GSK3βS9A, mAKT1 or both GSK3βS9A and mAKT. Cells were fixed and stained for PML, HIRA foci or SAHF. (d) Expression of proteins in (c) was verified by western blotting. (e) One hundred cells from (c) were scored for both HIRA foci and SAHF and a combined score plotted. The horizontal line inside the box is the median (50th percentile); the box itself encompasses the 25th and 75th percentiles (Inter Quartile Range (IQR)); the whiskers are the most extreme data points within 1.5×IQR; crosses outside the whiskers are outliers.
Figure 5. GSK3βps9, AKTpS473 and mTORpS2448 phosphorylation correlates with poor overall survival in human pancreatic cancer.
(a) Representative ‘high’(left panel) and ‘low’(right panel) scoring images of GSK3βps9 immunohistochemical staining from a human pancreatic adenocarcinoma tissue microarray. (b) Kaplan Meier curve representing patient survival per unit time. Survival is shown for patients with tumors with GSK3βps9 >100 (High) and <100 (Low). (c) Kaplan Meier curve for patients with low AKT1pS473/low mTORpS2448 and high AKT1pS473/high mTORpS2448. (d) Mean survival of indicated groups of patients. For each phospho-epitope in (b)-(d), the high staining group comprised 24 patients and the low staining group comprised 18 patients.
AKT pathway activation antagonizes RAS-induced proliferation arrest (with features of senescence) to drive tumorigenesis in the mouse pancreas
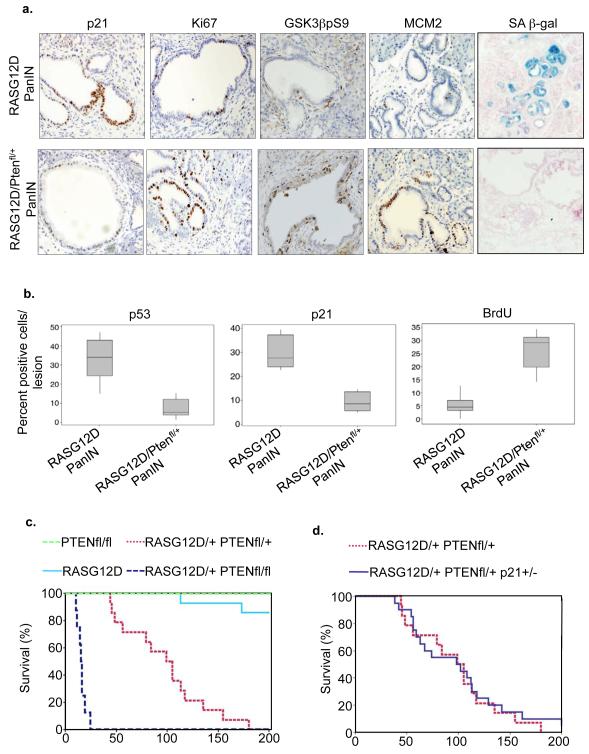
We next wanted to test whether activation of PIK3CA/AKT signaling is able to suppress activated RAS-induced senescence and accelerate tumor formation in vivo. To do this, we utilized a mouse model in which expression of activated RAS is restricted to the cells of the pancreas, by virtue of a conditional RAS allele (K-RASG12D) at its normal genomic locus that can be activated by Cre-mediated recombination, and pancreas specific expression of Cre recombinase under control of a PDX1 promoter (Hingorani et al., 2003). These PDX1-Cre/RASG12D animals develop normally, but develop benign precursor lesions termed pancreatic intraepithelial neoplasms (PanINs) that can, with long latency, progress to form PDAC. As shown previously (Morton et al., 2010b), these neoplastic lesions stain positively for markers of senescence, including SA β-gal and expression of p53 and p21CIP1 (Figure 6a, b). Conversely, they largely lack markers of proliferation, namely Ki67, MCM2 expression and incorporation of BrdU (Figure 6a-b). To test the impact of PIK3CA/AKT pathway activation on this activated RAS-induced in vivo senescence-like state, the PDX1-Cre/RASG12D animals were crossed to animals that have one or both PTEN alleles flanked by Cre recombination sites (Suzuki et al., 2001), to drive simultaneous activation of RAS and partial or biallelic inactivation of PTEN in the pancreas (PDX1-CRE/RASG12D/PTEN). Significantly, complete inactivation of PTEN in the mouse pancreas does not induce senescence (Stanger et al., 2005)(Supplementary Figure 5a). Comparing PanINs in the pancreata