Abstract
Background. Pentraxin-3 (PTX3), an inflammatory marker thought to be related to vascular inflammation, is elevated in advanced chronic kidney disease (CKD). Whether PTX3 is associated with mild to moderate kidney dysfunction is unknown.
Methods. We tested associations of proteins in the pentraxin family [PTX3, C-reactive protein (CRP) and serum amyloid protein (SAP)] with estimated glomerular filtration rate by cystatin C (eGFRcys) and microalbuminuria among 2824 participants in the Multi-Ethnic Study of Atherosclerosis. Associations were tested using multivariable linear regression with adjustment for demographics (age, gender, annual income), comorbidities (diabetes, hypertension, smoking, body mass index, low-density lipoprotein, high-density lipoprotein, triglycerides, ACE inhibitor and statin use) and systemic inflammation [interleukin-6 (IL-6)].
Results. Among the 2824 participants, mean age was 62 years and mean eGFRcys was 94 mL/min/1.73 m2; 25% were white, 25% Chinese, 25% African-American and 25% Hispanic. Among all participants after full adjustment, higher PTX3 was associated with lower eGFRcys independently of IL-6 (β − 3.0 mL/min/1.73 m2 per unit increase in lnPTX3, P < 0.001). In contrast, CRP and SAP were associated with eGFRcys in demographic adjusted models, but these associations were attenuated after adjustment for comorbidities and IL-6 (lnCRP β − 0.06, P = 0.9; lnSAP β − 0.35, P = 0.7). There was a significant interaction with race/ethnicity (P < 0.001) in the association of PTX3 and eGFRcys. After adjustment for demographics, comorbidities and IL-6, this association was significant in blacks (β − 5.7 mL/min/1.73 m2 per unit increase in lnPTX3, P = 0.002) but not in Hispanics (β − 2.4, P = 0.1), Chinese (β − 0.91, P = 0.5) or whites (β − 0.26, P = 0.9). PTX3 and CRP, but not SAP, had correlations with microalbuminuria in unadjusted models (Spearman coefficients PTX3 0.05, P = 0.005; CRP 0.07, P < 0.001; SAP 0.013, P = 0.5), but these were attenuated after full adjustment.
Conclusions. Endovascular inflammation may be an important mechanism associated with early kidney dysfunction, particularly among blacks. This mechanism appears to be independent of IL-6-regulated pathways.
Keywords: C-reactive protein, estimated glomerular filtration rate by cystatin, pentraxin-3, race/ethnicity, serum amyloid protein
Introduction
Endothelial dysfunction and arterial stiffness have been shown to be associated with moderate to severe chronic kidney disease (CKD), defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 [1–3]. Most recently, these findings have been extended to demonstrate that reduced small artery elasticity is associated with low estimated glomerular filtration rate by cystatin C (eGFRcys) in individuals with mild to moderate CKD [4]. One possible mechanism to explain these associations may involve direct endothelial injury that may be present even at early stages of kidney dysfunction.
Pentraxin-3 (PTX3), a long pentraxin, is a mediator of the innate immune system thought to be more specific to endovascular injury than the short pentraxins C-reactive protein (CRP) and serum amyloid protein (SAP). PTX3 is produced in endothelial cells as well as monocytes, macrophages, neutrophils, epithelial cells and fibroblasts. Unlike CRP and SAP, PTX3 is not produced in the liver and does not appear to be regulated by interleukin-6 (IL-6) [5]. PTX3 is found in atherosclerotic lesions [6] and has been shown to predict adverse cardiovascular outcomes independently of CRP [7,8]. PTX3 levels are increased in patients with advanced CKD and end-stage renal disease (ESRD), and PTX3 predicts mortality in these populations, independently of CRP [9,10]. However, the association of PTX3 with early kidney dysfunction has not been studied.
