Abstract
Background
Impairments in executive cognitive control, including a reduced ability to inhibit prepotent responses, have been reported in autism spectrum disorders (ASD). These deficits may underlie patterns of repetitive behaviors associated with the disorder.
Method
Eighteen individuals with ASD and 15 age- and IQ-matched healthy individuals performed an antisaccade task and a visually guided saccade control task, each with gap and overlap conditions. Measures of repetitive behaviors were obtained using the Autism Diagnostic Inventory – Revised (ADI-R) and examined in relation to neurocognitive task performance.
Results
Individuals with an ASD showed increased rates of prosaccade errors (failures to inhibit prepotent responses) on the antisaccade task regardless of task condition (gap/overlap). Prosaccade error rates were associated with the level of higher-order (e.g. compulsions, preoccupations) but not sensorimotor repetitive behaviors in ASD.
Conclusions
Neurocognitive disturbances in voluntary behavioral control suggest that alterations in frontostriatal systems contribute to higher-order repetitive behaviors in ASD.
Keywords: Antisaccade, neurocognition, oculomotor, prefrontal cortex, striatum
Introduction
The restricted and repetitive behaviors characteristic of autism spectrum disorders (ASD) can be the most disabling feature of the disorder (Bishop et al. 2007), although they have received far less research attention than social and communication impairments. Neurocognitive impairments affecting the voluntary control of behavior may contribute to repetitive behaviors in ASD. Successful voluntary behavioral inhibition is supported by frontostriatal systems (Sweeney et al. 1996; Kelly et al. 2004; Rubia et al. 2007) and disruption of this circuitry can impede the ability to suppress repetitive responses (Suvorov et al. 1997; Christ et al. 2003). Thus, reduced inhibitory control, mediated by alterations in frontostriatal systems, may contribute to rigid stereotypic preferences in ASD.
Eye movement studies offer a translational approach for examining disturbances in frontostriatal systems that support the ability to voluntarily suppress context-inappropriate responses. Studies applying antisaccade (ANTI) paradigms, in which individuals are instructed to generate eye movements away from rather than toward novel targets, have demonstrated deficits in ASD participants' ability to inhibit prepotent responses (Minshew et al. 1999; Goldberg et al. 2002; Manoach et al. 2004; Luna et al. 2007). These results are consistent with functional magnetic resonance imaging (fMRI) findings of atypical frontostriatal activation in ASD during visually guided saccade (Takarae et al. 2007) and ANTI paradigms (Liu et al. 2007; Thakkar et al. 2008), and suggest that oculomotor tasks may be useful paradigms for investigating response inhibition disturbances in ASD.
Lopez et al. (2005) and South et al. (2007) reported relationships between performance on neuropsychological tests of executive function and repetitive behaviors in autism, suggesting an involvement of impaired response inhibition and cognitive flexibility in the repetitive behaviors associated with ASD. Although executive dysfunction has been shown to be associated with social-communication impairments as well (McEvoy et al. 1993; Russell et al. 1999), no studies to date have indicated that atypical cognitive/behavioral inhibition is associated with the core social and communication abnormalities in ASD. Translational research examining potential causes of restricted and repetitive behaviors in ASD, including impaired inhibitory control supported by dorsolateral prefrontal cortical (DLPFC) and frontal eye field connections with the anterior cingulate cortex (Taylor et al. 2007) and striatum (Everling et al. 1999, 2000), can help to clarify specific cognitive mechanisms and the pathophysiology of this relatively underexplored dimension of ASD. Consistent with this hypothesis, Thakkar et al. (2008) recently reported that participants with ASD exhibit abnormally increased activation of rostral anterior cingulate cortex relative to healthy controls during correct antisaccade responses. This hyperactivation was associated with the level to which affected individuals exhibited repetitive behaviors, suggesting that anterior cingulate dysfunction during response monitoring may contribute to restricted and stereotyped behaviors in ASD.
In the present study, individuals with ASD (autistic disorder or Asperger's syndrome) and age- and IQ-matched healthy control participants performed an ANTI task. A visually guided saccade control task was also administered to assess automatic visual attention and sensorimotor components of voluntary saccades. We hypothesized that individuals with higher clinical ratings of repetitive behavior would have higher error rates on the ANTI task (as indexed by saccades toward rather than away from peripheral targets).
