Abstract
Estrogen therapy can promote cognitive function if initiated within a ‘critical window’ during the menopausal transition. However, in the absence of a progestogen, estrogens increase endometrial cancer risk which has spurred research into developing estrogenic alternatives that have the beneficial effects of estrogen but which are clinically safer. Soy protein is rich in isoflavones, which are a class of potential estrogenic alternatives. We sought to determine the effects of two diets, one with casein-lactalbumin as the main protein source and the other with soy protein containing isoflavones, on protein markers of hippocampal bioenergetic capacity in adult female cynomolgus macaques (Macaca fascicularis). Further, we assessed the effects of dietary soy isoflavones before or after ovariectomy. Animals receiving soy diet premenopausally then casein/lactalbumin post-ovariectomy had higher relative hippocampal content of glycolytic enzymes glyceraldehyde 3-phosphate dehydrogenase and pyruvate dehydrogenase subunit e1α. Post-ovariectomy consumption of soy was associated with higher succinate dehydrogenase α levels and lower levels of isocitrate dehydrogenase, both proteins involved in the tricarboxylic acid cycle, significantly decreased expression of the antioxidant enzyme peroxiredoxin-V, and a non-significant trend towards decreased manganese superoxide dismutase expression. None of the diet paradigms significantly affected expression levels of oxidative phosphorylation enzyme complexes, or of mitochondrial fission and fusion proteins. Together, these data suggest that long-term soy diet produces minimal effects on hippocampal expression of proteins involved in bioenergetics, but that switching between a diet containing primarily animal protein and one containing soy isoflavones before and after menopause may result in complex effects on brain chemistry.
Keywords: Alzheimer’s disease, hippocampus, menopause, mitochondria, phytoestrogens, soy isoflavone
1. Introduction
Alzheimer’s disease (AD) is a progressive, debilitating neurodegenerative disorder that is the leading cause of dementia worldwide. While there are many factors which have been hypothesized to underlie this disease (Prasad et al, 2002), one well-characterized antecedent to the development of AD pathology is brain hypometabolism (Reiman et al, 2004). Healthy neurons have an extremely high metabolic rate (Irwin et al, 2008), because they must produce a significant amount of ATP in order to generate action potentials and maintain synaptic plasticity (Kostanyan and Nazaryan, 1992). Considering that mitochondria are responsible for generating 90% of a cell’s total ATP, these organelles are critical for the function of cells with high energy demands such as neurons (for review, see Cadenas and Davies, 2000). Impairment of mitochondrial function would thus be expected to have serious consequences on neuronal viability and overall brain health.
Reproductive senescence (menopause) in both non-human primates (NHPs) and humans is also associated with a sharp decrease in circulating levels of endogenous estrogens (Boron and Boulpaep, 2009; Gilardi et al, 1997). A significant body of research has identified estrogen as a neuroprotective agent through its effects on mitochondria, including promotion of glucose utilization, modulating expression levels of glycolytic and TCA cycle enzymes, and enhancement of antioxidant systems (for review, see Simpkins et al, 2010). Further, mitochondrial function has been shown to decline markedly both at the time of natural reproductive senescence (Yao et al, 2009), and after ovariectomy (Irwin et al 2008; Nilsen et al, 2007) in female rodent models. This suggests that, in menopausal women, diminished endogenous estrogen levels could lead to mitochondrial decline and increased risk of developing neurodegenerative diseases. Notably, epidemiological data indicate that 68% of all individuals with AD are female, a statistic that holds true even when the greater longevity of women compared to men is taken into account in age-matched studies (Brinton, 2008a; Brookmeyer et al, 1998; Gao et al, 1998).
An obvious therapeutic tactic would be estrogen replacement after menopause; indeed, administration of 17β-estradiol immediately after ovariectomy in rodent models has been shown to increase the expression of enzymes involved in glycolysis, the TCA cycle, and oxidative phosphorylation (Irwin et al, 2008; Nilsen et al, 2007). While we recognize the difficulty in making trans-species comparisons, the majority of the research on mitochondrial bioenergetic function during aging has been conducted on ovariectomized mouse and rat models, and very little research has been carried out using an NHP model system. Considering that NHPs undergo reproductive senescence in a more similar manner to humans (Dumitriu et al, 2010), their inclusion in translational studies provides a useful bridge between rodents and humans (Shively and Clarkson, 2009). However, at least initially, we must rely on data generated using rodent models to indicate the potential effects of estrogen on mitochondrial enzymes. PET imaging studies have provided evidence of a link between brain metabolism and estrogen in humans, however: for example, one study showed that post-menopausal women taking estrogen therapy had improved brain metabolism in the hippocampus and middle temporal gyrus compared to women not taking estrogen therapy (Maki and Resnick, 2000). This indicates that estrogen likely has similar effects on mitochondrial enzyme function across species (for review, see Brinton, 2005).
Unfortunately, estrogen therapy is contraindicated in women with a uterus due to an increase in the risk of endometrial cancer (North American Menopause Society, 2010). Substantial research has focused on identifying sources of post-menopausal estrogenic alternatives that can provide the beneficial cognitive effects without the harmful proliferative effects. One such alternate class of compounds is the isoflavones (sometimes referred to as phytoestrogens), molecules that structurally resemble endogenous estrogens, but which are found in plants (for review, see Kurzer and Xu, 1997). Due to their molecular resemblance to mammalian estrogens, phytoestrogens are able to bind to estrogen receptors (ERs) with both estrogenic and anti-estrogenic effects, depending on the availability of endogenous estrogens and the expression levels of ER subtype α and β (for review, see Dixon, 2004; Zhao and Brinton, 2005).
Soybeans and soy products, which are relatively enriched in isoflavones, are of particular interest due to the fact that they make up a significant dietary protein source in some areas of the world (Zhao and Brinton, 2007). Studies examining dietary intake between various populations have found a vast difference in the amount of isoflavones consumed in Western vs. Asian diets (de Kleijn et al, 2001). These studies have shown that there is an association between the higher consumption of soy products and lower prevalence of hormone-related conditions such as breast cancer and hot flashes in Asian women (Henderson and Bernstein, 1991; Maskarinec, 2003; Ziegler, 2004). Additionally, prevalence rates for AD are significantly lower in Japan and China compared to countries where a Western-style diet is consumed (for review see Zhao and Brinton, 2007). Thus, the data indicate that consumption of phytoestrogens may help to counteract the drop in estrogen levels at menopause. By extension, this could be anticipated to protect against cognitive decline; however, data regarding the effects of soy intake on cognitive function are still unclear.