of 6 week old PDX1-Cre/RASG12D and PDX1-Cre/RASG12D/PTEN animals, we found that inactivation of PTEN largely abolished expression of senescence markers, p53, p21 and SA β-gal (Figure 6a-b). Consistent with the idea that inactivation of PTEN facilitates a complete bypass the senescence-like state, we found the PanINs of the PDX1-Cre/RASG12D/PTEN animals to be highly proliferative, as measured by an increase in immunohistochemical staining of Ki67, MCM2 and incoporation of BrdU (Figure 6a, b). Senescence bypass was associated with phosphorylation of GSK3 on serine 9, similar to the in vitro model (Figure 4b and 6a). In line with this senescence-like state being a potent tumor suppression mechanism in this in vivo model, expression of activated RAS and concurrent inactivation of PTEN resulted in rapid progression of PanINs into PDAC (Figure 6c and Supplementary Figure 5b), as reported recently (Hill et al., 2010). Previously, we have reported that inactivation of p21CIP1 accelerates tumorigenesis in this model, likely though inactivation of senescence (Morton et al., 2010a). Significantly, deficiency of p21CIP1 did not further accelerate tumorigenesis in PDX1-Cre/RASG12D/ PTENfl/+ animals (Figure 6d), indicating that loss of p21CIP1 and PTEN accelerate PDAC via the same pathway, further implicating loss of PTEN in abrogation of senescence in this model.
Figure 6. Inactivation of PTEN abrogates a senescence-like state in a mouse model of pancreatic cancer.
(a) Immunohistochemical staining of PanIN from pancreata of mice of indicated genotype. Markers of senescence include SA β-gal and p21; markers of proliferation include Ki67 and MCM2. (b) Quantitation of p53, p21 and BrdU from (a). Box plots as Figure 4e. (c) and (d) Kaplan Meier curve showing percentage of animals of indicated genotype surviving per unit time.
IHC analysis of PTEN indicated that tumors arising from PDX1-Cre/RASG12D/PTENfl/+ mice had lost the second allele of PTEN (Supplementary Figure 5c). Also, the effects of PTEN disruption were more marked when both, rather than one, alleles of PTEN were engineered for inactivation in the pancreas (Figure 6c and Supplementary Figure 5b). Loss of two alleles of PTEN led to an incredibly lethal acceleration of tumorigenesis, leading invariably to rapid death and a mean survival of 15 days (Figure 6c and Supplementary Figure 5b). In these mice, almost the entire pancreas was replaced by neoplastic tissue, with very little normal tissue remaining. Neoplastic tissue contained widespread mitoses, including some aberrant figures (Supplementary Figure 5d). In areas, there was loss of the normal pancreatic architecture with angulated glands, indicating invasive carcinoma (Supplementary Figure 5d). Tumors in these mice were large and exhibited a high proliferative index, as judged by Ki67 and BrdU incorporation (Supplementary Figure 5e-f). These observations suggest that the tumor suppressor function of PTEN in this model conforms to the Knudson “two-hit” paradigm for tumor suppressors.