Moreover, whether the association of endothelial dysfunction and kidney disease varies by race/ethnicity is unclear. Several studies have suggested that black race/ethnicity may be independently associated with reduced arterial elasticity [11,12]. It is well established that the burden of ESRD is greater in blacks than in whites. While this is partially attributable to the high rate of hypertensive kidney disease in blacks, the prevalence of hypertension among blacks [13] does not entirely explain their rate of ESRD [14–17]. It is possible that blacks may have increased vascular dysfunction [11,12], which may be associated with kidney damage, even among persons without clinical hypertension or CKD.
Therefore, we designed these analyses to evaluate the association of PTX3, CRP and SAP with kidney function in an ethnically diverse cohort free of cardiovascular disease and with a spectrum of kidney function from normal to moderate CKD. We hypothesized that PTX3, but not SAP or CRP, would be elevated in persons with lower estimated glomerular filtration rate determined by cystatin C (eGFRcys), independent of systemic inflammation. Moreover, we hypothesized that this association would be strongest among blacks.
Subjects
We examined 2824 participants in The Multi-Ethnic Study of Atherosclerosis (MESA). Sponsored by the National Heart, Lung, and Blood Institute, MESA is a large cohort study designed to investigate subclinical cardiovascular disease and its progression in a multi-ethnic cohort. Details on recruitment and design have been previously published [18]. Briefly, MESA enrolled 6814 men and women who were aged between 45 and 84 years, were free of cardiovascular disease and self-identified as white, African-American, Hispanic or Chinese American. Subjects were recruited from Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; northern Manhattan and the Bronx, NY; and St. Paul, MN between July 2000 and August 2002. Individuals were excluded if they had physician-diagnosed heart attack, angina, heart failure, stroke or transient ischemic attack or atrial fibrillation; if they had undergone coronary artery bypass grafting, angioplasty, valve replacement or pacemaker insertion; or if they weighed > 300 lb (> 136 kg). The institutional review boards at all participating centers approved the study, and all participants gave informed consent. We used data from the baseline visit from July 2000 to July 2002. A group of 2880 MESA subjects were selected to undergo novel biomarker testing with balanced representation from all four race/ethnic groups. Among these, 56 subjects were excluded due to missing measures of kidney function or PTX3. The remaining 2824 subjects who had measures of kidney function and serum PTX3 are thus included in these analyses.
Methods
Primary predictors
PTX3 was measured by human PTX3 ELISA Detection Set from Alexis Biochemicals (Axxora, LLC); analytical coefficient of variation (CV) 10.2%. SAP was measured at the University of Vermont Department of Pathology with in-house ELISA; analytical CV 10.8% [7]. CRP was measured using a BNII nephelometer (N high sensitivity CRP; Dade Behring, Inc., Deerfield, IL, USA); CV 2.1–5.7%. IL-6 was measured by ultra-sensitive ELISA (Quantikine HD Human IL-6 Immunoassay; R&D Systems, Minneapolis, MN, USA) with an analytical CV of 6.3%.
Primary outcomes
Glomerular filtration rate was estimated from serum cystatin C (eGFRcys). Cystatin C was measured using a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring). Cystatin C has been shown to be a better marker of kidney function at higher GFR levels [19], and it has stronger and more linear associations with adverse outcomes including cardiovascular events and death [20–22]. eGFRcys was calculated using the formula 76.7 × cys C − 1.19, which was developed from pooled data from cohorts with eGFR measured by iothalamate [23]. Urine albumin and creatinine were measured by nephelometry and the Jaffe reaction rate, respectively. Albuminuria is expressed as the urine albumin/creatinine ratio in milligrams per gram.
