Abstract
Background
Regulatory T cells (Treg) are present in atherosclerotic lesions and can modulate disease. In this study we characterized changes in Treg responses associated with prolonged hypercholesterolemia and lesion progression.
Methods and Results
Ldlr−/− mice in which Treg express green fluorescence protein (GFP) were fed a control or cholesterol-rich diet and GFP+ cells were enumerated in lymphoid tissues and in aorta. Splenic Treg numbers increased after 4, 8 and 20 weeks in cholesterol-diet fed mice, however, the number of circulating and lesional Treg peaked at 4 weeks and decreased significantly at 8 and 20 weeks, concomitant with increased numbers of CD4+ effector T cells and increased lesion size over this period. Treg expression of selectin ligands and their ability to bind to aortic endothelium decreased after prolonged hypercholesterolemia, and apoptosis of lesional Treg increased. After 4 weeks of cholesterol rich-diet, a switch to a control diet for 4 weeks reduced serum cholesterol stopped lesion growth, and the high aortic Treg content was maintained, compared to mice fed a cholesterol diet for 8 weeks. After the diet reversal, the splenic Treg retained the phenotype of Treg after 4 weeks of cholesterol diet.
Conclusions
Prolonged hypercholesterolemia impairs Treg but not effector T cell (Teff) accumulation in lesions, but reversal of hypercholesterolemia can prevent loss of lesional Treg. Therefore cholesterol lowering therapies may induce dynamic and beneficial changes in Treg:Teff ratios in atherosclerotic lesions.
Keywords: atherosclerosis, diet, hypercholesterolemia, T cells, regulatory T cells
Nonstandard abbreviations: CTLA-4, GITR, Ldlr, PSGL1, Treg
Introduction
T cells are present at all stages of atherosclerotic lesion development 1 and the majority of these are CD4+ T-helper cells (Th1) that produce IFN-γ2. Several lines of evidence indicate that Th1 cells promote lesion growth, as well as late plaque instability associated acute cardiovascular events 3. Consistent with a pro-atherogenic role for Th1 cells, the progression of atherosclerosis in low density lipoprotein receptor null (Ldlr−/−) mice is impaired by deficiency of IFN-γ or the T-bet transcription factor, which is required for Th1 differentiation 4, 5. The nature of the antigens recognized by T cells that promote atherosclerosis remains an open question, and may include modified or native self apoB-100 6, oxidized-LDL, β2-glycoprotein-1b, heat shock protein 60/65 7–9 and perhaps self proteins modified by dietary components 10.
Regulatory T cells (Treg) are essential for regulating T cell immune responses to prevent autoimmunity and possibly to prevent excessive responses to microbial pathogens 11. Treg can be identified by cell surface markers (CD3+CD4+CD25int/highCD127lo), as well as expression of glucocorticoid-induced tumor necrosis factor receptor (GITR) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) 12. The stable intracellular expression of the Foxp3 transcription factor is a defining feature of Treg13. The influence of Treg on atherosclerosis is of interest because these cells can block Th1 differentiation and effector function, and are of potential therapeutic importance 14. Treg may influence pro-atherogenic T cell responses in the lymphoid tissues, and/or human and mouse atherosclerotic lesions 15–17, modulating the local pro-inflammatory processes. For example, reduced or functionally impaired Treg leads to increased atherosclerosis 15, 18 and adoptive transfer of Treg into hypercholesterolemic mice reduces lesion development 19, 20.
Immunotherapy of atherosclerotic disease might be directed toward increasing Treg suppression of pro-atherogenic T cells 19–21. Because the ratio of regulatory to effector T cells (Teff) is critical in determining outcomes of T cell responses 22–25, we reasoned that therapeutic interventions aimed at increasing Treg suppression of pro-atherogenic T cell responses would require induced changes in Treg numbers within the arterial wall. In this study, we addressed the question of whether or not numbers of Treg within atherosclerotic aortas are dynamically regulated. We found that numbers of Treg and the Treg:Teff ratio within the atherosclerotic aorta progressively decreased during prolonged hypercholesterolemia, but the hypercholesterolemia-induced lesional Treg response could be maintained by dietary reversal of hypercholesterolemia.
Methods
Mice
Foxp3-eGFP knock in mice on a C57Bl/6 background 26, were crossed with Ldlr−/− mice on a C57Bl/6 background (Jackson Laboratories) to obtain Foxp3-eGFP+/Ldlr−/− mice. Treg in the Foxp3-eGFP mouse have functional Foxp3 and are identifiable by green fluorescence 26. Female and male Foxp3-eGFP/Ldlr−/− mice (all 8 weeks old at the start of experiments) were assigned to different groups in each experiment, and each group was fed a control or cholesterol-rich diet 27 for different time periods, depending on the experiments. A total number of 208 mice were used in this study. Exact numbers of mice per experimental group are indicated in figure legends. All mice were housed and bred in a pathogen-free facility at New Research Building (Harvard Medical School, Boston, Massachusetts, USA) in accordance with the guidelines of the Committee of Institutional Animal Care (IACUC) and the National Animal Research Guidelines. Additional Methods can be found in the online-only Data Supplement.
Serum Cholesterol Analysis
Blood from individual mice was collected by retro-orbital venous plexus sampling after 4, 8 and 20 weeks of diet, serum samples were prepared, and cholesterol was analyzed (see online data supplement).
Aortic atherosclerotic lesion quantification
Atherosclerotic lesion formation in aortic sinuses was analyzed in Oil Red O (ORO) stained cryosections as described 15, 17. See online data supplement for a detailed description of the methods of lesion analyses.
Immunohistochemistry of aortic lesions
Serial fixed cryostat sections of aortic-sinus adjacent to the ORO-stained sections were stained by standard immunohistochemical techniques, as described 28, with antibodies specific for CD4, smooth muscle cell (SMC) actin and F4/80. Quantification of SMC and macrophage staining was performed by digital image analysis 4 and expressed as percentage of intimal area. See online data supplement for a detailed description of antibodies and immunohistochemical techniques.
Flow cytometry
Multicolor flow cytometry was performed by standard protocols as described 17, to quantify Treg and Teff cells in spleen, lymph nodes, and collagenase digests of aortic walls from mice after various dietary regimens, and to characterize expression of adhesion molecules, chemokine receptors, activation molecules and apoptosis markers on Treg and Teff cells. See online data supplement for a detailed description of cell preparations, antibodies and flow cytometric analyses.
Immunofluorescence and confocal microscopy
Lesional GFP+ Treg were counted in confocal microscopic images of en face preparations of the aortic arches from Foxp3-eGFP+/Ldlr−/− mice prepared as described 29, stained with primary antibodies specific for CD4 or class II MHC (IA/IE), followed by Alexa-555 labeled secondary antibody and nuclear DAPI stain. Staining was analyzed by confocal microscopy. See online data supplement for a detailed description of the tissue preparation and confocal microscopic analyses.
Mouse aorta isolation and ex vivo adhesion assay
The ability of fluorescently-labeled Treg from hypercholesterolemic mice to adhere to the luminal surface of IL-1β plus TNFα-treated mouse aortas ex vivo was tested as described 30. Adherent fluorescently labeled Treg were detected by confocal microscopy. See online data supplement for a detailed description of this ex vivo adhesion assay.