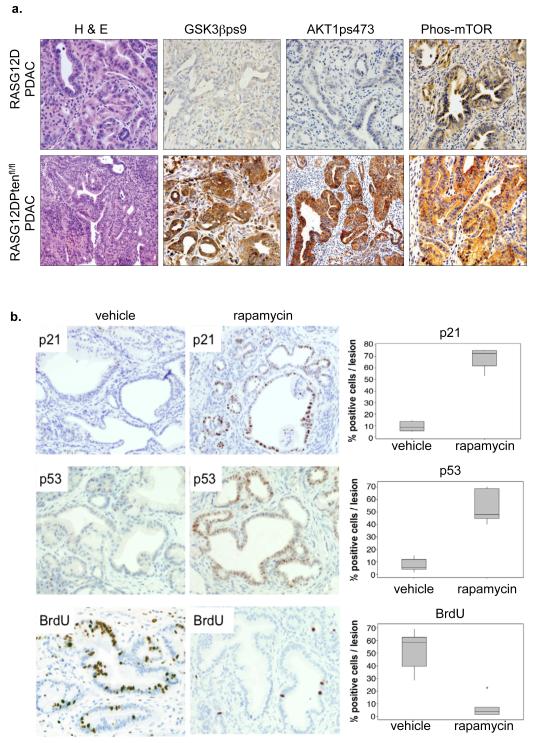
As expected, tumors that resulted from inactivation of PTEN exhibited a strongly activated AKT signaling pathway, as shown by immunohistochemical staining for activated phosphoserine 473 AKT (Figure 7a and data shown). Consistent with inactivation of PTEN and activation of AKT driving tumorigenesis through inactivation of GSK3β and activation of mTOR, tumors from PDX1-Cre/RASG12D/PTEN mice stained strongly for phosphoserine 9 GSK3β and phospho-mTOR (Figure 7a). Moreover, treatment of PDX1-Cre/RASG12D/ PTENfl/+ mice with rapamycin, a potent inhibitor of mTOR, restored cell senescence, as measured by proliferation arrest (BrdU) and p53 and p21 expression (Figure 7b and Supplementary Figure 6). Taken together, these in vivo data support our hypothesis that inactivation of PTEN and activation of AKT and its downstream effector, mTOR, is capable of antagonizing activated RAS-induced proliferation arrest (with features of senescence) leading to rapid acceleration of tumorigenesis.
Figure 7. Rapamycin reactivates senescence in PDAC harboring activated PIK3CA/AKT.
(a) Immunohistochemical staining of AKT pathway activation in pancreata of RASG12D/PTENfl/fl mice or RASG12D mice. (b) RASG12D/PTENfl/+ mice were treated with rapamycin for 7 days and then pancreata harvested and stained for p53, p21 and BrdU. Box plots as Figure 4e.
Discussion
Previous studies do not present a clear picture regarding the ability of activated PIK3CA/AKT to induce senescence. Some reports have indicated that activation of the PIK3CA/AKT pathway does induce senescence (Chen et al., 2005; Majumder et al., 2008; Miyauchi et al., 2004; Nogueira et al., 2008; Oyama et al., 2007). Other reports have concluded that PIK3CA/AKT activity is a weak inducer of senescence (Lin et al., 1998), is downregulated in senescence (Courtois-Cox et al., 2006; Young et al., 2009), and can antagonize senescence (Courtois-Cox et al., 2006; Kortlever et al., 2006; Tresini et al., 1998). A recent report on PTEN loss-induced senescence (PICS) supports our finding that senescence induced by PIK3CA/AKT activation is not associated with activation of DNA damage signaling, but did not examine chromatin changes, autophagy and the senescence secretome (Alimonti et al., 2010). In this study, by directly comparing activated RAS and PIK3CA/AKT, we find that the latter is not an efficient inducer of senescence. Specifically, we show that inactivation of PTEN and activation of AKT is impaired in its ability to induce senescence, as recorded by multiple effectors of senescence, including upregulation of p16, induction of DNA damage, recruitment of HIRA to PML bodies, formation of SAHF and upregulation of autophagy. Importantly, we also show that activation of PIK3CA/AKT is deficient in its ability to drive two functional outputs of the senescence program that are central to senescence-mediated tumor suppression, namely upregulation of the senescence secretome and efficient proliferation arrest. Most important, concurrent activation of both RAS and PIK3CA/AKT impairs RAS-induced senescence, both in vitro and in vivo.