Variables of interest
Blood pressure measurements were obtained by using an automated blood pressure device (DINAMAP PRO 100 monitor; General Electric Healthcare) following JNC guidelines [24]. Hypertension was defined as systolic pressure of ≥ 140 mmHg or diastolic pressure of ≥ 90 mmHg or the use of anti-hypertensive medication. Diabetes was defined as fasting glucose ≥ 126 or the use of hypoglycemic medication or insulin. Tobacco use was defined as self-reported current cigarette smoking. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight (kg)/height (m)2. Fasting blood was collected and stored at − 70°F (− 21°C) until needed for the appropriate assays, including high-density lipoprotein (HDL) cholesterol, triglycerides, glucose and IL-6. Low-density lipoprotein (LDL) cholesterol was calculated by using the Friedewald equation. These assays are previously described [25].
Statistical analysis
We compared sociodemographic characteristics, anthropometric measures and comorbidities by tertile of PTX3 using ANOVA or chi square where appropriate. Using multivariable linear regression, we analyzed the associations of PTX3, SAP and CRP with eGFRcys in staged models. To allow comparison of strengths of association, all three were log transformed to best approximate a normal distribution, then a one standard deviation of the log was evaluated as the continuous predictor variable. In companion analyses, we evaluated tertiles of PTX3. Of our three staged models, the first model adjusted for age, gender and socioeconomic status (specifically annual income); the second model added diabetes, hypertension, smoking, ACE inhibitor use, statin use, BMI, LDL, HDL and triglycerides; and the third model added IL-6 to represent systemic inflammation. We chose this approach to understand the relative importance of specific confounders that were either associated with PTX3 on univariate analysis or are known mediators of atherosclerosis. We tested for interaction by race/ethnicity and stratified where appropriate with both linear and categorical PTX3 models.
We also studied the associations of PTX3, SAP and CRP with albuminuria using Spearman coefficients for unadjusted associations. We used linear regression to examine adjusted associations between PTX3, SAP, CRP and log-transformed albumin to creatinine ratio. We log transformed the ratio to achieve normality. All calculations were performed in STATA version 11, and a two-tailed P < 0.05 was considered significant.
Results
Participants’ characteristics
Among the 2824 participants in this study, the mean age was 62 ± 10 years, 54% were female, 43% had hypertension and 13% had diabetes; 25% (n = 716) were white, 25% (n = 711) were Chinese, 25% (n = 704) African-American and 25% Hispanic (n = 707). Overall, 5% had CKD at baseline based on an eGFRcys < 60 mL/min/1.73 m2. Using cutoffs defining albuminuria as ≥ 17 mg/g for men and ≥ 25 mg/g for women, 15% had albuminuria [26].
Characteristics of participants by tertiles of PTX3 are described in Table 1. Subjects in the highest tertile of PTX3 were more likely to be older, have a lower income and have higher prevalence of diabetes, hypertension and current smoking. Blacks and Chinese had lower levels of PTX3 than whites and Hispanics. PTX3 mean (SD) in nanograms per milliliter were as follows: blacks 2.1 (1.5); Chinese 1.9 (1.2); whites 2.3 (1.5); Hispanics 2.3 (1.2) (ANOVA P-value < 0.001). These findings were not attenuated by adjustment for comorbidities.
Table 1.
