Abstract
Background. Renal calcium oxalate (CaOx) crystal deposition is associated with epithelial injury and movement of inflammatory cells into the interstitium. We have proposed that oxalate (Ox)- and CaOx crystal-induced injury is most likely caused by reactive oxygen species (ROS) produced by activation of membrane nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.
Methods. Present study was undertaken to determine the effect of NADPH oxidase inhibitor apocynin on the expression of kidney injury molecule-1 (KIM-1) and renal CaOx crystal deposition in rats with hyperoxaluria. We also investigated the urinary excretion of KIM-1, osteopontin (OPN) and monocyte chemoattractant protein-1 (MCP-1) and renal expression of OPN and ED-1. Male Sprague–Dawley rats were fed a diet containing 5% hydroxyl-L-proline (HLP) and 4 mmol apocynin to drink for 28 days. Urine was collected on Days 7, 14, 21 and 28. After that, rats were sacrificed and their kidneys processed for various microscopic and molecular investigations.
Results. HLP consumption produced heavy deposits of CaOx crystals. Renal expression of KIM-1 and OPN and urinary excretion of KIM-1, OPN, H2O2 and MCP-1 was significantly increased. ED-1-positive cells migrated into renal interstitium. Apocynin treatment caused significant reduction of crystal deposits, injured and dilated tubules; renal expression of KIM-1, OPN and ED-1 and urinary excretion of KIM-1, OPN, MCP-1 and H2O2. Apocynin had no effect on the urinary excretion of Ox.
Conclusions. This is the first study of urinary excretion and renal expression of KIM-1 in association with renal CaOx crystal deposition, experimental or clinical. The results indicate that NADPH oxidase inhibition leads to reduction in KIM-1 expression and urinary excretion as well as renal CaOx crystal deposition. KIM-1 is an important marker of renal epithelial injury. The results provide further support to our proposal that renal epithelial injury is critical for crystal retention and that injury is in part caused by the production of ROS with the involvement of NADPH oxidase.
Keywords: apocynin, calcium oxalate, kidney injury molecule-1, kidney stones, NADPH oxidase, oxalate, oxidative stress
Introduction
Nephrolithiasis, deposition of crystal in the kidneys and formation of kidney stones, is a common urological disorder. Most kidney stones are comprised of calcium oxalate (CaOx) mixed with calcium phosphate (CaP) crystals [1]. There is experimental evidence that crystal deposition in the kidneys is associated with the production of reactive oxygen species (ROS), development of oxidative stress [2], renal injury and inflammation [3–5]. There is also evidence for the activation of renin–angiotensin system and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase when cells are exposed to high oxalate (Ox) and CaOx/CaP crystals [6–9].
NADPH oxidase is composed of two membrane-binding subunits, gp91phox and p22phox, and several cytosolic subunits, including p47phox, p67phox, p40phox and the GTPase rac [10]. Phosphorylation of p47phox subunit has been considered to play a key role in the regulation of NADPH oxidase activity in the kidney. Under the hyperoxaluric condition, crystal deposition results in angiotensin II (Ang II) activation [8]. NADPH oxidase is stimulated by activated Ang II, and the cytosolic subunit p47phox is phosphorylated and translocated to the membrane assembling the catalytic complex of active oxidase leading to ROS production, which can damage renal cells [11, 12]. We have recently demonstrated (article in press, Nephrology Dialysis Transplantation) that oxalate and CaOx crystal exposures of renal epithelial cells in culture lead to changes in the expression of various subunits which in turn effect activation of NADPH oxidase. Significant correlation was seen between CaOx crystal-induced upregulation of p22phox and p47phox and NADPH oxidase activation and associated cell injury.
Apocynin (Apoc), an antioxidant and NADPH oxidase inhibitor, is a natural nontoxic compound isolated from a medicinal plant, Picrorhiza kurroa [13]. It prevents activation of NADPH oxidase by blocking the assembly of cytosolic units with the membrane complex [14]. We have developed an animal model in which CaOx nephrolithiasis is generated by feeding hydroxyl-L-proline (HLP), a physiological precursor of oxalate, to male Sprague–Dawley rats [15]. Rats treated with 5% HLP in a food for 28 days developed hyperoxaluria, CaOx crystalluria and CaOx nephrolithiasis. An increase in urinary excretion of Ox is associated with significantly high levels of urinary lactate dehydrogenase (LDH), H2O2 as well as lipid peroxides, indicating renal cell injury induced by oxidative stress.
It is our hypotheses that renal epithelial injury is a prerequisite to CaOx crystal deposition in the kidneys [16] and that injury is caused by high Ox/CaOx crystal-induced oxidative stress [2]. The purpose of this study is to evaluate the effect of apocynin on CaOx crystal-associated renal injury and inflammation as indicated by the expression of kidney injury molecule-1 (KIM-1), a marker of renal epithelial injury [17], and inflammatory mediators such as the monocyte chemoattractant protein-1 (MCP-1) and osteopontin (OPN) in HLP-induced hyperoxaluria and CaOx crystal deposition in the kidneys.
Materials and methods
Animals
Male Sprague–Dawley rats (120–130 g) were purchased from Harlan. After 1 week of acclimatization to our animal facilities, they were divided into four groups: control, apocynin, HLP diet and HLP diet and apocyin. All rats had free access to food and water, and their consumption was recorded daily. Food was ground, moistened with water and formed into small balls, which were individually weighed. Control rats received normal rat chow and regular drinking water, whereas experimental animals were given 4 mmol aqueous solution of apocynin to drink and/or 5% HLP (ICN Biochemicals, Aurora, OH) in the food. Food and water consumption was regularly monitored. Rats were weighed weekly to check their growth. Experimental procedures have been described in detail in our earlier publications [15, 18–20].
Urine was collected on a weekly basis and the volume, pH, protein and crystal count were recorded. The urine was analyzed for urinary ions and markers of cell injury and ROS production. Six rats from each group were sacrificed on Day 28. Their bladder urine was aspirated and examined for the presence of crystals. Their kidneys were processed for localization of crystals and various other morphological analyses.
Urine collection and analysis
Once a week, 24-h urine collection was made with 0.02% sodium azide to prevent bacterial growth. The rats were placed in metabolic cages for the collection of urine. After determining volume and pH, urine was aliquoted for various assays.
Urine was examined by light microscopy to analyze crystalluria. Urinary oxalate and calcium were determined using methods described in our previous studies. Urinary concentration of H2O2, a ROS indicating the development of oxidative stress, was determined using a colorimetric assay from Pierce (PeroXoquant Quantitative Peroxide Assay Kit; Pierce Biotechnology Inc., Rockford, IL).
Urinary excretion of KIM-1 (R&D Systems, Minneapolis, MN), OPN (R&D Systems) and MCP-1 (Pierce Biotechnology Inc.) were determined using ELISA kits according to the manufacturer’s instructions. The data were assessed from three rats in each group.
Kidney tissue processing
Six rats each from the normal control group and the experimental groups were sacrificed on Day 28. Details of tissue preparation for light microscopic analyses are available in earlier publications [15, 18, 19]. Paraffin-embedded kidneys were stained using hematoxylin and eosin or PAS. Calcium oxalate crystals were identified by Pizzolato staining and polarizing optics. Quantification of CaOx crystals was carried out by examining the sections at ×4, with five fields per section. The average crystal count was recorded as 0: no crystal deposits, 1: 1–2 crystal deposits per field, 2: 3–5 per field, 3: 6–10 deposits and 4: >10.
Immunohistochemistry
Kidney sections were processed for immunohistochemistry using specific antibodies against KIM-1 (R&D Systems), MCP-1 (US Biological, Swampscott, MA), OPN (US Biological, Swampscott) and ED-1 (Abcam, Cambridge, MA). The data were assessed from six rats in HLP-treated group, six rats in apocynin with HLP diet group and three rats in controls.