Regulatory T cells binding to E selectin under flow conditions
Binding of splenic Foxp3-GFP+ Treg from cholesterol fed mouse to recombinant mouse E-selectin was examined under laminar flow conditions in a flow chamber as described 31, 32. See online data supplement for a detailed description of the in vitro flow chamber assays.
Statistical analysis
For practical reasons, some experiments with multiple mice per group had to be performed in a two-part staggered fashion, in order to be able to complete all the technical aspects in a way that did not vary from mouse to mouse. When conducting each of the two chronologically different parts, all the different groups of mice were included in each part, with equal numbers in each group. Statistical analyses included the Students t test for experiments with two groups, one-way ANOVA for experiments that included cholesterol-diet for three different durations, and two-way ANOVA for experiments that included two diets (control, and cholesterol) for different durations (4, 8 and 20 weeks). Multiple pairwise comparisons were handled by Tukey's Multiple Comparison post test or Bonferroni post test. A value of P<0.05 was considered to be significant.
Results
Hypercholesterolemia increases the number of splenic regulatory T cells in Foxp3-eGFP+/Ldlr−/− mice
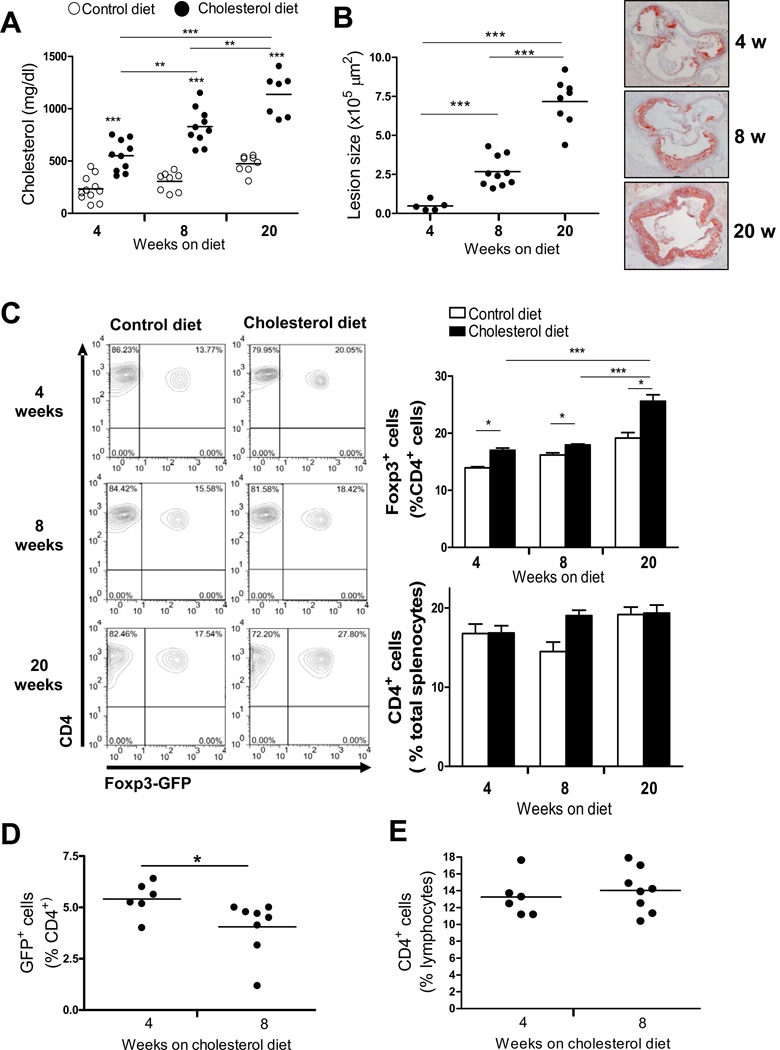
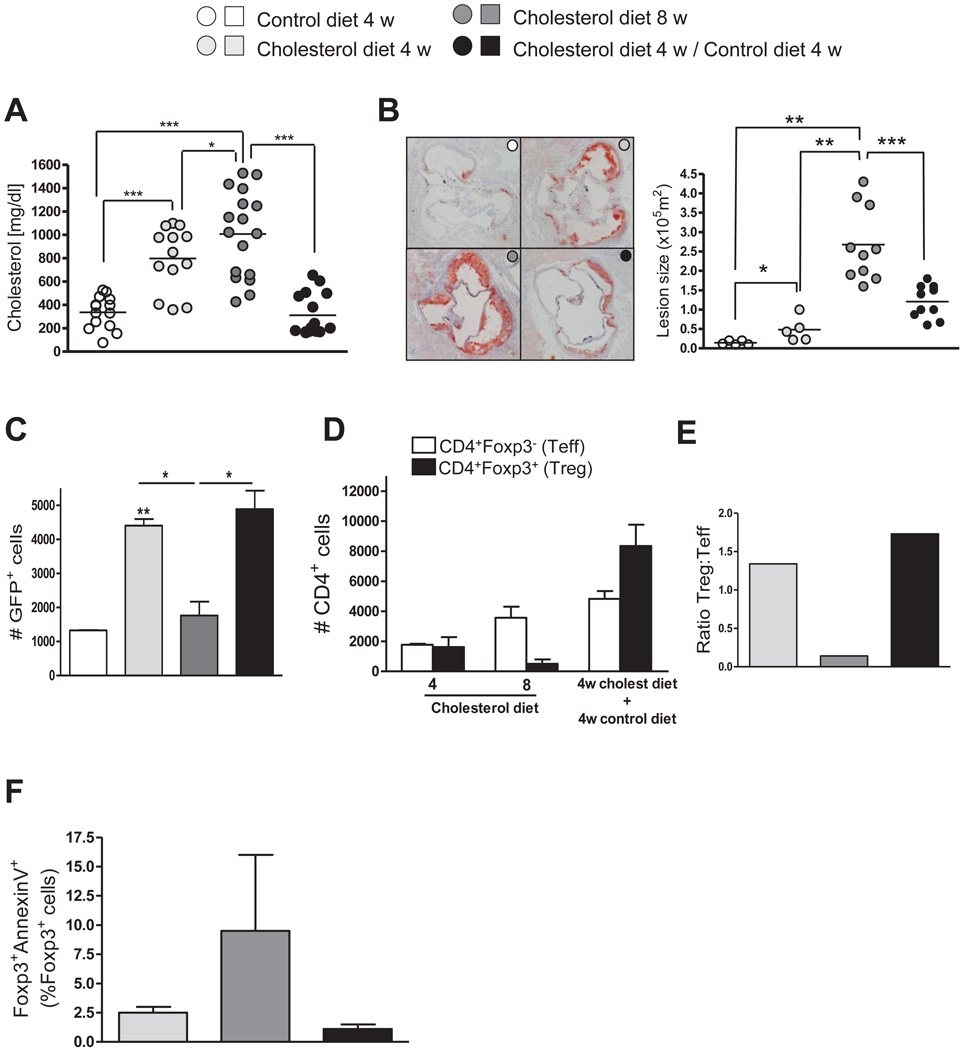
Foxp3-eGFP+/Ldlr−/− mice were fed a control or a cholesterol-rich diet, the latter causing a progressive significant increase of total cholesterol levels in serum between 4 and 20 weeks (Fig. 1A). Aortic root atherosclerotic lesions progressively increased in cholesterol diet-fed mice after 4, 8 and 20 weeks (Fig. 1B), while mice fed control diet had no significant lesions. To determine if hypercholesterolemia had a systemic effect on Treg, we quantified CD4+GFP+ T cells in the spleens of these mice. We observed a progressive increase in the percent of CD4+ cells that expressed GFP over the 20 weeks of cholesterol-diet feeding, and a smaller increase over time in control diet-fed mice, while the percent of total CD4+ T cells was not different between the two groups of mice at all time points (Fig. 1C). The small increase in percentage of splenic Treg in control-diet fed Ldlr−/− likely reflected the mild hypercholesterolemia in these mice (see Figure 1A), since we did not see any increase in splenic Treg in Ldlr+/+ Foxp3-eGFP+ mice fed control diet at 4, 8 and 20 weeks (data not shown). The percentage of CD4+ cells that expressed GFP in blood decreased slightly between 4 and 8 weeks of hypercholesterolemia (Fig. 1D), while the percentage of blood T cells expressing CD4 did not change (Fig. 1E).
