Abstract
Introduction:
Zebrafish are emerging as a powerful animal model for studying the molecular and physiological effects of nicotine exposure. The zebrafish have many advantageous physical characteristics, including small size, high fecundity rates, and externally developing transparent embryos. When combined with a battery of molecular–genetic tools and behavioral assays, these attributes enable studies to be conducted that are not practical using traditional animal models.
Methods:
We reviewed the literature on the application of the zebrafish model as a preclinical model to study the biological effects of nicotine exposure.
Results:
The identified studies used zebrafish to examine the effects of nicotine exposure on early development, addiction, anxiety, and learning. The methods used included green fluorescent protein–labeled proteins to track in vivo nicotine-altered neuron development, nicotine-conditioned place preference, and locomotive sensitization linked with high-throughput molecular and genetic screens and behavioral models of learning and stress response to nicotine. Data are presented on the complete homology of all known human neural nicotinic acetylcholine receptors in zebrafish and on the biological similarity of human and zebrafish dopaminergic signaling.
Conclusions:
Tobacco dependence remains a major health problem worldwide. Further understanding of the molecular effects of nicotine exposure and genetic contributions to dependence may lead to improvement in patient treatment strategies. While there are limitations to the use of zebrafish as a preclinical model, it should provide a valuable tool to complement existing model systems. The reviewed studies demonstrate the enormous opportunity zebrafish have to advance the science of nicotine and tobacco research.
Introduction
The World Health Organization has declared tobacco-caused disease a global epidemic leading to an estimated 8 million annual deaths worldwide by the year 2030 (World Health Organization, 2008). Morbidity and mortality from tobacco abuse and dependence are most profoundly impacted by prevention accomplished through the treatment of tobacco dependence. Advances in tobacco dependence research have identified genetic variants associated with nicotine addiction (Li, 2008) and three pharmacological agents (nicotine, bupropion, and varenicline) that have aided patients in achieving abstinence (Burke, Ebbert, & Hays, 2008; Ebbert, Wyatt, Hays, Klee, & Hurt, 2010; Jiménez-Ruiz, Berlin, & Hering, 2009). However, rates of relapse remain high (Lancaster, Hajek, Stead, West, & Jarvis, 2006), with up to 90% of cigarette smokers who quit resuming use within one year (Garrett, Rose, & Henningfield, 2001), and few innovative strategies exist to prevent relapse (Hajek, Tønnesen, Arteaga, Russ, & Tonstad, 2009). Prolonged success in treating patients for tobacco dependence may rely on expanding knowledge of the neurophysiologic changes that occur with nicotine exposure. Danio rerio (zebrafish) are a useful model for the preclinical study of nicotine and tobacco use.
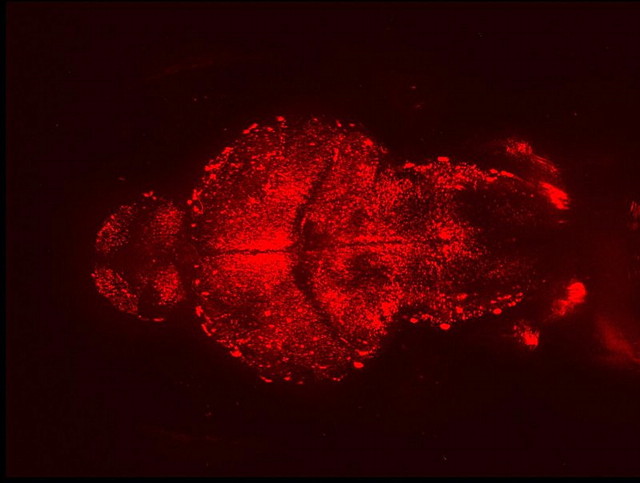
In the last eight years, zebrafish have emerged as an alternative preclinical model for behavioral studies of nicotine exposure (Figure 1). Zebrafish possess many innate characteristics that are advantageous in research models, including small physical size (∼2.5 cm in length), high reproduction rates (100–300 embryos per mating or “clutch”), and rapid cycle time (females can lay eggs every week) allowing for cost-effective investigations (Zon, 1999). Zebrafish embryos develop externally and are transparent through the larval stage, 14 days postfertilization (dpf). The transparency enables fluorescently labeled proteins to be used for in vivo monitoring of temporal and spatial protein expression patterns during development (Figure 2; Okamoto, Sato, & Aizawa, 2008). This method can be extended into adult fish assays using an available transparent adult zebrafish strain (White et al., 2008). In addition, an extensive set of molecular tools exists to manipulate the zebrafish genome for screening studies. Changes in phenotypes or behavioral assay responses can be linked to random DNA mutations (i.e., forward genetic screen) and site-specific mutations or gene knockdowns (i.e., reverse genetic screen). Zebrafish can also be used to screen for chemical compounds (e.g., pharmacotherapies) that may modulate disease states (Pardo-Martin et al., 2010; Zon & Peterson, 2010).
Figure 1.
A wild-type adult zebrafish. Figure reprinted with permission (Ekker, 2008).
Figure 2.
Example of specific protein labeling in larval zebrafish. This is a dorsal view of a day 5 larval zebrafish head with anterior side to the left. Brain expression of GABAB receptor protein is labeled by red fluorescent protein using a gene-trapping transposon.
Despite these advantages, several limitations exist when using zebrafish as a model for preclinical studies of nicotine and tobacco. As a nonmammalian vertebrate, the zebrafish is evolutionarily more distant from humans than rodent models but evolutionarily closer to humans than other nonvertebrate models, such as yeast, worm, or fruit fly. The zebrafish genome developed from an additional duplication event in fish, sometimes introducing a pair of genes arising from a single gene in the closest ancestor, where one of the two zebrafish genes are not represented in the human genome. Many of the traditional behavioral paradigms used in addiction research have only recently been introduced in zebrafish and thus lack the same rich history of development and publication such as that found in the rodent literature. The information available on drug absorption and metabolism rates in zebrafish is limited and requires more study. Morphologically, the neural anatomy of the zebrafish while described at a gross level is not fully defined at a detailed level, making comprehensive comparisons with mammalian structures currently difficult. For example, while the cholinergic system is conserved between humans and teleosts (Ninkovic & Bally-Cuif, 2006), specific neural structures, including the nuclear accumbens (NAc) and ventral tegmental area (VTA), have not been explicitly annotated in the zebrafish. These neural regions are involved in mammalian addiction and reward response, and identification of analogous structures would further legitimize zebrafish as a model for the study of addiction. However, the identity of the fish dopamine system that has functions similar to the mammalian mesolimbic (VTA-NAc) dopamine system is unclear. Rink and Wullimann (2001) propose that the posterior tubercle of teleosts may include cells functionally analogous to mesolimbic dopaminergic neurons in mammals; however, this is controversial (Panula et al., 2010). Conversely the habenula brain structure, which has recently been suggested to play a key regulatory role in addiction response, is well annotated and conserved between zebrafish and humans (Agetsuma et al.). The zebrafish as a preclinical model has advantages and limitations, which will define its optimal role in nicotine and tobacco research as its use matures in the field.
