Abstract
The complexity of micropatterned cell constructs has been limited by difficulties in patterning more than two surface components on a culture substrate. Photolithography using multiple aligned masks is well established for generalized multicomponent patterning, but is often too harsh for biomolecules. We report a two-mask photolithographic process that is tuned to preserve bioactivity in patterns composed of covalently coupled polyethylene glycol (PEG), adsorbed extracellular matrix protein (e.g. collagen I), and adsorbed serum proteins (e.g. vitronectin). Thereby, we pattern two cell types—primary hepatocytes and 3T3 fibroblasts—demonstrating control over contact and spacing (20–200 μm) between the two cell types for over one week. This method is applicable to the study of intercellular communication in cell biology and tissue engineering.
I. Introduction
Surface engineering of cell culture substrates has developed into a powerful tool for controlling multicellular organization at the micrometer scale.1 This new capability has brought valuable insight into the biological mechanisms by which the cellular microenvironment determines cell fate and function.2–6 However, studies requiring more complex tissue structures have been hindered by limitations in surface patterning. Typically, molecules that mediate cell attachment are patterned against a non-adhesive background, allowing arrays of a single cell type to be formed, with control of cell positioning and relative spacing.7 Alternatively, patterns composed of two different adhesive regions can be employed to form patterned co-cultures of two different cell types, as long as one cell type selectively attaches to a specific region.8 However, there have been few examples where multiple attachment chemistries have been successfully combined with non-adhesive surfaces in a multicomponent pattern. This has prevented the realization of configurations in which cell-cell contact and spacing between different cell types is controlled.
The most general approach to fabricating a surface with more than two components is to pattern each component individually, with each successive patterning step aligned to the previous. This has been the highly successful methodology of semiconductor microfabrication. Due to the harsh processing conditions of semiconductor manufacturing, an array of 'soft lithography' approaches have emerged as alternatives for biological micropatterning, including microcontact printing9 and microfluidic patterning,10 based on elastomeric stamps rather than photoresist-based patterning. Multistep patterning is problematic with this technology, however, due to the practical difficulties in accurately aligning multiple flexible stamps over a large area.11 While there have been limited demonstrations of aligned multistep microcontact printing,12, 13 the patterned areas were small, and simultaneous patterning of multiple cell types was not achieved. Certain groups have managed to circumvent the alignment issue by employing clever manipulations of a single elastomeric stamp to achieve multicomponent patterning.14, 15 Unlike multistep printing, however, the individual component patterns are not fully independent of each other as they must all be integrated onto a single stamp, and it is not clear that these methods can be generalized to arbitrary pattern geometries. In particular, although multiple cell types were patterned, along with non-adhesive regions, controlled variation in contact and spacing between cell populations was not shown. Finally, cell patterning on a uniform surface can be accomplished by utilizing a microfluidic network to deliver cells directly to desired positions. In this manner, multiple cell types have been patterned with defined microscale spacing.16 However, non-adhesive regions were not incorporated, and precise regulation of contact between cell populations was not demonstrated.
In contrast to soft lithography, photolithographic patterning using multiple masking steps, precisely aligned over large areas, is well established. Compatibility with biomolecules remains the critical hurdle to cell patterning. If only a single patterning step is required, the challenge is reduced since most of the processing can occur before biomolecules are introduced.8, 17, 18 However, additional patterning with subsequent masks exposes the first biomolecules to the full gamut of harsh processing conditions involved in photolithography. A number of groups have attempted to address this limitation, for example by using a temporary layer of sucrose as a chemical barrier for biomolecule protection19, 20 or through the development of more biocompatible resist chemistries.21, 22 Although these new processes hold promise, they will require substantial further development before approaching the level of refinement and reliability that has been achieved with semiconductor processes.
In this report, we demonstrate that biomolecular patterning via standard multimask photolithography is possible given proper reagent selection and process tuning to preserve the bioactivity of patterned species. Using standard reagents and equipment, two aligned photolithographic patterning steps are employed to pattern a three-component surface chemistry consisting of two cell-adhesive regions and one non-adhesive region with long-term non-fouling characteristics. Thereby, we demonstrate a generalizable method for the microscale regulation of cell-cell contact and spacing between two different cell types.