Activated PIK3CA/AKT suppresses senescence induced by activated RAS through multiple routes. First, activated AKT1 reversed the upregulation of p16INK4a induced by activated RAS. Second, GSK3β kinase is another key nodal point at which signals from activated RAS and PIK3CA/AKT interact. We and others have previously shown that activation of GSK3β kinase contributes to onset of senescence (Kortlever et al., 2006; Liu et al., 2008; Ye et al., 2007; Zmijewski and Jope, 2004). Specifically, we showed that activation of GSK3β phosphorylates the HIRA histone chaperone, thereby localizing this protein to PML bodies and instigating the formation of SAHF (Ye et al., 2007). Here we present evidence that activated PIK3CA/AKT suppresses RASG12V-induced HIRA relocalization and formation of SAHF through its ability to phosphorylate and inhibit GS3Kβ. The significance of the PIK3CA/AKT-GSK3β signaling axis in human cancer is underscored by our finding that a high level of AKTpS473 (with or without high mTORpS2448) or GSK3βpS9 is a predictor of poor survival in human pancreatic cancer, independent of other common prognostic indicators. Third, activated PIK3CA/AKT and activated RAS antagonize each other through mTOR signaling. mTOR is well-documented to be a potent repressor of autophagy (He and Klionsky, 2009). While activated RAS inhibits mTOR activity to upregulate autophagy and promote senescence (Young et al., 2009)(Figure), activated AKT1 was able to activate mTOR even in the presence of activated RAS, likely explaining the ability of mAKT1 to inhibit RASG12V-induced autophagy. To affirm this in vivo, in mice haboring activated RAS and activated PIK3CA/AKT signaling, the potent mTOR inhibitor, rapamycin, reactivated RAS-senescence. We conclude that activated PIK3CA/AKT suppresses RAS-induced senescence through its ability to intersect with and antagonize several outputs of chronic activated RAS, including upregulation of p16INK4a, activation of GSK3β and repression of mTOR. While activated PIK3CA/AKT signaling is known to have many targets in the cell, TMA analysis of human pancreatic cancer underscored GSK3β and mTOR as important targets in this disease. Phosphorylation of all three proteins was significantly directly correlated (Supplementary data), and high phosphorylation of each protein is a predictor of poor patient survival. Thus, the PIK3CA/AKTGSK3β/mTOR axis is an important driver of disease outcome in human pancreatic cancer.
Although activation of AKT1 impaired RASG12V-induced senescence in vitro by at least three criteria (suppression of p16INK4a, SAHF and autophagy), it did not completely abolish activated RAS-induced senescence, as measured by proliferation arrest (Supplementary Figure 3c). On the other hand, inactivation of PTEN did bypass activated RAS-induced senescence-like arrest in vivo (as measured by proliferation markers) and caused a dramatic acceleration of tumorigenesis. There are several possible explanations of this difference between the in vitro and in vivo models, including differences between cell types, use of RASG12V in vitro and RASG12D in vivo and influence of cellular microenvironment in vivo. It is also important to note that in the mouse model, we cannot conclude that inactivation of PTEN is sufficient to abrogate senescence in all of the RASG12D-expressing cells. Rather, inactivation of PTEN might weaken the senescence program enough to facilitate complete escape from senescence, but only in cooperation with additional acquired and selected mutations. Regardless, of the correct explanation, the in vitro and in vivo results are consistent in showing that inactivated PTEN/activated AKT can antagonize activated RAS-induced senescence and in vivo this facilitates tumorigenesis.
Our results show that all oncogenes are not equal in their abilities to induce senescence, and, surprisingly, a weak inducer of senescence can be dominant over a strong. This idea has important implications for understanding mechanisms of oncogene cooperation. Concurrent mutations of RAS and the PTEN/PIK3CA/AKT pathway have been described in a number of human tumor types, including colon, endometrium and ALL (Parsons et al., 2005; Yeang et al., 2008). Concurrent mutations are also probable in pancreatic cancer, as RAS mutations are thought to occur in >90% of cases (Tuveson and Hingorani, 2005) and functional inactivation of PTEN by promoter methylation (Asano et al., 2004), decreased mRNA levels (Ebert et al., 2002), loss of protein expression (Altomare et al., 2002; Asano et al., 2004) or loss of heterozygosity (Okami et al., 1998) has also been reported. Furthermore, amplification or activation of AKT2 kinase, related to AKT1, occurs in up to 60% of pancreatic cancers (Altomare et al., 2002; Ruggeri et al., 1998; Schlieman et al., 2003), and AKT is activated in pancreatic cancer based on IHC staining (Semba et al., 2003). Most strikingly, approximately 75% of human colon cancers that contain PIK3CA mutations also harbor mutations in K-RAS (Parsons et al., 2005). In addition, activating mutations of RAS and in the PTEN/PIK3CA/AKT pathway have been shown to cooperatively drive tumorigenesis in mouse models of glioblastoma, endometrium, thyroid and pancreas (Holland et al., 2000; Kim et al., 2010; Miller et al., 2009) (this study). To date, the molecular basis of cooperation between these mutations in human tumors and mouse models has been poorly understood. Here, we present evidence from both in vitro and in vivo studies to indicate that these mutations cooperate, at least in part, through the ability of PTEN/PIK3CA/AKT mutations to suppress RAS-induced senescence, thereby allowing for these oncogenic pathways to cooperate in tumorigenesis. Importantly, this new mechanistic understanding might be exploited as a pro-senescence cancer therapy. Rapamycin is a potent and specific inhibitor of mTOR, a key effector of activated PIK3CA/AKT signaling and is already used in the clinic. We found that rapamycin can reactivate senescence in mouse tumors haboring mutations in both RAS and PTEN, pointing to possible therapeutic activity against human tumors of this, or equivalent, genotype.