Figure 1. Splenic and circulating Treg numbers change under prolonged hypercholesterolemia.
A, Foxp3-eGFP+/Ldlr−/− mice (total 63) were fed a control or cholesterol rich diet and sacrificed after 4, 8 and 20 weeks on diet. Total cholesterol levels were measured in serum. B, Aortic root atherosclerotic lesions were quantified by analysis of ORO stained sections. A representative section from each group is also shown. C, Splenocytes were stained for CD4 and the percent of CD4+ cells dthat were GFP+ cells was determined by FACS. Horizontal bars and column heights represent the mean for each group. Error bars represent S.E.M.. N=6–10 mice per group. Each symbol in A and B represents one mouse. * P <0.05, ** P<0.01, *** P<0.001, analyzed by two way ANOVA with Bonferroni post test (A) or one way ANOVA with Tukey’s post test (B). In a separate experiment, Foxp3-eGFP+/Ldlr−/− mice (14 total) were fed a cholesterol rich diet and sacrificed after 4 and 8 weeks, and blood leukocytes were stained for CD4, and analyzed by FACS. D, The percent of CD4+ cells that were GFP+ is shown. E, the % total lymphocytes by scatter that were CD4+ is shown. Data are mean +/− S.E.M., N=6–8 mice per group. * P <0.05, analyzed by Students t test. Each symbol in D and E represents one mouse.
Regulatory T cell numbers transiently increase in the atherosclerotic aortas and then decrease during sustained hypercholesterolemia and lesion growth
We examined Treg accumulation in aortas of Foxp3-eGFP+/Ldlr−/− mice using two different methods. First, we used confocal microscopy of en face samples of the atherosclerotic prone area of the lesser curvature, as described 29. Prior studies have indicated that the retention of lipopoproteins and cholesterol in the arterial intima induces the initial monocyte and dendritic cell homing to the aorta 33 and this process amplifies further leukocyte recruitment during early atherogenesis 27, 34. In order to confirm that we were counting Treg in the location of the lesion development and inflammation in the aortic arch, we stained identically prepared samples for MHC class II (IA/IE), which is expressed on macrophages, DCs, endothelial cells and SMCs in response to IFN-γ. Consistent with previous studies, after 4 weeks of hypercholesterolemia, we detected IA/IE+ cells in these regions after 8 and 20 weeks of hypercholesterolemia (Supplementary data S1A). In control diet-fed mice, we also observed a small number of IA/IE+ cells in the aortic arch after 20 weeks, likely because these mice are mildly hypercholesterolemic, and do develop early lesions by 20 weeks (see insets showing ORO staining of the aortic sinus, Supplemental Figure S1A). Moreover, we verified the accumulation of F4/80+ macrophages in these samples. This accumulation increased when mice were fed a high cholesterol diet for 20 weeks (Supplemental Figure S1B).
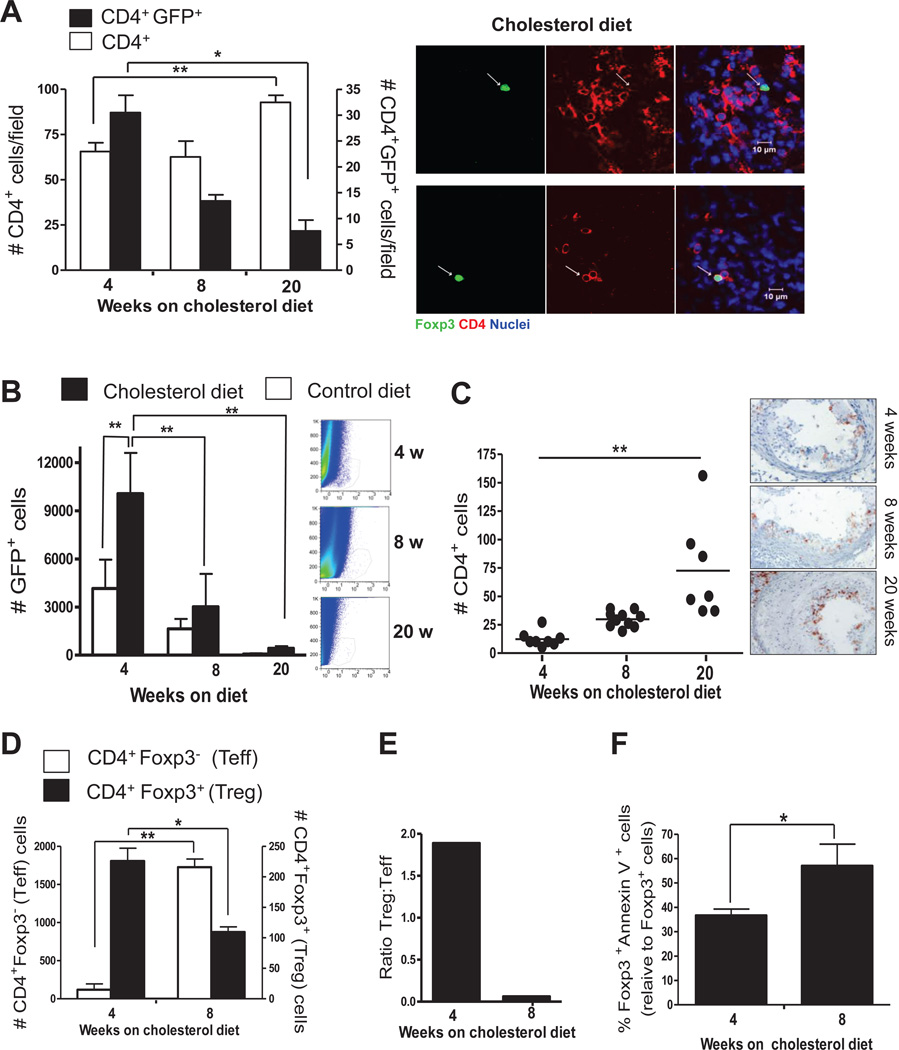
We quantified GFP+ Treg, from aortic arches of Foxp3-eGFP+/Ldlr−/− mice fed a cholesterol diet for 4, 8 and 20 weeks. The arches were stained with anti-CD4 and DAPI (nuclei), and were examined by confocal microscopy (Fig. 2A). Treg were present in lesions after 4, 8 and 20 weeks of diet. Remarkably, the number of Treg was maximum at 4 weeks of diet and decreased successively at 8 and 20 weeks, opposite to the increase of total CD4+ cells from 4 to 20 weeks.
