Abstract
Background
Procedural learning is an implicit process in which a behavioral response is refined through repeated performance. Neural systems supporting this cognitive process include specific frontostriatal systems responsible for the preparation and timing of planned motor responses. Evaluating performance on procedural learning tasks can provide unique information about neurodevelopmental disorders in which frontostriatal disturbances have been reported, such as autism.
Methods
Fifty-two individuals with autism and 54 age-, IQ-, and gender-matched healthy individuals performed an oculomotor serial reaction time task and a sensorimotor control task.
Results
Whereas the rate of procedural learning and the precision of planned motor responses were unimpaired in autism, a lateralized alteration in the ability to time predictive responses was observed. Rightward saccadic responses were speeded in individuals with autism relative to healthy control subjects.
Conclusions
Speeded rightward predictive saccades suggest atypical functioning of left hemisphere striatal chronometric systems in autism.
Keywords: Autism, left hemisphere, predictive saccade, prefrontal cortex, procedural learning, striatum
Autism is a pervasive neurodevelopmental disorder characterized by disturbances in social interactions, verbal and nonverbal communication, and restricted and repetitive patterns of interests and behavior. A broad pattern of neuropsychological and neurological impairments have been associated with autism (1).
One cognitive process that has received little attention in autism research is procedural learning, an implicit process in which behavioral responses are refined through repeated performance. Well-characterized frontostriatal and frontocerebellar loops subserve procedural learning (2,3), with the relative contribution of these systems determined in part by the duration of time intervals between component responses of behavioral sequences. Learning and enacting precise motor sequences depends on internal chronometric systems, neural time-keeping systems that maintain and regulate timed motor responding. Typically, the cerebellum controls the execution of rapid sequential motor responses with movement intervals of up to .5–1 sec, whereas the timing of slower motor sequences relies more on striatal chronometric systems (4,5). Deficits in striatum and cerebellum have been observed in neuroimaging (6–11) and histopathological studies (12,13) of autism, providing a rationale for investigating components of procedural learning in this disorder. Moreover, some but not all studies have found that timing abilities are impaired in patients with autism (14,15).
A common approach for investigating procedural learning is to study skill acquisition in serial reaction time tasks, in which the speed of performance of a motor sequence improves with practice (16). Mostofsky et al. (14) examined implicit learning of a repeating 10-component sequence and reported that adolescents with autism showed less decline than healthy control subjects in response latencies with repeated presentation of the sequence.
The predictive saccade task provides a simple and rapid test of procedural learning. It typically requires subjects to track visual targets that alternate between two locations at a fixed time interval, to which individuals quickly learn to anticipate the sequence as evidenced by rapid speeding of reaction times (17,18). Predictive saccades have latencies that are sufficiently brief (<90 msec) to indicate that they are planned and initiated in advance of target appearance.
The periodicity of the alternating target in the predictive saccade task is usually within the seconds range. Therefore, learning to accurately time predictive responses on this task depends more on frontostriatal systems than cerebellum, consistent with evidence from previous studies (19,20). Also, impaired performance on the predictive saccade task has been observed in disorders affecting the basal ganglia, including Parkinson’s (17) and Huntington’s diseases (21).
Several types of information can be derived from an analysis of predictive saccade task performance. The rate of learning over trials can be evaluated by examining the reduction in reaction times with practice. The ability to accurately time predictive saccades serves as an index of the integrity of striatal response-timing systems. Evaluating the accuracy of predictive saccades provides information on the precision of voluntary motor responses initiated without sensory guidance. Because of the strong lateralization of oculomotor systems, the presence of lateralized deficits in each of these processes can be identified. In this study, individuals with autism and matched healthy control participants performed a predictive saccade task and a sensorimotor control paradigm to assess the integrity of these three processes in autism.
Methods and Materials
Participants
Fifty-two high-functioning individuals with autism and 54 healthy control participants (5 female subjects/group) were matched on age [mean age (SD), range: 19.6 (11.3), 8–53 years, and 20.3 (12.2), 8–56 years, respectively] and full-scale IQ [mean IQ (SD): 108.0 (16.8) and 110.5 (15.4), respectively]. All participants had a full-scale IQ > 80 and far visual acuity of at least 20/40 (corrected or uncorrected).