Materials and Methods
Cell Culture
IMR90 and BJ (ATCC) cell lines were cultured according to ATCC guidelines in low oxygen (2%) unless otherwise indicated. Fibroblasts were cultured in Dulbecco’s modified Eagle’s medium supplemented with 20% fetal bovine serum.
Immunofluorescence, SAHF, and SA β-gal staining
Two color indirect immunofluorescence and SAHF assays were performed as described previously (Ye et al., 2007; Zhang et al., 2005). SA β-gal staining was performed as described previously (for in vitro studies) (Dimri et al., 1995).
Genetically modified mice
The Pdx1-Cre, LSL-K-RASG12D and PTENflox mice have been described previously (Hingorani et al., 2003; Suzuki et al., 2001). The p21CIP1−/− mice have been previously described (Deng et al., 1995). Conditional LSL-K-RASG12D/+ mice were from Tyler Jacks via MMHCC.
For additional methods see Supplementary Text and Figures.
Supplementary Material
Acknowledgements
We are indebted to Daniel Peeper, Masashi Narita and Chris Sell for communication of results prior to publication; to Michael Bouchard, Jane Clifford, Maureen Murphy and Matt Kennedy for helpful discussions and advice; to Stuart Pepper and the CR-UK Microarray Facility, Paterson Institute for microarray analyses; to Colin Nixon for immunohistochemistry; to Jane Hair for curating the NHSGGC biorepository. The lab of PDA is funded by CR-UK (C10652/A10250). Work in the lab of GHE was funded by NIH R01GM062281. The lab of OJS is funded by CR-UK. Thanks to the Scottish Executive Chief Scientist Office (CSO) for funding for NBJ, including the production of tissue microarrays (CSO ref: 41175). Additional support was from Think Pink Scotland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta JC, O’Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Adams PD. Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell. 2009;36:2–14. doi: 10.1016/j.molcel.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altomare DA, Tanno S, De Rienzo A, Klein-Szanto AJ, Skele KL, Hoffman JP, Testa JR. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem. 2002;87:470–476. doi: 10.1002/jcb.10287. [DOI] [PubMed] [Google Scholar]
- Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–8580. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- Banumathy G, Somaiah N, Zhang R, Tang Y, Hoffmann J, Andrake M, Ceulemans H, Schultz D, Marmorstein R, Adams PD. Human UBN1 is an ortholog of yeast Hpc2p and has an essential role in the HIRA/ASF1a chromatin-remodeling pathway in senescent cells. Mol Cell Biol. 2009;29:758–770. doi: 10.1128/MCB.01047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, Einhorn E, Herlyn M, Minna J, Nicholson A, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002;62:6997–7000. [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois-Cox S, Williams S.M. Genther, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein PE, MacCollin M, Cichowski K. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- d’Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. doi: 10.1038/nrc2440. [DOI] [PubMed] [Google Scholar]
- Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert MP, Fei G, Schandl L, Mawrin C, Dietzmann K, Herrera P, Friess H, Gress TM, Malfertheiner P. Reduced PTEN expression in the pancreas overexpressing transforming growth factor-beta 1. Br J Cancer. 2002;86:257–262. doi: 10.1038/sj.bjc.6600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C. Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 2009;28:889–901. doi: 10.1038/emboj.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha L, Ichikawa T, Anver M, Dickins R, Lowe S, Sharpless NE, Krimpenfort P, Depinho RA, Bennett DC, Sviderskaya EV, et al. ARF functions as a melanoma tumor suppressor by inducing p53-independent senescence. Proc Natl Acad Sci U S A. 2007;104:10968–10973. doi: 10.1073/pnas.0611638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, Dry S, Wu H. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010;70:7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- Kim TH, Wang J, Lee KY, Franco HL, Broaddus RR, Lydon JP, Jeong JW, Demayo FJ. The Synergistic Effect of Conditional Pten Loss and Oncogenic K-ras Mutation on Endometrial Cancer Development Occurs via Decreased Progesterone Receptor Action. J Oncol. 