Method
Subjects
Participants included 18 individuals with autism (n=13) or Asperger's syndrome (n=5) (four females) and 15 healthy control individuals (four females) who were matched on age and IQ (Table 1). Because of the known association between age and ANTI performance (Luna et al. 2007), we ensured that groups did not differ in the proportion of children aged 8–12 years [ASD: 39% (7); controls: 40% (6)], adolescents aged 13–17 years [ASD: 17% (3); controls: 20% (3)], or adults aged 18–55 years [ASD: 44% (8); controls: 40% (6)]. Diagnoses were established according to DSM-IV criteria using two structured research diagnostic instruments, the Autism Diagnostic Inventory – Revised (ADI-R; Lord et al. 1994) and the Autism Diagnostic Observation Schedule – Generic (ADOS-G; Lord et al. 2000). Individuals were excluded if they had a disorder with known etiology associated with ASD, including Fragile X syndrome and tuberous sclerosis.
Table 1. Demographic, intellectual and clinical characteristics of autism spectrum disorder (ASD) and healthy control subjects.
| ASD (n=18; 4 females) |
Controls (n=15; 4 females) |
||
|---|---|---|---|
| Age (years) | 17.7 (10.8), 8–54 | 19.9 (11.5), 8–55 | n.s. |
| Full-scale IQ | 109.2 (15.8), 81–140 | 109.5 (14.4), 80–140 | n.s. |
| Verbal IQ | 109.7 (17.0), 73–140 | 109.7 (11.5), 88–132 | n.s. |
| Performance IQ | 106.89 (13.9), 79–131 | 106.7 (15.7), 76–139 | n.s. |
| Repetitive behavior (ADI-R algorithm) | 7.23 (2.58), 2–11 | – | – |
ADI-R, Autism Diagnostic Inventory – Revised; n.s., not significant.
Values are given as mean (standard deviation), range.
Potential control participants were excluded for a personal history of psychiatric or neurological disorders, a family history of an ASD, or a first-degree relative with a history of severe psychopathology or developmental disorders, including schizophrenia, bipolar disorder, obsessive–compulsive disorder (OCD), attention deficit hyperactivity disorder or substance abuse. Both healthy control participants and individuals with ASD were excluded for a history of head injury, birth injury or seizure disorder. Individuals in both groups had full-scale IQs >80, and disparities between verbal and non-verbal IQs <20 points. Individuals were excluded if they reported taking medications known to affect eye movements or performance on cognitive tasks, including antipsychotics, methylphenidate, amphetamines or anticonvulsants. Far visual acuity was normal or corrected to at least 20/40. Informed consent was obtained from all adult participants; children and adolescents provided assent, and their parents provided consent. All study procedures were approved by the Institutional Review Board of the University of Illinois at Chicago.
Procedures
Participants were tested in a darkened black room and positioned in a chin and forehead rest with head strap and occipital restraints to minimize head movement. An examiner in an adjacent room provided instructions using an intercom and monitored eye movement activity during testing to ensure that participants were alert and performing tasks according to instructions. Visual stimuli subtending 1° of visual angle were presented in the horizontal plane at eye level on a 72′ × 96′ seamless rear projection screen (Stewart Techplex 150) using a high-resolution projector (Christie Digital Systems Marquee 8500 Ultra projector with 2500 × 2000 resolution and 120 Hz refresh rate). Participants were seated 55 inches from the screen.
Electrodes were placed at the lateral and nasal canthi of each eye to obtain direct current electrooculography (EOG) recordings (Grass Neurodata 12 Acquisition System, Astro-Med, Inc., West Warwick, RI, USA). EOG was selected over infrared (IR) recordings because of limitations in IR methods for measuring eye movements exceeding 12–15° from center. Blinks were monitored using electrodes placed above and below the left eye linked to an AC-coupled bioamplifier. Data from the right eye were scored unless prevented by signal clipping or high noise artifact; in these cases, recordings from the left eye were examined.