Figure 2. Aortic Treg of Ldlr−/− mice decreases during prolonged hypercholesterolemia and lesion development.
Foxp3-eGFP+/Ldlr−/− mice (54 total) were fed a cholesterol diet for 4, 8 or 20 weeks, aortic arches were harvested, and en face preparations were stained with DAPI plus anti-CD4/Alexa-555 for confocal microscopic examination. A, The mean number of total CD4+ cells (white bars) and Treg (black bars) per field among 5 fields was determined in 2 mice per diet/time. Images of two Treg in the same field of an atherosclerotic arch, but at two different z-stacks, are shown. B, Descending aortas from the same mice described in A were enzymatically digested and the GFP+ population was analyzed by FACs. Data in A and B represent mean +/− S.E.M, N=9 mice per group, * P<0.05, ** P<0.01 analyzed by one way ANOVA with Tukey’s post test (A) or two way ANOVA with Bonferroni post test (B). C, Frozen sections of aortic sinus from the cholesterol-fed mice described in A were stained for CD4 by immunohistochemistry. Data represent mean +/− S.E.M., N=7–9 mice per group. Each symbol represents one mouse. ** P<0.01, analyzed by one way ANOVA with Tukey’s post test. In a separate experiment, Foxp3-eGFP+/Ldlr−/− mice (18 total) were fed a cholesterol diet for 4 or 8 weeks. Aortic digest cells were stained for CD4, Foxp3, and Annexin V and analyzed by flow cytometry. D, The number of CD4+Foxp3− (white bars) and CD4+Foxp3+ cells (black bars) is shown. E, the CD4+Foxp3+: CD4+Foxp3− ratio is shown. F, the percent of apoptotic Treg is shown. Data represent mean +/− S.E.M, from N=9 mice per group, * P<0.05, ** P<0.01, analyzed by Students t test.
In order to confirm the confocal data, we performed flow cytometric analyses of cells collected by collagenase digestion of the aortic walls. This method will detect T cells throughout the aortic wall, including intimal lesions, media and adventitial cells. Changes in adventitial lymphoid populations are reflective of atherosclerotic disease progression and studies have demonstrated specific homing of lymphocytes to atherosclerotic aortic adventitia 35. Before digestion, we thoroughly removed para-aortic connective and adipose tissue and we have confirmed by histology that there is only a thin adventitia on the outer surface (not greater than 30 microns), which is devoid of any organized lymphoid tissue. Furthermore, we have never found adventitial or para-aortic tertiary lymphoid tissues on histological sections from aortas of Ldlr−/− mice fed cholesterol-diet up to 20 weeks. Aortas from control or cholesterol-fed Foxp3-eGFP+/Ldlr−/− mice were harvested after 4, 8 and 20 weeks, enzymatically digested and GFP expression was analyzed in the final cell suspensions by FACs. We were able to detect GFP+ Treg by this method and again, we observed an initial increase of Treg number in aortas of cholesterol diet-fed mice compared to control diet-fed mice after 4 weeks, but Treg numbers decreased at 8 and 20 weeks (Fig. 2B). Immunohistochemical staining of aortic sinuses in the same mice indicated that total lesional CD4+ T cells increased progressively from 4 to 20 weeks (Fig. 2C). We also performed digestion of aortas from different groups of Foxp3-eGFP+/Ldlr−/− mice fed cholesterol diet for 4 and 8 weeks, followed by