UNTIL recently carcinoma of the lung has been considered a relatively infrequent condition. However, recent studies demonstrate that pulmonary malignancy is not only a common occurrence but is one of the most frequent carcinomas of the body. This increase in incidence of bronchogenic malignancy is undoubtedly both apparent and real, as evinced by autopsy series from the German Clinics. Junghanns found an incidence of pulmonary carcinoma of 1.67 per cent of all autopsies performed from 1893 to 1927. The incidence of primary pulmonary carcinoma in all malignancies found at autopsy was 14.27 per cent during the period from 1893 to 1897. Seyfarth found increases in the incidence of pulmonary carcinoma from 5.01 per cent during 1900 to 1906 to 8.75 per cent from 1919 to 1923. During the first half of 1924 there was an incidence of 15.5 per cent. Jaff$eA, of Chicago, believes that pulmonary carcinoma represents 11.47 per cent of all carcinomas, and that pulmonary carcinoma was second in frequency only to carcinoma of the stomach and of the intestine. Frissel and Knox, in a series of 3,659 autopsies, found 588 cases of carcinoma of which 39 were carcinoma of the lung, thus representing 6.6 per cent of the total carcinomas and 1.06 per cent of the total autopsies. D'Aunoy, Pearson, and Halpert reported that of 6,000 necropsies on persons over 1 year of age, performed in the Charity Hospital in New Orleans, primary carcinoma of the lung occurred in 70, or 1.1 per cent, and was almost as frequent as primary carcinoma of the biliary tract or of the pancreas. In 1,244 autopsies performed at the Touro Infirmary in New Orleans there were 259 cases with carcinoma. Twenty-three of the carcinomas originated in the bronchus. The incidence of bronchial carcinoma in the total autopsies was 1.8 per cent, whereas that in all carcinomas was 8.8 per cent (36). It is evident, therefore, that the frequency of pulmonary carcinoma is high. The fact that in Jaff$eA's cases it was second only to carcinoma of the stomach emphasizes the necessity for its clinical consideration.
Although it is controversial whether the increase in pulmonary carcinoma in recent years is apparent or real, the German autopsy statistics would indicate that the increase is actual and not only apparent. There are several explanations for the actual increase in the incidence of pulmonary malignancies, most of which have not been satisfactory. A number of theories have been suggested. Winternitz, Wason, and McNamara, because of the presence of metaplasia in the bronchial mucosa of persons dying from influenza, suggested that this change is a precancerous lesion. The inhalation of irritating gases such as war gas or gas originating from the increased use of motor cars has been proposed as an etiological factor. In our opinion the increase in smoking with the universal custom of inhaling is probably a responsible factor, as the inhaled smoke, constantly repeated over a long period of time, undoubtedly is a source of chronic irritation to the bronchial mucosa. In addition to the actual increase in pulmonary malignancy, there is unquestionably a relative increase in those localities where routine postmortem examinations previously have not been made. This is due probably to the fact that the condition has not been suspected in many cases and adequate diagnostic procedures have not been employed. The recent development of thoracic surgery has stimulated interest in intrathoracic lesions. This, with the development of specialized methods of diagnosis, has facilitated the recognition of pulmonary malignancies.
PATHOLOGY
It is generally agreed that the lining cells of the alveoli rarely, if ever, give rise to malignant neoplastic growths. Consequently all carcinomas of the lung are bronchiogenic. Carcinomas of the lung have been variously classified according to their morphological appearance. However, the most logical classification to us is that proposed by B$eAla Halpert. This classification is based upon the development of the cells lining the bronchi and this adequately explains the histological structure of all primary pulmonary carcinomas. Normally, the cells lining the mucous membrane of the bronchial tree represent varying degrees of differentiation and specialization of the original entodermal cells. According to Halpert, “The epithelial cells covering the mucous membrane of the bronchial tree from stem to the minute branches are entodermal cells with a varying degree of differentiation and specialization. Apparently the undifferentiated entodermal ancestor cell is capable of developing into ciliated cylindrical epithelium, goblet cells, cuboidal cells, arranged into acinar and tubular structures producing a serous or mucous secretion, indifferent cells, lining the ducts of these glands, and into another kind of cuboidal or low cuboidal cells without cilia which line parts of the terminal bronchioles. In addition to the variety of cells just enumerated there are, beneath the ciliated cylindrical and goblet cells, a varying number of other epithelial cells which, like the basal cells in the epidermis, are lined up along the border toward the tunica propria. They are the cells from which the single layer of ciliated cylindrical and goblet cells are replenished. These cells, which may be called ‘reserve cells,’ are the parent cells of the ciliated, cylindrical, and goblet cells. In addition they naturally also possess the qualities of their ancestor cells in that they may differentiate into any kind of epithelium that an entodermal cell is capable of producing” (Fig. I). According to Halpert, carcinomas of the lung originate from these “reserve cells” by atypical proliferation.
Fig. 1.
Lining cells of tracheal mucosa of a fetus 12 centimeters long. Beneath the cylindrical cells covering the surface there are several rows of nuclei of “reserve cells.” Modified after D'Aunoy, Pearson, and Halpert (13, 26).