Visually guided saccade task
Subjects fixated a central target that was presented for a variable period (1500–2500 ms) prior to being extinguished and replaced by a peripheral target appearing for 2 s at 10°, 20° or 30° to the right or left of center. Multiple target locations were included to ensure that locations could not be anticipated. Target location was counterbalanced across hemifield and visual angle. Subjects were instructed to look at peripheral targets as soon as they appeared. Subjects understood the verbal instructions for the visually guided saccade task, so no practice trials were administered. Two conditions, gap and overlap, were presented to examine whether individuals with ASD are selectively impaired in conditions in which their attention is released prior to the appearance of a novel stimulus (gap trials) or during conditions in which they must disengage attention from a central stimulus (overlap trials). In gap trials, the central fixation target was extinguished 200 ms before the peripheral target appeared. In overlap trials, the central fixation remained illuminated for 200 ms after the appearance of the peripheral target. A temporal gap between the offset of the initial cue and the presentation of a novel peripheral stimulus allows the visual fixation system to be released, reducing the latency of shifts of attention and gaze. By contrast, overlap effects occur when the central target persists after the appearance of the peripheral cue, prolonging activity in visual fixation systems, and to the extent attention is engaged at central fixation there is a parallel increase in response latencies. Thirty-six trials of each condition (72 total trials) were interleaved and presented in a fixed pseudo-random order. Saccade latencies were measured.
ANTI trials
Stimulus parameters, including an interleaving of gap and overlap trials, were identical to those used in the visually guided saccade task. However, subjects were instructed to inhibit saccades toward the peripheral targets, and instead to look immediately to the mirror location of the targets in the opposite hemifield. To ensure that poor performance did not reflect inattention or confusion about task demands, subjects were reminded of task instructions if two consecutive prosaccade errors were made. Each subject also participated in 10 practice trials that were repeated if subjects did not perform two consecutive successful trials. Testing was discontinued if subjects were not able to successfully complete the practice session. Three participants with ASD were not able to successfully complete practice trials and are not included in the present study. The percentage of trials with prosaccade errors (trials in which subjects look toward rather than away from targets) and the latency of correct antisaccade responses were measured. ANTI trials were always presented following visually guided saccade trials.
ADI-R
The ADI-R is a semi-structured interview administered to parents or caregivers. The interview includes items related to early development, social behavior, communication skill, and repetitive behaviors. Algorithms for social, communication and repetitive behavior domains have been validated (Lord et al. 2000) and were examined in the present study. Based on prior work, repetitive behaviors were subdivided into ‘higher-order’ and ‘sensorimotor’ categories (see Turner, 1999 for a review). Higher-order repetitive behavior included algorithm items C1 (encompassing preoccupation or circumscribed pattern of interest) and C2 (apparently compulsive adherence to non-functional routines or rituals). Sensorimotor repetitive behaviors included algorithm items C3 (stereotyped and repetitive motor mannerisms) and C4 (preoccupations with parts of objects or non-functional elements of materials).
Analysis of oculomotor recordings
Recordings were digitized at 500 Hz (DI-210 16-bit A/D, DATAQ Instruments) and analyzed off-line using custom software developed in our laboratory. An algorithm identified saccade initiation when eye velocity rose above 30°/s, and saccade termination when eye velocity returned below that level. Trials were rejected if a blink occurred in the interval between 100 ms prior to presentation of peripheral targets and the end of primary saccades. Data were scored without knowledge of diagnostic or demographic participant characteristics.
Statistical analysis
Repeated-measures ANOVAs were used to compare ASD and control groups. The interactions of diagnostic group with target location (10°, 20° or 30° from center), visual hemifield (right versus left) and condition (gap versus overlap) were examined. There were no significant hemifield effects, or group by hemifield interactions, so data were pooled across hemifield for hypothesis testing. For analyses in which significant group interactions with target location or task condition were not significant, performance was pooled across locations and/or conditions. Because ANTI performance is known to improve with age (Luna et al. 2004), age was included as a covariate in all analyses.