intracellular staining for CD4 and Foxp3. Again, we observed a decrease in Treg from 4 to 8 weeks, while the total number of CD4+Foxp3− T cells increased over the same time period (Fig. 2D). By quantifying Foxp3+ and Foxp3− CD4+ T cells after digestion of the aortas we established a decreasing ratio between regulatory and effector CD4+ cells (Treg:Teff) in aortas over a period of sustained hypercholesterolemia and lesion growth (Fig. 2E). This result indicates that prolonged hypercholesterolemia inhibits the accumulation of Treg in atherosclerotic arteries, while effector T cell accumulation continues.
In order to explain loss of Treg that had already entered the lesion during the initial 4 weeks of cholesterol diet feeding, we explored the possibility that this loss was due to increased cell death by staining aortic digests for Foxp3, CD4 and the apoptosis marker Annexin V. The aortic digests were the same as those used to established decreasing Treg:Teff ratios shown in Fig. 2D and E. We found that the number of Annexin V+ Foxp3+ Treg was almost three times higher after 8 weeks of cholesterol diet than after 4 weeks (Fig. 2F), indicating that prolonged hypercholesterolemia induces Treg apoptosis in atherosclerotic aortas.
Hypercholesterolemia induces changes in functional phenotype of Foxp3-eGFP+ Treg in Ldlr−/− mice
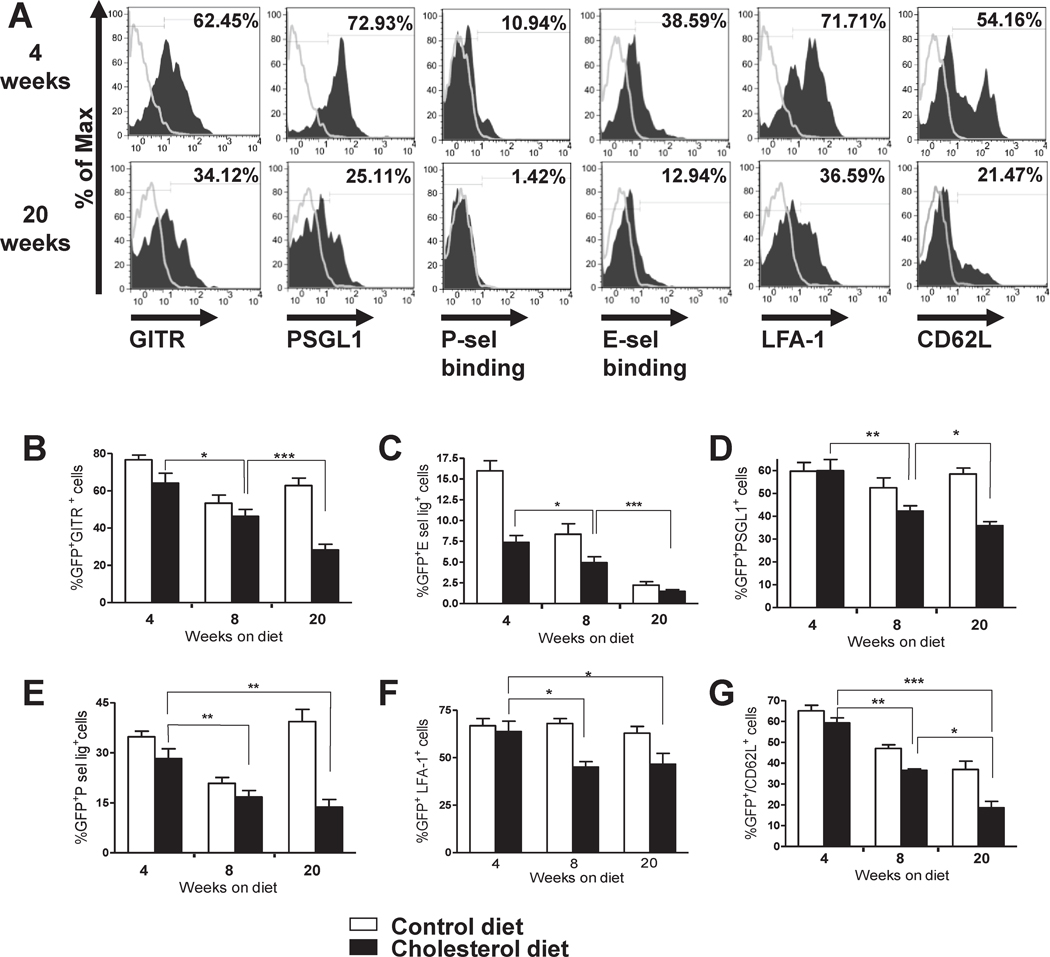
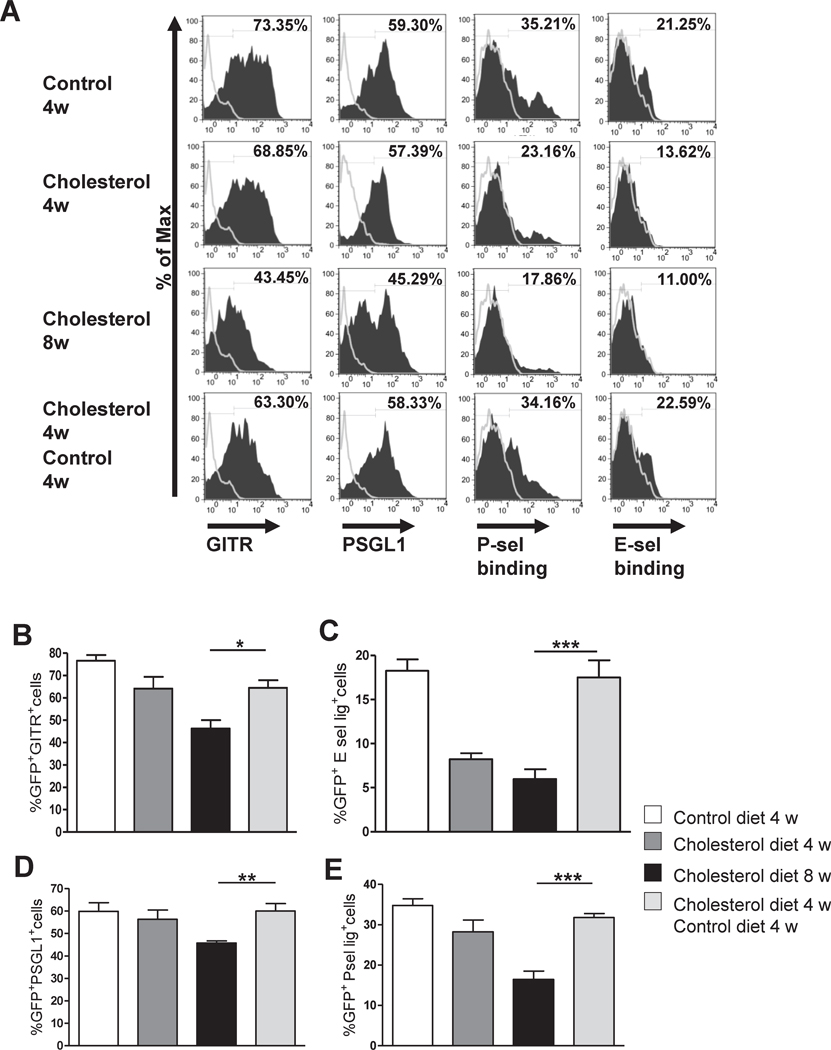
To address the possibility that the reduction of blood and lesional Treg over time in hypercholesterolemic mice reflected a change in migratory function, we analyzed the expression of cell surface molecules on splenic Treg, including chemokine receptors, integrins, and selectin ligands. We also analyzed expression of surface molecules related to function, including GITR, ICOS and CTLA4. Interestingly, we found that the expression of GITR, P-selectin glycoprotein ligand-1 (PSGL1), lymphocyte function associated-1 (LFA-1), L-selectin (CD62L) and binding of P-selectin and E-selectin IgG chimeras, all decreased significantly in CD4+GFP+ Treg after 20 weeks of cholesterol diet compared to Treg from control diet-fed mice (Fig. 3A–G). In contrast, CD4+GFP− lymphocytes (Teff) from cholesterol or control diet-fed mice had the same levels of PSGL-1, LFA-1 and binding of P/E selectin-Ig chimeras, although CD62L and GITR on the Teff did decreased over time (Supplemental Figure S2 A–F). There was also a reduction in Treg expression of CCR6, ICOS and CTLA4 from 4 to 8 to 20 weeks, but these changes were comparable in Treg from control and cholesterol diet-fed mice. The expression of glycosylated CD43 or CXCR4 on Treg did not change over time and did not differ in control and hypercholesterolemic mice (data not shown).
Figure 3. Hypercholesterolemia causes changes in phenotype of Treg.
Spleen cells from the same Foxp3-eGFP+/Ldlr−/− mice described in Figure 1 A–C, harvested after 4, 8 or 20 weeks of cholesterol or control diet, were stained for CD4, GITR, PSGL1, LFA-1 and CD62L or with P-selectin and E-selectin IgG chimeras, and analyzed by FACs gating on CD4+GFP+ cells. A, Histograms correspond to one representative mouse. B–G, FACS data represent mean +/− S.E.M, N= 6–9 mice per group. * P <0.05, ** P<0.01, analyzed by two way ANOVA with Bonferroni’s post test.