In the current study, we took advantage of an opportunity to investigate the functional outcome of long-term soy diet on glucose uptake and mitochondrial metabolism in the brains of adult female cynomolgus macaques (Macaca fascicularis). Based on our prior research implicating a decline in mitochondrial bioenergetics as an antecedent to development of AD (Yao et al, 2009), we were interested in evaluating whether a soy protein-based diet containing isoflavones would lead to enhanced mitochondrial bioenergetic function after reproductive senescence when compared to a casein + lactalbumin diet (modeled after a typical Western diet). Additionally, we sought to identify whether a ‘critical window’ might exist for beneficial effects of soy intake as has been hypothesized to exist for 17β-estradiol therapy (Henderson and Brinton, 2010), particularly with regard to whether the soy diet was more beneficial if initiated prior to or after the onset of menopause. To investigate this, we examined the effects of four diet paradigms on markers of mitochondrial bioenergetics in adult female NHP brains. Monkeys consumed one of the following: (1) a diet consisting of casein/lactalbumin as the primary protein source, both before and after ovariectomy (C/C); (2) a diet consisting of soy protein as the primary protein source, and containing isoflavones, both before and after ovariectomy (S/S); (3) a casein/lactalbumin diet prior to ovariectomy and a soy isoflavone diet following ovariectomy (C/S); or (4) a soy isoflavone diet prior to ovariectomy and a casein/lactalbumin diet following ovariectomy (S/C). Hippocampal tissues were analyzed to determine the expression of proteins involved in glucose uptake, glycolysis, the TCA cycle, oxidative phosphorylation, antioxidant function, and mitochondrial fission/fusion.
2. Results
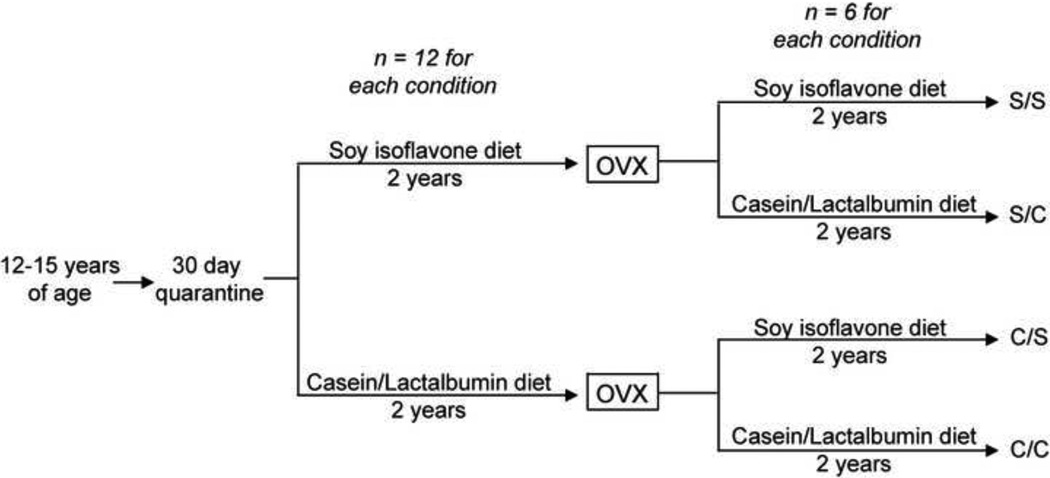
Female cynomolgus macaques (Macaca fascicularis) were obtained from Indonesia at an average age of 12 – 15 years (equivalent to 36 – 45 human years). After a 30-day quarantine, monkeys were randomized into one of two dietary paradigms: 1) a soy protein-based diet, which contained isoflavones, or 2) a diet where the primary source of protein was casein + lactalbumin. Monkeys consumed the diet for 2 years, and then underwent ovariectomy. Following ovariectomy, monkeys were again randomized to either remain on the pre-ovariectomy diet, or to switch to the opposite diet. This resulted in four diet paradigms (n = 6 monkeys in each paradigm), which allowed us to study whether the presence of soy isoflavones in diet and the timing of soy isoflavone consumption had an effect on hippocampal mitochondrial markers of glucose uptake, bioenergetics, antioxidant capacity, and fission/fusion. In the following section, diets are labeled as C/C (casein/lactalbumin before and after ovariectomy), C/S (casein/lactalbumin before and soy protein after ovariectomy), S/S (soy protein before and after ovariectomy), and S/C (soy protein before and casein/lactalbumin after ovariectomy) (Figure 1).
Figure 1. Experimental paradigm.
A detailed description of the experimental protocol. In this and all subsequent figures, diet paradigms are labeled as C/C (casein/lactalbumin before and after ovariectomy), C/S (casein/lactalbumin before and soy isoflavone after ovariectomy), S/S (soy isoflavone before and after ovariectomy), and S/C (soy isoflavone before and casein/lactalbumin after ovariectomy.)
Hippocampal punches encompassing the entire hippocampus (anterior to posterior, including all sub-regions) were used in these analyses. Expression levels of the proteins investigated in this study were quantified using Western blotting, and protein expression levels were normalized to β-actin or β-tubulin. Bar graphs are presented as an average of the protein levels measured from all samples within each group; however, it is worth noting that there was a fair amount of within-group variability, which affected measurements of statistically significant differences between groups. In certain conditions, variability was due to the fact that some monkeys would show an increase in enzyme expression, and others would not. In the results presented below, p-values for significant differences (p < 0.05) and trends (p < 0.35) are presented.