Results
Visually guided saccades
ASD subjects and healthy controls did not differ in the latency of visually guided saccades [F(1, 31)=1.22, p=0.29; see Table 2]. Latencies were shorter in the gap than the overlap condition [F(1, 31)=244.60, p<0.001] but there was no group difference in gap and overlap trials [F(1, 31)=0.37, p=0.55].
Table 2. Performance of autism spectrum disorder (ASD) and healthy subjects on visually guided saccade and antisaccade tasks.
| ASD (n = 18) | Controls (n = 15) | |||
|---|---|---|---|---|
| Gap | Overlap | Gap | Overlap | |
| Visually guided saccades | ||||
| Latency in ms (s.d.) | 198.8 (50.9) | 293.0 (68.0) | 175.1 (31.6) | 278.6 (40.7) |
| Antisaccades | ||||
| Latency in ms (s.d.) | 316.1 (51.7) | 419.8 (67.9) | 311.3 (37.4) | 416.2 (36.5) |
| Latency – trials following correct response (s.d.)a | 315.8 (57.6) | 423.9 (83.3) | 319.9 (49.0) | 416.5 (54.2) |
| Latency – trials following incorrect response (s.d.)a | 320.2 (77.8) | 416.2 (69.5) | 293.6 (50.1) | 433.4 (34.5) |
| Percentage of trials with pro-saccade errors (s.d.) | 42 (25) | 31 (23) | 27 (21) | 20 (16) |
s.d., Standard deviation.
The condition of the preceding trial is not indicated.
Antisaccades
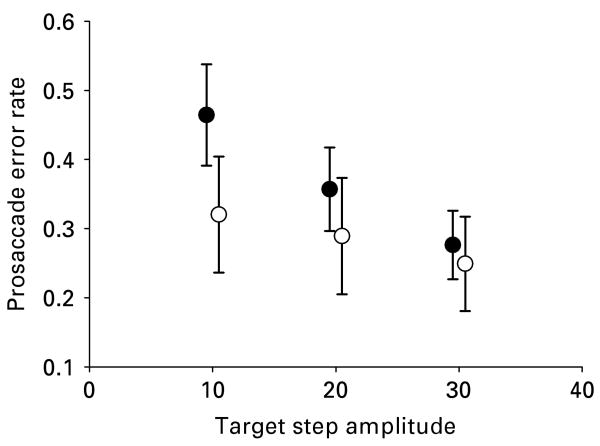
ASD subjects made more prosaccade errors than healthy individuals on the ANTI task [F(1, 30)=4.12, p=0.05]. For both groups, a greater number of prosaccade errors occurred on gap than overlap trials [F(1, 30)=11.42, p<0.01] and on trials with smaller target displacement [F(1, 30)=9.66, p=0.001]. No differential effect of gap versus overlap trials was observed between groups [F(1, 30)=0.49, p=0.49], but an interaction between location and group was observed as ASD subjects had disproportionately increased error rates on trials where targets were presented closer to central fixation [F(2, 29)=7.65, p=0.01; Fig. 1]. Differences between groups in error rates were significant [t(30)=2.54, p=0.03] at 10° of visual angle and approached significance at 20° of visual angle [t(30)=1.77, p=0.08]. Increased age was associated with fewer prosaccade errors (r=−0.45, p<0.001), but no age by group interactions were observed [F(2, 29)=0.73, p=0.49].
Fig. 1.
Mean rate of prosaccade errors for autism spectrum disorder (ASD, ●) and healthy participants (○) across various target step amplitudes from center fixation in degrees of visual angle on an antisaccade task. ASD participants made more prosaccade errors than healthy participants, and the magnitude of this effect was greater when targets were presented closer to center fixation. Error bars show standard error.
There were no group differences in response latencies for correctly performed ANTI trials [F(1, 31)=0.01, p=0.94]. Shorter latencies for gap relative to overlap trials were observed across groups [F(1, 31)=13.67, p=0.001] but the interaction of group and condition for latency data was not significant [F(1, 31)=0.20, p=0.66]. To examine whether participants adjusted their response timing following antisaccade errors, response latencies for correct responses following antisaccade errors were compared with response latencies for correct responses following a correct response. Overall, participants did not adjust their response rates following antisaccade errors [F(1, 28)=0.28, p=0.60; see Table 2]. The group by trial type (post-error versus post-correct) latency interaction was not significant [F(1, 28)=0.02, p=0.89].