Immunoblotting analysis
Immunoblotting was carried out using the labeled streptavidin–biotin method. Briefly, three kidney tissue samples (20–50 μg) from each group were subjected to electrophoresis; samples for sodium dodecyl sulfate–polyacrylamide gel electrophoresis were prepared in a 6-fold loading buffer (Bio-Rad, Hercules, CA). After electrotransfer, blotting membranes were blocked for 1 h at room temperature in 5% nonfat milk in TBST [20 mM Tris–HCl (pH 7.4), 150 mM NaCl and 0.05% (w/v) Tween-20] and then incubated with the primary antibody in TBST/5% milk at 4°C overnight. Primary antibodies used were anti-KIM-1 (R&D Systems), OPN (US Biological, Swampscott) and ED-1 (Abcam). The blot was then exposed to biotinylated secondary antibodies (GE Health) followed by a 30-min incubation with streptavidin-conjugated alkaline phosphatase (GE Health). Colorimetric development was performed with a one-step 5-bromo-4-chloro-3-indolyl phosphate reagent (Sigma). Semiquantitative evaluation of protein levels was performed with computer-assisted densitometric scanning using Image J program (Version 1.33) [21].
Statistical analysis
All analysis for statistical significance was performed using GraphPad Prism® software (GraphPad Software, San Diego, CA). Data were evaluated by one-way/two-way analysis of variance with post hoc Dunnett’s–Boneferroni Post test. All data are expressed as mean ± SE. Probability values <0.05 were required for statistical significance. Correlation coefficient r was obtained using a linear (Pearson’s) correlation test. Ps <0.05 were considered significant.
Results
Urinary changes
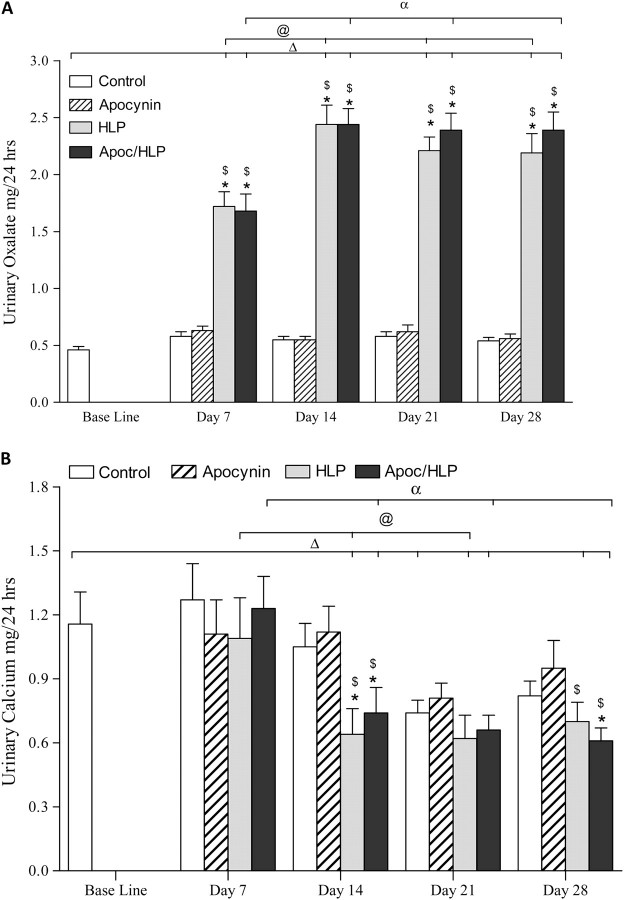
As expected, there was a significant increase in urinary excretion of Ox by rats that consumed HLP alone or in combination with apocynin, peaking on Day 14 and remaining significantly higher than excreted by the control rats or the baseline (Figure 1A). Urinary excretion of Ox by rats receiving only apocynin was similar to the baseline or control. HLP consumption also had a significant effect on urinary excretion of calcium. Rats treated with HLP alone or in combination with apocynin showed markedly reduced urinary calcium excretion on the 14th as well as 28th day (Figure 1B). Apocynin had no effect on the excretion of urinary Ox and urinary calcium in rats with normal diet for all time periods studied.
Fig. 1.
Urinary excretion of calcium and oxalate in normal control rats, apocynin-treated rats, HLP-consuming rats and HLP-consuming rats treated with apocynin (Apoc). (A) HLP consumption led to significant increase in urinary excretion of Ox. Apocynin had no effect on Ox excretion given to normal or HLP-consuming rats. (B) Urinary excretion of calcium by HLP-consuming rats was significantly decreased. Apocynin (Apoc) treatment did not have significant or consistent effect on urinary calcium. ΔP < 0.05 baseline versus HLP and HLP/Apoc on different days. @P < 0.05 HLP between various days. αP < 0.05 HLP/Apoc between different days. *P < 0.05 control versus HLP and HLP/Apoc within the time period. $P < 0.05 apocynin treated versus HLP consumption alone within the time period.
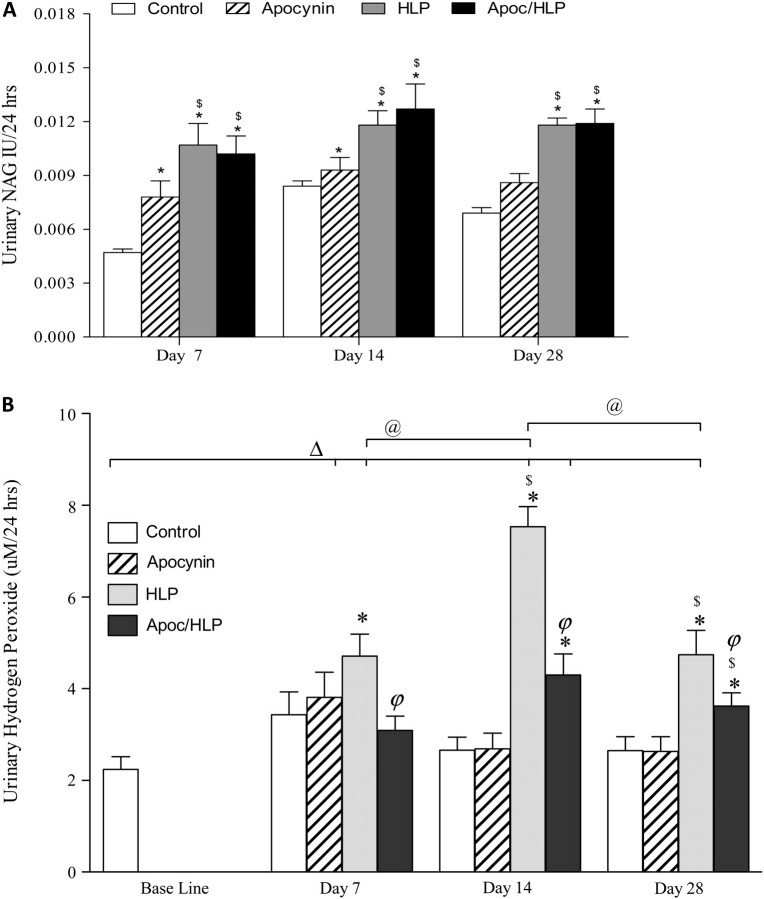
There was a marked increase in urinary excretion of N-acetyl-β-glucoseaminidase (NAG) for all time periods, by all treated rats compared to the controls (Figure 2A). Apocynin treatment to rats receiving HLP had no significant effect upon NAG excretion. HLP diet caused significant increase in the urinary excretion of hydrogen peroxide (HP) compared to the baseline and controls. Apocynin treatment produced significant reductions in HP excretion by HLP-consuming rats. Urinary excretion of HP by HLP-consuming rats also increased significantly compared to normal as well as baseline levels (Figure 2B). Largest increase was seen on Day 14. Apocynin treatment resulted in significant reduction of HP excretion.