Individuals with autism met DSM-IV criteria for autistic disorder on the basis of the Autism Diagnostic Interview–Revised (22) and Autism Diagnostic Observation Schedule-Generic (23). Participants diagnosed with a genetic or metabolic disorder known to be associated with autism were excluded (e.g., Fragile-X, tuberous sclerosis), as were those with a lifetime history of head injury or seizure disorder. Participants were free of medications known to affect cognitive or oculomotor abilities, including antipsychotics, methylphenidate, amphetamine, and anticonvulsants.
Healthy participants had no first- or second-degree relatives with a history of a neuropsychiatric disorder known to have a genetic component, including autism. Written informed consent or written assent from minors (in addition to written parental consent) was obtained from all participants. Study procedures were approved by the Institutional Review Board at the University of Pittsburgh.
Eye Movement Studies
Participants were seated in a darkened black room facing a black arc of 1 m radius containing red light-emitting diodes (LEDs) embedded in the horizontal plane at eye-level. The LEDs subtended approximately .2° of visual angle and were not visible unless illuminated. A chin and forehead rest with occipital restraints and head strap was used to minimize head movement.
Eye movements were recorded with infrared (IR) scleral-reflection sensors mounted on spectacle frames (Model 210; Applied Science Laboratories, Bedford, Massachusetts) or DC electro-oculography (EOG) for those whose uncorrected far visual acuity was < 20/40, so that subjects could use corrective lenses (Grass Neurodata 12, Astro-Med, West Warwick, Rhode Island). Blinks were monitored with AC-coupled electrodes placed above and below the left eye. Recordings were digitized at 500 Hz (DI-210 14-bit A/D; DATAQ Instruments, Akron, Ohio), stored for off-line analysis, and analyzed with custom software developed in our laboratory.
Visually Guided Saccade Task
A visually guided saccade control task was first administered to evaluate basic sensorimotor processes. Participants maintained central fixation for 1.5–2.5 sec at the start of each trial and then looked toward a peripheral target presented pseudo-randomly at one of six angular displacements in the horizontal plane (±10°, ±20°, or ±30°). Fifty-four trials were presented. Peripheral targets appeared concurrently with the offset of the central target. Latency (time from appearance of target to saccade initiation) and gain (% of distance moved to the target location) of primary saccades (the first saccade to the target) were measured.
Predictive Saccade Procedural Learning Task
During the predictive saccade task, participants visually tracked a target that stepped between two locations (±7.5° from center) every 1.5 sec. This sequence was repeated 10 times (i.e., 20 target presentations) (Figure 1). The first saccade, made from central fixation to one of the peripheral targets to start the task, was not included in analyses. Latency and gain of primary saccades were measured.
Figure 1.
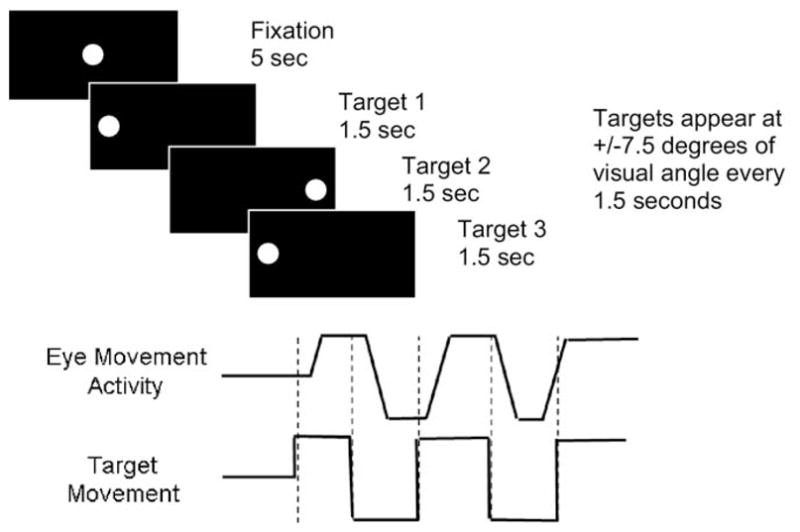
Schematic representation of the predictive saccade task. Participants were instructed to follow the dot with their eyes. Targets appear at +/− 7.5° of visual angle every 1.5 sec.
Primary saccades were classified qualitatively according to their latency. Sensory-guided saccades (>160 msec) represent reflexive responses to target appearance. Anticipatory saccades (<90 msec) reflect saccades made in anticipation of target appearance, before sensorimotor processes can respond to visual cues. We also considered an intermediate group of fast saccades (<160 msec but >90 msec). This classification of saccades is consistent with neurophysiological evidence (24) and previous oculomotor studies (20).