These malignant growths may, therefore, be classified into 3 types, depending upon the embryological direction of growth: (1) the “reserve-cell” carcinoma, (2) cylindrical-cell carcinoma, and (3) squamous-cell carcinoma. This conception of the embryological development of carcinomas is graphically illustrated by the development of the cells of the bronchial mucosa from a primitive entodermal cell (Fig. 2). According to D'Aunoy, Pearson, and Halpert the “reserve-cell” carcinomas consist of round, elongated, or polygonal cells growing in solid masses and forming no particular structure. Characteristically they have a palisade arrangement of the peripheral cells (Fig. 3). The cylindrical cell carcinomas are composed of cuboidal or columnar cells forming tubular or acinar structures, or are mounted on delicate connective tissue stalks in a papillary arrangement (Fig. 4). The squamous cell carcinomas have a tendency toward keratinization or to pearl formation with central keratinization (Fig. 5). A given tumor is as differentiated as its most differentiated part. If one accepts Halpert's classification, it is evident that only the “reserve-cell” carcinoma might be radiosensitive. As these represent, according to D'Aunoy, Pearson, and Halpert, approximately one-third of all carcinomas of the lung, it is obvious that relatively few pulmonary neoplasms should, on a theoretical basis at least, be radiosensitive. This is corroborated by the poor results obtained from this form of therapy. According to Halpert the gross characteristics of pulmonary carcinoma are in no way dependent upon the microscopic structures, but depend upon rapidity of growth and secondary changes which occur in the tumor, such as hemorrhage and necrosis.
Fig. 2.
The “reserve cell” is the parent cell of the ciliated cylindrical and goblet cells, and also possesses the qualities of its ancestor cell in that it may differentiate into any kind of epithelial cell that a primitive entodermal cell is capable of producing. Hence, the carcinomas of the lung may be: (1) “reserve-cell” carcinoma, (2) cylindrical-cell carcinoma, and (3) squamous-cell carcinoma. Modified after D'Aunoy, Pearson, and Halpert (13, 26).
Fig. 3.
“Reserve-cell” carcinomas grow in solid masses composed of round, elongated (oat cells) or polygonal cells forming no particular structure. Usually there is a palisade arrangement of the peripheral cells. Modified after D'Aunoy, Pearson, and Halpert (13, 26).
Fig. 4.
Cylindrical cell carcinomas are composed of cuboidal or columnar cells forming acinar, tubular, or papillary structures. The parent cells are the “reserve cells” which form haphazard imitations of the normal epithelial cell structure which composes the air passages. Modified after the method of D'Aunoy, Pearson, and Halpert (13, 26).
Fig. 5.
Squamous cell carcinomas grow in nests of cells in a concentric arrangement forming epithelial pearls with central keratinization. The entodermal cell of the air passages has the quality of producing stratified squamous epithelium. The parent cell of this carcinoma is a “reserve cell” of earlier ancestry than the ordinary. Modified after D'Aunoy, Pearson, and Halpert (13, 26).
As regards the location of primary neoplasia of the lung, the right side is involved slightly more often than the left. In Fischer's series of 3,735 cases of pulmonary carcinoma, the right side was involved in 53 per cent and the left in 45 per cent. In 2 per cent the lesions were bilateral. It is of interest that in 46, in which localization was given, of the 79 collected cases in which total pneumonectomy was performed for pulmonary neoplasm, the right side was involved in 19, or 41.3 per cent, whereas the left was involved in 27 patients, or 58.6 per cent. In 784 of Fischer's cases, in which the location according to the bronchus was designated, the findings were as follows: The right main bronchus was involved in 142; the left main bronchus in 115; the right upper lobe bronchus in 148; the left upper lobe bronchus in 130; the right lower lobe bronchus in 129; the left lower lobe bronchus in 105, and the middle lobe in 15. Most pulmonary neoplasms are centrally located, i.e., of hilar origin. According to Boyd, 90 per cent of these neoplasms are in the region of the hilum. In Frissel and Knox's series the incidence of hilar carcinomas was not so high, only 49.7 per cent. Seventeen and eight tenths per cent involved the parenchyma and were of the nodular variety; 6.5 per cent were peripheral; 23.9 per cent were diffuse, and 2.1 per cent were bilateral miliary.
SYMPTOMATOLOGY
There are few or no symptoms in the early course of bronchial carcinoma. The symptoms vary considerably and depend entirely upon the location and the extent of the lesion. Generally, the symptoms are not produced by the neoplasm itself, but are due to secondary changes resulting from its presence. The most constantly encountered symptoms are cough and thoracic discomfort. The latter may vary from a slight consciousness to actual pain within the thorax. In those cases in which ulceration occurs hemoptysis is a prominent manifestation. Whenever occlusion of the bronchus occurs atelectasis, with displacement of the mediastinum toward the affected side, and consequent infection produce marked symptoms. In the peripherally located tumors with extension to the pleura, evidences of pleurisy with effusion may be present. Symptoms which appear late are loss of weight and strength, dyspnea, and osteo-artropathy. Due to the circumferential growth the vascularity of the tumor in its central portion becomes impaired resulting in necrosis and abscess formation. Pulmonary abscess without an antecedent pneumonitis or foreign body aspiration should be considered of malignant origin until proved otherwise. Likewise an unexplained cough and hemoptysis in a patient past 40 years of age should be considered the result of carcinoma until this diagnosis is excluded. The physical findings in pulmonary malignancies are as protean as the symptoms and are dependent upon the location and extent of the lesion and secondary pulmonary changes which may be produced by it.