Despite these limitations, recent technological advances and several innate characteristics described previously render the zebrafish a remarkable tool for scientific advancement. Behavioral assays are actively being developed that facilitate the use of zebrafish to study many of the components associated with addiction. Zebrafish are used to study a wide range of human diseases, including but not limited to cardiovascular disease, leukemia, neurodegenerative disease, and solid tumor cancers (Chico, Milo, & Crossman, 2010; Payne & Look, 2009; Sager, Bai, & Burton, 2010; Taylor & Zon, 2009). Zebrafish are also being utilized to advance the field of nicotine research through investigations into neural nicotinic acetylcholine receptors (nAChRs) and studies of the behavioral effects of nicotine (Table 1). Rodent models (i.e., rats and mice) have traditionally dominated the preclinical study of addiction, including the rewarding effect of the addictive drug, tolerance, and withdrawal (O’Dell & Khroyan, 2009). The use of rodent models has been expertly reviewed elsewhere (Changeux, 2010; O’Dell & Khroyan, 2009; Zaniewska, Przegaliński, & Filip, 2009). To provide context to the existing body of research, specific examples of other models will be cited when presenting zebrafish models where warranted. The objective of this manuscript is to inform investigators of the advantages and disadvantages associated with using zebrafish as a preclinical model and to review the current published literature using zebrafish behavioral assays to study nicotine effects on biology.
Table 1.
Nicotine Research With the Zebrafish Model
| Study focus | Assay | Author | Year |
| Nicotine exposure | |||
| Embryonic development | Growth rate, swimming behavior, morphology | Svoboda | 2002 |
| Growth rate, swimming behavior, morphology | Parker | 2007 | |
| Morphology via green fluorescent protein-tagged imaging | Evodkia | 2008 | |
| Morphology, mutant testing | Welsh | 2009 | |
| Muscle-bend assay | Thomas | 2009 | |
| Startle response | Eddins | 2010 | |
| Addiction | Conditioned Place Preference | Kily | 2008 |
| Locomotive assay, mutant screening | Petzold | 2009 | |
| Anxiolytic effects | Swimming distance, tank localization | Levin | 2007 |
| Dive response | Bencan | 2008 | |
| Dive response (tank diving) | Bencan | 2009 | |
| Cognitive function | Spatial discrimination choice assay | Levin | 2004 |
| Three-chamber choice assay | Levin | 2006 | |
| Three-chamber choice assay | Eddins | 2009 | |
| Conditioned fear assay | CastRo | 2009 | |
| Nicotinic acetylcholine receptors | |||
| Receptor characterization | α4 and α6 by reverse transcription–polymerase chain reaction and in situ hybridization | Ackermann | 2009 |
| β2 receptor by immunohistochemistry | Welsh | 2009 | |
Embryonic Development
Human fetal and infant health hazards associated with maternal tobacco exposure include reduced birth weight, impaired growth, juvenile neurobehavioral disorders, stillbirths, and sudden infant death syndrome (; DiFranza, Aligne, & Weitzman, 2004; Rogers, 2008). Despite advances in identifying associations between tobacco exposure and abnormal fetal development, the mechanisms underlying these defects and potential causative role of nicotine, the addictive substance in tobacco (Stolerman & Jarvis, 1995), continue to be studied. Causality of deleterious developmental effects from nicotine can ethically be explored through the use of animal models (Dwyer, Broide, & Leslie, 2008). External development of transparent embryos make zebrafish an excellent model for studying developmental biology and embryogenesis (Fitch, 1970; Grunwald & Eisen, 2002). nAChRs are known to be expressed in zebrafish embryos and mediate nicotine-induced alterations in embryonic morphology (Parker & Connaughton, 2007).
A pioneering study of nicotine effects on neural development demonstrated that exposure early in embryonic development induced axons path-finding errors (Svoboda, Vijayaraghavan, & Tanguay, 2002). Zebrafish were exposed to 5, 15, and 33 μM nicotine, with 33 μM selected for most experiments because it elicited the strongest alteration in embryonic zebrafish behavior. At 33 μM, the embryos displayed a reduction in green fluorescent protein (GFP) expression in motorneurons, while behaviorally, the embryos could respond to touch but were unable to swim. Starting 22 hr postfertilization, just prior to extension of axons in secondary motor neurons, the nicotine-exposed zebrafish embryos displayed swimming paralysis but maintained physical response to tactile stimulation, prompting investigation into the effects of nicotine on motorneuron development (Svoboda et al., 2002). Neural cell development has been monitored using two immunohistochemical assays and one transgenic zebrafish line (islet-1) expressing GFP-labeled spinal secondary motoneurons (Higashijima, Hotta, & Okamoto, 2000). The use of GFP-tagged proteins in translucent embryos enables high-resolution imaging modalities to be used to detect cellular expression patterns. Imaging results suggest that nicotine delays differentiation of spinal motoneurons and induced axon path-finding errors. Results are supported by observations of neural differentiation at 120 hr postfertilization (hpf) in embryos extracted (i.e., rescued) from nicotine exposure at 66 hpf. The involvement of neural nAChRs was confirmed by the elimination of the nicotine-induced phenotype when using two nAChR antagonists. This study proved formative in the use of zebrafish as a general model for nicotine studies and provided the basis for subsequent work describing the impact of nicotine on embryonic development.
Nicotine exposure alters muscle development and swimming behavior in embryonic zebrafish. The physical response of embryonic zebrafish to nicotine is biphasic with acute exposure inducing rhythmic muscular bending and prolonged nicotine exposure inducing paralysis (Svoboda et al., 2002; Thomas, Welsh, Galvez, & Svoboda, 2009). The early muscular bend response, or twitch-like motion associated with early embryonic movement, may reflect exogenous nicotine activation of presumably dormant embryonic nAChRs in the spinal motor circuit (Thomas et al., 2009). The subsequent paralysis results from altered skeletal muscle development mediated by nicotine binding to muscular nAChRs (Welsh, Tanguay, & Svoboda, 2009). The involvement of nicotine binding to the muscular nAChRs has been confirmed by observations that paralysis is not induced by nicotine exposure in mutant zebrafish that lack muscular nAChRs (Ono, Higashijima, Shcherbatko, Fetcho, & Brehm, 2001). These findings reflect previously reported identification of nAChRs in spinal motor neurons in other vertebrates. Exogenous acetylcholine was reported to effect motor response in Xenopus embryos (Panchin Yu, Perrins, & Roberts, 1991) and dihydro-β-erythroidine (DHβE; an α4β2 antagonist) reported to inhibit spinal cord muscle response in embryonic chicks (Milner & Landmesser, 1999). The ease of in vivo visualization of developing neural structure in response to nicotine exposure presented in these studies highlights how the zebrafish model may provide advantages over existing mammalian model systems for the study of abnormal development.
Zebrafish studies have begun to highlight potential long-term effects of nicotine exposure during development. Persistent physiological changes in adult fish behavior resulting from embryonic nicotine exposure have been independently reported in two studies. The first study measured an increase in adult fish (140 dpf) startle response, when the fish were exposed to nicotine (15 and 25 μM) at 120 hpf (Eddins, Cerutti, Williams, Linney, & Levin, 2009). Startle response assays were used, which measure the time to response following an intense stimulation by a push-solenoid generated tap, and are often used as an indication of neurological changes following an event or exposure. In the second study, early neural differentiation and axon path-finding errors previously reported in embryonic zebrafish (Svoboda et al., 2002) were found to persist in juvenile fish (17–22 dpf) rescued from nicotine exposure at 72 hpf (Menelaou et al., 2008). Findings of long-term effects following prenatal nicotine exposure have been reported in rodents. Serotonin receptor response in adolescent rats to acute nicotine exposure and withdrawal was altered in rats exposed to nicotine throughout prenatal development (Slotkin, Tate, Cousins, & Seidler, 2006), and prenatal nicotine exposure induced greater locomotive activity in adolescent male rats following nicotine exposure (Shacka, Fennell, & Robinson, 1997). Similar observations of prenatal nicotine exposure impacting postnatal behavior across species suggests that this is a highly conserved phenomenon, supporting the use of zebrafish as a model to study the human health hazards of embryonic nicotine exposure.