2.1 Glycolytic effects of switching from a pre-ovariectomy soy diet to a casein diet after ovariectomy
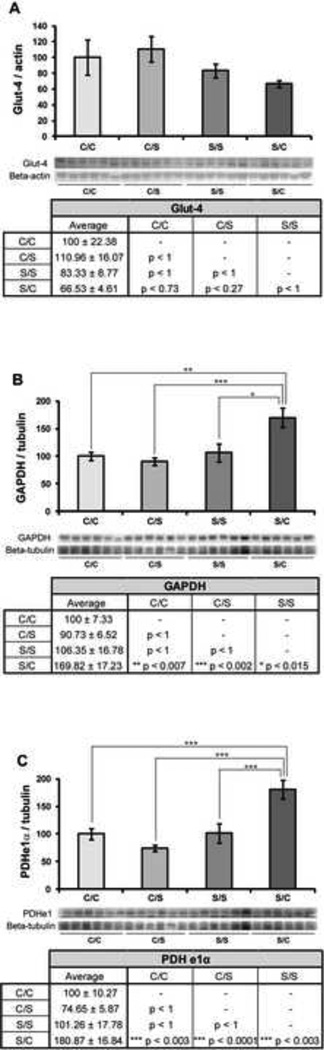
To determine the impact of the four different diet paradigms on proteins involved in glucose uptake and glycolysis, expression of three proteins (glucose transporter-4, the glycolytic enzyme GAPDH, and the pyruvate conversion enzyme PDH subunit e1α) was assessed. None of the diets had a significant effect on glucose transporter-4 (Glut-4) protein expression in the hippocampus (F3, 23 = 1.76, p < 0.19) (Figure 2A). There was, however, a trend towards decreased Glut-4 expression in hippocampal samples from NHPs on the S/C diet when compared to those on the C/S diet (p < 0.27). Diet did have a significant effect on GAPDH expression in the hippocampus (F3, 23 = 7.67, p < 0.05) (Figure 2B). GAPDH expression was higher in NHPs switched from a pre-ovariectomy soy diet to a post-ovariectomy casein diet (S/C paradigm), when compared to C/C (** p < 0.01), C/S (*** p < 0.005) and S/S (* p < 0.05) diet paradigms. Similarly, diet had a significant effect on PDH subunit e1α expression (F3, 23 = 11.5, p < 0.05) (Figure 2C). Hippocampal expression of PDH e1α was higher in the S/C group compared to C/C (*** p < 0.005), C/S (*** p < 0.005), and S/S (*** p < 0.005) groups.
Figure 2. Effect of diet on glucose uptake and glycolysis.
Western blot analysis of Glut-4 (A), GAPDH (B), and PDHe1α (C) expression was performed on NHP hippocampal samples from each of the four diet paradigms. Expression levels for each sample were normalized to beta-actin levels (Glut-4) and beta-tubulin levels (GAPDH and PDHe1α). Expression levels were then normalized to the C/C diet (C/C was set to 100%). Statistically significant differences were calculated using a two-tailed, one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc correction. Diet paradigm significantly affected GAPDH and PDHe1α expression in the NHP hippocampus. Bars represent % C/C ± S.E.M., n = 6 for each condition, p<0.05*, p<0.01**, p<0.005***.
2.2 Effect of pre-ovariectomy and post-ovariectomy diet on hippocampal IDH2 and SDHα expression
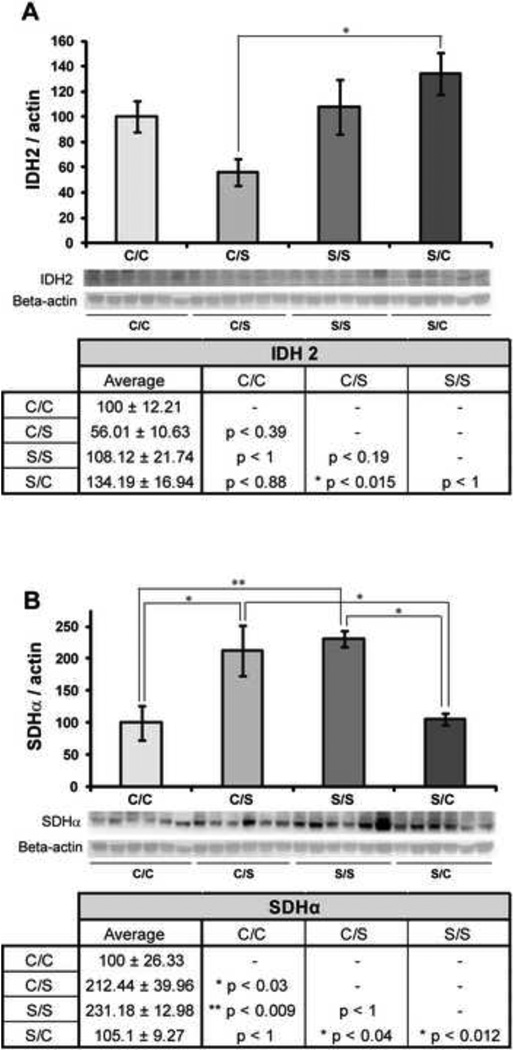
To determine the effects of diet on enzymes of the TCA cycle, we measured expression of isocitrate dehydrogenase (IDH2) and succinate dehydrogenase (SDHα). Diet was observed to exert a significant effect on IDH2 expression in the hippocampus (F3, 23 = 4.14, p < 0.05) (Figure 3A). NHPs in the C/S diet paradigm had significantly lower hippocampal IDH2 expression than those in the S/C diet paradigm (* p < 0.05). Although remaining on the same diet before and after ovariectomy had no effect on IDH2 expression (C/C compared to S/S, p < 1), switching from a soy to a casein diet showed a non-significant trend towards increased IDH2 expression when compared to remaining on a soy diet (p < 0.192).
Figure 3. Effect of diet on enzymes of the TCA cycle.
Western blot analysis of IDH2 (A) and SDHα (B) expression was performed on NHP hippocampal samples from each of the four diet paradigms. Expression levels for each sample were normalized to beta-actin levels. Expression levels were then normalized to the C/C diet (C/C was set to 100%). Statistically significant differences were calculated using a two-tailed, one-way analysis of variance (ANOVA), followed by a Bonferroni post-hoc correction. Diet paradigm significantly affected both IDH2 and SDHα expression in the NHP hippocampus. Bars represent % C/C ± S.E.M., n = 6 for each condition, p<0.05*, p<0.01**, p<0.005***.