DIAGNOSIS
The most important factor in the diagnosis of pulmonary carcinoma is the consideration of its possible presence. This lesion invariably should be suspected in every patient 40 years of age or older with unexplained cough, hemoptysis, or thoracic discomfort. While roentgenography, without the use of contrast media, is usually of little or no value in the early diagnosis of non-obstructive bronchial neoplasms, careful stereoroentgenographic studies are necessary in all such lesions. The roentgenographic interpretation of centrally located lesions is generally more difficult because of the confusion with hilar shadows produced by other lesions and normal structures. This is particularly significant because most pulmonary neoplasms occur in the hilar region. In centrally located lesions, after the condition has progressed to such an extent that bronchial obstruction occurs, atelectasis of one or more lobes develops, depending upon the degree of central location. The physical findings and particularly the roentgenographic manifestations of this condition are characteristic, i.e., dullness or shadow and displacement of the mediastinal structures toward the affected side (Fig. 6). In peripherally located pulmonary malignancy the roentgenographic diagnosis is dependent upon shadows produced by the infiltrating tumor (Fig. 7).
Fig. 6.
Anteroposterior roentgenogram of chest of patient with carconoma involving right lower lobe bronchus as characterized by slightly rounded but irregular shadow in lower right lung field adjacent to hilum. The mediastinum is displaced slightly toward the affected side (Case 3).
Fig. 7.
Anteroposterior roentgenogram of chest of patient with carcinoma of right upper lobe bronchus which had extended peripherally to involve practically the entire lobe as confirmed by subsequent examination. The area of increased density; occupying nearly the whole upper lobe of the right lung, is produced by the peripheral extension of the tumor and not by atelectasis which would cause displacement of the mediastinum toward the affected side (Case 2).
Of greatest importance, as regards accurate diagnosis, is bronchoscopic visualization of the tumor and biopsy obtained by this means. While the microscopic demonstration of malignancy of the bronchus is the ultimate ideal, there may be some difficulty in obtaining a satisfactory specimen. We are in complete accord with Jackson and Holinger that the incidence of correct diagnoses, made by bronchoscopic visualization of the lesion will probably be higher when made by one experienced in the bronchoscopic diagnosis of these tumors than by routine microscopic examination of biopsy specimens. This in part is due to the recalcitrance of most pathologists to diagnose carcinoma from the examination of a few cells; and it is also due to the fact that the inexperienced bronchoscopist may not obtain representative neoplastic tissue. In every instance, however, an attempt should be made to obtain tissue for microscopic examination, because, as mentioned, this is the ideal method of diagnostic proof. According to Jackson and Konzelmann, the incidence of correct diagnoses obtained bronchoscopically is approximately 75 per cent. This is about the incidence of hilar tumors and indicates that those cases, which are close enough to the main stem bronchi to be visualized, can be diagnosed by the trained bronchoscopist. Difficulty is likely to be encountered in eparterial bronchus tumors because of their location and because of the acute angle which they form with the right stem bronchus. Visualization usually can be obtained in those cases, however, in which the neoplasm is near the orifice of the eparterial bronchus.
The presence of malignant cells in expectorated material can frequently be demonstrated microscopically. By this method of examination Dudgeon found carcinoma cells in 60 per cent of patients in whom a diagnosis of pulmonary neoplasm subsequently was proved. Similarly, the demonstration, according to Mandlebaum's technique of malignant cells in the pleural fluid of those cases in which there has been extension to the pleura, is of diagnostic importance. The latter is of little use early in the disease, however, because of the relatively late extension to the periphery, except in peripherally located lesions. The importance of this method of diagnosis lies principally in its prognostic value.
Thoracoscopic examination is another method of diagnosis in selected cases of pulmonary carcinoma and is particularly valuable in those cases with peripheral extension with or without pleural effusion. This method of examination is useful in determining the cause of the pleural effusion and the operability of the case. In those cases in which there is extensive seeding in the pleural cavity, an attempt at radical extirpation is obviously not justified. In cases in which the lesion is located peripherally, obviating bronchoscopic visualization of the tumor, aspiration biopsy can be performed with relative safety and with a fair degree of accuracy. Martin and Ellis, in 1930, reported their results in a large series of aspiration biopsies done for a variety of conditions. In 2 pulmonary carcinomas microscopic diagnoses of the lesions were positive. Sharp reports 3 cases in which a positive microscopic diagnosis of pulmonary neoplasm was made on material obtained by aspiration. He believes that the method is particularly valuable in upper lobe malignancies. Binkley reported 92 aspiration biopsies of the thorax performed in the Memorial Hospital. In 56 bronchogenic carcinomas a positive diagnosis was made by aspiration biopsy in 60 per cent, whereas bronchoscopic examination gave a positive diagnosis in only 43 per cent of this group. An accurate diagnosis is possible, following aspiration biopsy, only if the examining pathologist is capable of interpreting the changes occurring in the few cells obtained by this method, and if he is willing to commit himself on the basis of these findings.
TREATMENT
The treatment of carcinoma of the lung, as that of most carcinomas elsewhere, consists ideally of complete surgical extirpation. Surgical removal is particularly indicated in pulmonary malignancies, because of the almost hopeless outlook following other types of therapy. While in a number of reported cases resections of the involved lobes have been performed, it seems to us that any procedure, short of total removal of the involved lung, is irrational. Only by complete excision of the entire lung can the primary focus be adequately removed, and lobectomy does not permit removal of the regional lymph nodes. The performance of simple lobectomy in carcinoma of the lung is just as illogical as partial removal of the breast in mammary carcinoma with no attempted extirpation of the regional lymph nodes. Another reason for total pneumonectomy is that approximately 75 per cent of pulmonary neoplasms originate in the proximal bronchi. As shown by Bonniot, Monod, and Evrard, it is not possible to apply a tourniquet high enough on the pedicle of the lung to permit division of the main bronchus without injuring the pericardium or other mediastinal structures. Moreover, from a technical standpoint, total pneumonectomy is a much more surgical and anatomical procedure than is lobectomy. The latter at best consists more or less of a makeshift operation. It is necessary almost invariably to cut through pulmonary tissue because of the incomplete division of the lung down to the hilum by the fissures which does not permit individual ligation of the bronchus and pulmonary vessels.