Fig. 2.
Sign of injury in the urine. (A) Urinary excretion of NAG increased significantly by HLP consumption for all time periods. Apocynin treatment had no effect on NAG excretion. *P < 0.05 control versus HLP and HLP/Apoc within the time period. $P < 0.05 apocynin treated versus HLP consumption alone within the time period. (B) There was a significant increase in urinary HP excretion, which was significantly reduced by apocynin treatment. ΔP < 0.05 baseline versus HLP and HLP/Apoc on different days. @P < 0.05 HLP between various days. P < 0.05 HLP/Apoc between different days. *P < 0.05 control versus HLP and HLP/Apoc within the time period. $P < 0.05 apocynin treated versus HLP consumption alone within the time period. φP < 0.05 HLP versus HLP/Apoc within the time period.
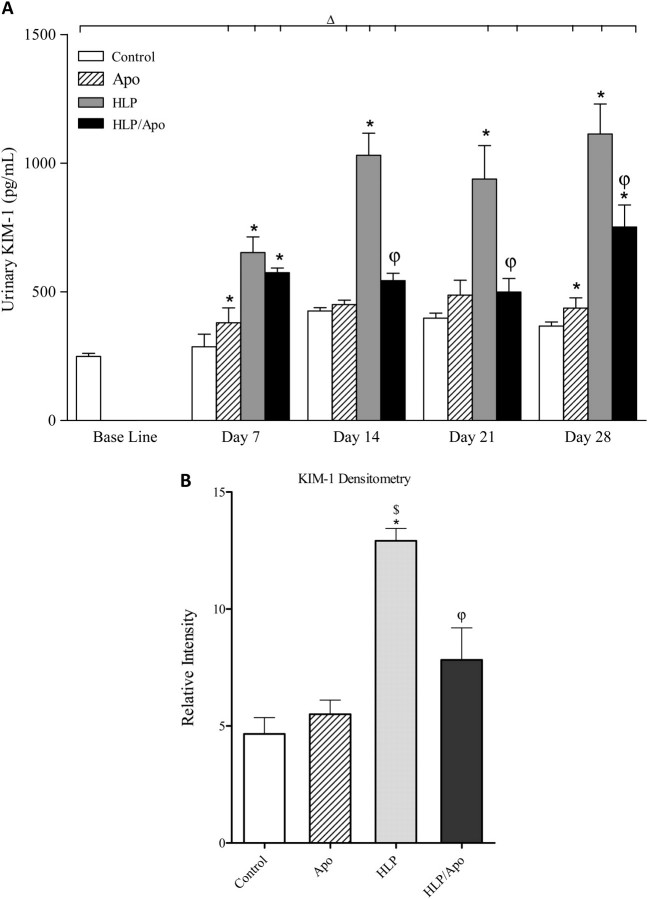
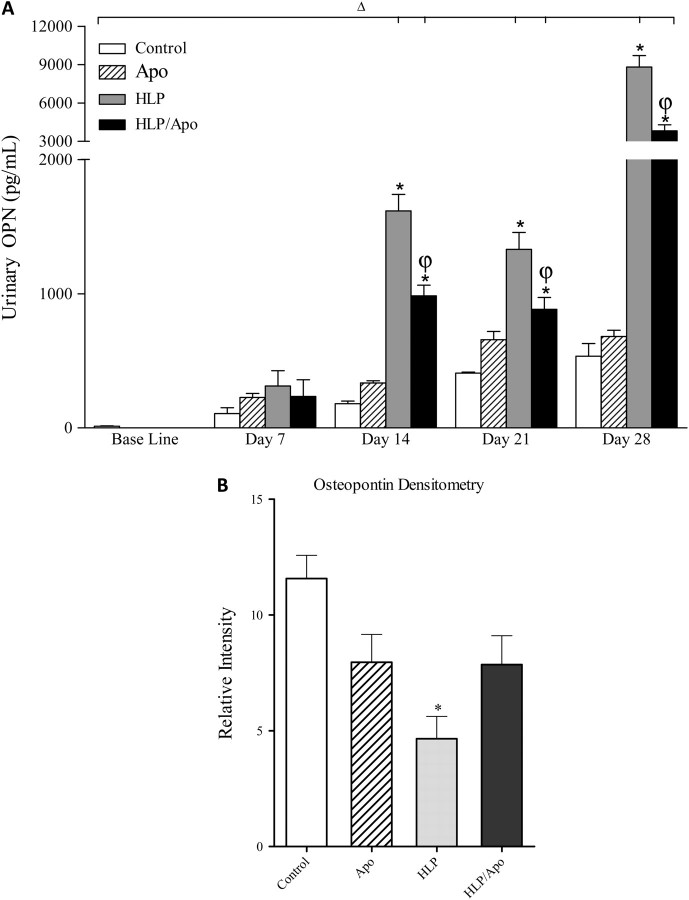
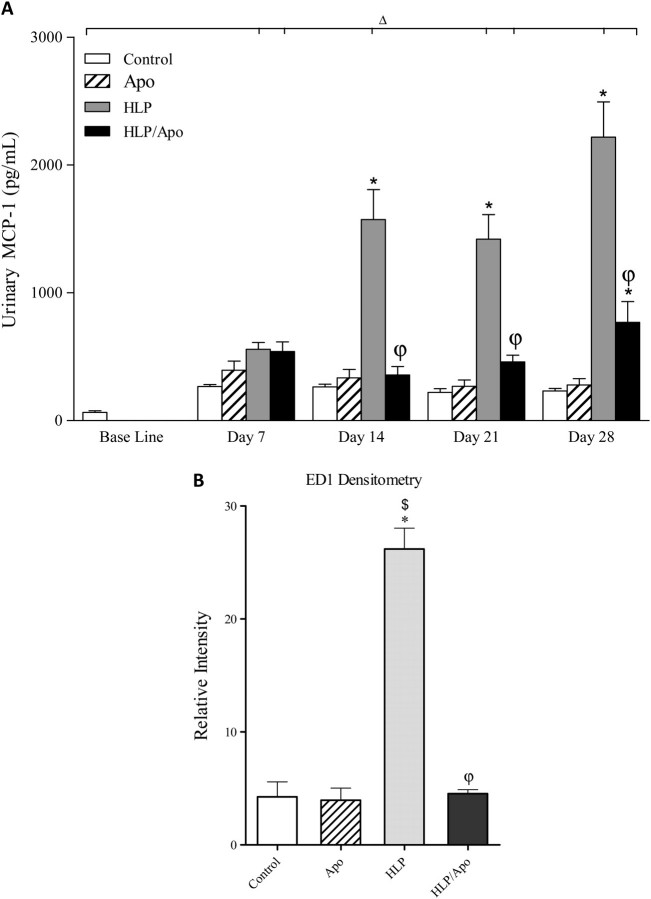
Enzyme-linked immunosorbent assay showed significant increase in urinary levels of KIM-1 by rats who received HLP in their diet (Figure 3A). KIM-1 excretion reached its peak by 14 days and remained at that high level for the next 14 days. Apocynin treatment resulted in highly significant reduction of KIM-1 excretion by HLP-consuming rats. Urinary excretion of both OPN (Figure 4A) and MCP-1 (Figure 5A) by rats on HLP also increased significantly, with the highest levels reached on 28th day of the experiment. Apocynin treatment resulted in significant reduction of OPN and MCP-1 excretion in the urine of rats on HLP.
Fig. 3.