Demographic and clinical characteristics of 2824 MESA participants by PTX3 tertile
| PTX3 median, (SD) range (ng/mL) | Lower tertile n = 947 | Middle tertile n = 946 | Upper tertile n = 945 | P-value (ANOVA or χ2) |
|---|---|---|---|---|
| 1.24, (0.27) 0.42–1.56 | 1.90, (0.21) 1.57–2.28 | 2.9, (1.71) 2.30–8.9 | ||
| Characteristicsa | ||||
| Age (years) | 61 (10) | 61 (10) | 63 (11) | < 0.001 |
| Female | 502 (53%) | 520 (55%) | 501 (53%) | 0.45 |
| Race | ||||
| Black | 250 (26%) | 248 (26%) | 206 (22%) | < 0.001 |
| Hispanic | 180 (19%) | 236 (25%) | 291 (31%) | |
| Chinese | 324 (34%) | 202 (21%) | 185 (20%) | |
| White | 193 (20%) | 260 (27%) | 263 (28%) | |
| Income | ||||
| <$20k | 246 (26%) | 246 (26%) | 284 (30%) | 0.10 |
| $20k–50k | 331 (35%) | 321 (34%) | 340 (36%) | 0.68 |
| $50k–100k | 340 (36%) | 350 (37%) | 293 (31%) | 0.01 |
| Diabetes | 104 (11%) | 123 (13%) | 151 (16%) | 0.03 |
| Impaired fasting glucose | 256 (27%) | 246 (26%) | 274 (29%) | 0.30 |
| Hypertensionb | 401 (42%) | 452 (48%) | 473 (50%) | 0.003 |
| Current smoker | 104 (11%) | 132 (14%) | 151 (16%) | 0.03 |
| ACE-I or ARB | 127 (13%) | 132 (14%) | 163 (17%) | 0.04 |
| Statin | 141 (15%) | 131 (14%) | 125 (13%) | 0.60 |
| SBP (mmHg) | 124 (20) | 127 (22) | 127 (22) | 0.002 |
| Fasting glucose (mg/dL) | 96 (26) | 98 (33) | 100 (33) | 0.06 |
| BMI | 28 (5.5) | 28 (5.3) | 28 (5.6) | 0.14 |
| LDL (mg/dL) | 117 (31) | 118 (31) | 117 (32) | 0.44 |
| HDL (mg/dL) | 50.1 (13.6) | 50.9 (14.6) | 50.7 (14.7) | 0.47 |
| Triglycerides (mg/dL) | 138 (96) | 131 (78) | 137 (99) | 0.26 |
| IL-6 (pg/mL) | 1.42 (1.14) | 1.46 (1.13) | 1.63 (1.36) | < 0.001 |
| CRP (mg/L) | 3.1 (4.6) | 3.3 (4.3) | 4.5 (8.3) | < 0.001 |
| SAP (μg/mL) | 45 (17) | 47 (18) | 48 (21) | < 0.001 |
| eGFRcys (mL/min/m2) | 96 | 94 | 91 | 0.002 |
All measures presented as mean (SD) or N (%).
SBP ≥ 140 mmHg or on anti-hypertensive medication.
Pentraxins and kidney function
We found that each SD increase in log-transformed PTX3 was associated with a 1.7 mL/min/1.73 m2 decrease in eGFRcys after adjusting for age, gender and income (P < 0.001). This association was only modestly attenuated by adjustment for comorbidities and IL-6, such that each SD increase in log-transformed PTX3 was associated with a 1.4 mL/min/1.73 m2 decrease in eGFRcys in the final model (P < 0.001). CRP and SAP were associated with eGFRcys in demographic adjusted models, but this association was strongly attenuated with adjustment for comorbidities and fully attenuated after adjustment for IL-6 (Table 2).
Table 2.
Cross-sectional associations of proteins in the pentraxin family with eGFRcys among MESA participantsa
| Model 1 | P | Model 2 | P | Model 3 | P | |
|---|---|---|---|---|---|---|
| PTX3 | − 1.65 (− 2.4, − 0.91) | < 0.001 | − 1.5 (− 2.2, − 0.79) | < 0.001 | − 1.39 (− 2.1, − 0.68) | < 0.001 |
| CRP | − 3.52 (− 4.3, − 2.8) | < 0.001 | − 0.90 (− 1.7, − 0.09) | 0.03 | − 0.07 (− 1, 0.82) | 0.89 |
| SAP | − 2.5 (− 3.2, − 1.7) | < 0.001 | − 0.53 (− 1.3, 0.23) | 0.17 | − 0.13 (− 0.91, 0.64) | 0.74 |
β coefficients represent difference in eGFRcys (mL/min/1.73 m2) per SD increase in lnPTX3, lnSAP and lnCRP, with 95% confidence intervals.
Model 1 = age, gender, yearly income; Model 2 = Model 1 + HTN, diabetes, impaired fasting glucose, current smoking, ACE inhibitor use, statin use, LDL, HDL, triglycerides; Model 3 = Model 2 + IL-6.