Analyses of Eye Movements
Eye position recordings obtained during fixation of targets in each trial were used to convert voltage recordings to eye position in degrees of visual angle. This “within-trial” calibration minimizes artifacts resulting from drift in DC-EOG signals over the course of testing. Performance was examined to identify primary saccades, artifacts (e.g., blinks, signal clipping), and failures of software algorithms to correctly identify saccades. Before analysis, digitized eye movement signals were smoothed with a linear-phase, finite-impulse response low-pass filter.
Statistical Analyses
For the visually guided saccade task, repeated-measures analyses of variance (ANOVAs) were used to examine effects of target step amplitude (10°, 20°, 30°), direction (left, right), and participant group (autism, control) on saccade latency and gain.
For the predictive saccade task, mixed-effects regression models were used to accommodate repeated measurements across trials that were correlated to different degrees, as is the case when learning occurs. Latency and gain data were each modeled as quadratic functions over trials, allowing for expected nonlinear rates of learning. Initial models included group (autism, control), linear and quadratic terms for change across trials, response direction (left, right), and all two- and three-way interactions. Terms were eliminated from the model with a backwards elimination procedure to arrive at the most parsimonious model. Mixed-effects models were analyzed with SAS (v.8.02 for Windows; SAS, Cary, North Carolina).
Repeated-measures ANOVAs were used to compare the proportion, gain, and latency of each saccade type as a function of group and saccade direction. Correlations of age with all parameters were nonsignificant in both participant groups.
Results
Visually Guided Saccade Task
There were no differences between individuals with autism and healthy control subjects in visually guided saccade latencies [F(1,104) = .43, p = .51] or gain [F(1,104) = .66, p = .42]. No group interactions with target location or direction were significant.
Predictive Saccade Task
Accuracy
Primary saccade gain did not differ between groups [F(1,1699) = .61, p = .43], and there were no group differences in the gain of different saccade types (Table 1).
Table 1.
Saccade Tasks Results
| Autism
|
Control
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | Latency
|
Gain
|
% | Latency
|
Gain
|
|||||
| M | SD | M | SD | M | SD | M | SD | |||
| Predictive Saccade Task | ||||||||||
| Right | ||||||||||
| Sensory-guided | 43.64a | 228.59 | 60.02 | .98 | .09 | 58.57 | 210.03 | 33.64 | .95 | .11 |
| Fast | 16.73 | 131.45 | 14.21 | .94 | .13 | 16.33 | 137.73 | 15.49 | .91 | .16 |
| Anticipatory | 39.64a | −247.68a | 152.43 | .78 | .22 | 25.10 | −170.97 | 126.83 | .77 | .26 |
| Left | ||||||||||
| Sensory-guided | 48.88 | 231.77 | 68.10 | .97 | .07 | 56.36 | 201.77 | 43.52 | .91 | .10 |
| Fast | 18.57 | 134.24 | 12.59 | .93 | .10 | 18.60 | 140.09 | 8.84 | .90 | .16 |
| Anticipatory | 32.54 | −185.72 | 159.51 | .82 | .19 | 25.04 | −146.05 | 127.56 | .80 | .25 |
| Visually Guided Saccade Task | ||||||||||
| Right | ||||||||||
| 10 | 218.51 | 47.45 | .95 | .07 | 218.73 | 34.38 | .95 | .08 | ||
| 20 | 239.01 | 45.27 | .92 | .06 | 232.37 | 36.07 | .91 | .07 | ||
| 30 | 243.48 | 53.61 | .91 | .07 | 245.53 | 40.38 | .92 | .06 | ||
| Left | ||||||||||
| 10 | 219.89 | 35.94 | .95 | .07 | 219.48 | 37.02 | .99 | .06 | ||
| 20 | 235.77 | 40.20 | .92 | .06 | 229.35 | 37.42 | .94 | .05 | ||
| 30 | 264.80 | 47.98 | .91 | .07 | 250.56 | 41.00 | .93 | .06 | ||
Percentage of each of the three saccade types (sensory-guided, fast, and anticipatory) among rightward and leftward responses, and the mean (M) and standard deviation (SD) of saccade latency and gain for saccades of each type during the predictive saccade task and for rightward and leftward saccades to each of the three target locations in the visually guided saccade task. Percentages of saccade types for the predictive saccade task are presented separately as a proportion of all scorable leftward and rightward saccades for each subject group. Saccades were classified as follows: sensory-guided (>160 msec); anticipatory (<90 msec); fast (<160 msec but >90 msec).