Changes in KIM-1. (A) Urinary excretion of KIM-1 as determined by enzyme-linked immunosorbent assay was significantly increased by HLP consumption and was significantly reduced by apocynin treatment. ΔP < 0.05 baseline versus Apoc, HLP and Apoc/HLP on different days. *P < 0.05 control versus HLP and HLP/Apoc within the time period. φP < 0.05 HLP versus HLP/Apoc within the time period. (B) Renal expression of KIM-1 as determined by densitometric analysis of the immunoblots. Kidneys of HLP-treated rats had significantly higher amounts of KIM-1. Apocynin treatment resulted in significant reduction of KIM-1. *P < 0.05 HLP versus control, $ P < 0.05 apocynin versus HLP and φP < 0.05 HLP versus HLP/Apoc.
Fig. 4.
Changes in OPN. (A) Urinary excretion of OPN was significantly increased by on Days 14, 21 and 28 compared to controls and was significantly reduced by apocynin treatment on 14, 21 and 28 days of the experiment. ΔP < 0.05 baseline versus HLP and Apoc/HLP on different days. *P < 0.05 control versus HLP and HLP/Apoc within the time period. φP < 0.05 HLP versus HLP/Apoc within the time period. (B) Renal expression of OPN-1 as determined by densitometric analysis of the immunoblots. Kidneys of HLP-treated rats had significantly less amounts of OPN. Apocynin treatment had no significant effect on OPN production. *P < 0.05 HLP versus control, Apoc and HLP/Apoc.
Fig. 5.
Changes in inflammatory molecules. (A) Urinary excretion of MCP-1 was significantly increased on Days 14, 21 and 28 compared to controls and was significantly reduced by apocynin treatment on 14, 21 and 28 days of the experiment. ΔP < 0.05 baseline versus HLP and Apoc/HLP on different days. *P < 0.05 control versus HLP and HLP/Apoc within the time period. φP < 0.05 HLP versus HLP/Apoc within the time period. (B) Renal expression of ED-1 as determined by densitometric analysis of the immunoblots. Kidneys of HLP-treated rats had significantly high amounts of ED-1. Apocynin treatment resulted in significant reduction, back to the control levels. *P < 0.05 HLP versus control, $ HLP versus apocynin and φP < 0.05 HLP/Apoc versus HLP.
Crystalluria
Light microscopic analyses revealed that urine of control rats as well as rats on apocynin alone was either devoid of any crystals or held amorphous materials and/or struvite type (STR) crystals. By Day 28, all experimental rats in Groups 3 and 4, who consumed HLP, produced CaOx dihydrate (COD) and monohydrate (COM) crystals along with STR and amorphous CaP crystals (Figure 6). STR crystals were typical coffin shaped. The amorphous material was most likely calcium phosphate as reported earlier [15]. COD crystals were typically bipyramidal, while COM crystals were plate like, often with characteristic twinning and dumbbell-shaped morphology. Apocynin treatment did not appear to have an effect on crystalluria since both apocynin-treated as well as nontreated hyperoxaluric rats produced similar types of crystals. Over time, however, the crystalluria pattern appeared to change. The number of STR crystals decreased, while that of COM and COD increased.
Fig. 6.
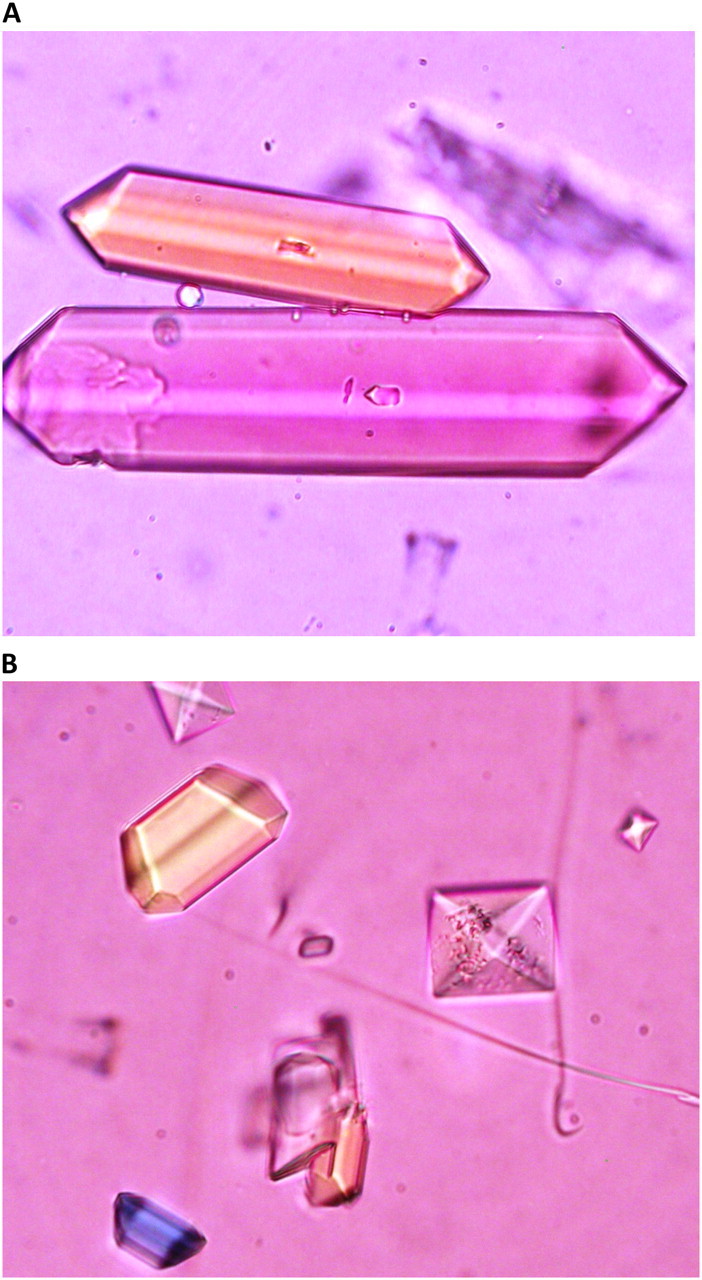
Urinary crystals. (A) Urine of control rats contained coffin-shaped STR crystals. (B) Urine of HLP-treated rats contained both STR as well as CaOx crystals. Picture shows pyramidal CaOx dehydrate.
Renal histology
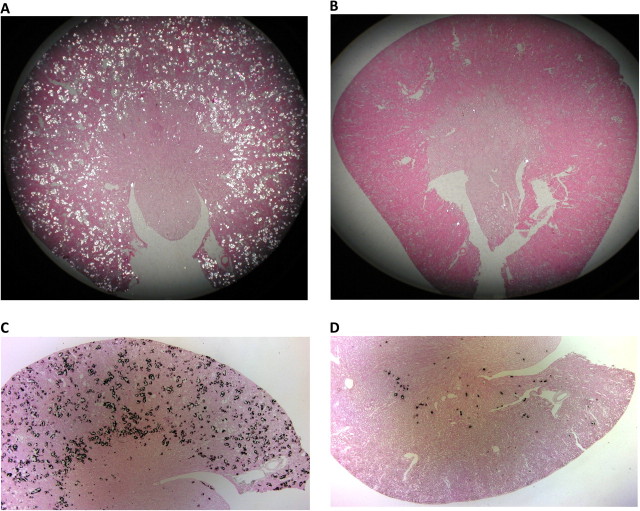
Pizzolato staining and examination of tissue sections using polarizing optics showed CaOx crystal deposits in five of six rats on HLP. Crystals were present in all segments of the kidney, including cortex, medulla and papilla (Figure 7A and C). Most crystals were, however, seen in the cortex and outer medulla with smaller numbers in the inner medulla. Most tubules that contained crystals were dilated and showed destruction of the lining epithelium. The crystals often appeared to completely occlude tubular lumen with the loss of the tubular epithelium. Neighboring tubules appeared normal with no overt signs of injury. Due to tissue damage during processing for light microscopic examination, the precise location of crystals within various segments of the renal tubules was difficult to determine. However, careful examination showed that after 4 weeks of treatment, crystals were mostly present in the tubular lumens of the distal tubules and cortical and outer medullary collecting ducts. However, a few crystals were also seen in proximal tubules.