On the basis of our experience we are convinced that preliminary pneumothorax should be attempted in all cases of malignancies of the lung. This should be done preferably in stages, increasing the amount of intrapleural pressure gradually until the pressure is definitely upon the positive side. Most surgeons agree that a preliminary pneumothorax is desirable. The procedure was originally advocated by Kuemmel who performed the first pneumonectomy in November, 1910. Pre-operative pneumothorax is of diagnostic importance in determining the presence, extent, and location of adhesions, thus permitting the pre-operative planning of the operative procedure. In those cases in which extensive basal adhesions are present, a posterolateral approach is preferable to an anterior one, whereas, conversely, a patient with adhesions involving the apex can best be operated upon through an anterior approach. Another decided advantage of pre-operative pneumothorax is the gradual compression of the pulmonary capillary bed, giving the right heart a chance to compensate for the increased peripheral resistance in this area, rather than permitting a sudden cutting off of the blood to the involved lung at the time of the ligation of the pulmonary vessels. This is particularly true in elderly patients whose cardiac reserve is diminished, and in whom malignancies of the lung are likely to occur.
Rienhoff (46) advocates the pre-operative introduction of beef broth bouillon into the pleural cavity in order to produce a serofibrinous pleurisy, which he believes decreases the incidence of infection following the operation.
Patients with pulmonary carcinoma usually have an anemia because of the associated infection and loss of blood. In such instances pre-operative unmodified blood transfusions are desirable. It is also imperative that two or more donors be available at the time of operation, because prolonged bleeding may follow division of extensive adhesions, and because of possible accidental massive hemorrhage in which the administration of blood during the operation is frequently life-saving.
Anesthesia. Although pneumonectomy may be performed under local and spinal analgesia, the latter being particularly popular in Canada and England, we prefer cyclopropane inhalation anesthesia. Because of the wide opening in the chest wall, it is necessary that the anesthetic be administered under positive pressure. All of our earlier cases were done under positive pressure, intratracheal tubes being used, but we now are convinced and agree with Rienhoff (46) that the use of the intratracheal tube is deleterious, because of the likelihood of the introduction of infection and the increased secretion resulting from trauma, which in the presence of a single lung following pneumonectomy, is particularly dangerous. One of our fatalities 14 hours after operation undoubtedly was due to trauma of the trachea by the intratracheal tube, resulting in such excessive secretion that the patient virtually drowned in her own secretions despite almost continuous aspiration (Case 5).
In those cases in which the pleural cavity is free, containing no adhesions, the anterior approach advocated by Rienhoff (47, 48) is very satisfactory. Instead of the intercostal incision suggested by Rienhoff, we are of the opinion that resection of the third rib from the lateral border of the sternum to the anterior axillary line is preferable (Figs. 8, 9). This gives a better exposure and permits a more satisfactory closure because it facilitates accurate approximation without tension of the pleura and intercostal muscles. The anterior approach is also applicable to those cases in which the lesion is located in the periphery of the upper lobe, and in which there are adhesions between the upper lobe and the parietal pleura. This approach, however, is not recommended for those patients in whom there are adhesions between the lower lobe and the posterior parietal pleura, because of the great difficulty encountered in mobilizing the lung. In such cases resection of the fifth rib through the incision suggested by Crafoord has been more satisfactory in our hands (Figs. 10, 11, 12).
Fig. 8.
Drawing illustrating site of skin incision over third rib from chondrosternal junction to anterior axillary line in anterior approach for pneumonectomy.
Fig. 9.
Drawing illustrating anterior approach for pneumonectomy. The third rib is resected subperiosteally from chondrosternal junction to anterior axillary line. Incision of the pleura is made in the bed of the third rib. Inset shows exposure and immediate ligation of internal mammary vessels.
Fig. 10.
Drawing showing position of patient in posterior approach for pneumonectomy.
Fig. 11.
Drawing illustrating skin incision in posterior approach for penumonectomy as devised by Crafoord. The incision begins over the fourth rib about 7 to 8 centimeters from the posterior midline, is extended downward beneath the angle of the scapula, and then up toward the midaxillary line to the level of the fifth rib which is followed anteriorly to the costal cartilage.
Fig. 12.
Drawing showing method of elevating angle of scapula so as to expose the fifth rib along its entire length. The fifth rib is subperiosteally resected throughout its entire length and the pleura is opened by incising in the furrow left by the rib.
A distinct advantage of the latter approach, in addition to permitting the division of adhesions between the lower lobe and the lateral and posterior parietal pleuras under direct vision, is that the hilum can be approached from behind, thus allowing initial mobilization of the relatively fixed bronchus. Following division of the bronchus, which normally holds the hilar structures quite rigidly, dissection of the other hilar structures, the pulmonary artery and veins, is greatly facilitated (Fig. 13). In the posterior approach also, rib resection is preferable to intercostal incision. After the pleural cavity is opened and the lung is mobilized by division of adhesions by sharp dissection, the hilum is exposed in the mediastinum by incising the mediastinal pleura anteriorly and superiorly in the anterior approach, and posteriorly and superiorly in the posterior approach (Fig. 14). The flap of mediastinal pleura thus formed is mobilized, thus exposing the hilar structures. The mobilization is greatly facilitated by the use of long, ball-tipped, slightly curved scissors (Fig. 14). The pulmonary artery, pulmonary veins, and bronchus are isolated individually (Fig. 15).