Diet also had a significant effect on SDHα expression in the hippocampus (F3, 23 = 7.55, p < 0.05), and SDHα expression levels appeared to be dependent on consumption of a soy diet after ovariectomy in NHPs (Figure 3B). A significant increase in SDHα expression was observed between hippocampal samples from NHPs in the C/C and C/S diet paradigms (* p < 0.05), the C/C and S/S diet paradigms (** p < 0.01), the S/C and S/S paradigms (* p < 0.05) and the S/C and C/S paradigms (* p < 0.05). Put more simply, both the diet paradigms in which NHPs consumed soy after ovariectomy (C/S and S/S) showed a significant increase in SDHα expression over post-ovariectomy casein diets.
2.3 Effects of diet after ovariectomy on hippocampal levels of the enzyme complexes of oxidative phosphorylation
To determine how diet paradigm affected energy generation by the mitochondria, we measured hippocampal protein expression of complex I, complex II, complex III, complex IV, and complex Vα. We observed no significant effect of diet paradigm on expression levels of complex I (F3, 23 = 0.82, p < 0.5) (Figure 4A), complex II (F3, 23 = 0.65, p < 0.6) (Figure 4B), or complex III (F3, 23 = 0.79, p < 0.52) (Figure 4C). Similarly, results showed no overall significant effect of diet on hippocampal expression of complex IV (F3, 23 = 1.92, p < 0.16) (Figure 4D); however, there was a trend towards increased complex IV expression in hippocampal samples from NHPs in the S/C diet paradigm compared to those in the S/S diet paradigm (p < 0.27). Again, there was no significant effect of diet on hippocampal expression of complex Vα (F3, 23 = 1.95, p < 0.15) (Figure 4E), but there was a trend towards increased complex Vα expression in hippocampal samples from NHPs in the C/S diet group compared to the C/C diet group (p < 0.15).
Figure 4. Effect of diet on enzyme complexes involved in oxidative phosphorylation.
Western blot analysis of complex-I (A), complex-II (B), complex-III (C), complex-IV-II (D) and complex-Vα (E) expression was performed on NHP hippocampal samples from each of the four diet paradigms. Expression levels for each sample were normalized to beta-actin levels. Expression levels were then normalized to the C/C diet (C/C was set to 100%). Statistically significant differences were calculated using a two-tailed, one-way analysis of variance (ANOVA), followed by a Bonferroni post-hoc correction. Diet paradigm did not significantly affect expression of any of the oxidative phosphorylation enzyme complexes in the NHP hippocampus. Bars represent % C/C ± S.E.M., n = 6 for each condition.
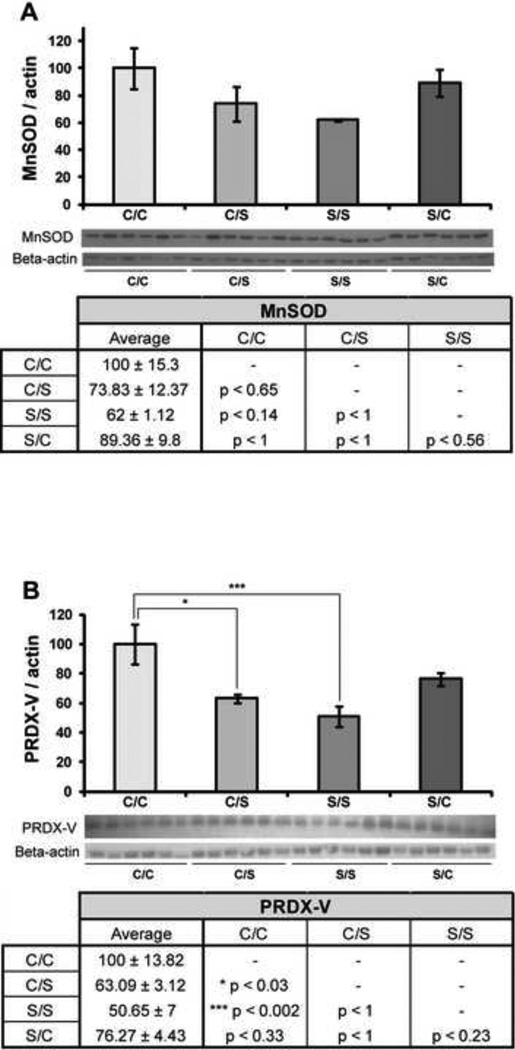
2.4 Soy diet after ovariectomy decreases expression of antioxidant enzymes
To determine the effects of diet in NHPs on antioxidant enzymes, we measured hippocampal expression of manganese superoxide dismutase (MnSOD) and peroxiredoxin-V (PRDX-V). There was no significant change in the hippocampal expression of MnSOD across the four diet paradigms (F3, 23 = 2.32, p < 0.11) (Figure 5A). However, a trend towards decreased MnSOD expression was observed in the NHPs which consumed a S/S diet compared to those which consumed a C/C diet (p < 0.14). Diet did exert a significant effect on PRDX-V expression in the hippocampus (F3, 23 = 6.62, p < 0.05) (Figure 5B). There was a significant decrease in PRDX-V expression between NHPs on a C/C diet and those on an S/S diet (*** p < 0.005). We also observed a significant decrease in PRDX-V expression between NHPs on a C/C diet and a C/S diet (* p < 0.05) (Figure 5B). There was a trend towards decreased PRDX-V expression in the S/S condition when compared to the S/C condition (p < 0.23) and in the S/C condition when compared to the C/C condition (p < 0.33), but neither change was significant.
Figure 5. Effect of diet on mitochondrial antioxidant enzymes.
Western blot analysis of MnSOD (A) and PRDX-V (B) expression was performed on NHP hippocampal samples from each of the four diet paradigms. Expression levels for each sample were normalized to beta-actin levels. Expression levels were then normalized to the C/C diet (C/C was set to 100%). Statistically significant differences were calculated using a two-tailed, one-way analysis of variance (ANOVA), followed by a Bonferroni post-hoc correction. Diet paradigm significantly affected PRDX-V expression in the NHP hippocampus. Bars represent % C/C ± S.E.M., n = 6 for each condition, p<0.05*, p<0.01**, p<0.005***.