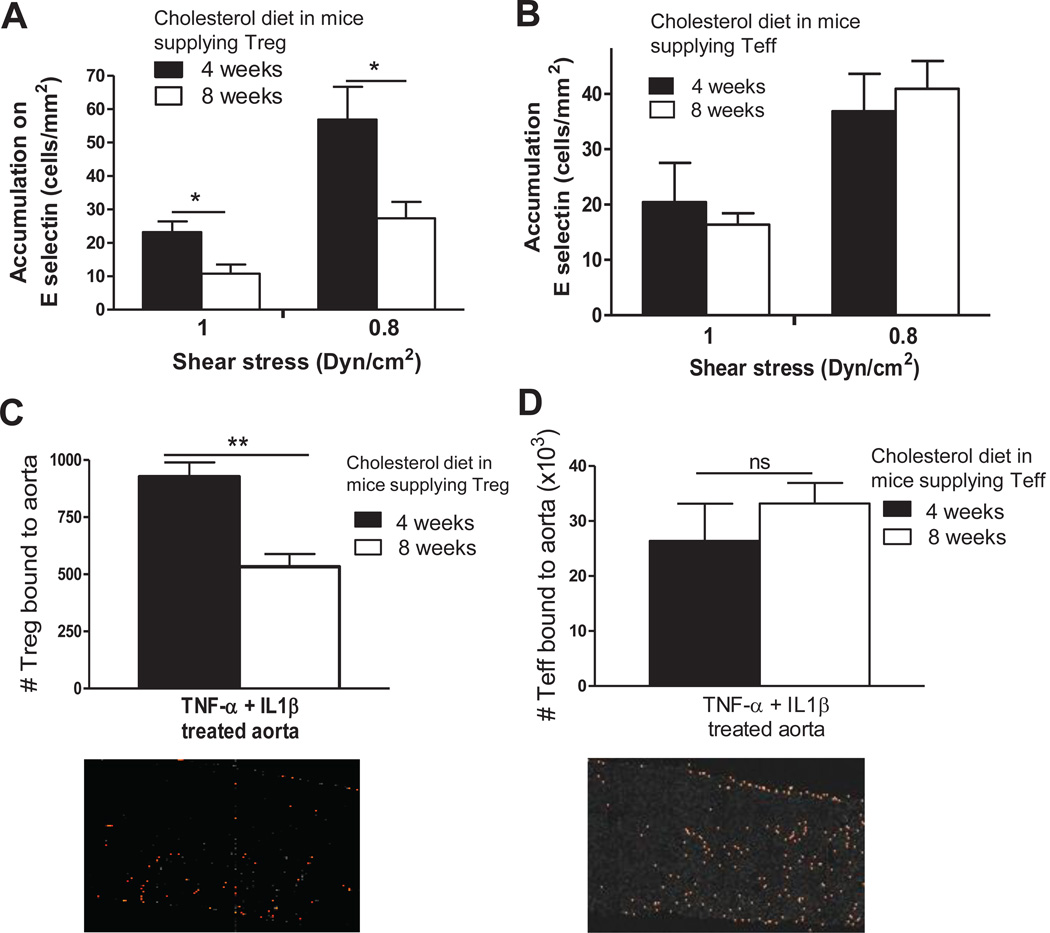
The changes in Treg but not Teff phenotype that we observed in response to prolonged hypercholesterolemia suggested that Treg might also have a reduced ability to adhere to endothelial adhesion molecules in comparison to Teff. We analyzed the ability of Treg and Teff from Foxp3-eGFP+/Ldlr−/− mice to bind to E-selectin under physiologic flow conditions in an in vitro flow chamber assay. The results showed that prolonged hypercholesterolemia reduced the ability of Treg to bind to E-selectin under conditions of shear stress (1 and 0.8 dynes/cm2) (Figure 4A). In contrast, we did not find significant differences in binding of Teff from 4 vs. 8 weeks cholesterol diet fed mice (Figure 4B). We also tested the effect of prolonged hypercholesterolemia on the ability of Treg and Teff to bind to the endothelial lining of intact aortas ex vivo. Aortas from C57BL/6 wild-type mice were harvested and stimulated with TNFα and IL1β to induce the expression of receptors involved in inflammation and migration. Consistent with the above findings from the flow assays, significantly less splenic Treg from Foxp3-eGFP+/Ldlr−/− mice fed a cholesterol diet for 8 weeks bound to the aortas than did Treg from mice fed the diet for 4 weeks (Fig.4C). In contrast, Teff taken after 4 and 8 weeks of cholesterol diet bound equally well (Fig. 4D). These results indicate that prolonged hypercholesterolemia significantly reduces Treg but not Teff binding to the aortic endothelium.
Figure 4. Prolonged hypercholesterolemia inhibits Treg adhesion to E selectin and aortic endothelium.
A–B, Splenic CD4+GFP+ (Treg) and CD4+GFP− (Teff) cells were isolated from the same Foxp3-eGFP+/Ldlr−/− mice fed a cholesterol diet for 4 and 8 weeks described in Figure 1 D–F, and were perfused over glass coverslips coated with recombinant E-selectin (100 µg/ml) at 1 and 0.8 Dynes/cm2. Data are mean ± S.E.M. of values from different fields; N=3–5 mice per group. C–D, The binding of same Treg and Teff to the lumena of IL-1β and TNFα treated aortas of C57Bl/6 mice was detected by spinning disk confocal microscopy. Adherent Treg and Teff were counted on deconvoluted images (an example is shown). Binding to unstimulated aortas was less than 25% of binding to cytokine treated aortas, confirming efficacy of the cytokine activation (not shown). Data represent mean ± SEM of N=4–5 aortas per group. *P<0.05, **P<0.01, analyzed by Students t test.
We also tested if hypercholesterolemia can modulate splenic Treg suppressive activity. Using a standard ex vivo Treg suppression assay in which Treg, responder T cells, and splenic APCs are cultured with anti-CD3, we observed that Treg isolated from Foxp3-eGFP+/Ldlr−/− mice fed a cholesterol diet for 8 weeks were as effective in inhibiting responder T cell (CD4+CD25−) proliferation as Treg isolated after only 4 weeks of cholesterol diet (Supplemental Figure S3A). The ability to suppress IFNγ secretion was also not impaired by prolonged hypercholesterolemia (data not shown). In addition, when the assay was run with DCs as the APCs, recovered from the spleen of the same animals from which CD4+GFP+ Treg were isolated, no effects of prolonged hypercholesterolemia on Treg suppression were found (Fig. S3B). These results support the idea that Treg remain functional in the setting of hypercholesterolemia, even if their capacity to bind to aortic endothelium is impaired.
Reversal of hypercholesterolemia by change in diet prevents reduction in the initial Treg response and change in Treg phenotype
In order to prove that the reduction in the initial Treg response was due to prolonged hypercholesterolemia, we designed a study in which mice were fed a cholesterol diet for 4 weeks, and then the mice were fed a cholesterol-free diet for an additional 4 weeks, in order to reverse the hypercholesterolemia. We reasoned that this would allow the initial Treg response to be maintained. The diet reversal mice were compared with mice fed a cholesterol diet for 8 weeks. We confirmed that the diet switch resulted in significantly reduced serum cholesterol compared to the mice fed cholesterol diet continuously for 8 weeks (Fig. 5A). There was significant inhibition of lesion progression between 4 and 8 weeks in mice changed to a control diet compared to mice fed cholesterol diet for 8 weeks (Fig. 5B). Flow cytometric analyses of aortic digestions indicated that the diet reversal resulted in sustained peak Treg numbers in the aorta, which were approximately the same numbers observed after 4 weeks of cholesterol diet (Fig. 5C). Moreover, staining of CD4 and Foxp3 cells in the aortic digests confirmed that the decrease in dietary and blood cholesterol resulted in a sustained high number of Treg (CD4+Foxp3+) within the aorta, which was higher than the number of Teff cells (CD4+Foxp3−) (Fig. 5D). Quantification of Treg and Teff showed again a decreasing ratio Treg:Teff in aortas over a period of sustained hypercholesterolemia that was prevented in mice changed to a control diet (Fig. 5E). In addition, diet reversal also prevented the increase in Treg apoptosis seen in mice with prolonged hypercholesterolemia (Fig. 5F). We also found, by imunohistochemical analysis, a lower number of total CD4+ cells in aortic sinus lesions in the diet reversal group (Fig. S4A). SMCs content in the lesion did not show any difference between the groups (Fig. S4B), suggesting that changes in SMC content are not as readily altered with changes in diet.
Figure 5. Changes in diet prevent reduction in aortic Treg numbers.