Fig. 13.
Drawing showing relation of hilar structures and steps of operation in posterior approach for pneumonectomy. The hilar structures are exposed by incising mediastinal pleura posteriorly and superiorly. The azygos vein is first exposed, doubly transfixed and ligated, and then divided between these ligatures. This permits easy access to the bronchus which is doubly clamped by means of crushing forceps and divided between the clamps. Following division of bronchus, which normally holds the hilar structures quite rigidly, dissection of the other hilar structures, the pulmonary artery and veins, is greatly facilitated.
Fig. 14.
Drawing showing incision of mediastinal pleura in anterior approach for pneumonectomy. Mobilization of flaps of mediastinal pleura is greatly facilitated by use of long ball-tipped, slightly curved scissors.
Fig. 15.
Drawing showing individual isolation of pulmonary artery, pulmonary vein, and bronchus in anterior approach for pneumonectomy.
Mass ligation of the hilum is to be condemned as an unsurgical procedure and one which will give bad results in the majority of cases because of the incomplete extirpation. It does not permit removal of the mediastinal lymph nodes in which metastases are likely to occur, and in many instances does not permit the complete removal of the tumor. This is particularly exemplified in our first case in which the tumor was located just beyond the bifurcation of the trachea in the left main-stem bronchus. Although individual ligation of the hilar structures was performed, the bronchus was divided insufficiently high to include the tumor. The pathologist, who was present at the operation, noticed the absence of the tumor before the mediastinal wound was closed, permitting further dissection of the bronchus up to the carina and the high removal of the bronchus and the adjacent wall of the trachea (Fig. 16). The tumor was situated in the small segment of bronchus which was approximately 2 centimeters in length. The posterior approach is particularly applicable and desirable in those cases of right-sided malignancies because of the greater difficulty in approaching the hilum on this side due to the presence of the vena azygos extending over the eparterial bronchus. This is especially true in cases in which the lesion originates in the eparterial bronchus and in which extension to the mediastinum in this area is likely to be present. In 1 of our cases the inability to free the bronchus anteriorly necessitated ligation of the vena azygos. Subsequent slippage of the ligature resulted in fatal hemorrhage (Case 2). Through the posterior approach the vena azygos can be more easily and more safely ligated before the bronchus is isolated (Fig. 13). Separate ligation of the pulmonary vessels is imperative, and is perfected by double transfixion sutures (Fig. 17). The use of No. 2 silk, which is sufficiently strong to compress the vessels and not too large to interfere with the tying of the knot, is considered preferable to other suture materials. The bronchus is doubly clamped by means of crushing forceps and divided between the clamps (Fig. 13). Before the bronchus is closed by means of interrupted No. 1 silk sutures, approximating the mucosal edges, the distal cartilaginous ring is removed from the end of the bronchus. The removal of all the mediastinal lymph nodes is an essential part of the operation and can be accomplished either through the anterior or the posterior approach. Obviously mass ligation of the hilar structures will not permit this.
Fig. 16.
Diagrammatic illustration of futility of mass ligation and necessity of high section of bronchus as exemplified in authors' first case. Although individual ligation of hilar structures was done, the bronchus was divided insufficiently high to include the tumor. The pathologist, who was present at the operation, observed absence of tumor before mediastinal wound was closed, permitting further dissection of bronchus up to carina and high removal of bronchus to include tumor.
Fig. 17.
Diagrammatic illustration of technique which is employed in individual isolation of hilar structures. Division of the pulmonary artery and of the pulmonary veins is performed between double ligation and transfixion sutures.
Following the complete extirpation of all the mediastinal lymph nodes careful pleuralization of the mediastinum is imperative. The edges of the divided mediastinal pleura are approximated, and the stump of the ligated vessels and bronchus is covered with pleura (Fig. 18). This is important to minimize the danger of infection and augment prompt healing of the bronchial stump.
Fig. 18.
Drawing showing pleuralization of mediastinum following complete extirpation of all mediastinal lymph nodes. The edges of the divided mediastinal pleura are approximated covering the stump of the ligated vessels and bronchus with pleura.
In our cases we have not resorted to drainage, because we believe with Rienhoff (49) that filling of the pleural cavity with fibrinous exudate is important in the obliteration of the cavity. Obliteration of the cavity also is facilitated by elevation of the diaphragm which follows crushing of the phrenic nerve at the beginning of the operation. The thoracic wound is tightly closed, using interrupted No. 1 silk sutures for the pleura and the intercostal muscles. The superficial muscles and the skin are closely approximated by means of the same material. A compression sea sponge bandage is applied over the wound to obliterate the dead space and to lend support to the wound.
ANALYSES OF CASES
There have been 79 reported cases of total pneumonectomy for neoplastic disease. In addition to this number, the authors have performed total pneumonectomy for malignant disease of the lung in 7 cases making a total of 86 cases1 (Table I, Graph 1). Of the 86 collected cases, including those of the authors, 55 (63.9 per cent) died and 31 (36 per cent) recovered. Of the 31 patients who recovered there were 5, or 5.8 per cent of the entire group of 86, who subsequently died either of metastases or of other causes. In 50 the age of the patient was stated. Two (4 per cent) were in the first decade of life, the younger being 3½ years of age with a lymphosarcoma; 2 (4 per cent) in the second decade; 3 (6 per cent) in the third decade; 7 (14 per cent) in the fourth decade; 18 (36 per cent) were in the fifth decade; 14 (28 per cent) in the sixth decade; and 4 (8 per cent) in the seventh decade (Graph 2). Of the 50, 32 (64 per cent) occurred in the fifth and sixth decades. Of significance also is the fact that 14 (28 per cent) were younger than forty years of age.