II. Experimental Section
Materials
Collagen I was purified from rat tails as previously described.23 PEG-disilane (molecular weight 3,400 Da) was purchased from Nektar Therapeutics (Huntsville, AL); reconstituted solutions were prepared 1 hr prior to use. Details regarding cell culture and assays can be found online as supporting information.
Photolithographic Patterning of Collagen and PEG-disilane
34-mm glass coverslips were cleaned in acetone, deionized water, and methanol, then dehydrated (75 ºC, 5 min). Photoresist (Microposit S1818, Shipley, Marlborough, MA) was applied by spin coating (5000 rpm, 30 s), soft baked (75 ºC, 30 s), exposed through a commercially printed photomask using a contact mask aligner (HTG System 3HR 2–3, 300 nm, 100 s, 10 mW/cm2), and developed (Microposit MF-321, Shipley, 90 s). Substrates were rinsed in deionized water and dried under a stream of nitrogen.
Following a hard bake (90 ºC, 5 min; then 130 ºC, 45 min), substrates were etched (10% hydrofluoric acid, 30 s) to remove roughly 0.5 μm of glass in the exposed areas. After thorough rinsing, substrates were incubated in collagen (500 μg/ml, 37 ºC, 45 min). Resist was removed by sonicating in acetone for 2 min, followed by rinsing and drying.
Again, substrates were dehydrated, coated with photoresist, and soft baked. A second photomask was aligned to the first pattern, and the resist was exposed and developed. Oxygen plasma (Technics 500 Asher, 200 mT, 200 W, 5 min) was applied, removing any protruding collagen in the exposed areas. The substrates were immersed in PEG-disilane (10mM, 10 min) and dried on a photoresist spinner (1000 rpm, 120 s) to ensure uniform coating, followed by baking (75 ºC, 10 min; then 25 ºC, 1 hr). Finally, the resist was removed by sonicating in acetone for 15 sec, and the substrates were rinsed and dried.
Seeding of cells onto patterned substrates
Patterned coverslips were sterilized by soaking in 70% ethanol for 1 hr and then washed twice in distilled water. The substrates were incubated in bovine serum albumin for 2 hr at 37 ºC to block non-specific attachment to bare glass regions, then washed with serum-free hepatocyte culture medium. Primary hepatocytes (106 cells/ml) were seeded in serum-free medium and incubated for 1 hr at 37 ºC, with shaking every 15 min to resuspend unattached cells. After the first hour, unattached cells were aspirated, the substrate was washed with serum-free medium, and seeding was repeated with a second hepatocyte suspension. After the second hour, unattached cells were aspirated, and serum-containing hepatocyte medium was added. After 24 hr, 3T3-J2 fibroblasts (375,000 cells/ml) were seeded in fibroblast medium (with serum) and incubated for 1 hr. Unattached cells were then aspirated. The patterned cells were cultured in hepatocyte medium.
III. Results and Discussion
The goal of this work, essentially, was to maintain long-term separation of two different cell populations by patterning a non-fouling surface chemistry between two different adhesive regions. A number of non-fouling surface chemistries, which resist protein adsorption and thus cell migration, have been demonstrated, with polyethylene glycol (PEG) being one of the most widely employed.1 We chose a PEG-disilane previously employed by Irimia and coworkers for covalent coupling to glass since this formulation had been shown to be amenable to photolithographic patterning.18 In the adhesive regions, collagen I was patterned against unmodified glass in order to mediate selective attachment of hepatocytes and 3T3 fibroblasts.8 It is known that liver-specific functions of primary hepatocytes can be maintained in vitro through co-cultivation with stromal cell types such as 3T3 fibroblasts, however the mechanisms behind this effect remain poorly understood.5, 24 Control of cell-cell contact could thus serve as a useful tool for helping to elucidate intercellular communication in this system.
Multimask photolithography was employed to pattern the multicomponent substrate, as outlined in Fig. 1. Briefly, photoresist was applied to a glass substrate, photopatterned, and then employed to mask collagen adsorption.8 After photoresist removal in acetone, a second photoresist coating was applied and patterned in order to mask the conjugation of aqueous PEG-disilane.18 Mask-to-mask registration of the two patterns was accomplished using a standard contact mask aligner. In order to facilitate alignment, a brief hydrofluoric acid etch was employed after the first photolithographic step to imprint visible ridges onto the glass substrate (figure available online as supporting information). The etch depth was less than a micron, minimal compared to cell diameters of roughly 25 microns, hence cell culture planarity was not significantly affected. Also, in order to negate alignment errors between the two mask patterns, the collagen regions were drawn larger than required, so as always to overlap the PEG regions; prior to PEG-disilane treatment, oxygen plasma was used to remove the protruding collagen (Fig. 1). PEG coating thickness was measured via profilometry to average roughly 0.2 μm, with a 0.8-μm ridge along the perimeter of the patterned regions. More detailed characterization of a similar method by atomic force microscopy has been previously reported.18
Fig. 1. Schematic of patterning process.