The discovery of adverse effects of nicotine during embryonic development and altered neural adult states following brief nicotine exposure in zebrafish may have important human health implications. These findings complement reports in mammalian models on fetal nicotine exposure causing reduction in growth rates (Wang et al., 2009) and brain development problems (Mao et al., 2008 and continue to strengthen the case for limiting fetal exposure to maternal smoking. Zebrafish models also provide an avenue for studying the isolated effects of other toxic compounds found in cigarette smoke and exploring the effect of environmental tobacco smoke on early fetal development.
Drug Addiction and Dependence
The reinforcing effects of nicotine acting through neural nAChRs are believed to induce persistent neurological changes leading to tobacco dependence (Wonnacott, Sidhpura, & Balfour, 2005). Substantial knowledge regarding the neural molecular properties of addiction has been obtained using animal models (Changeux, 2010; O’Dell & Khroyan, 2009; Zaniewska et al., 2009). Behavioral assays have been developed in a number of animal models to measure many of the known physiological manifestations associated with drug addiction, including locomotor sensitization (Benwell & Balfour, 1992; Kalivas & Stewart, 1991), conditioned place preference (CPP; Tzschentke, 1998), self-administration (Stairs, Neugebauer, & Bardo, 2010), and withdrawal (André, Gulick, Portugal, & Gould, 2008). Behavioral assays define changes induced by drug exposure and enable differential molecular analysis of the resulting transcriptional (i.e., gene expression) and epigenetic states (Kane, Konu, Ma, & Li, 2004; Kumar et al., 2005; Levine et al., 2005; Nestler, 2008; Renthal et al., 2007; Romieu et al., 2008; Shen et al., 2008). As the reinforcing properties of addictive drugs are highly conserved, behavioral assays have been adapted to zebrafish to study addiction to cocaine (Darland & Dowling, 2001), ethanol (Lockwood, Bjerke, Kobayashi, & Guo, 2004; Peng et al., 2009), amphetamines (Ninkovic & Bally-Cuif, 2006; Webb et al., 2009), and opiates (Bretaud et al., 2007; Lau, Bretaud, Huang, Lin, & Guo, 2006; Sanchez-Simon & Rodriguez, 2008). A broad perspective review of zebrafish use in the study of addiction and other neuropsychiatric disorders has been published (Mathur & Guo, 2010).
Zebrafish behavioral models have been used to study the molecular and genetic components of nicotine addiction (Kily et al., 2008; Petzold et al., 2009). CPP is a behavioral model used to study the rewarding and reinforcing properties of drugs of addiction. This model measures how exposing animals to addictive drugs in a training environment with discernable cues changes the choice of preferred location (i.e., preference) during the testing phase in an environment that has the same cues in only a subset of the total space. A CPP assay using zebrafish to study the effects of nicotine and ethanol exposure has identified responsive changes in gene expression common to both drugs (Kily et al., 2008). In this study, testing tanks were divided into two distinct locations using different visual cues and a baseline-preferred location was determined. Fish were subsequently restricted to the nonpreferred side of the tank and exposed to either nicotine or ethanol. Place preference testing was performed the next day during which fish displayed a change in preference, spending more time on the side of the tank where the drug exposure had occurred (i.e., nonpreferred side at baseline). This place preference persisted even when fish were exposed to adverse stimuli, such as removal from water for 3 s. However, preference induced by ethanol exposure was diminished by the adverse stimuli. Interestingly, zebrafish exhibited no decrease in CPP to nicotine when subjected to adverse stimuli. Place preference following repeated exposures to the addictive drugs (4-week training session) persisted even following prolonged periods of nicotine or alcohol abstinence (3 weeks); however, nicotine-induced preference for tank locations decreased with increasing period of abstinence but continued to be statistically significant after 21 days of abstinence from the nicotine exposure. The differential expression analysis identified 128 genes with similar changes (twofold) in transcriptional activity following nicotine and ethanol CPP assays. This included genes associated with nine Gene Ontology Biological Processes (http://www.geneontology.org/), including protein modification/ubiquitination, transcription/translation, neurotransmission/synaptic plasticity, synaptic plasticity/structural, signal transduction, cell cycle apoptosis, steroid metabolism, ion/protein transport, and metabolism. Many of these processes, including synaptic plasticity and neurotransmission, are proposed to be involved in the establishment of long-term modification associated with exposure to drugs of abuse. These observations demonstrate the ability of adult zebrafish to incur nicotine-mediated molecular alterations and exhibit behaviors consistent with drug dependence.
Genetic loci modifying the response of zebrafish to nicotine-induced locomotive sensitization have been identified in forward genetic screening (i.e., random DNA mutations) study (Petzold et al., 2009). Locomotive sensitization is an observed phenomenon describing higher drug-induced locomotive activity among animal models previously exposed to the drug compared with animals receiving the drug for the first time. This study used transposons to genetically mutate zebrafish and screened for induced changes in the nicotine-induced locomotive response. The transposon-mediated mutagenesis with fluorescently tagged reporters (see Figure 2) generated two mutant fish lines, bdav and hbog, with significantly attenuated nicotine locomotive response. Mutations were found in the chaperonin-containing protein 8 (cct8) gene and a GABAB receptor ortholog, gabbr1.2 gene. These genes encode proteins involved with nAChR modulation and GABAB receptor structure, respectively. Identification of GABAB receptor involvement in nicotine response provides strong evidence for the role of the GABAergic system in nicotine reward reinforcement and increased physiological activity. This study highlights how the zebrafish model can be used in the study of in vivo molecular–visual readouts to facilitate forward genetic screens in a more rapid and efficient manner than possible with traditional mammalian models.
Published studies demonstrate that the zebrafish is a viable model for studying drug addiction. The data presented illustrates robust responses in two behavioral assays commonly used to study addiction. The integration of these behavioral assays with cost-effective and efficient forward genetic screening methods creates a powerful tool for discovery of novel genetic components associated with addiction. Despite these advantages, the zebrafish model is currently limited in its ability to study certain brain mechanisms associated with addiction. While the zebrafish does have a well-described habenula brain region, which has recently been implicated in the response to addictive drugs. Furthermore, the prefrontal cortex, a major focus of addiction research, is not well defined in the zebrafish. This compromises the experimental utility of zebrafish as a model for the study of all of the neural pathways implicated in addiction. Investigators need to understand the strengths and limitations of the zebrafish when considering it as a model of addiction response.
Anxiolytic Effects
The relationship between tobacco use and perceived stress in humans is not well understood. High stress levels may lead to increased tobacco use, but tobacco use may not actually decrease stress (Heishman, 1999). Anxiolytic effects of nicotine have been suggested to influence the onset of tobacco use in adolescents and the maintenance of tobacco use in adults (Byrne, Byrne, & Reinhart, 1995). In rodent models, chronic nicotine self-administration has been linked to a reduction in hypothalamic paraventricular nucleus norepinephrine release and reduced c-Fos expression during induced stress (Yu & Sharp, 2010). Understanding the phenotypic and molecular effects nicotine may advance our understanding of the link between stress and tobacco. Zebrafish are capable of displaying anxiety (Blaser, Chadwick, & McGinnis, 2010) when subjected to new locations or conditions (novelty) and demonstrating a cortisol response to the stress (Barcellos et al., 2007). This has led to the development of stress response behavioral assays (Levin, Bencan, & Cerutti, 2007) used to test anxiolytic compounds and molecularly characterize the response (Egan et al., 2009).