2.5 Effects of pre- and post-ovariectomy diet on expression of mitochondrial fission and fusion proteins
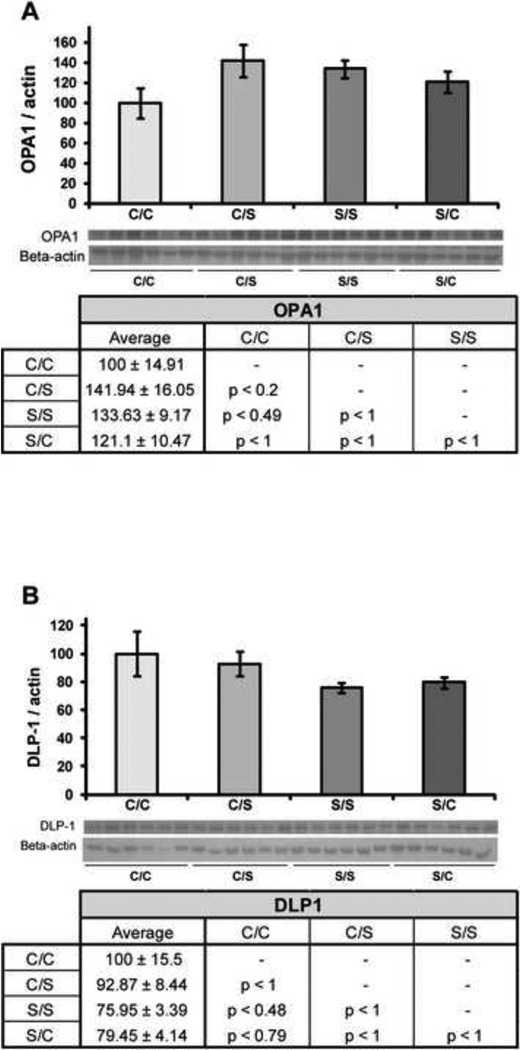
Effects of the four diet paradigms on mitochondrial dynamics were measured by looking at the expression of a protein which promotes fusion (optic atrophy 1; OPA1) and a protein which promotes fission (dynamin-like protein; DLP1). There was no significant effect of diet on hippocampal expression of OPA1 (F3, 23 = 1.98, p < 0.15) (Figure 6A), although a trend towards decreased hippocampal expression of OPA 1 was seen in NHP samples from the C/C diet paradigm when compared to both the C/S diet paradigm (p < 0.20). Likewise, there was no significant effect of diet on hippocampal expression of DLP1 (F3, 23 = 1.50, p < 0.25) (Figure 6B).
Figure 6. Effect of diet on mitochondrial fission and fusion.
Western blot analysis of OPA1 (A) and DLP-1 (B) expression was performed on NHP hippocampal samples from each of the four diet paradigms. Expression levels for each sample were normalized to beta-actin levels. Expression levels were then normalized to the C/C diet (C/C was set to 100%). Statistically significant differences were calculated using a two-tailed, one-way analysis of variance (ANOVA), followed by a Bonferroni post-hoc correction. Diet paradigm did not significantly affect expression of either protein involved in mitochondrial fission/fusion in the NHP hippocampus. Bars represent % C/C ± S.E.M., n = 6 for each condition.
3. Discussion
The effects of estrogens on brain biochemistry and function, alone or in combination with progestogens, are both complex and controversial. Recent reanalyses of data collected in the WHI and WHIMS trials (Henderson et al, 2005; Zandi et al, 2002) suggest that there are beneficial cognitive and neuroprotective effects of hormone therapy after menopause, but that these effects may be limited to women who begin taking hormone therapy during perimenopause or soon after menopause. Additionally, hormone therapy in postmenopausal women has been shown to increase the risk of breast cancer and stroke, and menopausal women must take these risks into consideration when deciding whether to initiate hormone therapy. Considering that soy isoflavones have the potential to bind and activate estrogen receptors, investigations within both the research and clinical communities have sought to determine whether dietary soy could serve as an alternative to estrogen therapy to alleviate hormone-related symptoms of menopause such as hot flashes, without the adverse proliferative effects. Further, there was interest as to whether soy isoflavones might be able to mimic the neuroprotective effects of 17β-estradiol seen in perimenopausal or early menopausal women.
Estrogen associates with estrogen receptors on neuronal membranes, exerting effects on neurons through PI3K signaling pathways which converge on the mitochondria to promote mitochondrial bioenergetics (for reviews see Brinton, 2008 and Brinton, 2009; Mannella and Brinton, 2006). Two general classes of estrogen receptors, ERα and ERβ, are found in the brain (Ishunina and Swaab, 2008). While ERα is generally understood to mediate the proliferative effects of estrogen signaling, and has a greater role in regulating reproduction, ERβ is more involved in regulating aspects of brain function related to cognition and is considered to oppose the proliferative effects of ERα (Brinton, 2009). Aging is known to affect signaling responses through these two receptor subtypes; in particular, ERα is more highly expressed in the brains of young female rats than old female rats (Waters et al, 2011), and old female rats are more responsive to estrogen signaling through the ERβ receptor (Zhao et al, 2002). Soy isoflavones are capable of signaling through both ERα and ERβ. Genistein (one of the principle soy isoflavones) has a chemical structure that is similar to 17β-estradiol, and although it has only a weak affinity for ERα, it is able to bind ERβ with nearly the same affinity as 17β-estradiol (reviewed in Kim et al, 2000; Kuiper et al, 1997; Kuiper et al, 1998). Thus, in a physiological state such as menopause when endogenous 17β-estradiol levels are low, soy isoflavones would be expected to be capable of acting as estrogen alternatives and exerting ERβ-mediated effects on neurons.
Zhao and Brinton reviewed eight randomized, placebo-controlled studies in humans investigating the effects of soy isoflavones on cognitive function (Zhao and Brinton, 2007). Data from these were inconclusive; of the eight studies, three showed no effect of soy isoflavones on all cognitive measures investigated, and five showed positive effects of soy isoflavones on some cognitive measures and neutral effects on others (Zhao and Brinton, 2007). In particular, the studies which indicated a positive effect of soy diet saw the greatest response on measures of verbal memory and executive function (Zhao and Brinton, 2007). Notably, these are two domains of cognitive function which are affected early in the development of AD (Henderson and Brinton, 2010).