Significant group differences, p = .05.
Latency
Whereas there was a significant overall reduction in response latencies over trials [F(1,1664) = 16.91, p < .001], the rate of learning (i.e., the overall latency reduction over trials) did not differ across groups [F(1,1664) = .03, p = .90]. However, the three-way interaction was significant [F(1,1664) = 5.97, p = .01], driven by progressively faster rightward responses over trials in individuals with autism, relative to control subjects. Follow-up analyses confirmed that the interaction was significant for rightward but not leftward saccades [F(1,779) = 6.70, p = .01, and F(1,677) = 1.25, p = .26, respectively] (Figures 2 and 3).
Figure 2.
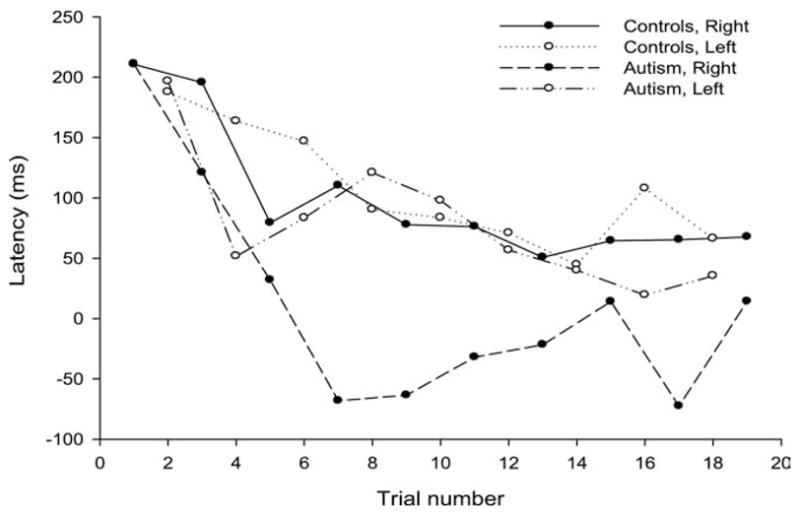
Average saccade latency (msec) for rightward and leftward saccades in participants with autism and healthy control subjects over trials in the predictive saccade task. The percentage of scorable saccades included in each curve (of the possible number of trials presented in each condition) is: Control Subjects, Right: 95.6, Left: 96.1; Autism, Right: 86.0, Left: 89.0. There was no significant group difference between the percentages of saccades included in each condition.
Figure 3.
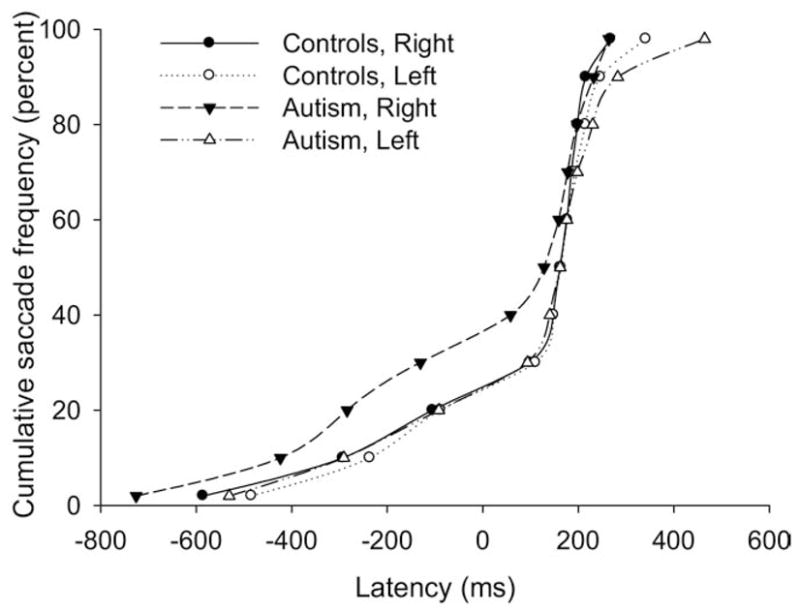
Cumulative frequency of saccade latencies over all trials during the predictive saccade task presented separately for leftward and rightward saccades for participants with autism and healthy control subjects.