Two aligned photolithographic steps are used to pattern collagen I and PEG-disilane on a glass substrate. Hepatocytes seeded in serum-free medium attach preferentially to collagen-coated regions. 3T3 fibroblasts are subsequently seeded in serum-enriched medium. Serum proteins adsorb to regions of bare glass to mediate fibroblast attachment. PEG-treated regions resist protein adsorption for at least one week, thus preventing cell migration and maintaining spatial separation between the two cell types. The hydrofluoric acid etch (step 2) imprints a visible ridge in the glass substrate to facilitate visual alignment to the second mask. The edge of the collagen mask is intentionally offset to extend into PEG region (step 5), and the protruding collagen is removed by oxygen plasma (step 6), ensuring that the patterned collagen region abuts the PEG region. (Dimensions not to scale.)
Following substrate preparation, cells were seeded, with hepatocytes attaching specifically to collagen-coated regions, while subsequently seeded 3T3 fibroblasts attached to regions of bare glass via adsorbed serum proteins from the culture medium (Fig. 2). Although hepatocyte-filled regions generally excluded fibroblast attachment, a small number of fibroblasts were able to attach to hepatocyte regions in areas where the hepatocytes were not fully confluent. Multiple hepatocyte seedings were thus utilized to increase confluency and decrease fibroblast contamination. PEGylated regions resisted cell migration for at least one week of culture in the presence of 10% serum, comparing favorably to other non-fouling chemistries in terms of long-term pattern fidelity.25 It was observed that narrower regions could be bridged by fibroblastic processes, at widths of 40 μm and below (figure available online as supporting information).
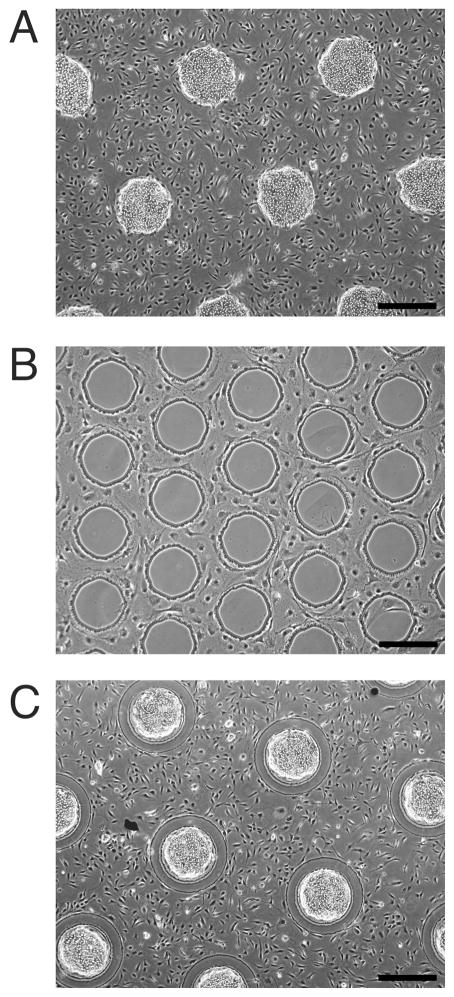
Fig. 2. Control of cell organization via patterned surface chemistry.
(A) Phase contrast image of hepatocytes selectively adhered to islands of collagen, with 3T3-J2 fibroblasts filling the remaining bare glass regions (day 2). (B) 3T3-J2 fibroblasts excluded from islands of PEG-disilane, (day 5). (C) Hepatocytes and 3T3-J2 fibroblasts patterned on a combination of collagen and PEG-disilane, (day 2). Scale bars 500 μm.