Table I.
SUMMARY OF REPORTED AND AUTHORS' CASES OF PNEUMONECTOMY FOR PULMONARY NEOPLASMS
Graph 1.
Graphic representation of total mortality in 86 collected cases of pneumonectomy for neoplastic disease, including 7 performed by the authors.
Graph 2.
Graphic representation of age incidence according to decades in 50 collected cases of pneumonectomy including authors'
The sex was stated in 51 cases, including the authors' cases, of which 36 (70.5 per cent) were males and 15 (29.4 per cent) were females (Graph 3).
Graph 3.
Graphic representation of sex incidence in 51 collected cases of pneumonectomy including authors'.
The localization as regards the side involved was stated in 53 cases. The left side was involved in 29 (54.7 per cent) and the right in 24 (45.2 per cent) (Graph 4). In 31 cases, including all of the authors', individual ligation of the hilar structures was done. Of this group, 11 (35.4 per cent) recovered, and 20 (64.5 per cent) died. Of 17 cases in which mass ligation was done and so stated, 4 (23.5 per cent) recovered and 13 (76.4 per cent) died. If we included in the fatal group having mass ligation 1 of the patients recovering, who subsequently died of metastases, and in whom death may have been the result of incomplete removal of the lung, these respective figures would be 17.6 per cent and 82.3 per cent (Graph 5). This difference in the mortality percentage in the two techniques of treatment of the hilar structures demonstrates the superiority of individual ligation of the bronchus and pulmonary vessels combined with extirpation of the mediastinal lymph nodes.
Graph 4.
Graphic representation of localization, according to side involved, of pulmonary neoplasms in 53 collected cases of pneumonectomy including authors'.
Graph 5.
Graphic representation of mortality in 48 collected cases of pneumonectomy including authors', according to treatment of hilar structures.
In 38 collected cases, including the authors' series, death as a result of the operation was due to the following: 6 (15.7 per cent) hemorrhage; 8 (21 per cent) cardiac failure; 19 (50 per cent) infection; 2 (5.2 per cent of the total) late hemorrhage as a result of the infection; 3 (7.8 per cent) thrombosis and embolism; 1 (2.6 per cent) asphyxia; and 1 (2.6 per cent) peritonitis (Graph 6).
Graph 6.
Graphic representation of cause of death in 38 collected cases of pneumonectomy including authors'.
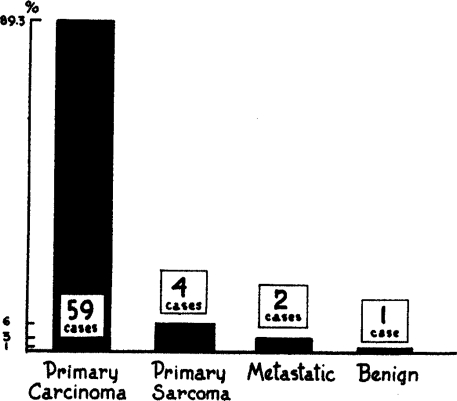
The type of neoplasm was stated in 66 cases including the authors'. Fifty-nine (89.3 per cent) were carcinoma; 4 (6 per cent) were primary sarcoma; 2 (3 per cent) were metastatic lesions, (1, a metastatic sarcoma from a primary focus in the uterus, and 1, a melanoma in which the primary lesion had previously been removed. It was the surgeon's opinion in the latter case that the lung extirpation had resulted in recovery). In 1 (1.5 per cent) the lesion was found to be benign (Graph 7).
Graph 7.
Graphic representation of type of neoplasm in 66 collected cases of pneumonectomy including authors'.
It has been the authors' experience that a total pneumonectomy on the right side is technically more difficult than that on the left, due principally to the fact that on the right side the vena azygos crosses over the eparterial bronchus, usually making the mobilization of the right mainstem bronchus difficult. This is borne out by the results obtained in right and left-sided lesions in the collected cases including the authors'. Of 24 patients with right-sided lesions, 6 (25 per cent) recovered, and 18 (75 per cent) died. Of 29 patients who were afflicted with left-sided lesions, 12 (41.3 per cent) recovered and 17 (58.6 per cent) died (Graph 8).
Graph 8.
Graphic representation of mortality in 53 collected cases of pneumonectomy including authors', according to side of operation.
Of the authors' 7 cases, 5 died and 2 recovered, giving a mortality rate of 71.4 per cent and a recovery incidence of 28.5 per cent. Of the 5 fatal cases, 1 died on the table of hemorrhage as a result of slippage of the ligature from the vena azygos shortly after it was applied. In this case there was infiltration of the mediastinum in the region of the vena azygos, the tumor extending from the eparterial bronchus (Case 2). One died of asphyxia about an hour after the completion of the operation, because of the tongue dropping back before the patient had sufficiently recovered from the anesthetic (Case 4). One patient died as a result of severe tracheitis and pulmonary edema in the opposite lung which, in the authors' opinion, was the result of trauma to the trachea by the intratracheal tube (Case 5). One died of cardiac failure 5 days after the operation. Pre-operatively there was electrocardiographic evidence of considerable myocardial damage. During the first 4 postoperative days his convalescence was very satisfactory. On the fifth day the pulse became irregular but slow, and about 10 hours after this developed the patient died. Postmortem examination revealed no other cause of death, except myocarditis (Case 6). One patient died of peritonitis from rupture of the intestine as a result of gangrene complicating a periarteritis nodosa of the mesenteric vessels 10 days after operation. The pulmonary wound was well healed, and there was no evidence of any disturbance in the chest (Case 3). One patient is living 2½ years after operation (Case 1). The remaining patient survived the operation but the operation was done so recently that it is too soon to draw any conclusions concerning the outcome (Case 7).