Nicotine exposure has been shown to reduce stressed behaviors in zebrafish. Novelty-induced anxiety in zebrafish can be detected by changes in swimming behavior manifesting as increased propensity to swim near tank edges and decreased preference for open water (Peitsaro, Kaslin, Anichtchik, & Panula, 2003). The anxiety response diminishes as the novelty of a situation diminishes and the zebrafish returns to normal swimming behavior. This observation was used to design a novel tank dive–response stress assay for zebrafish (Levin et al., 2007). Control zebrafish initially introduced into the testing tank showed a propensity to localize to the bottom third of the tank with general migration into the entire tank occurring over time. Acute nicotine exposure (100 mg/L by immersion for 3 min) given 5 min prior to testing significantly reduced the time the zebrafish spent in the tank bottom (Levin et al., 2007). This effect was blocked by the coadministration of the nonselective and noncompetitive nAChR antagonist mecamylamine, the α7 receptor antagonist methyllycaconitine (MLA), and the α4β2 antagonist DHβE, suggesting that the anxiolytic effects of nicotine involve a direct response from α4β2 and α7 receptor binding (Bencan & Levin, 2008; Levin et al., 2007). The assay has also been used to study other anxiolytic drugs (Bencan, Sledge, & Levin, 2009) and to develop a drug withdrawal model in zebrafish (Cachat et al., 2010). These studies present a functional stress–response assay that can be used with mutagenized and transgenic zebrafish to further characterize the molecular aspects of nicotine's anxiolytic effects. Advancement in understanding of these phenomenon will complement the work being pursued in embryonic development and addiction science research, and could ultimately impact how nicotine dependence is addressed clinically.
Cognitive Function
An association between nicotine exposure and improved cognitive function has been reported in many studies. Rodent animal models have demonstrated promnesic effects of nicotine following acute exposure manifest as improvements in memory and selective attention (Swan & Lessov-Schlaggar, 2007). In humans, enhanced cognitive function following nicotine exposure has been reported in case–control studies with patients suffering from schizophrenia (Barr et al., 2008; Jubelt et al., 2008). This has lead to the consideration of nicotine or other nAChR agonists as therapeutics for the treatment of patients with schizophrenia (Smith, Singh, Infante, Khandat, & Kloos, 2002). Diminished cognitive function has been observed in tobacco users experiencing tobacco withdrawal followed by an improvement in cognitive function with tobacco readministration (Bell, Taylor, Singleton, Henningfield, & Heishman, 1999). The real or perceived effect of nicotine exposure on cognitive function may be an important factor influencing relapse to tobacco use. A meta-analysis of the recent literature suggests that nicotine may have positive effects in several motor and cognitive performance domains (Heishman, Kleykamp, & Singleton, 2010). However, the relationship between tobacco use and cognitive function remains controversial (Heishman, 1998, 1999) and poorly understood (Heishman et al., 2010; Poorthuis, Goriounova, Couey, & Mansvelder, 2009).
Zebrafish are trainable animals (Williams, White, & Messer, 2002), enabling assays to be developed to measure memory and learning (Levin & Chen, 2004). Acute nicotine exposure has been observed to improve discrimination and memory function in zebrafish following a 20- to 40-min latency but not immediately following exposure (Eddins, Petro, Williams, Cerutti, & Levin, 2009; Levin & Chen, 2004; Levin, Limpuangthip, Rachakonda, & Peterson, 2006). The observed nicotine-induced cognitive enhancement was reduced by mecamylamine (i.e., nonselective and noncompetitive nAChR antagonist) administration immediately prior to the learning assays but not when coadministered with nicotine 40 min prior to the testing. This observation led to the hypothesis that nicotine improves cognitive function through nAChR receptor desensitization and resensitization with heighted response to native acetylcholine (Levin et al., 2006). The increased cognitive function was also correlated with increased concentrations of dihydroxyphenylacetic acid (DOPAC). This dopamine (DA) metabolite, created during DA synaptic reuptake, acts as a DA level surrogate and suggests that potentiated release of DA following nicotine exposure is linked to cognitive improvements (Eddins, Cerutti, et al., 2009). The cognitive stimulation of nicotine and its mitigation by preadministration of mecamylamine was also observed in a study of memory using a place preference assay (de Castro et al., 2009). Many similar studies have also been carried out using mammalian models. The α7 nAChR activation has been linked with improved cognitive function in several species, including rodents (Levin et al., 2009; Rushforth, Allison, Wonnacott, & Shoaib, 2010) and primates, and is under evaluation as a potential therapeutic focus for Alzheimer's disease (Bitner et al., 2010). Establishment of conserved cognitive effects of nicotine in zebrafish would facilitate the use of this model to complement studies in mammalian systems, providing a rapid and inexpensive method for advancing understanding in this field.
Dopamine
DA neurons in mammals form the core of the neural circuitry of addiction (Corbett & Wise, 1980) and regulate the function of brain areas, such as the nucleus accumbens, prefrontal cortex, and hippocampus (Hyman, Malenka, & Nestler, 2006; Wise, 2002; Wise & Bozarth, 1984). While defined brain regions such as the nucleus accumbens or the prefrontal cortex have not been distinguished in the zebrafish brain, the neuroanatomical organization of DA neurons in zebrafish and mammals is similar. In larval and adult zebrafish, tyrosine hydroxylase immunoreactive cells have been characterized using immunostaining techniques in whole mount and sectioned brain tissue of larval and adult zebrafish (Panula et al., 2010; Rink & Wullimann, 2001; Sallinen et al., 2009; Yamamoto, Ruuskanen, Wullimann, & Vernier, 2010). DA cells are arranged in 13 clusters within olfactory, telencephalic, preoptic, postoptic, thalamic, tubercular, diencephalic periventricular, and hypothalamic complexes. The first DA cells can be labeled at 18–19 hpf (Holzschuh, Ryu, Aberger, & Driever, 2001), and the complete set of DA neurons is detectable by 8 dpf. While the organization of zebrafish DA neurons has been described in detail, the homologous system of the DA neuron reward pathway of mammals and humans has not been identified in zebrafish. No electrophysiological recordings from DA neurons in zebrafish have been performed. An increase in DOPAC levels in a nicotine-enhanced learning task indicates that nicotine increases DA activity (Eddins, Petro, et al., 2009). Using forward genetics and gene-breaking transposons, zebrafish mutants with changed GABAB receptor expression and changed nicotine response indicate similar synaptic regulation of the reward pathway in zebrafish and mammals (Petzold et al., 2009). Future studies in zebrafish can employ methods such as c-fos labeling, electrophysiological recording, and optogenetic monitoring to characterize the zebrafish reward pathway.
Nicotinic Acetylcholine Receptors
Nicotine acts on nAChRs, which are pentameric, ligand-gated ion channels existing in three functional states: resting, active, and desensitized (Monod, Wyman, & Changeux, 1965). Human neural nAChR receptors consist of alpha and beta subunits encoded by 11 genes (α2–α7, α9, α10, and β2–β4; Jensen, Frølund, Liljefors, & Krogsgaard-Larsen, 2005). Heteromeric receptors consist of three alpha subunits and two beta subunits with the most common heteromeric nAChR containing three α4 subunits and two β2 subunits (α4β2; Gotti et al., 2009). Physiological responses to nicotine result from receptor activation and receptor desensitization and have been shown to stimulate the release of many brain neurotransmitters (McGehee & Role, 1995).
Published literature supports the use of the zebrafish model for exploring nicotine effects on the central nervous system and nAChRs. Transcript expression of α2, α7, and β3 subunits has been observed in early zebrafish embryos between 2 and 8 hpf by reverse transcription–polymerase chain reaction (Zirger, Beattie, McKay, & Boyd, 2003). The α4 and α6 receptor subunits were also characterized in embryonic zebrafish revealing a dynamic pattern of expression across neural regions in fish 3–96 hpf (Ackerman, Nakkula, Zirger, Beattie, & Boyd, 2009). Expression of the nAChR β2 subunit was identified in central nervous system elements of embryonic zebrafish via immunohistochemistry using an anti-β2 nAChR polyclonal antibody (Welsh et al., 2009). These findings suggest that nAChR's in early zebrafish development have common positional and temporal expression patterns between zebrafish and humans (i.e., conservation).