Fig. 7.
Calcium oxalate crystals deposits identified using polarizing optics and Pizzolato staining of paraffin-embedded kidneys. (A) Kidney of a rat consuming only HLP. Polarized light showed a large number of CaOx crystal deposits. (B) Kidney of a HLP-consuming rat treated with apocynin. There is minimal CaOx crystal deposition. (C) Kidney of a rat consuming HLP. Results of Pizzolato staining are similar to that of examination using polarized light. (D) Kidney of a HLP-consuming rat treated with apocynin. Results of Pizzolato staining are similar to that of examination using polarized light. Crystal deposition is markedly reduced. Only a few crystals are present, mostly at corticomedullary junction.
CaOx crystal deposition was also seen in four of six rats in the group receiving apocynin in addition to HLP (Figure 7B and D). The kidneys for most part, however, appeared normal. The extent and amount of crystal deposition were markedly less than in the HLP-treated rats, who did not receive apocynin (Table 1). There were fewer crystal deposits with individual deposits being much smaller (compare Figure 7A with Figure 7B). Figure 7 shows sections of kidneys with most crystals, Figure 7A and C of rats that consumed HLP only and Figure 7B and D of rats that consumed HLP and treated with apocynin. The examination of paraffin-embedded sections at a magnification of ×4 showed that five of six hyperoxaluric rats without apocynin treatment had ≥6–10 crystal deposits per field, while four of six rats that additionally received apocynin had ≤5 crystal deposits per field. There was no renal CaOx crystal deposition in one of six hyperoxaluric rats without apocynin treatment and two of six hyperoxaluric rats with apocynin treatment. Even though some crystal deposits in the apocynin-treated hyperoxaluric rats were large enough to obliterate the tubular epithelium, most tubules with crystals had intact epithelium.
Table 1.
Crystal deposition in rat kidneysa
| Rat treatment | Cortex | Medulla | Papilla |
| HLP | 0 | 0 | 0 |
| HLP | 0 | 0 | +++ |
| HLP | ++++ | +++ | ++ |
| HLP | +++ | 0 | 0 |
| HLP | ++ | + | ++ |
| HLP | ++++ | +++ | + |
| HLP/Apoc | 0 | 0 | 0 |
| HLP/Apoc | 0 | 0 | 0 |
| HLP/Apoc | + | + | + |
| HLP/Apoc | + | + | + |
| HLP/Apoc | ++ | + | + |
| HLP/Apoc | + | + | + |
Rats became hyperoxaluric by consuming HLP. Six rats received only HLP, while other six were treated with apocynin (Apoc). Quantification of CaOx crystals was carried out by examining the sections at ×4, with five fields per section. The average crystal count was recorded as 0: no crystal deposits, 1: 1–2 crystal deposits per field, 2: 3–5 per field, 3: 6–10 deposits and 4: >10.
Immunohistochemical analysis of the kidneys
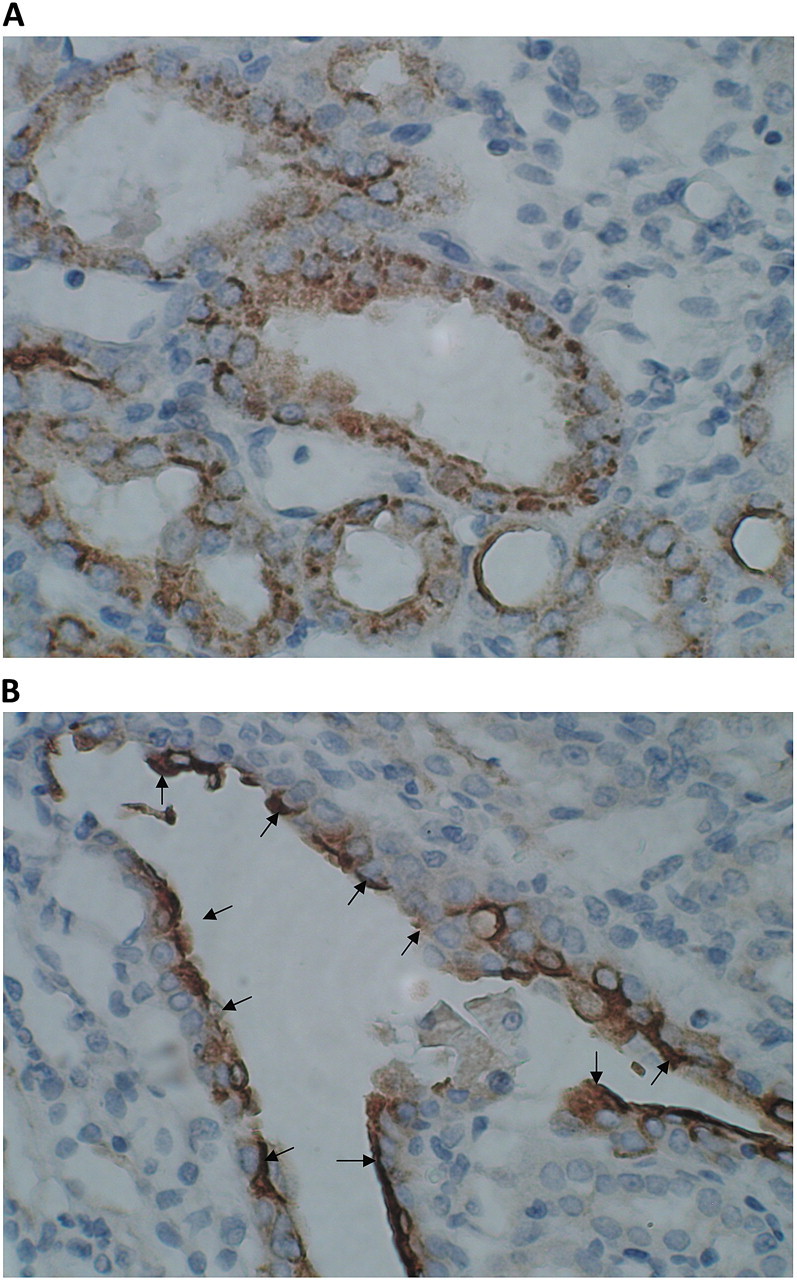
Immunostaining was used to determine the expression of KIM-1, ED-1 and OPN in the kidneys. OPN expression was almost nonexistent in the normal renal tubules but unmistakable in renal tubules of the rats consuming HLP. Epithelial staining was heavy in the crystal-associated sections of the tubules even though crystal free tubules were also stained (Figure 8A and B). The surface of the papillary urothelium was heavily stained for OPN (Figure 8A). Tubules with crystal deposits were surrounded by ED-1-positive cellular infiltrates in the adjacent interstitium (Figure 9A). KIM-1 was also highly expressed in the tubules associated with the crystals and, where clearly discernible, appeared to be present at the luminal side of the epithelial cells (Figure 9B). No KIM-1 or ED-1 staining was seen in the control kidneys. Staining for OPN, ED-1 as well as KIM-1 was markedly reduced in the kidneys of HLP-consuming rats treated with apocynin.
Fig. 8.
Expression of OPN in kidneys of rats that consumed HLP and produced CaOx crystal deposits. Crystal deposition caused tissue disruption. Tubules with as well as without crystals showed heavy expression of OPN. (A) Expression of OPN in cortical tubules. OPN is present both on the luminal surface of epithelium as well as inside the cells. (B) Surface of the urothelium covering papillary surface was heavily stained. The picture shows expression of OPN on urothelial surface in the renal fornix.
Fig. 9.