SUMMARY
Primary pulmonary carcinoma is an important clinical entity because of its frequent occurrence. It occurs in approximately 1 to 2 per cent of all autopsies and from 10 to 15 per cent of all carcinomas.
Chronic irritation of the bronchial mucosa is probably the most important etiological factor. Repeated inhalation of smoke over long periods of time is believed to be a prominent, irritative factor.
All pulmonary carcinomas probably originate in the bronchial mucosa. A classification based upon the embryological derivation of the tumor cell is presented.
Persistent cough with expectoration and hemoptysis, and thoracic discomfort are the most prominent symptoms and when present in a person past 40 years of age should always be considered as due to pulmonary neoplasm until proved otherwise.
Roentgenographic examination is particularly valuable in peripherally located lesions with parenchymal infiltration and in centrally located lesions with bronchial obstruction. Because most bronchial malignancies occur in the primary bronchi, bronchoscopy is especially valuable as a diagnostic procedure.
Treatment of pulmonary malignancy consists of total extirpation of the involved lung and removal of the mediastinal lymph nodes. Lobectomy and mass ligation of the hilar structures are condemned because they do not permit complete eradication of the lesion. Preliminary pneurnothorax always should be attempted. Depending upon the location of the lesion, the presence and extent of adhesions, either the anterior or posterior approach with rib resection should be used in the treatment of these patients.
An analysis of 79 collected and 7 personal cases of total pneumonectomy for neoplastic disease is presented.
Footnotes
Reprinted with permission from the American College of Surgeons (Journal of the American College of Surgeons, 1939, Vol 68, 435-451).
Presented in the Symposium on Cancer, before the Clinical Congress of the American College of Surgeons, New York, October 17-21, 1938.
1Since this presentation 2 more patients have been operated upon. One, a male, age 56 years, had carcinoma of lower left bronchus. Pneumonectomy of left lung was performed November 17, 1938. Patient made uneventful recovery. The second patient was a white female with a metastatic melanoma of left main stem bronchus having its origin from a melanoma of right eye removed 23 years previously. Pneumonectomy of left lung was done October 12, 1938. Patient died 12 days after operation of uremia. Thus of total series of 9 cases 3 have recovered and 6 have died, giving a total mortality of 66 per cent.
References
- 1.Alexander J. Personal communication to Haight. Surg., Gynec. & Obst., 1934;58 [Google Scholar]
- 2.Idem. Observations on total pulmonary lobectomy and pneumonectomy. Ann. Surg. 1935;101 doi: 10.1097/00000658-193501000-00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arce M. J. Pneumectomie totale (Le tampon-drainage en chirurgie endothoracique). Mém. Acad. de Chir., 1936;62 [Google Scholar]
- 4.Idem. Tratamiento quirurgico de los quiste y tumores del pulmón (metodos y procedemuntos generates) Soc. Internat de Chir., 11th Congress, Sept. 1938.
- 5.Archibald E. The technique of total unilateral pneumonectomy. Ann. Surg., 1934;100 doi: 10.1097/00000658-193410000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigger I. A. The diagnosis and treatment of primary carcinoma of the lung. South. Surg. 1935;4 [Google Scholar]
- 7.Binkley J. S. Aspiration biopsy of lung. Presented at 21st annual meeting of the Am. Soc. Thoracic Surg., 1938(in press)-J. Thoracic Surg. Reviewed by I. A. Bigger, Surgery. 1938;4 [Google Scholar]
- 8.Bonniot O. Monod, Evrard H. Considerations anatomiques pour servir à l'abord des pédicules pulmonaires par voie antérieure. J. de chir. 1936;48 [Google Scholar]
- 9.Boyd W. Pathology of primary carcinoma of the lung. Canadian M. Ass. J. 1930;23 [PMC free article] [PubMed] [Google Scholar]
- 10.Churchill E. D. Discussion of Overholt's paper. J. Thoracic Surg. 1935-36;5 [Google Scholar]
- 11.Ibid. Lobectomy and pneumonectomy in bronchiectasis and cystic disease. 1936-37;6 [Google Scholar]
- 12.Crafoord C. On the Technique of Pneumonectomy in Man. Stockholm: Tryckiri Aktiebolaget Thule. 1938.
- 13.D'Aunoy R., Pearson B., Halpert B. Personal communications.