Evidence of nAChR conservation in the zebrafish has also been indirectly demonstrated through known nAChR antagonist studies to determine the reversibility of nicotine effects (Bencan & Levin, 2008; Eddins, Cerutti, et al., 2009; Levin et al., 2006; Svoboda et al., 2002). Mecamylamine (a nonspecific nAChR receptor antagonist) modulates α4β2, α3β4, α3β2, and α7 receptors (Papke, Sanberg, & Shytle, 2001) and has been observed to reverse a broad range of nicotine-mediated effects in zebrafish, including locomotive sensitization, improved learning, and anxiety reduction (Eddins, Cerutti, et al., 2009; Levin et al., 2006, 2007; Petzold et al., 2009). Specific nAChR antagonists, including DHβ3, MLA, and conotoxin ImI (McIntosh, Santos, & Olivera, 1999; α7 receptor antagonist), have also effectively reversed nicotine-induced changes in zebrafish (Bencan & Levin, 2008; Svoboda et al., 2002).
The identification of sequence similarity (homology) between human and zebrafish nAChR genes (or proteins) provides evidence of evolutionarily conserved receptor function complementing the direct and indirect experimental findings previously discussed with respect to nAChRs (Fitch, 1970). Computational comparisons of the human nAChR gene sequences to the zebrafish genome identified candidate conserved genes (i.e., orthologs) for the 11 genes encoding human neural nAChRs (Table 2). As shown in Table 2, a high degree of protein sequence conservation exists between human and zebrafish orthologs. At the protein level, identical amino acid structure is found for greater than 60% of the residues across more than 90% of the entire peptide strand. High coverage values reflect sequence conservation of the complete protein and not just a single protein domain. The Zebrafish Model Organism Database (Sprague et al., 2006) provides additional evidence of amino acid sequence similarity in eight putative orthologs and conserved genomic location for four putative orthologs. During the evolution of ray-finned fish, which includes zebrafish, a genome-wide duplication event occurred, resulting in zebrafish genome sequence redundancy (Meyer & Van de Peer, 2005). This can lead to one human gene being homologous to more than one zebrafish gene as observed for CHRNA2, CHRNB2, and CHRNB3. Together, the sequence homology data provides additional evidence of conservation of the nAChR receptors between zebrafish and humans.
Table 2.
Orthology of Nicotinic Acetylcholine Receptor Genes
| Human |
Zebrafish |
ZFIN orthology | Protein sequencea |
|||
| Gene name | Protein ID | Gene name | Protein ID | Evidence codes | % identityb | % coveragec |
| CHRNA2 | NP_000733.2 | chrna2b | XP_697298.2 | AA, CL | 69 | 97 |
| chrna2a | NP_001035417.1 | – | 73 | 96 | ||
| CHRNA3 | NP_000734.2 | LOC568467 (chrna3) | XP_001921314.1 | AA, CL | 74 | 95 |
| CHRNA4 | NP_000735.1 | chrna4 | NP_001041528.1 | AA | 62 | 95 |
| CHRNA5 | NP_000736.2 | chrna5 | NP_001017885.1 | – | 75 | 92 |
| CHRNA6 | NP_004189.1 | chrna6 | NP_001036149.1 | AA, CL | 67 | 98 |
| CHRNA7 | NP_000737.1 | chrna7 | NP_957513.1 | AA | 76 | 99 |
| CHRNA9 | NP_060051.2 | LOC568807 | XP_001920894.1 | – | 67 | 99 |
| CHRNA10 | NP_065135.2 | si:dkey-24p1.3 | NP_001038269.1 | – | 66 | 84 |
| CHRNB2 | NP_000739.1 | chrnb2a(si:ch211 -240p12.2-001) | OTTDARP00000033350 (VEGA) | AA | 67 | 98 |
| chrnb2b | XP_690400.4 | AA, CL | 75 | 96 | ||
| CHRNB3 | NP_000740.1 | chrnb3a | NP_957514.1 | – | 77 | 95 |
| chrnb3b | NP_775394.1 | AA | 77 | 96 | ||
| CHRNB4 | NP_000741.1 | LOC568566 | XP_696993.2 | – | 64 | 96 |
Note.
Alignments performed using NCBI BLAST (blastp) with default parameters.
Percentage of identical amino acids between human and zebrafish protein across region of alignment.
Percent of human protein matched to zebrafish protein—region of alignment.
Discussion
The zebrafish model organism has been established as an important vertebrate model for the study of developmental biology and an emerging model for disease etiology. A number of recent studies have reported results using zebrafish to study the effects of nicotine exposure. While the application of zebrafish in this field of study is still in its early stages, a number of advantages exists that should promote the expanded use of the zebrafish as an alternative or complementary model to advance our understanding of and treatment for tobacco dependence. Zebrafish facilitate large cost-effective studies that can be combined with a battery of advanced molecular tools for genetic modulation and powerful in vivo molecular–visual readouts to carry out forward genetic or chemical screens in a more rapid and efficient manner than possible with traditional mammalian models. The use of behavioral assays commonly associated with addiction research to study nicotine response in the zebrafish is also being developed. However, assays are not readily available for all phases of addiction, and this is one area where the zebrafish model currently lags behind traditional mammalian models. The lack of a fully annotated neural anatomy in the zebrafish with clearly functional homologous structures to human neural makeup is another current limitation. However, considerable research is ongoing with the zebrafish that may, in time, mitigate some of these limitations. In the immediate future, the use of zebrafish to study nicotine and tobacco use is likely to continue expanding, establishing itself as the primary model in some areas of research, which most effectively utilize the innate advantages associated with the zebrafish, and acting as a secondary or complementary model in research studies better suited for mammalian model systems.
Funding
This work was supported by the National Institute of Drug Addiction at the National Institutes of Health (144546) to SCE; the Mayo Clinic's Clinical and Translational Science Award (RR024151) to EWK; and DePauw University, Greencastle, Indiana, to HS.
Declaration of Interests
None declared.
Acknowledgments
We would like to thank Andy Petzold for providing the red fluorescent protein-labeled GABA(B) zebrafish image. We would also like to thank Karl Clark for his review and editorial comments on the manuscript.