Findings from the Brinton group indicate that a mitochondrial bioenergetic crisis precedes the development of pathology in the triple-transgenic mouse model of AD (Yao et al, 2009). Additionally, Yao and colleagues found that the decline in the bioenergetic capacity of the brain, represented by a decline in aerobic glycolysis and mitochondrial respiration, coincided with reproductive senescence in their mouse model. Mice showed a significant decline in the activity of PDH and complex-IV, indicating that the neurons are no longer metabolizing glucose and generating ATP efficiently after estrogen depletion. There is also evidence showing that estrogen can act upon the mitochondria to increase glucose metabolism and sustain enzymatic activity of many of the mitochondrial proteins involved in ATP generation (for reviews see Brinton, 2008b and Simpkins, 2010). Based on this data linking estrogen, mitochondrial function, and neurodegenerative disease, as well as the fact that soy isoflavones are capable of signaling through estrogen receptors and exerting estrogenic effects, the present study evaluated the effects of dietary soy protein containing isoflavones vs. casein-lactalbumin on hippocampal content of proteins involved in mitochondrial bioenergetics in a NHP model. Additionally, this study assessed the effects of consuming a diet containing soy isoflavones prior to or after reproductive senescence (ovariectomy) in female NHPs. Our data suggest that (a) timing of soy diet initiation (i.e., before or after ovariectomy) influenced steady-state expression of enzymes involved in glycolysis and the TCA cycle, (b) soy diet had no effect on the evaluated protein markers of energy-generating capacity of the mitochondria, and (c) soy diet reduced hippocampal levels of antioxidant enzymes, perhaps indicating a reduction in oxidative stress.
Results from the current study indicate that consuming a soy diet prior to ovariectomy and switching to a casein/lactalbumin diet after ovariectomy may increase hippocampal levels of GAPDH, an enzyme involved in the breakdown of glucose, and PDHe1α, the enzyme which links glycolysis and the TCA cycle through conversion of pyruvate to acetyl-CoA. The significance of this finding is unclear, although it is important to note that dietary soy protein produced an increase in insulin sensitivity in these monkeys during the postmenopausal phase (Walker et al, 2008). Results also indicate an increase in SDHα expression when the diet included soy isoflavones after ovariectomy. Overall, these increases and decreases in glycolytic/TCA cycle protein expression may balance out, as there was no ensuing increase or decrease in the expression of any of the oxidative phosphorylation enzyme complexes. As such, more research would be necessary to fully clarify these findings.
We observed no effect of diet paradigm on expression levels of mitochondrial enzyme complexes I–Vα in the hippocampus. Complexes I–III and Vα showed a trend towards increased expression in the hippocampus of NHPs consuming the C/S diet compared to the other three diet paradigms; however, the increase was minimal and not statistically significant.
In contrast, antioxidant expression did appear to be influenced by diet paradigm. In both diets containing soy isoflavones after ovariectomy, NHPs showed a significant decrease in hippocampal expression of the antioxidant enzyme PRDX-V, which is involved in catalysis of hydrogen peroxide (H2O2). Further, there was a moderate (but not statistically significant) decrease in expression of MnSOD, which catalyzes the breakdown of superoxide anion into water and H2O2. This decrease in antioxidant enzymes may be the result of the natural antioxidant properties of soy decreasing the cell’s reliance on its mitochondrial antioxidant mechanisms.
Notably, previous studies of iliac artery biopsies obtained at the time of ovariectomy from these same animals demonstrated that arterial expression levels of several inflammatory markers (monocyte chemotactic protein-1, intercellular adhesion molecule-1, and interleukin-6) were lower the group which consumed the soy protein diet pre-ovariectomy (Walker et al, 2008). This may further explain the reduction in antioxidant enzyme expression that we observed in our study, as a decrease in inflammation of the cardiovascular system would be expected to have a positive impact on brain by reducing exposure to inflammatory cytokines. In addition, the soy isoflavone-based diet was shown to have beneficial effects on atherosclerosis, and it decreased atherosclerotic plaque size, total plasma cholesterol, and non-high-density lipoprotein (HDL) cholesterol independent of its effects on plasma lipoproteins (Walker et al, 2008). This led to NHPs having better cardiovascular health at ovariectomy (Walker et al, 2008). There is strong evidence of a connection between vascular health and neurological health, and cardiovascular disease is known to affect cognitive networks and increase with the risk of AD, particularly in cases where AD pathology is still mild (Chui, 2006). Thus, a diet which promotes cardiovascular health would be expected to also promote cognitive health.
In summary, data from our current study suggest that a soy diet did not significantly alter the measured indices of mitochondrial bioenergetics in vivo. Nevertheless, soy diet may be associated with decreased oxidant stress on hippocampal neurons, as indicated by lower expression of antioxidant enzymes. This effect may be mediated by the decreased vascular inflammation and improvement in cardiovascular health that occur with consumption of a soy protein-based diet containing isoflavones.
4. Experimental Procedures
4.1 Animals and diet design
Female cynomolgus macaques (Macaca fascicularis) were obtained as adults (average age of 12–15 years; equivalent to 36 – 45 human years) from Indonesia. Adult status was confirmed by evidence of epiphyseal closure. Following their arrival, monkeys were individually housed during a 30-day quarantine, after which they were placed into social groups of 5–6 animals.
During the quarantine period, monkeys were fed monkey chow (Ralston Purina). Once they were placed into social groups, they were randomized to be fed either 1) a casein/lactalbumin diet with wheat protein, or 2) a soy protein diet with 1.88 mg aglycone isoflavones/g protein (SUPRO® SOY isolated Soy protein, Solae St. Louis MO) (Figure 1A). Both diets were formulated to be atherogenic, and they contained the same calorie consumption for carbohydrates (46%), lipids (35%), cholesterol (0.20 mg/kg mass) and protein (19%) with 120 calories consumed/kg body weight (Table 1). NHP consumed the diet that they were initially randomized to for 2 years, after which point they underwent ovariectomy (OVX). Following ovariectomy, monkeys either remained on the same diet or were switched to the alternate diet. This resulted in four different diet paradigms: 1) casein/lactalbumin prior to and after OVX ((C/C); n = 6); 2) casein/lactalbumin prior to and soy isoflavone after OVX ((C/S); n = 6) S); 3) soy isoflavone prior to and after OVX ((S/S); n = 6); 4) soy isoflavone prior to and casein/lactalbumin after OVX ((S/C); n = 6) (Figure 1).
TABLE 1.