Consistent with these observations, individuals with autism had a higher proportion of rightward than leftward anticipatory saccades [F(1,104) = 9.77, p = .02] (Table 1), and the mean latency of anticipatory saccades in the autism group was significantly faster than control subjects for rightward movements only F [ (1,320) = 4.73, p = .03].
Discussion
We examined procedural learning with a predictive saccade task known to engage frontostriatal systems in a relatively large group of individuals with autism. We did not observe abnormalities in the overall rate of procedural learning in autism (i.e., the reduction in response latencies over trials). However, individuals with autism displayed a speeding of rightward predictive/anticipatory responses. Internal clocks, in the form of striatal temporal oscillators (25), are the means by which precise interval timing is perceived and used to initiate planned motor sequences (26). The specific speeding of rightward saccades exhibited by individuals with autism on the predictive saccade task suggests a lateralized acceleration of the striatal temporal coding system that is used to time internally generated motor response sequences by the contralateral (left) hemisphere.
Neuroimaging (27) and animal studies (28) have demonstrated the importance of the basal ganglia in tasks requiring precisely timed responses, including predictive saccades (20), and in the implicit judgment of time intervals (29). Patients with cerebellar lesions typically have a reduced ability to acquire learned motor sequences during predictive saccade tasks rather than specific deficits in response timing (30). The integrity of visually guided saccades in this sample of individuals with autism also argues against a cerebellar explanation for our results. Alterations in response timing on our predictive saccade task are within the response interval range regulated by striatal chronometric systems. Thus, our findings suggest that a speeded chronometric system in the left striatum is responsible for the observed pattern of results.
The neurophysiology of the striatal clock is known to be plastic, with regulation mediated by neurochemical systems. In rodent studies, acceleration and deceleration of interval timing has been demonstrated with striatal administration of dopamine agonists and antagonists respectively (31) and with systemic administration of haloperidol and clozapine (32). Thus, our findings are both consistent with striatal abnormalities observed in functional and structural neuroimaging studies of autism (7,10,11) and might be related to neurochemical as well as neurodevelopmental factors.
An alternative explanation for our findings is that they might represent an inability to withhold planned motor responses until they are appropriate to execute. Deficits in prefrontally mediated inhibitory control in autism have been suggested by neuropsychological (1) and oculomotor studies using the antisaccade task (33). However, deficits on antisaccade tasks reflect a reduced ability to suppress responses to external stimuli rather than internally generated responses, and lateralization of these inhibitory deficits has not been observed. Furthermore, behavioral responses in some cognitive paradigms, including some oculomotor tasks, are slowed rather than accelerated in autism (33,34). Together, these findings suggest that reduced prefrontal inhibition of planned behavior is likely not the cause of speeded predictive saccades in autism.
It is, of course, noteworthy that speeding selectively affected rightward anticipatory saccades, indicating a lateralized neurobiological alteration in individuals with autism. Although autism clearly affects functions localized in both hemispheres, our observation of a left-lateralized alteration leading to speeded rightward saccades is consistent with findings of greater left hemisphere abnormalities in some studies of autism (35). Also, language deficits are a core feature of autism, whereas spatial and musical abilities are often less impaired in higher-functioning patients (36,37). Neuroimaging studies have found abnormal growth trajectories (38) and increased disorder and density of white matter bundles in left frontal language regions and superior temporal gyrus in autism (39,40). Electroencephalography studies of autism have reported left fronto-temporal abnormalities (41) and altered connectivity of left frontal and temporal cortex (42). Manual (43) and pursuit eye-movement performance (44) and some neuroimaging findings (45) provide evidence for left-lateralized disturbances of sensorimotor systems in autism. Thus, our findings add to a growing body of literature suggesting that some left hemisphere brain systems and the cognitive abilities they support are more compromised in at least some individuals with autism.