References
- Ackerman KM, Nakkula R, Zirger JM, Beattie CE, Boyd RT. Cloning and spatiotemporal expression of zebrafish neuronal nicotinic acetylcholine receptor alpha 6 and alpha 4 subunit RNAs. Developmental Dynamics. 2009;238:980–992. doi: 10.1002/dvdy.21912. doi:10.1002/dvdy.21912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agetsuma M, Aizawa H, Aoki T, Nakayama R, Takahoko M, Goto M, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nature Neuroscience. 2010;13:1354–1356. doi: 10.1038/nn.2654. doi:10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- André JM, Gulick D, Portugal GS, Gould TJ. Nicotine withdrawal disrupts both foreground and background contextual fear conditioning but not pre-pulse inhibition of the acoustic startle response in C57BL/6 mice. Behavioural Brain Research. 2008;190:174–181. doi: 10.1016/j.bbr.2008.02.018. doi:10.1016/j.bbr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos L, Ritter F, Kreutz L, Quevedo R, da Silva L, Bedin A, et al. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture. 2007;272:774–778. doi:10.1016/j.aquaculture.2007.09.002. [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008;33:480–490. doi: 10.1038/sj.npp.1301423. doi:10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreases tobacco craving in drug abusers. Nicotine & Tobacco Research. 1999;1:45–52. doi: 10.1080/14622299050011141. doi:10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiology & Behavior. 2008;95:408–412. doi: 10.1016/j.physbeh.2008.07.009. doi:10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacology Biochemistry and Behavior. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. doi:10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJ. The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. British Journal of Pharmacology. 1992;105:849–856. doi: 10.1111/j.1476-5381.1992.tb09067.x. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1908718/?tool=pubmed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Decker MW, Drescher KU, Kohlhaas KL, Markosyan S, et al. In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: Preclinical considerations in Alzheimer's disease. Journal of Pharmacology and Experimental Therapeutics. 2010;334:875–886. doi: 10.1124/jpet.110.167213. doi:10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- Blaser RE, Chadwick L, McGinnis GC. Behavioral measures of anxiety in zebrafish (Danio rerio) Behavioural Brain Research. 2010;208:56–62. doi: 10.1016/j.bbr.2009.11.009. doi:10.1016/j.bbr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Bretaud S, Li Q, Lockwood BL, Kobayashi K, Lin E, Guo S. A choice behavior for morphine reveals experience-dependent drug preference and underlying neural substrates in developing larval zebrafish. Neuroscience. 2007;146:1109–1116. doi: 10.1016/j.neuroscience.2006.12.073. doi:10.1016/j.neuroscience.2006.12.073. [DOI] [PubMed] [Google Scholar]
- Burke MV, Ebbert JO, Hays JT. Treatment of tobacco dependence. Mayo Clinic Proceedings. 2008;83:479–483. doi: 10.4065/83.4.479. quiz: 483–1474. doi:10.4065/83.4.479. [DOI] [PubMed] [Google Scholar]
- Byrne DG, Byrne AE, Reinhart MI. Personality, stress and the decision to commence cigarette smoking in adolescence. Journal of Psychosomatic Research. 1995;39:53–62. doi: 10.1016/0022-3999(94)00074-f. doi:10.1016/0022-3999(94)00074-F. [DOI] [PubMed] [Google Scholar]
- Cachat J, Canavello P, Elegante M, Bartels B, Hart P, Bergner C, et al. Modeling withdrawal syndrome in zebrafish. Behavioural Brain Research. 2010;208:371–376. doi: 10.1016/j.bbr.2009.12.004. doi:10.1016/j.bbr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Changeux J-P. Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nature Reviews Neuroscience. 2010;11:389–401. doi: 10.1038/nrn2849. doi:10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Chico TJA, Milo M, Crossman DC. The genetics of cardiovascular disease: New insights from emerging approaches. Journal of Pathology. 2010;220:186–197. doi: 10.1002/path.2641. doi:10.1002/path.2641. [DOI] [PubMed] [Google Scholar]
- Corbett D, Wise RA. Intracranial self-stimulation in relation to the ascending dopaminergic systems of the midbrain: A moveable electrode mapping study. Brain Research. 1980;185:1–15. doi: 10.1016/0006-8993(80)90666-6. doi:10.1016/0006-8993(80)90666-6. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. doi:10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro MR, Lima JV, de Freitas DPS, Valente R.d. RS., Dummer NS, de Aguiar RB, et al. Behavioral and neurotoxic effects of arsenic exposure in zebrafish (Danio rerio, Teleostei: Cyprinidae) Comparative Biochemistry and Physiology—Part C: Toxicology and Pharmacology. 2009;150:337–342. doi: 10.1016/j.cbpc.2009.05.017. doi:10.1016/j.cbpc.2009.05.017. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113(4 Suppl.):1007–1015. Retrieved from http://pediatrics.aappublications.org/cgi/content/full/113/4/S1/1007. [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Research Part C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. doi:10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Ebbert JO, Wyatt KD, Hays JT, Klee EW, Hurt RD. Varenicline for smoking cessation: Efficacy, safety, and treatment recommendations. Patient Preference Adherence. 2010;4:355–362. doi: 10.2147/ppa.s10620. doi:10.2147/PPA.S10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Cerutti DT, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicology and Teratology. 2009;32:99–108. doi: 10.1016/j.ntt.2009.02.005. doi:10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddins D, Petro A, Williams P, Cerutti DT, Levin ED. Nicotine effects on learning in zebrafish: The role of dopaminergic systems. Psychopharmacology (Berlin) 2009;202:103–109. doi: 10.1007/s00213-008-1287-4. doi:10.1007/s00213-008-1287-4. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behavioural Brain Research. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. doi:10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker SC. Zinc finger-based knockout punches for zebrafish genes. Zebrafish. 2008;5:121–123. doi: 10.1089/zeb.2008.9988. doi:10.1089/zeb.2008.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch WM. Distinguishing homologous from analogous proteins. Systematic Zoology. 1970;19:99–113. doi:10.2307/2412448. [PubMed] [Google Scholar]
- Garrett BE, Rose CA, Henningfield JE. Tobacco addiction and pharmacological interventions. Expert Opinion on Pharmacotherapy. 2001;2:1545–1555. doi: 10.1517/14656566.2.10.1545. doi:10.1517/14656566.2.10.1545. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochemical Pharmacology. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. doi:10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Headwaters of the zebrafish—Emergence of a new model vertebrate. Nature Reviews Genetics. 2002;3:717–724. doi: 10.1038/nrg892. doi:10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Hajek P, Tønnesen P, Arteaga C, Russ C, Tonstad S. Varenicline in prevention of relapse to smoking: Effect of quit pattern on response to extended treatment. Addiction. 2009;104:1597–1602. doi: 10.1111/j.1360-0443.2009.02646.x. doi:10.1111/j.1360-0443.2009.02646.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. What aspects of human performance are truly enhanced by nicotine ? Addiction. 1998;93:317–320. doi: 10.1080/09652149835864. doi:10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- Heishman SJ. Behavioral and cognitive effects of smoking: Relationship to nicotine addiction. Nicotine & Tobacco Research. 1999;1(Suppl. 2):S143–S147. doi: 10.1080/14622299050011971. Discussion: S165–S146. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berlin) 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. doi:10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. Journal of Neuroscience. 2000;20:206–218. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mechanisms of Development. 2001;101:237–243. doi: 10.1016/s0925-4773(01)00287-8. doi:10.106/S0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. doi:10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Frølund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: Structural revelations, target identifications, and therapeutic inspirations. Journal of Medicinal Chemistry. 2005;48:4705–4745. doi: 10.1021/jm040219e. doi:10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ruiz C, Berlin I, Hering T. Varenicline: A novel pharmacotherapy for smoking cessation. Drugs. 2009;69:1319–1338. doi: 10.2165/00003495-200969100-00003. doi:10.2165/00003495-200969100-00003. [DOI] [PubMed] [Google Scholar]
- Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology (Berlin) 2008;199:89–98. doi: 10.1007/s00213-008-1133-8. doi:10.1007/s00213-008-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research Reviews. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. doi:10.1016/0165-0173(91)90007-U. [DOI] [PubMed] [Google Scholar]
- Kane JK, Konu O, Ma JZ, Li MD. Nicotine coregulates multiple pathways involved in protein modification/degradation in rat brain. Brain Research Molecular Brain Research. 2004;132:181–191. doi: 10.1016/j.molbrainres.2004.09.010. doi:10.1016/j.molbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Kily LJM, Cowe YCM, Hussain O, Patel S, McElwaine S, Cotter FE, et al. Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro-adaptation pathways. Journal of Experimental Biology. 2008;211(Pt 10):1623–1634. doi: 10.1242/jeb.014399. doi:10.1242/jeb.014399. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi K-H, Renthal W, Tsankova NM, Theobald DEH, Truong H-T, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. doi:10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of relapse after quitting smoking: A systematic review of trials. Archives of Internal Medicine. 2006;166:828–835. doi: 10.1001/archinte.166.8.828. doi:10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- Lau B, Bretaud S, Huang Y, Lin E, Guo S. Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes, Brain, and Behavior. 2006;5:497–505. doi: 10.1111/j.1601-183X.2005.00185.x. doi:10.1111/j.1601-183X.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiology & Behavior. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. doi:10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicology and Teratology. 2004;26:731–735. doi: 10.1016/j.ntt.2004.06.010. doi:10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology (Berlin) 2006;184:547–552. doi: 10.1007/s00213-005-0162-9. doi:10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Petro A, Rezvani AH, Pollard N, Christopher NC, Strauss M, et al. Nicotinic alpha7- or beta2-containing receptor knockout: Effects on radial-arm maze learning and long-term nicotine consumption in mice. Behavioural Brain Research. 2009;196:207–213. doi: 10.1016/j.bbr.2008.08.048. doi:10.1016/j.bbr.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. doi:10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD. Identifying susceptibility loci for nicotine dependence: 2008 update based on recent genome-wide linkage analyses. Human Genetics. 2008;123:119–131. doi: 10.1007/s00439-008-0473-0. doi:10.1007/s00439-008-0473-0. [DOI] [PubMed] [Google Scholar]
- Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: A genetic system for large-scale screening. Pharmacology Biochemistry and Behavior. 2004;77:647–654. doi: 10.1016/j.pbb.2004.01.003. doi:10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Mao C, Yuan X, Zhang H, Lv J, Guan J, Miao L, et al. The effect of prenatal nicotine on mRNA of central cholinergic markers and hematological parameters in rat fetuses. International Journal of Developmental Neuroscience. 2008;26:467–475. doi: 10.1016/j.ijdevneu.2008.02.007. doi:10.1016/j.ijdevneu.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Guo S. Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiology of Disease. 2010;40:66–72. doi: 10.1016/j.nbd.2010.05.016. doi:10.1016/j.nbd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. doi:10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Santos AD, Olivera BM. Conus peptides targeted to specific nicotinic acetylcholine receptor subtypes. Annual Review of Biochemistry. 1999;68:59–88. doi: 10.1146/annurev.biochem.68.1.59. doi:10.1146/annurev.biochem.68.1.59. [DOI] [PubMed] [Google Scholar]
- Menelaou E, Husbands EE, Pollet RG, Coutts CA, Ali DW, Svoboda KR. Embryonic motor activity and implications for regulating motoneuron axonal pathfinding in zebrafish. European Journal of Neuroscience. 2008;28:1080–1096. doi: 10.1111/j.1460-9568.2008.06418.x. doi:10.1111/j.1460-9568.2008.06418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Van de Peer Y. From 2R to 3R: Evidence for a fish-specific genome duplication (FSGD) Bioessays. 2005;27:937–945. doi: 10.1002/bies.20293. doi:10.1002/bies.20293. [DOI] [PubMed] [Google Scholar]
- Milner LD, Landmesser LT. Cholinergic and GABAergic inputs drive patterned spontaneous motoneuron activity before target contact. Journal of Neuroscience. 1999;19:3007–3022. doi: 10.1523/JNEUROSCI.19-08-03007.1999. Retrieved from http://www.jneurosci.org/cgi/reprint/19/8/3007.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: A plausible model. Journal of Molecular Biology. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. doi:10.1016/S0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Review. Transcriptional mechanisms of addiction: Role of DeltaFosB. Philosophical Transactions of the Royal Society of London Series B Biological Sciences. 2008;363:3245–3255. doi: 10.1098/rstb.2008.0067. doi:10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 2006;39:262–274. doi: 10.1016/j.ymeth.2005.12.007. doi:10.1016/j.ymeth.2005.12.007. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: What do they tell us about tobacco abuse in humans. Pharmacology Biochemistry and Behavior. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. doi:10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Sato T, Aizawa H. Transgenic technology for visualization and manipulation of the neural circuits controlling behavior in zebrafish. Development, Growth & Differentiation. 2008;50(Suppl. 1):S167–S175. doi: 10.1111/j.1440-169X.2008.01003.x. doi:10.1111/j.1440-169X.2008.01003.x. [DOI] [PubMed] [Google Scholar]
- Ono F, Higashijima S, Shcherbatko A, Fetcho JR, Brehm P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. Journal of Neuroscience. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. Retrieved from http://www.jneurosci.org/cgi/reprint/21/15/5439.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchin Yu Y, Perrins RJ, Roberts A. The action of acetylcholine on the locomotor central pattern generator for swimming in Xenopus embryos. Journal of Experimental Biology. 1991;161:527–531. doi: 10.1242/jeb.161.1.527. Retrieved from http://www.jneurosci.org/cgi/reprint/21/15/5439.pdf. [DOI] [PubMed] [Google Scholar]
- Panula P, Chen Y-C, Priyadarshini M, Kudo S, Semenova S, Sundvik M, et al. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiology of Disease. 2010;40:46–57. doi: 10.1016/j.nbd.2010.05.010. doi:10.1016/j.nbd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. Journal of Pharmacology and Experimental Therapeutics. 2001;297:646–656. Retrieved from http://jpet.aspetjournals.org/content/297/2/646.full. [PubMed] [Google Scholar]
- Pardo-Martin C, Chang T-Y, Koo BK, Gilleland CL, Wasserman SC, Yanik MF. High-throughput in vivo vertebrate screening. Nature Methods. 2010;7:634–636. doi: 10.1038/nmeth.1481. doi:10.1038/nmeth.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B, Connaughton VP. Effects of nicotine on growth and development in larval zebrafish. Zebrafish. 2007;4:59–68. doi: 10.1089/zeb.2006.9994. doi:10.1089/zeb.2006.9994. [DOI] [PubMed] [Google Scholar]
- Payne E, Look T. Zebrafish modelling of leukaemias. British Journal of Haematology. 2009;146:247–256. doi: 10.1111/j.1365-2141.2009.07705.x. doi:10.1111/j.1365-2141.2009.07705.x. [DOI] [PubMed] [Google Scholar]
- Peitsaro N, Kaslin J, Anichtchik OV, Panula P. Modulation of the histaminergic system and behaviour by alpha-fluoromethylhistidine in zebrafish. Journal of Neurochemistry. 2003;86:432–441. doi: 10.1046/j.1471-4159.2003.01850.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1046/j.1471-4159.2003.01850.x/pdf. [DOI] [PubMed] [Google Scholar]