- 14.Dudgeon L. S., Wrigley C. H. On demonstration of particles of malignant growth in sputum by means of wet-film method. J. Laryngol. & Otol. 1935;50 [Google Scholar]
- 15.Duval P., Monad R. Discussion of Lambret's paper: Pneumectomie totale pour cancer du poumon gauche. Bull. et mém. Soc. nat. de chir. 1935;61 [Google Scholar]
- 16.Edwards A. T. Malignant diseases of the lung. J. Thoracic Surg., 1934-35;4 [Google Scholar]
- 17.Idem. Quoted by Crafoord, Ref. No. 12
- 18.Fischer W. In Henke and Lubarsch: Handbuch der speziellen pathologischen Anatomie und Histologie. Vol. 3. Vol. 3. Berlin: Springer; 1931. 509 pp. [Google Scholar]
- 19.Flick J. B., Gibbon, J. H., Jr. Total removal of the left lung for carcinoma. Ann. Surg. 1936;103 [Google Scholar]
- 20.Freedlander S. O. Total pneumonectomy. Ohio State. M. J., Columbus, 1937;33 [Google Scholar]
- 21.Frissel L. E., Knox J. C. Primary cancer of the lung. Am. J. Cancer. 1937;30 [Google Scholar]
- 22.Goldman Alfred. Cytology of serous effusions, with special reference to tumor cells. Arch. Surg. 1929;19 [Google Scholar]
- 23.Graham E. A., Singer J. J. Successful removal of an entire lung for carcinoma of bronchus. J. Am. M. Ass. 1933;101 [Google Scholar]
- 24.Graham E. A. Primary carcinoma of the lung or bronchus. Ann. Surg. 1936;103 doi: 10.1097/00000658-193601000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haight C. From discussion on “Pneumonectomy for malignant and suppurative disease of the lung,”. by R. Overholt J. Thoracic Surg. 1935-36. 5 77 [Google Scholar]
- 26.Halpert B. Pathology of bronchogenic carcinoma. N. Orleans M. & S. J. (in press)
- 27.Hinz R. Totale extirpation der lunken hunge Wegen bronchial Carcinom. Arch. f. klin. Chir. 1923;124 [Google Scholar]
- 28.Holinger P. Personal communication
- 29.Holst J. Lobektomi og pulmeklomi ved bronchiektosi. Förhandl. ved nord. Kirurg. Forenings moote. 1937;411 [Google Scholar]
- 30.Ivanissevich O., Ferrari R. C. La pneumectomia en el hombre. Bol. y trab. Soc. de cirug. de Buenos Aires. 1933. p. 17. July 5.
- 31.Jackson C. W. C., Konzelmann F. W. Bronchial carcinoma; bronchus biopsy in a series of 32 cases. J. Thoracic Surg. 1935-36;4 [Google Scholar]
- 32.Jaffé R. H. Primary carcinoma of lung. J. Lab. & Clin. Med. 1935;20 [Google Scholar]
- 33.Junghanns H. Der Krebs der lunger bronchien und oberen luftwege. Ztschr. f. Krebsforsch. 1929;28 [Google Scholar]
- 34.Kuemmel H. Discussion of W. Mueller: Demonstration zur extirpation ganzer Lungenlappen. Verhandl. d. deutsch. Gesellsch. f. Chir. 1911;40 [Google Scholar]
- 35.Lambret M. O. Pneumectomie totale pour cancer du poumon gauche. Bull, et mém. Soc. nat. de chir. 1935;61 [Google Scholar]
- 36.Lanford J. A. Personal communication
- 37.Lilienthal H. Pneumonectomy for sarcoma of the lung in a tuberculous patient. J. Thoracic Surg. 1932-33;2 [Google Scholar]
- 38.Lyle H. H. M. Carcinoma of the right lung: Pneumectomy in one stage. Ann. Surg. 1936;103 [Google Scholar]
- 39.Mandlebaum F. S. Quoted by Goldman, Ref. No. 22
- 40.Martin H. E., Ellis E. B. Biopsy by needle puncture and aspiration. Ann. Surg. 1930;92 doi: 10.1097/00000658-193008000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matson R. C., Roberts J. M., Bisaillon M. Total removal of the right lung for bronchogenic carcinoma. Dis. of the Chest. 1938;4 [Google Scholar]
- 42.Meyer W. Observations in lung suppuration and its treatment. Arch. Surg. 1923;6 [Google Scholar]
- 43.Overholt R. H. The total removal of right lung for carcinoma—report of a successful case. J. Thoracic Surg. 1934-35;4 [Google Scholar]
- 44.Ibid. Pneumonectomy for malignant and suppurative diseases of the lung. J. Thoracic Surg. 1935-36;5 [Google Scholar]
- 45.Idem. Clinical studies and treatment of primary carcinoma of the lung. J. Connecticut Med. Soc, 1938;2:122. [Google Scholar]
- 46.Rienhoff W. F., Jr. The treatment of carcinoma of the lung. Surg. Clin. N. America. 1936;16 [Google Scholar]
- 47.Idem. Pneumonectomy. A preliminary report on the operative technic in two successful cases. Bull. Johns Hopkins Hosp., Balt. 1933;53 [Google Scholar]
- 48.Idem. The surgical technic of total pneumonectomy. Arch. Surg. 1936;32 [Google Scholar]
- 49.Idem. Intrathoracic anatomical readjustments following complete ablation of one lung. J. Thoracic Surg. 1937;6 [Google Scholar]
- 50.Santy Bonniot, Dargent Corajod, Berard Lobectomie et pneumonectomie pour neoplasmes bronchopulmonaires. Lyon méd. 1936;157:485, 521. [Google Scholar]
- 51.Seyfarth C. Lungenkarzionon in Leipzig. Deutsche Med. Wchnschr. 1924;50 [Google Scholar]
- 52.Sharp G. The diagnosis of primary carcinoma of the lung by aspiration. Am. J. Cancer. 1931;15 [Google Scholar]
- 53.Winternitz M. A., Wason, I. M., McNamara F. P. The Pathology of Influenza. New Haven: Yale University Press; 1920. [Google Scholar]