Some (14) but not all (46) studies of procedural learning provide evidence for deficits in autism. Laterality effects were not investigated in these studies, because all responses were made with one hand. Variations in task complexity might account for these differences. Mostofsky et al. (14) used a much more difficult serial reaction time task and reported reduced procedural learning in autism. The simplicity of our oculomotor paradigm might place less demand on prefrontally mediated skills, such as maintaining a longer stimulus sequence in working memory during the learning process. Also, Mostofsky et al. studied adolescents, whereas the present study used a wider age range, mostly adults. Future studies are needed to address the importance of task complexity and developmental trends in relation to response timing and motor learning deficits in autism, as well as whether lateralized speeding in autism is seen in other tasks requiring precisely timed responses. Nonetheless, our findings are notable for demonstrating that the rate of procedural learning, at least on simple tasks, might be a relatively intact cognitive domain in autism in the context of widespread deficits in cognitive function (1).
Our results identify functional abnormalities in an important cognitive process, coding temporal information for anticipatory behavior. Our findings implicate left striatal chronometric systems and might have clinical and developmental implications for impaired higher-order functions such as praxis and imitation learning.
Acknowledgments
This study was supported by HD35469, HD055751, the National Alliance for Autism Research, T32 MH085391, and the University of Illinois Graduate Student Fellowship. We also gratefully acknowledge the participation of the individual subjects and their families.
Footnotes
The authors report no biomedical financial interests or potential conflicts relevant to this manuscript.
References
- 1.Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: Profile of a complex information processing disorder. J Int Neuropsychol Soc. 1997;3:303–316. [PubMed] [Google Scholar]
- 2.Joseph RM, Tanaka J. Holistic and part-based face recognition in children with autism. J Child Psychol Psychiatry. 2003;44:529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- 3.Hubert V, Beaunieux H, Chetelat G, Platel H, Landeau B, Danion JM, et al. The dynamic network subserving the three phases of cognitive procedural learning. Hum Brain Mapp. 2007;28:1415–1429. doi: 10.1002/hbm.20354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivry RB. The representation of temporal information in perception and motor control. Curr Opin Neurobiol. 1996;6:851–857. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- 5.Meck WH, Benson AM. Dissecting the brain’s internal clock: how frontal–striatal circuitry keeps time and shifts attention. Brain Cogn. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- 6.Courchesne E, Saitoh O, Townsend JP, Yeung-Courchesne R, Press GA, Lincoln AJ, et al. Cerebellar hypoplasia and hyperplasia in infantile autism. Lancet. 1994;343:63–64. doi: 10.1016/s0140-6736(94)90923-7. [DOI] [PubMed] [Google Scholar]
- 7.Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann WE, Cooper KL, Mostofsky SH, Capone GT, Kates WR, Newschaffer CJ, et al. Specificity of cerebellar vermian abnormalities in autism: A quantitative magnetic resonance imaging study. J Child Neurol. 2003;18:463–470. doi: 10.1177/08830738030180070501. [DOI] [PubMed] [Google Scholar]
- 9.Muller RA, Pierce K, Ambrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: A functional magnetic resonance study. Biol Psychiatry. 2001;49:665–676. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- 10.Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 11.Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Res. 2007;156:117–127. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 13.Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- 14.Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: Implications for cerebellar contribution. J Int Neuropsychol Soc. 2000;6:752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- 15.Sears LL, Finn PR, Steinmetz JE. Abnormal classical eye-blink conditioning in autism. J Autism Dev Disord. 1994;24:737–751. doi: 10.1007/BF02172283. [DOI] [PubMed] [Google Scholar]
- 16.Robertson EM. The serial reaction time task: Implicit motor skill learning? J Neurosci. 2007;27:10073–10075. doi: 10.1523/JNEUROSCI.2747-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muslimovic D, Post B, Speelman JD, Schmand B. Motor procedural learning in Parkinson’s disease. Brain. 2007;130:2887–2897. doi: 10.1093/brain/awm211. [DOI] [PubMed] [Google Scholar]