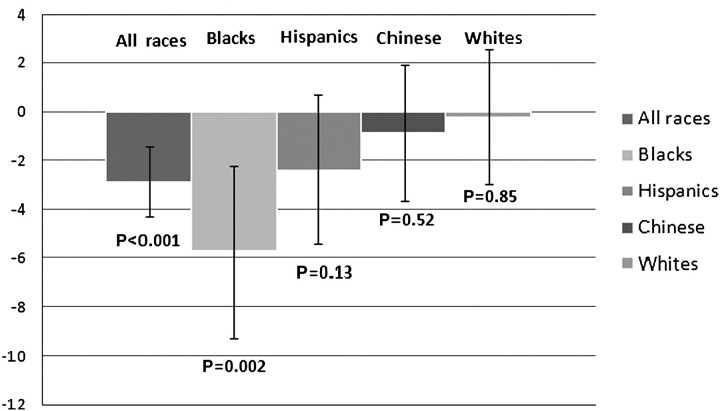
In the fully adjusted model, we found a significant interaction by race/ethnicity in the association of PTX3 and kidney function whereby this association was strongest among blacks (P-value for interaction < 0.001). There was a similar pattern among Hispanics, although the association did not reach statistical significance. There was no significant association between PTX3 and eGFRcys among whites or Chinese (Figure 1). We conducted a sensitivity analysis excluding the 32 subjects with eGFRcys < 60 mL/min/1.73 m2, and findings were not materially different.
Fig. 1.
Association of PTX3 and eGFRcys among MESA cohort participants adjusted for IL-6 and stratified by race (P-value for interaction = < 0.001). Bars represent β coefficients for change in eGFRcys (mL/min/1.73 m2) per natural log of PTX3, brackets represent 95% CI.
We also studied the associations between PTX3 tertiles and eGFRcys. Higher PTX3 was associated with lower eGFRcys. This association was significant only in blacks and was not attenuated by adjustment for comorbidities or systemic inflammation (Table 3).
Table 3.
The associations of PTX3 tertiles with eGFRcys among 2824 MESA participants, overall and stratified by race
| eGFRcys (mL/min/1.73 m2)a | ||||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
| Difference (95% CI) | P-value | Difference (95% CI) | P-value | Difference (95% CI) | P-value | |
| All n = 2824 | ||||||
| PTX3 tertile 1 (0.31–1.56, median 1.24, SD 0.28 ng/mL) | Reference | Reference | Reference | |||
| PTX3 tertile 2 (1.57–2.29, median 1.90, SD 0.21 ng/mL) | − 2.2 (− 4.0, − 0.36) | 0.02 | − 1.9 (− 3.6, − 0.21) | 0.03 | − 1.9 (− 3.7, − 0.23) | 0.03 |
| PTX3 tertile 3 (2.30–28.57, median 2.91, SD 1.71 ng/mL) | − 2.8 (− 4.0, − 0.35) | 0.003 | − 2.6 (− 4.3, − 0.9) | 0.003 | − 2.3 (− 4.0, − 0.56) | 0.009 |
| Blacks (n = 700) | ||||||
| PTX3 tertile 2 | − 3.6 (− 7.6, 0.32) | 0.07 | − 3.9 (− 7.7, − 0.14) | 0.04 | − 3.9 (− 7.8, − 0.08) | 0.045 |
| PTX3 tertile 3 | − 4.6 (− 8.8, − 0.4) | 0.03 | − 4.4 (− 8.4, − 0.31) | 0.04 | − 4.1 (− 8.2, 0.06) | 0.05 |
| Hispanics (n = 704) | ||||||
| PTX3 tertile 2 | 0.3 (− 3.4, 4.0) | 0.9 | 0.19 (− 3.3, 3.7) | 0.9 | 0.39 (− 3.1, 3.9) | 0.8 |
| PTX3 tertile 3 | − 2.1 (− 5.6, 1.5) | 0.3 | − 2.4 (− 5.9, 1.0) | 0.2 | − 1.6 (− 5.1, 1.8) | 0.3 |
| Chinese (n = 708) | ||||||
| PTX3 tertile 2 | − 1.5 (− 5.0, 2.0) | 0.4 | − 1.3 (− 4.6, 2.1) | 0.5 | − 1.4 (− 4.8, 1.9) | 0.4 |
| PTX3 tertile 3 | − 0.08 (− 3.6, 3.4) | 1.0 | − 0.36 (− 3.8, 3.1) | 0.8 | − 0.17 (− 3.6, 3.3) | 0.9 |
| Whites (n = 712) | ||||||
| PTX3 tertile 2 | 0.9 (− 2.6, 4.3) | 0.6 | − 0.45 (− 3.7, 2.7) | 0.8 | − 0.36 (− 3.6, 2.8) | 0.8 |
| PTX3 tertile 3 | 1.3 (− 2.2, 4.7) | 0.5 | 0.02 (− 3.2, 3.2) | 1.0 | 0.1 (− 3.1, 2.8) | 1.0 |
β coefficients represent difference in eGFRcys (mL/min/1.73 m2) compared with the lowest tertile of PTX3.
Model 1 = age, gender, yearly income; Model 2 = Model 1 + HTN, diabetes, impaired fasting glucose, smoking, ACE-I, statin, LDL, HDL, triglycerides; Model 3 = Model 2 + IL-6.
Pentraxins and albuminuria