Foxp3-eGFP+/Ldlr−/− mice (56 total) were fed control or cholesterol diet for 4 or 8 weeks, or cholesterol diet for 4 weeks and then control diet for 4 weeks. A, Total cholesterol levels were measured in serum at time of sacrifice. N=11–17 mice per group. B, Frozen sections of aortic sinuses were stained with ORO to determine lesion size. Horizontal bars represent the mean value of the entire group of mice. N=4–10 per group. C, GFP+ cells in aortic digests were enumerated by FACS. Data represent mean +/− S.E.M, N=8 mice. Data represent mean +/− S.E.M. * P <0.05, ** P<0.01, *** P< 0.001, analyzed by one way ANOVA with Tukey’s post test. In a separate experiment, Foxp3-eGFP+/Ldlr−/− mice (21 total) were fed a cholesterol diet for 4 or 8 weeks, or a cholesterol diet for 4 weeks followed by a control diet for 4 weeks, and aortic digest cells were stained for CD4, Foxp3 and Annexin V, and analyzed by FACs to determine: D, number of CD4+Foxp3− (white bars) and CD4+Foxp3+ cells (black bars); E, the CD4+Foxp3+:CD4+Foxp3− ratio; and F, the percent of apoptotic cells. Data represent mean +/− S.E.M.
We compared phenotypic markers on GFP+ lymphocytes from spleens of mice changed to a control diet and mice fed a cholesterol diet continuously. The decreased expression of GITR, PSGL1 and P/E-selectin IgG chimera binding that we observed in the GFP+ population of hypercholesterolemic mice were not seen in the mice changed to control diet at 4 weeks (Fig. 6A–E). In other words, splenic Treg harvested 4 weeks after mice were changed to a control diet had the same levels of expression of these molecules as Treg in continuously control-diet fed mice. However, the 4 week level of LFA-1 expression was not maintain by dietary reversal of hypercholesterolemia (Supplemental Figure S5A and B); suggesting that the changes in selectin ligand expression were more important for Treg accumulation. These results show that Treg which increase in numbers and accumulate in the aortic wall along with CD4+ effector T cells during the first 4 weeks of hypercholesterolemia, selectively undergo phenotypic due to prolonged hypercholesterolemia beyond 4 weeks, which result in their absolute reduction in lesions compared to effector T cells.
Figure 6. Changes in diet prevent reduction in the functional phenotype of Treg.
Spleen cells from mice in the diet reversal study described in Figure 5A–E were stained for CD4, GITR, PSGL1, and P-selectin and E-selectin IgG chimeras, and analyzed by flow cytometry gating on CD4+GFP+ population. A, Histograms correspond to one representative mouse. B–E, FACS analysis data represent mean +/− S.E.M, N= 5–9 mice per group. * P <0.05, ** P<0.01, *** P< 0.001, analyzed by one way ANOVA with Tukey’s post test.
Discussion
Emerging evidence indicates that Treg can suppress proatherogenic T cell responses, similar to their established function in regulating autoreactive T cells in immune mediated/inflammatory disease. Studies from mouse and humans show that both induced Treg and effector T cells are increased in numbers in many immune responses to foreign and self antigens, and the ratio of Treg to effector T cells determines the outcome of these responses 22–24. We reasoned that therapeutic interventions aimed increasing Treg to effector T cell ratios in atherosclerotic lesions might favor control of or reduction in growth of the lesions and would diminish inflammation. Such a therapeutic approach would be feasible only if lesional Treg content could be dynamically changed by those interventions.
One important finding from our studies is that hypercholesterolemia induces an accumulation of Treg in the atherosclerotic aorta, but the Treg content is not maintained over time under sustained hypercholesterolemic conditions. Significantly, effector T cell content in the aorta increases while Treg content goes down. Therefore pro-inflammatory effector T cells within lesions will be subject to decreased local regulation as the disease progresses. Possible mechanisms for this decrease over time are the loss of the ability of Treg to migrate into lesions after prolonged hypercholesterolemia. In support of this, we observed an increase in Treg in peripheral lymphoid tissue (spleen) concomitant with the decrease of Treg in lesions. This finding indicates that there is not a failure to generate or expand Treg systemically, nor a systemic decrease in their survival, under hypercholesterolemic conditions. However, the expression of functional selectin ligands decreases on splenic Tregs between 4 weeks and 20 weeks of hypercholesterolemia. Previous studies have shown that T cell accumulation in atherosclerotic lesions is selectin-dependent 34–37, and that Treg migration is also selectin dependent 38, 39. Interestingly, selectin ligands did not decrease on other lymphocytes in the spleens of the hypercholesterolemic mice, which might explain why effector T cells continue to accumulate in the atherosclerotic aortas of the mice. The basis for the effect of hypercholesterolemia on Treg selectin binding ability and the selectiveness of the effect for Treg but not T effector cells requires further investigation. One possibility is that hypercholesterolemia alters dendritic cells in a way that alters Treg differentiation. We have observed that naïve T cells from Foxp3-eGFP+ mice fail to bind selectins, but Treg derived in vitro from naive Foxp3-eGFP+ T cells acquire selectin ligand capabilities (unpublished data). We have also shown that dendritic cells become foam cells and retain antigen –presenting functions 40. It is possible that cholesterol-loaded dendritic cells are less capable of inducing expression of the glycosyltransferase enzymes required for functional selectin ligand synthesis.
A loss of ability of Treg to enter lesions after prolonged hypercholesterolemia may explain a lack of continued Treg accumulation, but does not explain loss of cells that had already entered the aortic wall at 4 weeks. Increased Treg death after exposure to the increasingly altered microenvironment of the atherosclerotic arterial wall could be the basis of the increased Treg loss at 8 and 20 weeks. Our finding that there were significantly more apoptotic aortic Treg after 8 weeks than 4 weeks supports this explanation.
One limitation of the data shown in Figures 1 and 2 is that they cannot distinguish effects of prolonged hypercholesterolemia from effects of age, since the longer the mice were kept on the cholesterol diet, the older they were at time of sacrifice. However, we did not find any age-related change in selectin binding by Treg from normocholesterolemic C57BL/6 mice at the equivalent ages as those in our experiments with Ldlr−/− mice (data not shown). Furthermore, in the diet reversal study (Figures 5, 6), the mice were the exact same age at sacrifice, and difference in Treg numbers and phenotype correlated with cholesterol content of diet during the final 4 weeks of life.
An important finding in this study is that changes in induced Treg content in atherosclerotic aortas are linked to hypercholesterolemia and can indeed be prevented by changes in diet-dependent blood cholesterol levels. Moreover, these changes are inversely correlated with total effector T cell content in lesions and lesion size. Thus, changing a cholesterol-rich diet to a cholesterol-free diet we were able to maintain high Treg numbers in the aorta and decrease the inability of Treg to bind to selectins and bind to aortic endothelium. Our finding supports the interpretation that therapeutic reversal of hypercholesterolemia would enhance Treg migration into the lesions.
Clinical Summary.
CD4+ effector T cells have multiple pro-inflammatory properties that contribute to the chronic inflammatory phenotype of evolving atherosclerotic lesions, as well as to the destabilization of plaques associated with acute coronary events. Regulatory T cells (Treg) actively suppress T cell-mediated immune responses, and reduced Treg function or numbers is associated with immune-mediated inflammatory disease. The influence of regulatory T cells (Treg) in atherosclerosis has become a central area of interest because of potential therapeutic implications. This study demonstrates that in a mouse model of atherosclerosis, a Treg response is induced by hypercholesterolemia, but the response declines while the effector T cells response is maintained when hypercholesterolemia is prolonged. The decline in the Treg response is associated with selective decrease homing properties and increased apoptosis of Treg but not Teff cells during prolonged hypercholesterolemia. The Treg response is sustained by dietary reversal of hypercholesterolemia early after the initial response is induced. Our data suggest that an important therapeutic goal in atherosclerotic patients is to re-establish favourable lesional Treg:Teff ratios, and this may be one of the mechanisms of benefit of profound cholesterol lowering.