2010;2010:139087. doi: 10.1155/2010/139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Lin AW, Barradas M, Stone JC, van Aelst L, Serrano M, Lowe SW. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 1998;12:3008–3019. doi: 10.1101/gad.12.19.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Fang X, Hall H, Yu S, Smith D, Lu Z, Fang D, Liu J, Stephens LC, Woodgett JR, et al. Homozygous deletion of glycogen synthase kinase 3beta bypasses senescence allowing Ras transformation of primary murine fibroblasts. Proc Natl Acad Sci U S A. 2008;105:5248–5253. doi: 10.1073/pnas.0704242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Grisanzio C, O’Connell F, Barry M, Brito JM, Xu Q, Guney I, Berger R, Herman P, Bikoff R, et al. A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression. Cancer Cell. 2008;14:146–155. doi: 10.1016/j.ccr.2008.06.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res. 2009;69:3689–3694. doi: 10.1158/0008-5472.CAN-09-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JP, Jamieson NB, Karim SA, Athineos D, Ridgway RA, Nixon C, McKay CJ, Carter R, Brunton VG, Frame MC, et al. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology. 2010a;139:586–597. 597 e581–586. doi: 10.1053/j.gastro.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B, Jamieson NB, Oien KA, Lowy AM, Brunton VG, et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010b;107:246–251. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/s0092-8674(03)00401-x. [DOI] [PubMed] [Google Scholar]
- Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okami K, Wu L, Riggins G, Cairns P, Goggins M, Evron E, Halachmi N, Ahrendt SA, Reed AL, Hilgers W, et al. Analysis of PTEN/MMAC1 alterations in aerodigestive tract tumors. Cancer Res. 1998;58:509–511. [PubMed] [Google Scholar]
- Oyama K, Okawa T, Nakagawa H, Takaoka M, Andl CD, Kim SH, Klein-Szanto A, Diehl JA, Herlyn M, El-Deiry W, et al. AKT induces senescence in primary esophageal epithelial cells but is permissive for differentiation as revealed in organotypic culture. Oncogene. 2007;26:2353–2364. doi: 10.1038/sj.onc.1210025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, Silliman N, Ptak J, Szabo S, Willson JK, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Meltzer PS. A genome-based strategy uncovers frequent BRAF mutations in melanoma. Cancer Cell. 2002;2:5–7. doi: 10.1016/s1535-6108(02)00089-2. [DOI] [PubMed] [Google Scholar]
- Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–86. [PubMed] [Google Scholar]
- Sarkisian CJ, Keister BA, Stairs DB, Boxer RB, Moody SE, Chodosh LA. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Stiles B, Lauwers GY, Bardeesy N, Mendoza M, Wang Y, Greenwood A, Cheng KH, McLaughlin M, Brown D, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Sun P, Yoshizuka N, New L, Moser BA, Li Y, Liao R, Xie C, Chen J, Deng Q, Yamout M, et al. PRAK is essential for ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Tresini M, Mawal-Dewan M, Cristofalo VJ, Sell C. A phosphatidylinositol 3-kinase inhibitor induces a senescent-like growth arrest in human diploid fibroblasts. Cancer Res. 1998;58:1–4. [PubMed] [Google Scholar]
- Tuveson DA, Hingorani SR. Ductal pancreatic cancer in humans and mice. Cold Spring Harb Symp Quant Biol. 2005;70:65–72. doi: 10.1101/sqb.2005.70.040. [DOI] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Zerlanko B, Kennedy A, Banumathy G, Zhang R, Adams PD. Downregulation of Wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Mol Cell. 2007;27:183–196. doi: 10.1016/j.molcel.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeang CH, McCormick F, Levine A. Combinatorial patterns of somatic gene mutations in cancer. FASEB J. 2008;22:2605–2622. doi: 10.1096/fj.08-108985. [DOI] [PubMed] [Google Scholar]
- Young AR, Narita M, Ferreira M, Kirschner K, Sadaie M, Darot JF, Tavare S, Arakawa S, Shimizu S, Watt FM. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, et al. Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell. 2005;8:19–30. doi: 10.1016/j.devcel.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Zmijewski JW, Jope RS. Nuclear accumulation of glycogen synthase kinase-3 during replicative senescence of human fibroblasts. Aging Cell. 2004;3:309–317. doi: 10.1111/j.1474-9728.2004.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.