An important key to the multimask photolithographic patterning process is ensuring that the processing steps following collagen adsorption do not negatively impact its ability to mediate hepatocyte attachment. Possible mechanisms by which collagen may be denatured include chemical (photoresist solvent and developer), thermal (photoresist bake), or ultraviolet radiation (photoresist exposure). Process steps were thus examined individually as well as in combination in order to determine their effect on adsorbed collagen patterns (Table 1). Hepatocyte attachment onto collagen following treatment served as a stringent measure of retained receptor binding activity. Our findings indicated that ultraviolet exposure was acceptable at the levels that were used for photopatterning, as well as photoresist baking at hotplate settings of up to 100 ºC. Use of a photoresist developer based on sodium hydroxide was detrimental, rendering the substrate nonadhesive to hepatocytes, however an alternative developer based on tetramethylammonium hydroxide was found to be acceptable. Therefore, a suitable process window was identified to allow photolithographic patterning over an existing pattern of adsorbed collagen. Though some collagen degradation likely occurs, evidently there is still sufficient preservation of receptor-binding subdomains to mediate hepatocyte attachment. Process flows in which PEG conjugation was performed before collagen adsorption were unsuccessful.
Table 1. Biocompatibility of photolithographic processing steps.
Patterns of adsorbed collagen (500 μg/ml) were exposed to various photolithographic factors and evaluated on whether the ability to mediate hepatocyte attachment was preserved. These results demonstrate that photolithographic patterning can be performed on collagen-patterned substrates, using a tetramethylammonium hydroxide developer and restricting photoresist bake temperatures to below 100 ºC.
| Photolithographic Process Element | Collagen bioactivity retained? |
|---|---|
|
Photoresist Solvent: propylene glycol methyl ether acetate Binder: novalac resin Photoactive compound: diazonapthoquinone |
Yes |
|
Photoresist exposure 300 nm, 10 mW/cm2, 35 s |
Yes |
|
Developer (Microposit MF-319) Tetramethylammonium hydroxide (TMAH), 2 min |
Yes |
|
Developer (Microposit 354) Sodium hydroxide, 2 min |
No |
| Photoresist + ultraviolet exposure + TMAH | Yes |
|
50 ºC 10 min on hotplate |
Yes |
|
100 ºC 10 min on hotplate |
Yes |
|
200 ºC 10 min on hotplate |
No |
|
Photoresist removal Acetone, 2 min |
Yes |
A primary benefit of employing photolithography is the ability to produce a diverse array of precisely aligned patterns across a large substrate (we demonstrated diameters up to 2 in). In this way, numerous variations in cell organization and composition can be screened in parallel in a single experiment (Fig. 3). Our platform thus enables an iterative approach in which multiple configurations are screened initially (Fig. 3, B-D), followed by secondary designs focusing on the most biologically interesting configurations. In situ functional assays such as immunofluorescence staining can be employed for the initial screen (Fig. 3E), while precise quantitative bulk assays can be added during the follow-up experiments, using multiple repeats of the same configuration reproduced across a single substrate. Use of photolithography also produced high-resolution patterning, achieving minimum feature sizes on the order of 10 μm using printed masks. Smaller feature sizes could be achieved by using chrome masks, however patterning via photoresist liftoff for features approaching 1 μm could be problematic given that the thickness of the PEG coating was as great as 0.8 μm.
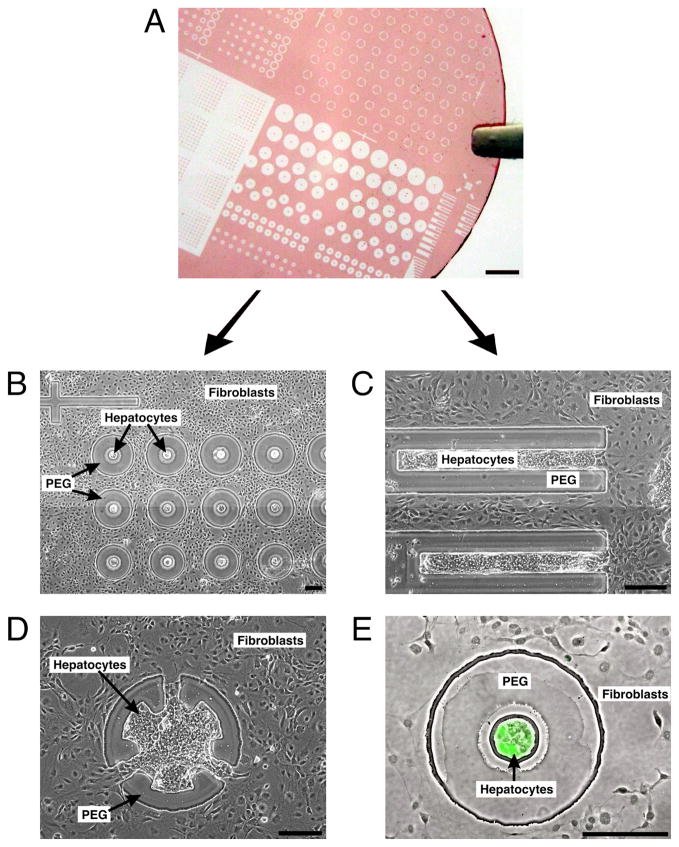
Fig. 3. Parallel screening of multiple cell configurations.