We found small but significant correlations between PTX3 and CRP with albumin/creatinine ratio in unadjusted models (Spearman coefficient 0.05, P = 0.005 for PTX3 and 0.07, P < 0.001 for CRP). SAP was not significantly correlated with albumin/creatinine ratio (Spearman coefficient 0.013, P = 0.5). In multivariable models, none of these inflammatory markers had significant associations with albuminuria. In models adjusted for demographics, comorbidities and IL-6, β coefficients (95% CI) were as follows: PTX3 0.01 (− 0.03, 0.05), P = 0.6; CRP 0.02 (− 0.03, 0.07), P = 0.4; and SAP − 0.02 (− 0.06, 0.02), P = 0.5.
Discussion
In a large, multi-ethnic cohort free of cardiovascular disease and comprised mainly of subjects without CKD (eGFR > 60 mL/min/1.73 m2), we found that PTX3, an inflammatory marker thought to be related to vascular inflammation, was significantly associated with eGFRcys. The association remained significant after adjustment for demographics, comorbidities and IL-6. In contrast, other proteins in the pentraxin family (CRP and SAP) were associated with kidney function in demographic adjusted models, but these associations were attenuated after adjustment for comorbidities and systemic inflammation. Moreover, the association between PTX3 and kidney function was strongest among blacks, moderate among Hispanics and non-significant among whites or Chinese. Our results suggest that endovascular inflammation may be an important mechanism associated with kidney dysfunction, particularly among blacks.
Our findings are in concordance with prior studies that found an association between PTX3 and advanced kidney disease [9,10]. We extend these observations to a broader spectrum of kidney function. The fact that adjustment for IL-6 attenuated the associations of SAP and CRP but not PTX3 with eGFRcys suggests that the association of PTX3 and kidney function is independent of IL-6-dependent pathways. The mechanism by which PTX3 is associated with impaired kidney function remains unclear. One possible explanation is that elevated PTX3 represents endovascular inflammation, which may be a mechanism involved in initiation and progression of GFR loss. However, whether PTX3 itself is a direct effector of this injury or is part of a protective response is unclear. PTX3 has diverse functions, including activation and regulation of the complement cascade, agglutination, opsonization and regulation of inflammation. PTX3 has been shown in one mouse model to be required for inflammation following intestinal ischemia and reperfusion [27], but two different mouse models suggest a protective effect of PTX3 after myocardial infarction [28] and in the development of atherosclerosis [29]. In clinical studies, higher PTX3 has been shown to predict adverse cardiovascular outcomes independently of CRP [7,8], but it is unknown whether PTX3 has a causal effect. Alternatively, PTX3 is relatively small (42 kDa [30]); it is possible that levels of PTX3 are increased due to accumulation in the setting of reduced renal clearance. Whether PTX3 is filtered in the glomerulus in humans is unknown. However, if decreased renal clearance were the only mechanism for increased PTX3, it would be difficult to explain why an association between PTX3 and eGFRcys would predominate in blacks. Further studies should focus on understanding whether PTX3 is associated with kidney function decline and on the mechanisms that may underlie these associations.