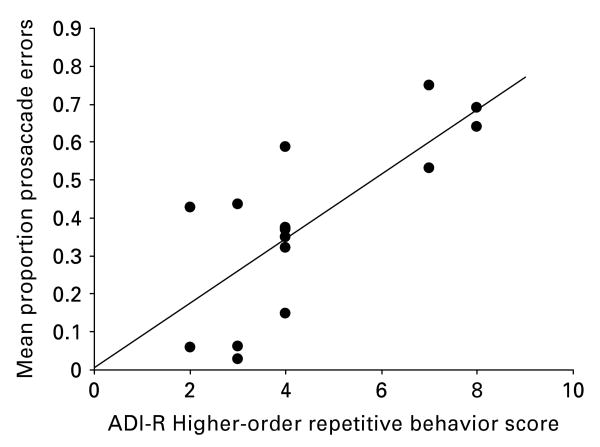
Relationship between ANTI performance and repetitive behaviors (Fig. 2)
Fig. 2.
Relationship between prosaccade error rates and scores on algorithm items C1 and C2 on the restricted, repetitive behavior domain of the Autism Diagnostic Inventory – Revised (ADI-R) for autism spectrum disorder (ASD) participants. This relationship was significant both before and after controlling for age and IQ (r=0.65, p=0.01).
Prosaccade errors were related to higher-order repetitive behaviors both before (r=0.65, p=0.01) and after controlling for age (partial r=0.73, p=0.004). Prosaccade errors were not associated with sensorimotor repetitive behaviors (r=0.11, p=0.62; partial r=0.08, p=0.79). The relationship between prosaccade errors on the ANTI task and social and communication scores from the ADI-R were not significant (r's <0.2). The relationship between ADI-R ratings of repetitive behaviors and age was not significant (r=0.04, p= 0.86).
Discussion
The current study provides evidence that impairments in inhibitory control are associated with an increased severity of higher-order repetitive behaviors in ASD patients but not with sensorimotor repetitive behaviors or communication and social disturbances. These findings highlight a distinct pattern of neurocognitive dysfunction associated with behavioral inflexibility involving preoccupations or compulsions in ASD. Specifically, these data suggest that higher-order repetitive behaviors in ASD are not exclusively a result of intense emotional attachment to an object or activity of interest, but also involve fundamental problems in behavioral flexibility. In the context of previous findings from non-human primate neurophysiology (Schlag-Rey et al. 1997; Johnston & Everling, 2006) and human fMRI studies (Sweeney et al. 2002; Manoach et al. 2007; Polli et al. 2007) with the ANTI task, the present findings suggest that deficits in inhibitory control mediated by alterations in frontostriatal systems may have an important role in the pathophysiology of higher-order restricted and repetitive behaviors in ASD.
Inhibitory control in ASD
Participants with ASD made a greater number of prosaccade errors on an ANTI task than healthy control participants. The rate of response inhibition failures varied as a function of target location for both participants with ASD and healthy controls. However, the effect of target location was disproportionate for the ASD participants, who made increased errors relative to controls at the two locations closest to center, but not at the location furthest from center. These findings are similar to those reported on children with OCD (Rosenberg et al. 1997) and suggest that inhibitory control networks are more susceptible to perturbations when visual information that should be ignored appears closer to the preceding focus of attention and fixation.
The finding of increased prosaccade errors on an ANTI task in ASD is consistent with prior work from our laboratory with independent samples (Minshew et al. 1999; Luna et al. 2007) and with other published work (Goldberg et al. 2002; Manoach et al. 2004; Thakkar et al. 2008). The mechanistic understanding of this pattern of deficits involves an alteration in top down signals from DLPFC to frontal eye fields, striatum and superior colliculus that enable steady fixation on objects of interest as relevant to ongoing behavioral plans, and the ability to override effects of novel sensory events that tend to automatically elicit shifts of gaze toward them (Everling et al. 1999, 2000). Impaired inhibitory control on the ANTI task implicates this specific frontostriatal system in ASD, and parallels previous fMRI findings of DLPFC and anterior cingulate alterations during tasks requiring a voluntary suppression of prepotent behavioral responses in individuals with ASD (Schmitz et al. 2006; Liu et al. 2007). Consistent with this hypothesis, Thakkar et al. (2008) recently reported associations in individuals with ASD between anterior cingulate cortical hyperactivation during antisaccade trials, decreased white matter microstructural integrity in right rostral anterior cingulate cortex, and repetitive behavior.