- Peng J, Wagle M, Mueller T, Mathur P, Lockwood BL, Bretaud S, et al. Ethanol-modulated camouflage response screen in zebrafish uncovers a novel role for cAMP and extracellular signal-regulated kinase signaling in behavioral sensitivity to ethanol. Journal of Neuroscience. 2009;29:8408–8418. doi: 10.1523/JNEUROSCI.0714-09.2009. doi:10.1523/JNEUROSCI.0714-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold AM, Balciunas D, Sivasubbu S, Clark KJ, Bedell VM, Westcot SE, et al. Nicotine response genetics in the zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18662–18667. doi: 10.1073/pnas.0908247106. doi:10.1073/pnas.0908247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD. Nicotinic actions on neuronal networks for cognition: General principles and long-term consequences. Biochemical Pharmacology. 2009;78:668–676. doi: 10.1016/j.bcp.2009.04.031. doi:10.1016/j.bcp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, Xiao G, Kumar A, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. doi:10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon. (posterior tuberculum) Brain Research. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. doi:10.1016/S0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rogers JM. Tobacco and pregnancy: Overview of exposures and effects. Birth Defects Research Part C Embryo Today. 2008;84:1–15. doi: 10.1002/bdrc.20119. doi:10.1002/bdrc.20119. [DOI] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. Journal of Neuroscience. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. doi:10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacott S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: A novel use of the odour span task. Neuroscience Letters. 2010;471:114–118. doi: 10.1016/j.neulet.2010.01.022. doi:10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Sager JJ, Bai Q, Burton EA. Transgenic zebrafish models of neurodegenerative diseases. Brain Structure and Function. 2010;214:285–302. doi: 10.1007/s00429-009-0237-1. doi:10.1007/s00429-009-0237-1. [DOI] [PubMed] [Google Scholar]
- Sallinen V, Torkko V, Sundvik M, Reenilä I, Khrustalyov D, Kaslin J, et al. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. Journal of Neurochemistry. 2009;108:719–731. doi: 10.1111/j.1471-4159.2008.05793.x. doi:10.1111/j.1471-4159.2008.05793.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Simon FM, Rodriguez RE. Developmental expression and distribution of opioid receptors in zebrafish. Neuroscience. 2008;151:129–137. doi: 10.1016/j.neuroscience.2007.09.086. doi:10.1016/j.neuroscience.2007.09.086. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Fennell OB, Robinson SE. Prenatal nicotine sex-dependently alters agonist-induced locomotion and stereotypy. Neurotoxicology and Teratology. 1997;19:467–476. doi: 10.1016/s0892-0362(97)00063-9. doi:10.1016/S0892-0362(97)00063-9. [DOI] [PubMed] [Google Scholar]
- Shen H-Y, Kalda A, Yu L, Ferrara J, Zhu J, Chen J-F. Additive effects of histone deacetylase inhibitors and amphetamine on histone H4 acetylation, cAMP responsive element binding protein phosphorylation and DeltaFosB expression in the striatum and locomotor sensitization in mice. Neuroscience. 2008;157:644–655. doi: 10.1016/j.neuroscience.2008.09.019. doi:10.1016/j.neuroscience.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Prenatal nicotine exposure alters the responses to subsequent nicotine administration and withdrawal in adolescence: Serotonin receptors and cell signaling. Neuropsychopharmacology. 2006;31:2462–2475. doi: 10.1038/sj.npp.1300988. doi:10.1038/sj.npp.1300988. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. doi:10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, Frazer K, et al. The Zebrafish Information Network: The zebrafish model organism database. Nucleic Acids Research. 2006;34(Database issue):D581–D585. doi: 10.1093/nar/gkj086. doi:10.1093/nar/gkj086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stairs DJ, Neugebauer NM, Bardo MT. Nicotine and cocaine self-administration using a multiple schedule of intravenous drug and sucrose reinforcement in rats. Behavioural Pharmacology. 2010;21:182–193. doi: 10.1097/FBP.0b013e32833a5c9e. doi:10.1097/FBP.0b013e32833a5c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berlin) 1995;117:2–10. doi: 10.1007/BF02245088. Discussion: 14–20. doi:10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Vijayaraghavan S, Tanguay R. Nicotinic receptors mediate changes in spinal motoneuron development and axonal pathfinding in embryonic zebrafish exposed to nicotine. Journal of Neuroscience. 2002;22:10731. doi: 10.1523/JNEUROSCI.22-24-10731.2002. Retrieved from http://www.jneurosci.org/cgi/reprint/22/24/10731.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychology Review. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. doi:10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Zon LI. Zebrafish tumor assays: The state of transplantation. Zebrafish. 2009;6:339–346. doi: 10.1089/zeb.2009.0607. doi:10.1089/zeb.2009.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LT, Welsh L, Galvez F, Svoboda KR. Acute nicotine exposure and modulation of a spinal motor circuit in embryonic zebrafish. Toxicology and Applied Pharmacology. 2009;239:1–12. doi: 10.1016/j.taap.2008.08.023. doi:10.1016/j.taap.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. doi:10.1016/S0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen M, Yan Y-E, Xiao F-Q, Pan X-L, Wang H. Growth retardation of fetal rats exposed to nicotine in utero: Possible involvement of CYP1A1, CYP2E1, and P-glycoprotein. Environmental Toxicology. 2009;24:33–42. doi: 10.1002/tox.20391. doi:10.1002/tox.20391. [DOI] [PubMed] [Google Scholar]
- Webb KJ, Norton WH, Trümbach D, Meijer AH, Ninkovic J, Topp S, et al. Zebrafish reward mutants reveal novel transcripts mediating the behavioral effects of amphetamine. Genome Biology. 2009;10:R81. doi: 10.1186/gb-2009-10-7-r81. doi:10.1186/gb-2009-10-7-r81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh L, Tanguay RL, Svoboda KR. Uncoupling nicotine mediated motoneuron axonal pathfinding errors and muscle degeneration in zebrafish. Toxicology and Applied Pharmacology. 2009;237:29–40. doi: 10.1016/j.taap.2008.06.025. doi:10.1016/j.taap.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. doi:10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. WHO Report on the Global Tobacco Epidemic. WHO Press, Switzerland: Geneva; 2008. [Google Scholar]
- Williams FE, White D, Messer WS. A simple spatial alternation task for assessing memory function in zebrafish. Behavioural Processes. 2002;58:125–132. doi: 10.1016/s0376-6357(02)00025-6. doi:10.1016/S0376-6357(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: Insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. doi:10.1016/S0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. Brain reward circuitry: Four circuit elements “wired” in apparent series. Brain Research Bulletin. 1984;12:203–208. doi: 10.1016/0361-9230(84)90190-4. doi:10.1016/0361-9230(84)90190.4. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJK. Nicotine: From molecular mechanisms to behaviour. Current Opinion in Pharmacology. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. doi:10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ruuskanen JO, Wullimann MF, Vernier P. Two tyrosine hydroxylase genes in vertebrates new dopaminergic territories revealed in the zebrafish brain. Molecular and Cellular Neuroscience. 2010;43:394–402. doi: 10.1016/j.mcn.2010.01.006. doi:10.1016/j.mcn.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Yu G, Sharp BM. Nicotine self-administration diminishes stress-induced norepinephrine secretion but augments adrenergic-responsiveness in the hypothalamic paraventricular nucleus and enhances adrenocorticotropic hormone and corticosterone release. Journal of Neurochemistry. 2010;112:1327–1337. doi: 10.1111/j.1471-4159.2009.06551.x. doi:10.1111/j.1471-4159.2009.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaniewska M, Przegaliński E, Filip M. Nicotine dependence—Human and animal studies, current pharmacotherapies and future perspectives. Pharmacological Reports. 2009;61:957–965. doi: 10.1016/s1734-1140(09)70157-4. Retrieved from http://www.if-pan.krakow.pl/pjp/pdf/2009/6_957.pdf. [DOI] [PubMed] [Google Scholar]
- Zirger JM, Beattie CE, McKay DB, Boyd RT. Cloning and expression of zebrafish neuronal nicotinic acetylcholine receptors. Gene Expression Patterns. 2003;3:747–754. doi: 10.1016/s1567-133x(03)00126-1. doi:10.1016/S1567-133X(03)00126-1. [DOI] [PubMed] [Google Scholar]
- Zon LI. Zebrafish: A new model for human disease. Genome Research. 1999;9:99–100. doi:10.1101/gr.9.2.99. [PubMed] [Google Scholar]
- Zon LI, Peterson R. The new age of chemical screening in zebrafish. Zebrafish. 2010;7:1. doi: 10.1089/zeb.2010.9996. doi:10.1089/zeb.2010.9996. [DOI] [PubMed] [Google Scholar]