Composition of the experimental diets
| Ingredient | Casein/lactalbumin diet, g/100g |
Soy protein isolate diet, g/100g |
|---|---|---|
| Casein, USP | 8.5 | - |
| Lactalbumin | 8.5 | - |
| Soy protein isolate | - | 17.09 |
| DL-Methionine | - | 0.30 |
| Wheat flour, self-rising | 35.76 | 35.76 |
| Dextrin | 9.00 | 8.90 |
| Sucrose | 7.00 | 7.00 |
| Alphacel | 8.03 | 8.23 |
| Lard | 5.00 | 5.00 |
| Beef tallow | 4.00 | 4.00 |
| Butter, lightly salted | 3.10 | 3.10 |
| Safflower oil (linoleic) | 3.00 | 2.56 |
| Crystalline cholesterol | 0.06 | 0.06 |
| Complete vitamin mix | 2.50 | 2.50 |
| Ausman-Hayes mineral mix | 5.00 | 5.00 |
| Calcium carbonate | 0.40 | 0.36 |
| Calcium phosphate, monobasic | 0.15 | 0.15 |
Soy protein was derived from SUPRO® SOY Isolated Soy Protein (Solae, St. Louis, MO)
After 2 years on the post-OVX diet, NHP were euthanized and brains were collected, with the right hemisphere formalin fixed and the left hemisphere cut into 2 mm slices and snap frozen. Hippocampal punches were taken from the left hemisphere slices to be analyzed by Western blot. The punches encompassed the whole anterior-to-posterior extent of the left hippocampus, including all sub-regions, and measured 1 cm thick by 2 mm deep. All procedures were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and regulations and guidelines established by the Wake Forest University Animal Care and Use Committee. Wake Forest University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
4.2 Western blot
Protein was extracted from hippocampal tissues using Tissue Protein Extraction Reagent (Thermoscientific, Rockford, IL) with phosphatase and protease inhibitors (Sigma, St. Louis, MO), and protein concentrations were determined with the Bio-Rad Bradford assay. 20–50 mg of protein was loaded per well on 12.5% SDS PAGE criterion gels (Bio-Rad, Hercules, CA). Proteins were transferred to PVDF membranes and probed with primary antibodies as listed in Table 2. Antibodies were then each probed with their corresponding HRP-conjugated secondary antibody (1:10000 dilution, Vector Laboratories, Burlingame, CA) for 1 hour at room temperature. All membranes were stripped once for 15–20 minutes with stripping buffer, and were then re-probed with antibody/secondary antibody as described above for either another mitochondrial protein or β-actin/β-tubulin. β-actin (1:5000, mouse) (MS1501, Millipore, Billerica MD) or β-tubulin (1:3000, mouse) (ABCAM, Cambridge, MA, (AB6046)) was used as a loading control. Bands were visualized using an ECL kit (Thermoscientific, Pittsburgh PA) followed by a TMB colorimetric kit (Vector Laboratories, Burlingame, CA). The relative intensities of the immunoreactive bands were captured using a Molecular Imager ChemiDoc XRS+ System (Bio-Rad, Hercules, CA) and quantified with Quantity One Analysis Software, Version 4.6.4 (Bio-Rad, Hercules, CA).
Table 2.
List of antibodies used for Western blotting
| Antibody | Size | Catalog # | Company | Dilution | Species | Reference |
|---|---|---|---|---|---|---|
| aComplex I | 20 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | Liang et al, 2008 |
| aComplex II-FeS subunit | 30 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | |
| aComplex III subunit, Core 2 | 46 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | |
| aComplex IV subunit II | 22 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | |
| aComplex Vα | 55 KDa | MS601 | Mitosciences, Eugene, OR | 1/1000 | Mouse | |
| Dynamin-like protein-1 (DLP-1) | 79–84 KDa | 611113 | BD Biosciences, San Jose, CA | 1/1000 | Mouse | Wang et al, 2008 |
| Glucose transporter-4 (Glut-4) | 55 KDa | 07-140455 | Millipore, Billerca, MD | 1/1000 | Rabbit | Weisová et al, 2009 |
| Glyceraldehyde phosphate dehydrogenase (GAPDH) | 38 KDa | AB8245 | ABCAM, Cambridge, MA | 1/1000 | Mouse | Thomson et al, 2010 |
| Isocitrate dehydrogenase 2 (IDH2) | 44 KDa | H00003418 | Novus Biologicals, Littleton, CO | 1/1000 | Mouse | Brinton Lab, unpublished data |
| Manganese superoxide dismutase (MnSOD) | 25 KDa | 611581 | BD Biosciences, San Jose, CA | 1/1000 | Mouse | Irwin et al, 2008 |
| Optic atrophy protein-1 (OPA1) | 80, 100 KDa | 612607 | BD Biosciences, San Jose, CA | 1/1000 | Mouse | Wang et al, 2008 |
| Peroxiredoxin-V (PRDX-V) | 15 KDa | 612085 | BD Biosciences, San Jose, CA | 1/1000 | Mouse | Irwin et al, 2008 |
| Pyruvate dehydrogenase e1α subunit | 44 KDa | MSP03 | Mitosciences, Eugene, OR | 1/1000 | Mouse | Yao et al, 2009 |
| Succinate dehydrogenase α (SDHα) | 78 KDa | SC-59687 | Santa Cruz Biotechnology, Santa Cruz, CA | 1/1000 | Mouse | Costford et al, 2008 |
These antibodies were purchased from MitoSciences as MitoProfile® Total OXPHOS Human WB Antibody Cocktail (Catalog # MS601).
4.3 Statistical analysis
Data are presented as group mean normalized to the casein/casein (C/C) group ± S.E.M. Statistically significant differences between diet paradigms were obtained by a two-tailed one-way analysis of variance (ANOVA) assuming unequal variances, followed by a Bonferroni post-hoc correction. All statistical analysis was conducted using Origin data analysis and graphing software (OriginLab Corporation, Northampton, MA).
Acknowledgements
The authors would like to thank Dr. Jon Nilsen for his contributions to this study. This research was supported by HL 45666 (JRK), HL 079421 (JRK), NIA USC ADRC pilot grant to RDB (P50AG005142 H. Chui PI), and Norris Foundation to RDB.