- 18.Harris MS, Wiseman CL, Reilly JL, Keshavan MS, Sweeney JA. Effects of risperidone on procedural learning in antipsychotic-naive first-episode schizophrenia. Neuropsychopharmacology. 2009;4:468–476. doi: 10.1038/npp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: A selective overview. Neuropsychologia. 2001;39:742–756. doi: 10.1016/s0028-3932(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 20.Simo LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: Dissociation between sensory-driven and memory-driven systems. Cereb Cortex. 2005;15:1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- 21.Lasker AG, Zee DS. Ocular motor abnormalities in Huntington’s disease. Vision Res. 1997;37:3639–3645. doi: 10.1016/S0042-6989(96)00169-1. [DOI] [PubMed] [Google Scholar]
- 22.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 23.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 24.Smit AC, Van Gisbergen JA. A short-latency transition in saccade dynamics during square-wave tracking and its significance for the differentiation of visually-guided and predictive saccades. Exp Brain Res. 1989;76:64–74. doi: 10.1007/BF00253624. [DOI] [PubMed] [Google Scholar]
- 25.Schoner G. Timing, clocks, and dynamical systems. Brain Cogn. 2002;48:31–51. doi: 10.1006/brcg.2001.1302. [DOI] [PubMed] [Google Scholar]
- 26.Hinton SC, Meck WH. The ‘internal clocks’ of circadian and interval timing [erratum] Endeavour. 1997;21:82–87. doi: 10.1016/s0160-9327(97)01043-0. [DOI] [PubMed] [Google Scholar]
- 27.Ferrandez AM, Hugueville L, Lehéricy S, Poline JB, Marsault C, Pouthas V. Basal ganglia and supplementary motor area subtend duration perception: An fMRI study. NeuroImage. 2003;19:1532–1544. doi: 10.1016/s1053-8119(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 28.Desbonnet L, Temel Y, Visser-Vandewalle V, Blokland A, Hornikx V, Steinbusch HWM. Premature responding following bilateral stimulation of the rat subthalamic nucleus is amplitude and frequency dependent. Brain Res. 2004;1008:198–204. doi: 10.1016/j.brainres.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 29.Hinton SC, Meck WH. Frontal-striatal circuitry activated by human peak-interval timing in the supra-seconds range. Brain Res Cogn Brain Res. 2004;21:171–182. doi: 10.1016/j.cogbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Molinari M, Leggio M, Solida A, Ciorra R, Misciagna S, Silveri M, et al. Cerebellum and procedural learning: Evidence from focal cerebellar lesions. Brain. 1997;120:1753–1762. doi: 10.1093/brain/120.10.1753. [DOI] [PubMed] [Google Scholar]
- 31.Meck WH. Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald CJ, Meck WH. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacology (Berl) 2005;182:232–244. doi: 10.1007/s00213-005-0074-8. [DOI] [PubMed] [Google Scholar]
- 33.Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biol Psychiatry. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DGM. Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Rinehart NJ, Bradshaw JL, Brereton AV, Tonge BJ. Lateralization in individuals with high-functioning autism and Asperger’s disorder: A frontostriatal model. J Autism Dev Disord. 2002;32:321–331. doi: 10.1023/a:1016387020095. [DOI] [PubMed] [Google Scholar]
- 36.Heaton P. Pitch memory, labeling and disembedding in autism. J Child Psychol Psychiatry. 2003;44:543–551. doi: 10.1111/1469-7610.00143. [DOI] [PubMed] [Google Scholar]
- 37.Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? J Child Psychol Psychiatry. 1997;38:527–534. doi: 10.1111/j.1469-7610.1997.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 38.Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, et al. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- 39.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, Ekstein PM, Hendler T, Tarrasch R, et al. Accelerated maturation of white matter in young children with autism: A high b value DWI study. NeuroImage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 40.Spencer MD, Moorhead TWJ, Lymer GKS, Job DE, Muir WJ, Hoare P, et al. Structural correlates of intellectual impairment and autistic features in adolescents. NeuroImage. 2006;33:1136–1144. doi: 10.1016/j.neuroimage.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Gomot M, Giard M, Adrien J, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: Electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39:577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- 42.Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol Psychiatry. 2007;62:270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawson G, Warrenburg S, Fuller P. Hemisphere functioning and motor imitation in autistic persons. Brain Cogn. 1983;2:346–354. doi: 10.1016/0278-2626(83)90018-0. [DOI] [PubMed] [Google Scholar]
- 44.Takarae Y, Minshew NJ, Luna B, Krisky CM, Sweeney JA. Pursuit eye movement deficits in autism. Brain. 2004;127:2584–2594. doi: 10.1093/brain/awh307. [DOI] [PubMed] [Google Scholar]
- 45.Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain. 2007;130:2117–2122. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- 46.Barnes KA, Howard JH, Jr, Howard DV, Gilotty L, Kenworthy L, Gaillard WD, et al. Intact implicit learning of spatial context and temporal sequences in childhood autism spectrum disorder. Neuropsychology. 2008;22:563–570. doi: 10.1037/0894-4105.22.5.563. [DOI] [PubMed] [Google Scholar]