The finding that the association between PTX3 and eGFRcys is strongest among blacks is noteworthy. PTX3 is highly conserved through evolution, with homology evident between human and mouse [31]; thus, a genetic difference in PTX3 expression between races of the same species seems unlikely. Furthermore, PTX3 levels were lower in blacks than in whites and Hispanics. It is important to consider alternative hypotheses to explain our findings. It is possible that part of the innate immune system that regulates PTX3 differs across races/ethnicity, and that this accounts for the stronger association of PTX3 and eGFRcys in blacks. Alternatively, this race/ethnicity interaction may represent higher renal susceptibility in blacks to damage by vascular inflammation, or conversely, higher sensitivity of the endothelium in blacks to become inflamed in the presence of kidney disease. There may be environmental influences that we did not adjust for in our analysis that account for this difference. Future studies on the role of early, pre-clinical endothelial injury in blacks and its association with CKD progression are needed.
Strengths of this study are the unique setting, as MESA is a large, well-characterized cohort including four major race/ethnic groups. In addition, the inclusion of both specific and non-specific markers of vascular injury in the pentraxin family makes our analysis more robust. However, our study has important limitations. Since the study was cross-sectional, we are not able to assess the direction of the associations. We must also consider that PTX3 increases during systemic inflammation or infection. As these conditions were not assessed in our cohort, residual confounding may still be present. However, our findings were robust even after adjustment for systemic inflammation and comorbidities. Moreover, the MESA cohort is a healthy cohort and the prevalence of chronic inflammatory or infectious conditions is likely to be low. As with most population-based epidemiological studies, we had no gold standard GFR measure. However, cystatin C has been shown to be a better marker of GFR than creatinine at GFR > 60 mL/min/1.73 m2 [19]. Furthermore, our analysis of albuminuria may be limited by power due to the low prevalence of albuminuria in MESA, thus increasing the probability of type 2 error.
In summary, our findings that higher PTX3 is significantly and linearly associated with lower eGFRcys may represent a link between endovascular injury and early kidney disease, particularly in blacks. In addition, these associations may point to an important mechanism linking kidney disease and cardiovascular disease, given the strong association of PTX3 as a risk factor for cardiovascular events [7,8]. Further studies should include longitudinal studies of decline in kidney function and physiological studies of the determinants of PTX3 levels.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. C.P. is supported with NIH funding by NIDDK Grant #1K23DK082793-01.
Conflict of interest statement. The abstract of this paper was published by American Society of Nephrology in October 2010.