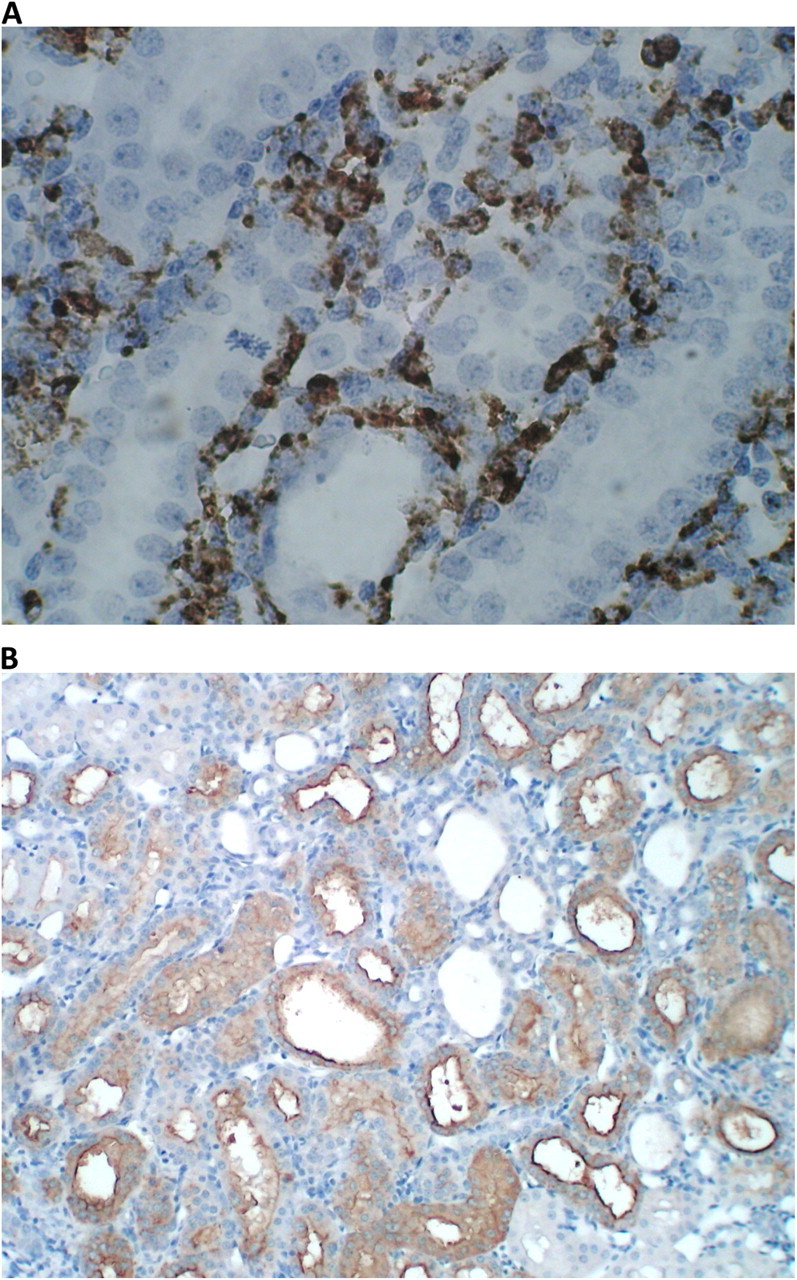
Expression of ED-1 and KIM-1 in kidneys of rats that consumed HLP and produced CaOx crystal deposits. (A) Heavy ED-1 staining around renal tubules in the cortex. The picture shows areas with intact tubular epithelium. (B) Heavy expression of KIM-1 in renal cortex. Tubules are dilated and their epithelia are heavily stained. Intertubular area is invaded by inflammatory cells.
Renal expression of protein
Compared to rats in the control group, significantly high levels of KIM-1 were detected in kidneys of rats receiving HLP. Apocynin treatment resulted in significant reduction of KIM-1 in kidneys (Figure 3B). OPN expression was, however, significantly lower in kidneys of rats receiving HLP compared to the rats in other groups (Figure 4B). On the other hand, ED-1 levels were highly increased in kidneys of HLP-treated rats and decreased by apocynin treatment (Figure 5B).
Discussion
Randall [22] emphasized the importance of renal damage in stone formation when he suggested that interstitial subepithelial deposits of calcium phosphate or calcium carbonate arising from pathological conditions of the renal papilla erode through to the papillary surface forming a lesion that could serve as focal points for stone development. Since then, clinical observations and results of experimental studies have provided further information about the association between renal injury and the formation of kidney stones. Higher than normal levels of renal enzymes, γ-glutamyl transpeptidase, angiotensin-1-converting enzyme (ACE), β-galactosidase and NAG were found in the urine of idiopathic CaOx stone formers [23]. Elevation of these enzymes in urine is considered as an indication of renal proximal tubular injury, suggesting that the stone patients had damaged renal tubules. Animal model studies have confirmed an association between CaOx crystal deposition in the kidneys and renal epithelial injury [20, 24]. Rats with experimentally induced hyperoxaluria showed increased urinary excretion of lipid peroxides in association with LDH [25]. Results of tissue culture studies indicate that exposure of renal epithelial cells to high levels of oxalate and/or CaOx crystals leads to changes in the epithelial function and structure conducive to crystal adherence and their retention within the kidneys [16, 26–30]. Results further indicate that these changes are brought about by the production of ROS. Renal cells exposed to CaOx crystals secrete superoxide in real time as measured by an electrochemical superoxide biosensor [31]. Renal fibroblasts are also stimulated by the exposures to Ox and CaOx crystals. NRK49F exposed to Ox or CaOx crystals showed signs of injury- and ROS-induced lipid peroxidation [32] The generation of a large amount of ROS plays a major role in cell injury, but generation of low concentration of ROS represents a second messenger system for the generation of Ox/CaOx/CaP-induced macromolecules, such as MCP-1 and OPN.
Recent clinical studies also describe kidneys of CaOx kidney stone patients to be under oxidative stress [33, 34]. Urine from stone patients had increased NAG and significantly higher α-glutathione S-transferase, malondialdehyde and thiobarbituric acid-reactive substances, indicating that CaOx kidney stone-associated renal injury is most likely caused by the production of ROS. Urinary 8-hydroxydeoxyguanosine, a marker of oxidative damage of DNA, was increased in stone patients and was positively correlated with tubular damage as assessed by urinary excretion of NAG.
Support for the involvement of oxidative stress in CaOx nephrolithiasis was also provided by treating the hyperoxaluric rats with antioxidants. Administration of vitamin E to the hyperoxaluric rats resulted in the amelioration of their renal tubular injury and reduction in CaOx crystal deposition [35, 36]. Taurine treatment of the hyperoxaluric rats improved antioxidant status and resulted in a reduction of CaOx crystal deposition in the kidneys [37]. NADPH oxidase is a major source of ROS in the kidneys [38, 39], particularly in the presence of Ang II [11]. Ang II is implicated in causing oxidative stress by stimulating membrane-bound NADPH oxidase leading to increased generation of superoxide [12]. A significant reduction in hyperoxaluria-induced production of renal lipid peroxides after administration of angiotensin II receptor type I blockers [8] or ACE inhibitors [6] was seen in hyperoxaluric rats. A reduction in renal epithelial injury and CaOx crystal deposition was also seen in hyperoxaluric rats treated with atorvastatin [40].
Activation of NADPH oxidase requires docking of the cytoplasmic units with the membrane units which is selectively inhibited by apocynin [10]. Apocynin has been effective in ameliorating oxidative stress and associated renal injury in various animal models of nephrotoxicity [41]. It has also been effective in reducing oxidative stress induced by hyperoxaluria [42]. Administration of apocynin to rats with ethylene glycol-induced hyperoxaluria reduced urinary excretion of 8-isoprostane, a product of lipid peroxidation. We investigated the efficacy of apocynin treatment in reducing the production of ROS, associated renal injury and crystal deposition in the kidneys of rats with HLP-induced hyperoxaluria. Since ROS are also involved in the production of MCP-1 and OPN, we also studied the effect of apocynin treatment on these macromolecules.