Frontostriatal dysfunction and repetitive behaviors
ASD participants' response inhibition errors were related to clinically rated severity of higher-order, but not sensorimotor, repetitive behaviors. Higher-order repetitive behaviors involve a disturbance in context-dependent behavioral flexibility whereas sensorimotor stereotypies involve repetitive motor actions or sensory behaviors (Cuccaro et al. 2003). Moreover, higher-order behavioral inflexibility is unique to ASD in that it is not typically observed in other developmental disorders, whereas sensorimotor repetitive behaviors are evident in many developmental disorders. Previous findings (Lopez et al. 2005; South et al. 2007) and the present data indicate that inhibitory control deficits are associated with a resistance to change behavior according to dynamic environmental demands. Previous visually guided saccade studies (Minshew et al. 1999; Takarae et al. 2004), and the present study, each failed to find disturbances in the ability to automatically redirect visual attention in ASD. This consistent pattern of impaired performance on ANTI but not visually guided saccade tasks suggests a deficit that is specific to situations that require voluntary inhibitory control and, by inference, that engage rostral frontostriatal systems. These mechanisms associated with higher-order repetitive behaviors are distinct from those involved in sensorimotor stereotypies.
Clinical observations suggest that intense emotional attachment to specific stimuli or activities also contributes to repetitive behaviors in ASD. In this regard, it is important to note that the results of the present study were obtained in a laboratory setting with simple ‘dot’ targets having little emotional salience. It is possible that reduced cognitive flexibility may prove to have a greater behavioral impact during situations with high emotional or motivational salience. However, its manifestation in situations with modest motivational considerations, as in the context of the current laboratory studies, points to a fundamental deficit in inhibitory control supporting behavioral flexibility as a significant contributing factor. Thus, the phenomenon of higher-order repetitive behaviors in ASD seems to result not only from issues related to intense emotional engagement but also to a fundamental neurocognitive alteration in the dynamic control of behavioral flexibility that implicates prefrontal systems.
The observed connection between inhibitory control deficits and repetitive behaviors is consistent with studies of individuals with OCD, a disorder that, like ASD, is characterized by compulsive and rigid behaviors (APA, 2000). Tien et al. (1992) and Rosenberg et al. (1997) observed deficits in response inhibition on an ANTI task among individuals with OCD. Although both groups seem to have inhibitory control deficits, OCD patients also have been noted to have increased antisaccade latencies (Maruff et al. 1999; van der Wee et al. 2006). This suggests that patients with OCD show either a slowing of psychomotor function or an attempt to compensate for inhibitory deficits by slowing reaction times to better inhibit reflexive responses. The degree of similarity in the causes of inflexible behavior in ASD and OCD remains to be established, but observations that inhibitory control deficits are evident in both groups and are reduced by serotonergic drugs in neuropsychiatric disorders (Rosenberg et al. 2000; Moresco et al. 2007) suggest that frontostriatal disruption is one commonality in these behavioral disturbances.
Frontostriatal alterations and repetitive behaviors in ASD and OCD may be related to alterations in brain serotonin systems. Whitaker-Azmitia (2005) and Boylan et al. (2007) have argued that a loss of 5-hydroxytryptamine (5-HT) terminals in cerebral cortex, especially in forebrain regions, may result in social, repetitive and sensorimotor behaviors that parallel those seen in individuals with ASD. Studying rat pups injected with a 5-HT neurotoxin in the major afferent pathway to the forebrain, Boylan et al. (2007) showed that neonatal 5-HT depletion results in increased repetitive behaviors. A role for serotonergic alterations in frontostriatal system disturbances in ASD is also suggested by reports of decreased serotonin synthesis within frontostriatal circuitry in ASD (Chugani et al. 1997), elevated whole-blood serotonin levels (Cook et al. 1988), and reduced repetitive behaviors in ASD following therapy with selective serotonin reuptake inhibitors (DeLong et al. 1998; Moore et al. 2004; Owley et al. 2005). Investigating the effects of serotonin alterations on voluntary inhibitory control deficits in ASD is therefore a strategy that may be important both for developing models of the pathophysiology of repetitive behaviors in ASD and for new treatments targeting these behaviors.