Abbreviations
- AD
Alzheimer’s disease
- DLP-1
Dynamin like protein-1
- ER
Estrogen receptor
- Glut-4
Glucose transporter type 4
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- ICAM-1
Intercellular adhesion molecule-1
- IL-6
Interleukin-6
- IDH2
Isocitrate dehydrogenase-2
- MnSOD
Manganese superoxide dismutase
- NHP
Non-human primate
- OPA-1
Optic atrophy-1
- OVX
Ovariectomy
- PRDX-V
Peroxiredoxin-V
- PDHe1α
Pyruvate dehydrogenase e1α subunit
- SDHα
Succinate dehydrogenase α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boron WF, Boulpaep EL. Medical Physiology. second ed. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008a;31(10):529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: Therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev. 2008b;60(13–14):1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–222. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Chui HC. Vascular cognitive impairment: today and tomorrow. Alzheimers Dement. 2006;(3):185–194. doi: 10.1016/j.jalz.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Costford SR, Chaudhry SN, Crawford SA, Salkhordeh M, Harper ME. Long-term high-fat feeding induces greater fat storage in mice lacking UCP3. Am J Physiol Endocrinol Metab. 2008;295(5):E1018–E1024. doi: 10.1152/ajpendo.00779.2007. [DOI] [PubMed] [Google Scholar]
- de Kleijn MJ, van der Schouw YT, Wilson PW, Adlercreutz H, Mazur W, Grobbee DE, Jacques PF. Intake of dietary phytoestrogens is low in postmenopausal women in the United States: the Framingham study(1–4) J Nutr. 2001;131:1826–1832. doi: 10.1093/jn/131.6.1826. [DOI] [PubMed] [Google Scholar]
- Dixon RA. Phytoestrogens. Annu Rev Plant Biol. 2004;55:225–261. doi: 10.1146/annurev.arplant.55.031903.141729. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57(2):335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Bernstein L. The international variation in breast cancer rates: an epidemiological assessment. Breast Cancer Res Treat. 1991;5:901–906. doi: 10.1007/BF02633520. [DOI] [PubMed] [Google Scholar]
- Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA MIRAGE Study Group. Postmenopausal hormone therapy and Alzheimer's disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76(1):103–105. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, Brinton RD. Menopause and mitochondria: windows into estrogen effects on Alzheimer's disease risk and therapy. Prog Brain Res. 2010;182:77–96. doi: 10.1016/S0079-6123(10)82003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149:3167–3175. doi: 10.1210/en.2007-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Swaab DF. Estrogen receptor-alpha splice variants in the human brain. Gynecol Endocrinol. 2008;24(2):93–98. doi: 10.1080/09513590701705148. [DOI] [PubMed] [Google Scholar]
- Kim H, Xia H, Li L, Gewin J. Attenuation of neurodegeneration-relevant modifications of brain proteins by dietary soy. Biofactors. 2000;12(1–4):243–250. doi: 10.1002/biof.5520120137. [DOI] [PubMed] [Google Scholar]
- Kostanyan A, Nazaryan K. Rat brain glycolysis regulation by estradiol-17 beta. Biochim Biophys Acta. 1992;1133:301–306. doi: 10.1016/0167-4889(92)90051-c. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Kurzer MS, Xu X. Dietary phytoestrogens. Annu Rev Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, Niedzielko TL, Schneider LE, Mastroeni D, Caselli R, Kukull W, Morris JC, Hulette CM, Schmechel D, Rogers J, Stephan DA. Alzheimer's disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci U S A. 2008;105(11):4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Resnick SM. Longitudinal effects of estrogen replacement therapy on PET cerebral blood flow and cognition. Neurobiol Aging. 2000;21(2):373–383. doi: 10.1016/s0197-4580(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci. 2006;26:9439–9447. doi: 10.1523/JNEUROSCI.1443-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskarinec S. The effect of phytoestrogens on hot flashes. Nutrition Bytes. 2003;9(2):5. [Google Scholar]
- Nilsen J, Irwin RW, Gallaher TK, Brinton RD. Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci. 2007;27:14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of The North American Menopause Society. Menopause. 2010;17(2):242–255. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Cole WC, Prasad KC. Risk factors for Alzheimer's disease: role of multiple antioxidants, non-steroidal anti-inflammatory and cholinergic agents alone or in combination in prevention and treatment. J Am Coll Nutr. 2002;21(6):506–522. doi: 10.1080/07315724.2002.10719249. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U.S.A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Clarkson TB. The unique value of primate models in translational research. Nonhuman primate models of women's health: introduction and overview. Am J Primatol. 2009;71(9):715–721. doi: 10.1002/ajp.20720. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yang SH, Sarkar SN, Pearce V. Estrogen actions on mitochondria--physiological and pathological implications. Mol Cell Endocrinol. 2008;29:51–59. doi: 10.1016/j.mce.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins JW, Yi KD, Yang SH, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Biochim Biophys Acta. 2010;1800(10):1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson SE, McLennan SV, Hennessy A, Boughton P, Bonner J, Zoellner H, Yue DK, Twigg SM. A novel primate model of delayed wound healing in diabetes: dysregulation of connective tissue growth factor. Diabetologia. 2010;53(3):572–583. doi: 10.1007/s00125-009-1610-6. [DOI] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011 doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SE, Register TC, Appt SE, Adams MR, Clarkson TB, Chen H, Isom S, Franke AA, Kaplan JR. Plasma lipid-dependent and -independent effects of dietary soy protein and social status on atherogenesis in premenopausal monkeys: implications for postmenopausal atherosclerosis burden. Menopause. 2008;15(5):950–957. doi: 10.1097/gme.0b013e3181612cef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105(49):19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisová P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci. 2009;29(9):2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC. Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. Structure-based virtual screening for plant-based ERβ-selective ligands as potential preventative therapy against age-related neurodegenerative diseases. J Med Chem. 2005;48:3463–3466. doi: 10.1021/jm0490538. [DOI] [PubMed] [Google Scholar]
- Zhao L, Brinton RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Rev Neurother. 2007;7(11):1549–1564. doi: 10.1586/14737175.7.11.1549. [DOI] [PubMed] [Google Scholar]
- Ziegler RG. Phytoestrogens and breast cancer. Am J Clin Nutr. 2004;79:183–184. doi: 10.1093/ajcn/79.2.183. [DOI] [PubMed] [Google Scholar]