Results presented here confirm our earlier observations that HLP consumption leads to hyperoxaluria [15] which reached highest levels by Day 14 and then stayed about the same for the next 14 days. Apocynin treatment did not have a significant effect on hyperoxaluria. Hyperoxaluria led to the production of ROS as determined by urinary excretion of HP, which was significantly increased, reaching highest levels on Day 14. An increase in urinary HP is indicative of increased production of superoxide and reduction in antioxidant capacity of the cells. Apocynin treatment resulted in a significant reduction of HP production for all time periods but the treatment did not bring it completely down to the control levels suggestive of sources other than NADPH oxidase being involved in the production of ROS.
Hyperoxaluric rats excreted significant amounts of KIM-1 in their urine, reaching highest levels on Day 14 and then basically staying the same. Increase in urinary KIM-1 was positively and significantly related to increase in urinary oxalate (r = 0.73, two-tailed P = 0.0004). Urinary KIM-1 was also related its renal expression. KIM-1 protein was increased in the kidneys as determined by western analysis as well as immunohistochemical staining. Apocynin treatment significantly reduced not only urinary KIM-1 excretion but its expression in the kidneys as well. Urinary KIM-1 level is considered as a sensitive biomarker of renal tubular injury [17, 43]. It is conserved across species and is upregulated after renal injury in zebrafish, mice, rats, nonhuman primates as well as humans.
Urinary excretion of OPN and MCP-1 by the hyperoxaluric rats also increased and followed a pattern similar to KIM-1 excretion. However, on Day 28, there was an additional increase in urinary OPN and MCP-1. Increase in urinary OPN and MCP-1 was positively and significantly related to increase in urinary oxalate (OPN, r = 0.46, two-tailed P = 0.0327 and MCP-1, r = 0.66, two-tailed P = 0.002). We have shown previously that hyperoxaluria leads to cell injury followed by regeneration of cells. The second burst of OPN and MCP-1 production may represent their production by regenerating cells. Alternatively or additionally, infiltrating cells may be responsible for this increase. As we mentioned above, there was a significant increase in ED-1 protein as well as ED-1-positive cells in the interstitium. Apocynin treatment resulted in a significant reduction in OPN and MCP-1 excretion. Even though expression of OPN in the renal tissue, as determined by immunostaining, appeared significantly increased, western analysis showed significant reduction in OPN production. This can, however, be explained by OPN's tendency to bind to the CaOx crystals [44, 45] and their removal from the tissue during processing for western analysis.
Hyperoxaluria was associated with significantly increased urinary excretion of NAG. Urinary NAG levels on 14 and 28 days of hyperoxaluria were not significantly different from those on Day 7 and were unaffected by the apocynin treatment. This indicates the involvement of injury causing mechanisms in addition to the activation of NADPH oxidase. Interestingly, both clinical [23, 34] and animal model studies [46, 47] have shown increased urinary excretion of NAG during the development of CaOx nephrolithiasis. An increase in urinary NAG is considered a sign of injury to renal proximal tubular epithelium, which is generally more susceptible to nephrotoxic challenges particularly high oxalate.
Of six rats in the group consuming HLP, five rats had deposits of CaOx crystals in their kidneys at the time of sacrifice on Day 28. Crystal deposition was associated with cell injury and death. ED-1-positive inflammatory cells moved into the adjoining interstitium. Even though the number of apocynin-treated rats with renal crystal deposits was similar to that in the untreated rats group, apocynin treatment led to a significant reduction in the number of crystal deposits. We have suggested that renal tubular crystals retention requires cell damage and dysfunction. It is our suggestion that apocynin treatment reduced oxidative stress and cell damage resulting in declining crystal deposition. Retention of some crystals and unchanged excretion of NAG in the apocynin-treated rats indicates involvement of additional deleterious mechanisms in hyperoxaluria-induced renal injury.
We investigated the effect of NADPH oxidase inhibition by apocynin treatment on the expression of KIM-1 and renal CaOx crystal deposition in hyperoxaluric male Sprague–Dawley rats. This is the first report on urinary excretion and renal expression of KIM-1 in association with renal CaOx crystal deposition, experimental or clinical. Results indicate that NADPH oxidase inhibition leads to reduction in KIM-1 expression and its urinary excretion as well as renal CaOx crystal deposition. As mentioned above, KIM-1 is an important urinary biomarker of renal injury [43]. Results presented here pertain to direct contact between renal epithelial cells and CaOx crystals which have been deposited in the renal tubules of hyperoxaluric rats. Clinically, tubular deposits of CaOx crystals have been seen in primary hyperoxaluria and hyperoxaluria secondary to bariatric surgery for obesity as well as brushite stone formers and renal tubular acidosis [48]. We have also shown that renal epithelial cells are susceptible to oxidative stress when exposed to apatite or brushite crystals [5]. In addition, NADPH oxidase inhibitor, diphenileneiodium chloride, has the capacity to reduce the production of ROS as well as MCP-1 [9]. Antioxidant treatment with apocynin may prove effective in reducing crystal-induced injury and inflammation and perhaps end-stage renal disease.
Acknowledgments
Research was supported by NIH grant #RO1-DK078602 and University of Florida Center for the Study of Lithiasis. Ms Anum Khan and Lauren Streifel provided technical assistance.
Conflict of interest statement. None declared.
References
- 1.Finlayson B. Symposium on renal lithiasis. Renal lithiasis in review. Urol Clin North Am. 1974;1:181–212. [PubMed] [Google Scholar]
- 2.Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res. 2005;33:349–357. doi: 10.1007/s00240-005-0492-4. [DOI] [PubMed] [Google Scholar]
- 3.Khan SR. Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res. 2006;34:86–91. doi: 10.1007/s00240-005-0016-2. [DOI] [PubMed] [Google Scholar]
- 4.Jonassen J, Cao LC, Honeyman T, et al. Intracellular events in the initiation of calcium oxalate stones. Nephron Exp Nephrol. 2004;98:e61–e64. doi: 10.1159/000080258. [DOI] [PubMed] [Google Scholar]
- 5.Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol. 2004;8:75–88. doi: 10.1007/s10157-004-0292-0. [DOI] [PubMed] [Google Scholar]
- 6.Toblli JE, Ferder L, Stella I, et al. Protective role of enalapril for chronic tubulointerstitial lesions of hyperoxaluria. J Urol. 2001;166:275–280. [PubMed] [Google Scholar]
- 7.Toblli JE, Ferder L, Stella I, et al. Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol. 2002;168:1550–1555. doi: 10.1016/S0022-5347(05)64519-3. [DOI] [PubMed] [Google Scholar]
- 8.Umekawa T, Hatanaka Y, Kurita T, et al. Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol. 2004;15:635–644. doi: 10.1097/01.asn.0000113321.49771.2d. [DOI] [PubMed] [Google Scholar]
- 9.Umekawa T, Byer K, Uemura H, et al. Diphenyleneiodium (DPI) reduces oxalate ion- and calcium oxalate monohydrate and brushite crystal-induced upregulation of MCP-1 in NRK 52E cells. Nephrol Dial Transplant. 2005;20:870–878. doi: 10.1093/ndt/gfh750. [DOI] [PubMed] [Google Scholar]