Conclusions
The present study has demonstrated that individuals with ASD show deficits in inhibitory control that are associated with the clinically rated intensity of higher-order repetitive behaviors. Deficits in inhibitory control were not associated with other core features of ASD, indicating that the reported relationship is specific to a subset of repetitive behaviors. These findings implicate disturbances in frontostriatal circuitry as a cause of reduced behavioral flexibility in ASD.
Acknowledgments
This study was supported by the NICHD Collaborative Program of Excellence in Autism HD35469, the NIH Autism Center of Excellence Project award HD055751, and the National Alliance for Autism Research. We also gratefully acknowledge the participation of the individual subjects and their families in these studies.
Footnotes
Declaration of Interest: None.
References
- APA. Diagnostic and Statistical Manual. 4th. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Bishop SL, Richler J, Cain AC, Lord C. Predictors of perceived negative impact in mothers of children with autism spectrum disorder. American Journal of Mental Retardation. 2007;112:450–461. doi: 10.1352/0895-8017(2007)112[450:POPNII]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Boylan CB, Blue ME, Hohmann CF. Modeling early cortical serotonergic deficits in autism. Behavioural Brain Research. 2007;176:94–108. doi: 10.1016/j.bbr.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ SE, White DA, Brunstrom JE, Abrams RA. Inhibitory control following perinatal brain injury. Neuropsychology. 2003;17:171–178. [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Rothermel R, Behen M, Chakraborty P, Mangner T, da Silva EA, Chugani HT. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Annals of Neurology. 1997;42:666–669. doi: 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Leventhal BL, Freedman DX. Free serotonin in plasma: autistic children and their first-degree relatives. Biological Psychiatry. 1988;24:488–491. doi: 10.1016/0006-3223(88)90192-8. [DOI] [PubMed] [Google Scholar]
- Cuccaro ML, Shao Y, Grubber J, Slifer M, Wolpert CM, Donnelly SL, Abramson RK, Ravan SA, Wright HH, DeLong GR, Pericak-Vance MA. Factor analysis of restricted and repetitive behaviors in autism using the Autism Diagnostic Interview-R. Child Psychiatry and Human Development. 2003;34:3–17. doi: 10.1023/a:1025321707947. [DOI] [PubMed] [Google Scholar]
- DeLong GR, Teague LA, McSwain KM. Effects of fluoxetine treatment in young children with idiopathic autism. Developmental Medicine and Child Neurology. 1998;40:551–562. doi: 10.1111/j.1469-8749.1998.tb15414.x. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. Journal of Neuroscience. 1999;19:2740–2754. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and antisaccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40:2039–2049. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Johnston K, Everling S. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade-antisaccade tasks. Journal of Cognitive Neuroscience. 2006;18:749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. European Journal of Neuroscience. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Liu R, Sweeney J, Minshew N, Geier C, Garver K, Luna B. Developmental improvements in brain function supporting response inhibition from adolescence to adulthood in autism. Paper presented at the Annual Meeting of the Society for Neuroscience; San Diego, CA. 2007. [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of autistic disorder. Journal of Autism and Developmental Disorders. 2005;35:445–460. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview – Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biological Psychiatry. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Lindgren KA, Barton JJS. Deficient saccadic inhibition in Asperger's disorder and the social-emotional processing disorder. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:1719–1726. doi: 10.1136/jnnp.2003.025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Cain MS, Polli FE, Edelman JA, Fischl B, Barton JJ. Neural activity is modulated by trial history: a functional magnetic resonance imaging study of the effects of a previous antisaccade. Journal of Neuroscience. 2007;27:1791–1798. doi: 10.1523/JNEUROSCI.3662-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruff P, Purcell R, Tyler P, Pantellis C, Currie J. Abnormalities of internally generated saccades in obsessive-compulsive disorder. Psychological Medicine. 1999;29:1377–1385. doi: 10.1017/s0033291799008843. [DOI] [PubMed] [Google Scholar]