Supplementary Material
Acknowledgment
We are grateful to Dr. Vijay Kuchroo (Center for Neurologic Diseases Brigham & Women's Hospital) for providing Foxp3-eGFP knock in mice. We thank George Stavrakis (Morphology Core, Pathology, Brigham and Women’s Hospital) for the technical assistance with IHC staining. We thank Pilar Alcaide and Francis Luscinskas (Brigham and Women’s Hospital) for helping with in vitro flow studies. We also thank Harvard Neurodiscovery Optical Imaging Program (Harvard Medical School) for the contribution helping with the immunofluorescence images acquisition.
Funding Sources
This work was supported by NIH grant HL087282 and Foundation Antonio Martín Escudero.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Zhou X, Stemme S, Hansson GK. Evidence for a local immune response in atherosclerosis. Cd4+ t cells infiltrate lesions of apolipoprotein-e-deficient mice. Am J Pathol. 1996;149:359–366. [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a t helper (th) 1/th2 switch of the autoimmune response in atherosclerotic apo e-knockout mice. J Clin Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 4.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the ldlr-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 5.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of t cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameli S, Hultgardh-Nilsson A, Regnstrom J, Calara F, Yano J, Cercek B, Shah PK, Nilsson J. Effect of immunization with homologous ldl and oxidized ldl on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler Thromb Vasc Biol. 1996;16:1074–1079. doi: 10.1161/01.atv.16.8.1074. [DOI] [PubMed] [Google Scholar]
- 8.George J, Yacov N, Breitbart E, Bangio L, Shaish A, Gilburd B, Shoenfeld Y, Harats D. Suppression of early atherosclerosis in ldl-receptor deficient mice by oral tolerance with beta 2-glycoprotein i. Cardiovasc Res. 2004;62:603–609. doi: 10.1016/j.cardiores.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Maron R, Sukhova G, Faria AM, Hoffmann E, Mach F, Libby P, Weiner HL. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation. 2002;106:1708–1715. doi: 10.1161/01.cir.0000029750.99462.30. [DOI] [PubMed] [Google Scholar]
- 10.Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, Chen X, Witztum JL, Varki NM, Varki A. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114:5225–5235. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing K, Sakaguchi S. Regulatory t cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 12.Vignali D. How many mechanisms do regulatory t cells need? Eur J Immunol. 2008;38:908–911. doi: 10.1002/eji.200738114. [DOI] [PubMed] [Google Scholar]
- 13.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: Regulatory t cell development and the forkhead family transcription factor foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 14.Allan SE, Broady R, Gregori S, Himmel ME, Locke N, Roncarolo MG, Bacchetta R, Levings MK. Cd4+ t-regulatory cells: Toward therapy for human diseases. Immunol Rev. 2008;223:391–421. doi: 10.1111/j.1600-065X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 15.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory t-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 16.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of foxp3 positive regulatory t cells are present in all developmental stages of human atherosclerotic lesions. PLoS One. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotsman I, Gupta R, Lichtman AH. The influence of the regulatory t lymphocytes on atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2493–2495. doi: 10.1161/ATVBAHA.107.153064. [DOI] [PubMed] [Google Scholar]
- 18.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory t cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 19.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G, George J. Role of naturally occurring cd4+ cd25+ regulatory t cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 20.Yang K, Li D, Luo M, Hu Y. Generation of hsp60-specific regulatory t cell and effect on atherosclerosis. Cell Immunol. 2006;243:90–95. doi: 10.1016/j.cellimm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Mallat Z, Gojova A, Brun V, Esposito B, Fournier N, Cottrez F, Tedgui A, Groux H. Induction of a regulatory t cell type 1 response reduces the development of atherosclerosis in apolipoprotein e-knockout mice. Circulation. 2003;108:1232–1237. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 22.Brusko TM, Putnam AL, Bluestone JA. Human regulatory t cells: Role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 23.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, Mugyenyi P, Cao H. Depletion of regulatory t cells in hiv infection is associated with immune activation. J Immunol. 2005;174:4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 24.Finney OC, Nwakanma D, Conway DJ, Walther M, Riley EM. Homeostatic regulation of t effector to treg ratios in an area of seasonal malaria transmission. Eur J Immunol. 2009;39:1288–1300. doi: 10.1002/eji.200839112. [DOI] [PubMed] [Google Scholar]
- 25.Koyama K, Kagamu H, Miura S, Hiura T, Miyabayashi T, Itoh R, Kuriyama H, Tanaka H, Tanaka J, Yoshizawa H, Nakata K, Gejyo F. Reciprocal cd4+ t-cell balance of effector cd62llow cd4+ and cd62lhighcd25+ cd4+ regulatory t cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14:6770–6779. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector th17 and regulatory t cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Cybulsky MI, Lichtman AH, Hajra L, Iiyama K. Leukocyte adhesion molecules in atherogenesis. Clin Chim Acta. 1999;286:207–218. doi: 10.1016/s0009-8981(99)00102-3. [DOI] [PubMed] [Google Scholar]
- 28.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of cd40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]
- 29.Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- 30.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17a results in reduced atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim YC, Henault L, Wagers AJ, Kansas GS, Luscinskas FW, Lichtman AH. Expression of functional selectin ligands on th cells is differentially regulated by il-12 and il-4. J Immunol. 1999;162:3193–3201. [PubMed] [Google Scholar]
- 32.Alcaide P, King SL, Dimitroff CJ, Lim YC, Fuhlbrigge RC, Luscinskas FW. The 130-kda glycoform of cd43 functions as an e-selectin ligand for activated th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J Invest Dermatol. 2007;127:1964–1972. doi: 10.1038/sj.jid.5700805. [DOI] [PubMed] [Google Scholar]
- 33.Bobryshev YV. Monocyte recruitment and foam cell formation in atherosclerosis. Micron. 2006;37:208–222. doi: 10.1016/j.micron.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially l-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 37.Maffia P, Zinselmeyer BH, Ialenti A, Kennedy S, Baker AH, McInnes IB, Brewer JM, Garside P. Images in cardiovascular medicine. Multiphoton microscopy for 3-dimensional imaging of lymphocyte recruitment into apolipoprotein-e-deficient mouse carotid artery. Circulation. 2007;115:e326–e328. doi: 10.1161/CIRCULATIONAHA.106.658492. [DOI] [PubMed] [Google Scholar]
- 38.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like cd4+ regulatory t cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venturi GM, Conway RM, Steeber DA, Tedder TF. Cd25+cd4+ regulatory t cell migration requires l-selectin expression: L-selectin transcriptional regulation balances constitutive receptor turnover. J Immunol. 2007;178:291–300. doi: 10.4049/jimmunol.178.1.291. [DOI] [PubMed] [Google Scholar]
- 40.Packard RR, Maganto-Garcia E, Gotsman I, Tabas I, Libby P, Lichtman AH. Cd11c(+) dendritic cells maintain antigen processing, presentation capabilities, and cd4(+) t-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.