- 10.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 11.Hanna IR, Taniyama Y, Szocs K, et al. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal. 2002;4:899–914. doi: 10.1089/152308602762197443. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox CS, Welch WJ. Oxidative stress: cause or consequence of hypertension. Exp Biol Med (Maywood) 2001;226:619–620. doi: 10.1177/153537020222600702. [DOI] [PubMed] [Google Scholar]
- 13.Lafeber FP, Beukelman CJ, van den Worm E, et al. Apocynin, a plant-derived, cartilage-saving drug, might be useful in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 1999;38:1088–1093. doi: 10.1093/rheumatology/38.11.1088. [DOI] [PubMed] [Google Scholar]
- 14.Touyz RM. Apocynin, NADPH oxidase, and vascular cells: a complex matter. Hypertension. 2008;51:172–174. doi: 10.1161/HYPERTENSIONAHA.107.103200. [DOI] [PubMed] [Google Scholar]
- 15.Khan SR, Glenton PA, Byer KJ. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int. 2006;70:914–923. doi: 10.1038/sj.ki.5001699. [DOI] [PubMed] [Google Scholar]
- 16.Khan SR. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res. 1995;23:71–79. doi: 10.1007/BF00307936. [DOI] [PubMed] [Google Scholar]
- 17.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 18.Khan SR. Calcium phosphate/calcium oxalate crystal association in urinary stones: implications for heterogeneous nucleation of calcium oxalate. J Urol. 1997;157:376–383. [PubMed] [Google Scholar]
- 19.Khan SR, Finlayson B, Hackett RL. Experimental calcium oxalate nephrolithiasis in the rat. Role of the renal papilla. Am J Pathol. 1982;107:59–69. [PMC free article] [PubMed] [Google Scholar]
- 20.Khan SR, Shevock PN, Hackett RL. Acute hyperoxaluria, renal injury and calcium oxalate urolithiasis. J Urol. 1992;147:226–230. doi: 10.1016/s0022-5347(17)37202-6. [DOI] [PubMed] [Google Scholar]
- 21.Maslamani S, Glenton PA, Khan SR. Changes in urine macromolecular composition during processing. J Urol. 2000;164:230–236. [PubMed] [Google Scholar]
- 22.Randall A. The origin and growth of renal calculi. Ann Surg. 1937;105:1009–1027. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baggio B, Gambaro G, Ossi E, et al. Increased urinary excretion of renal enzymes in idiopathic calcium oxalate nephrolithiasis. J Urol. 1983;129:1161–1162. doi: 10.1016/s0022-5347(17)52619-1. [DOI] [PubMed] [Google Scholar]
- 24.de Water R, Boeve ER, van Miert PP, et al. Experimental nephrolithiasis in rats: the effect of ethylene glycol and vitamin D3 on the induction of renal calcium oxalate crystals. Scanning Microsc. 1996;10:591–601. discussion-3. [PubMed] [Google Scholar]
- 25.Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol. 1997;157:1059–1063. [PubMed] [Google Scholar]
- 26.Verkoelen CF, Romijn JC, de Bruijn WC, et al. Association of calcium oxalate monohydrate crystals with MDCK cells. Kidney Int. 1995;48:129–138. doi: 10.1038/ki.1995.276. [DOI] [PubMed] [Google Scholar]
- 27.Verkoelen CF, Van Der Boom BG, Romijn JC. Identification of hyaluronan as a crystal-binding molecule at the surface of migrating and proliferating MDCK cells. Kidney Int. 2000;58:1045–1054. doi: 10.1046/j.1523-1755.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- 28.Vervaet BA, Verhulst A, D'Haese PC, et al. Nephrocalcinosis: new insights into mechanisms and consequences. Nephrol Dial Transplant. 2009;24:2030–2035. doi: 10.1093/ndt/gfp115. [DOI] [PubMed] [Google Scholar]
- 29.Khan SR, Thamilselvan S. Nephrolithiasis: a consequence of renal epithelial cell exposure to oxalate and calcium oxalate crystals. Mol Urol. 2000;4:305–312. [PubMed] [Google Scholar]
- 30.Khan SR, Byer KJ, Thamilselvan S, et al. Crystal-cell interaction and apoptosis in oxalate-associated injury of renal epithelial cells. J Am Soc Nephrol. 1999;10(Suppl 14):S457–S463. [PubMed] [Google Scholar]
- 31.Gaspar S, Niculite C, Cucu D, et al. Effect of calcium oxalate on renal cells as revealed by real-time measurement of extracellular oxidative burst. Biosens Bioelectron. 2010;25:1729–1734. doi: 10.1016/j.bios.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Umekawa T, Iguchi M, Uemura H, et al. Oxalate ions and calcium oxalate crystal-induced up-regulation of osteopontin and monocyte chemoattractant protein-1 in renal fibroblasts. BJU Int. 2006;98:656–660. doi: 10.1111/j.1464-410X.2006.06334.x. [DOI] [PubMed] [Google Scholar]
- 33.Tungsanga K, Sriboonlue P, Futrakul P, et al. Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res. 2005;33:65–69. doi: 10.1007/s00240-004-0444-4. [DOI] [PubMed] [Google Scholar]
- 34.Boonla C, Wunsuwan R, Tungsanga K, et al. Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res. 2007;35:185–191. doi: 10.1007/s00240-007-0098-0. [DOI] [PubMed] [Google Scholar]
- 35.Thamilselvan S, Menon M. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int. 2005;96:117–126. doi: 10.1111/j.1464-410X.2005.05579.x. [DOI] [PubMed] [Google Scholar]
- 36.Huang HS, Chen J, Chen CF, et al. Vitamin E attenuates crystal formation in rat kidneys: roles of renal tubular cell death and crystallization inhibitors. Kidney Int. 2006;70:699–710. doi: 10.1038/sj.ki.5001651. [DOI] [PubMed] [Google Scholar]
- 37.Li CY, Deng YL, Sun BH. Taurine protected kidney from oxidative injury through mitochondrial-linked pathway in a rat model of nephrolithiasis. Urol Res. 2009;37:211–220. doi: 10.1007/s00240-009-0197-1. [DOI] [PubMed] [Google Scholar]
- 38.Li N, Yi FX, Spurrier JL, et al. Production of superoxide through NADH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol. 2002;282:F1111–F1119. doi: 10.1152/ajprenal.00218.2001. [DOI] [PubMed] [Google Scholar]
- 39.Geiszt M, Kopp JB, Varnai P, et al. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsujihata M, Momohara C, Yoshioka I, et al. Atorvastatin inhibits renal crystal retention in a rat stone forming model. J Urol. 2008;180:2212–2217. doi: 10.1016/j.juro.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 41.Chirino YI, Sanchez-Gonzalez DJ, Martinez-Martinez CM, et al. Protective effects of apocynin against cisplatin-induced oxidative stress and nephrotoxicity. Toxicology. 2008;245:18–23. doi: 10.1016/j.tox.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Li CY, Deng YL, Sun BH. Effects of apocynin and losartan treatment on renal oxidative stress in a rat model of calcium oxalate nephrolithiasis. Int Urol Nephrol. 2009;41:823–833. doi: 10.1007/s11255-009-9534-0. [DOI] [PubMed] [Google Scholar]
- 43.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 44.Gokhale JA, Glenton PA, Khan SR. Localization of tamm-horsfall protein and osteopontin in a rat nephrolithiasis model. Nephron. 1996;73:456–461. doi: 10.1159/000189110. [DOI] [PubMed] [Google Scholar]
- 45.McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res. 1995;10:1913–1929. doi: 10.1002/jbmr.5650101211. [DOI] [PubMed] [Google Scholar]
- 46.Khan SR, Shevock PN, Hackett RL. Urinary enzymes and calcium oxalate urolithiasis. J Urol. 1989;142:846–849. doi: 10.1016/s0022-5347(17)38928-0. [DOI] [PubMed] [Google Scholar]
- 47.Khan SR, Hackett RL. Hyperoxaluria, enzymuria and nephrolithiasis. Contrib Nephrol. 1993;101:190–193. [PubMed] [Google Scholar]
- 48.Coe FL, Evan AP, Lingeman JE, et al. Plaque and deposits in nine human stone diseases. Urol Res. 2010;38:239–247. doi: 10.1007/s00240-010-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]