- McEvoy RE, Rogers SJ, Pennington BF. Executive function and social communication deficits in young autistic children. Journal of Child Psychology and Psychiatry. 1993;34:563–578. doi: 10.1111/j.1469-7610.1993.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52:917–922. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ML, Eichner SF, Jones JR. Treating functional impairment of autism with selective serotonin-reuptake inhibitors. Annals of Pharmacotherapy. 2004;38:1515–1519. doi: 10.1345/aph.1D543. [DOI] [PubMed] [Google Scholar]
- Moresco RM, Pietra L, Henin M, Panzacchi A, Locatelli M, Bonaldi L, Carinelli A, Gobbo C, Bellodi L, Perani D, Fazio F. Fluvoxamine treatment and D2 receptors: a PET study on OCD drug-naïve patients. Neuropsychopharmacology. 2007;32:197–205. doi: 10.1038/sj.npp.1301199. [DOI] [PubMed] [Google Scholar]
- Owley T, Walton L, Salt J, Guter SJ, Jr, Winnega M, Leventhal BL, Cook EH., Jr An open-label trial of escitalopram in pervasive developmental disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:343–348. doi: 10.1097/01.chi.0000153229.80215.a0. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Averbach DH, O'Hearn KM, Seymour AB, Birmaher B, Sweeney JA. Oculomotor response inhibition abnormalities in pediatric obsessive-compulsive disorder. Archives of General Psychiatry. 1997;54:831–838. doi: 10.1001/archpsyc.1997.01830210075008. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, McMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J, Saltmarsh R, Hill E. What do executive factors contribute to the failure on false belief tasks by children with autism? Journal of Child Psychology and Psychiatry. 1999;40:859–868. [PubMed] [Google Scholar]
- Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DG. Neural correlates of executive function in autistic spectrum disorders. Biological Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism. 2007;11:437–451. doi: 10.1177/1362361307079606. [DOI] [PubMed] [Google Scholar]
- Suvorov NF, Shuvaev VT, Voilokova NL, Chivileva OG, Shefer VI. Corticostriatal mechanisms of behavior. Neuroscience and Behavioral Physiology. 1997;27:653–662. doi: 10.1007/BF02461923. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Levy D, Harris MS. Commentary: eye movement research with clinical populations. Progress in Brain Research. 2002;140:507–522. doi: 10.1016/S0079-6123(02)40072-6. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintum MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Oculomotor abnormalities parallel cerebellar histopathology in autism. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:1359–1361. doi: 10.1136/jnnp.2003.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Research. 2007;156:117–127. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WW. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJS, Manoach DS. Response monitoring, repetitive behavior and anterior cingulate abnormalities in ASD. Brain. 2008;131:2464–2478. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien AY, Pearlson GD, Machlin SR, Bylsma FW, Hoehn-Saric R. Oculomotor performance in obsessive-compulsive disorder. American Journal of Psychiatry. 1992;149:641–646. doi: 10.1176/ajp.149.5.641. [DOI] [PubMed] [Google Scholar]
- Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. Journal of Child Psychology and Psychiatry. 1999;40:839–849. [PubMed] [Google Scholar]
- van der Wee NJ, Hardeman HH, Ramsey NF, Raemakers M, Van Megen HJ, Denys DA, Westenberg HG, Kahn RS. Saccadic abnormalities in psychotropic-naive obsessive-compulsive disorder without co-morbidity. Psychological Medicine. 2006;36:1321–1326. doi: 10.1017/S0033291706007926. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Behavioral and cellular consequences of increasing serotonergic activity during brain development: a role in autism? International Journal of Developmental Neuroscience. 2005;23:75–83. doi: 10.1016/j.ijdevneu.2004.07.022. [DOI] [PubMed] [Google Scholar]