References
- 1.Briet M, Bozec E, Laurent S, et al. Arterial stiffness and enlargement in mild-to-moderate chronic kidney disease. Kidney Int. 2006;69:350–7. doi: 10.1038/sj.ki.5000047. [DOI] [PubMed] [Google Scholar]
- 2.Foster MC, Keyes MJ, Larson MG, et al. Relations of measures of endothelial function and kidney disease: the Framingham Heart Study. Am J Kidney Dis. 2008;52:859–67. doi: 10.1053/j.ajkd.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghiadoni L, Cupisti A, Huang Y, et al. Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol. 2004;17:512–9. [PubMed] [Google Scholar]
- 4.Peralta CA, Adeney KL, Shlipak MG, et al. Structural and functional vascular alterations and incident hypertension in normotensive adults: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2010;171:63–71. doi: 10.1093/aje/kwp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Garlanda C, Bottazzi B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine. 2003;21:S43–S47. doi: 10.1016/s0264-410x(03)00199-3. [DOI] [PubMed] [Google Scholar]
- 6.Rolph MS, Zimmer S, Bottazzi B, et al. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–e14. doi: 10.1161/01.atv.0000015595.95497.2f. [DOI] [PubMed] [Google Scholar]
- 7.Jenny NS, Arnold AM, Kuller LH, et al. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2009;29:594–599. doi: 10.1161/ATVBAHA.108.178947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latini R, Maggioni AP, Peri G, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- 9.Suliman ME, Qureshi AR, Carrero JJ, et al. The long pentraxin PTX-3 in prevalent hemodialysis patients: associations with comorbidities and mortality. QJM. 2008;101:397–405. doi: 10.1093/qjmed/hcn019. [DOI] [PubMed] [Google Scholar]
- 10.Tong M, Carrero JJ, Qureshi AR, et al. Plasma pentraxin 3 in patients with chronic kidney disease: associations with renal function, protein-energy wasting, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2007;2:889–897. doi: 10.2215/CJN.00870207. [DOI] [PubMed] [Google Scholar]
- 11.Bhuiyan AR, Li S, Li H, et al. Distribution and correlates of arterial compliance measures in asymptomatic young adults: the Bogalusa Heart Study. Am J Hypertens. 2005;18:684–691. doi: 10.1016/j.amjhyper.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Duprez DA, Jacobs DR, Jr, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity—results of the multi-ethnic study of atherosclerosis (MESA) Ethn Dis. 2009;19:243–250. [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler JA, Sorlie PD, Wolz M, et al. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 14.McClellan W, Tuttle E, Issa A. Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis. 1988;12:285–90. doi: 10.1016/s0272-6386(88)80221-x. [DOI] [PubMed] [Google Scholar]
- 15.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13:2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 16.USRDS . USRDS 2004 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Digestive and Diabetes and Kidney Diseases; 2004. 2004. [Google Scholar]
- 17.Whittle JC, Whelton PK, Seidler AJ, et al. Does racial variation in risk factors explain black–white differences in the incidence of hypertensive end-stage renal disease? Arch Intern Med. 1991;151:1359–1364. [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 21.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Ix JH, Shlipak MG, Chertow GM, et al. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 25.Keller C, Katz R, Cushman M, et al. Association of kidney function with inflammatory and procoagulant markers in a diverse cohort: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis (MESA) BMC Nephrol. 2008;9:9. doi: 10.1186/1471-2369-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Foundation NK. NKF KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification, Table 15: Definitions of Proteinuria and Albuminuria. 2002; http://www.kidney.org/professionals/KDOQI/guidelines_ckd/p4_class_g1.htm] [PubMed]
- 27.Souza DG, Amaral FA, Fagundes CT, et al. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am J Pathol. 2009;174:1309–1318. doi: 10.2353/ajpath.2009.080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salio M, Chimenti S, De Angelis N, et al. Cardioprotective function of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2008;117:1055–1064. doi: 10.1161/CIRCULATIONAHA.107.749234. [DOI] [PubMed] [Google Scholar]
- 29.Norata GD, Marchesi P, Pulakazhi Venu VK, et al. Deficiency of the long pentraxin PTX3 promotes vascular inflammation and atherosclerosis. Circulation. 2009;120:699–708. doi: 10.1161/CIRCULATIONAHA.108.806547. [DOI] [PubMed] [Google Scholar]
- 30. ExPASy. ExPASy Proteomics Server. Available from: http://www.expasy.ch/tools/pi_tool.html.
- 31.Bottazzi B, Doni A, Garlanda C, et al. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2009;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]