(A) Glass coverslip with patterned photoresist (PEG mask) illustrating the variety of designs possible on a single substrate. Scale bar is 2 mm. (B) Phase contrast image showing variations in hepatocyte-fibroblast separation (day 6). (C and D) Spatially restricted contact between hepatocytes and fibroblasts (day 6). (E) Phase contrast and fluorescence overlay showing expression of intracellular albumin in green after 8 days of culture, indicating retained hepatocyte function. This in situ assay identifies an interesting experimental condition that can be further pursued in larger formats. (B-E) Scale bars are 200 μm.
In conclusion, this work demonstrates that multimask photolithography can be an effective method for surface engineering and cell patterning when complex multicomponent structures are required, particularly when precise alignment over large areas is needed. For our chosen model system, we have shown that biocompatibility concerns can be circumvented through appropriate selection of reagents and process parameters. Whether this method can be extended to other extracellular matrix proteins or non-fouling chemistries is dependent on their sensitivity to photolithographic processing. Of particular concern is the exposure to acetone, an organic solvent, during photoresist removal. This may be addressed in the future through the use of biocompatible photoresist chemistries.21, 22 Still, even the substrates demonstrated here may be suitable for patterning other cells types, provided that the first cell type adheres to collagen but not glass, and the second cell type does not adhere significantly on top of the first type. As a biological tool, multicomponent patterning has potential application to the study of many aspects of heterotypic intercellular communication including contact-mediated signaling, soluble signaling over various distances, and functional variations within a homogeneous cell population dependent on distance from the heterotypic contact interface.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Salman Khetani and Jennifer Felix for valuable technical assistance. Funding was generously provided by NSF CAREER, NIH NIDDK, and the David and Lucile Packard Foundation. E.E.H. was supported by a Ruth L. Kirschstein National Research Service Award.
Footnotes
Supporting Information Available. Details regarding cell culture and assays, a micrograph of the alignment features, and a micrograph showing bridging of narrow PEG regions are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Falconnet D, Csucs G, Michelle Grandin H, Textor M. Biomaterials. 2006;27:3044–3063. doi: 10.1016/j.biomaterials.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 2.El-Ali J, Sorger PK, Jensen KF. Nature. 2006;442:403–411. doi: 10.1038/nature05063. [DOI] [PubMed] [Google Scholar]
- 3.Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Proc Natl Acad Sci U S A. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu WF, Nelson CM, Pirone DM, Chen CS. J Cell Biol. 2006;173:431–441. doi: 10.1083/jcb.200510087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia SN, Balis UJ, Yarmush ML, Toner M. FASEB J. 1999;13:1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- 6.Doh J, Irvine DJ. Proc Natl Acad Sci U S A. 2006;103:5700–5705. doi: 10.1073/pnas.0509404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia SN, Yarmush ML, Toner M. J Biomed Mater Res. 1997;34:189–199. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Whitesides GM. Appl Phys Lett. 1993;63:2002–2004. [Google Scholar]
- 10.Delamarche E, Bernard A, Schmid H, Michel B, Biebuyck H. Science. 1997;276:779–781. doi: 10.1126/science.276.5313.779. [DOI] [PubMed] [Google Scholar]
- 11.Rogers JA, Paul KE, Whitesides GM. J Vac Sci & Tech B. 1998;16:88–97. [Google Scholar]
- 12.Wheeler BC, Corey JM, Brewer GJ, Branch DW. J Biomech Eng Trans ASME. 1999;121:73–78. doi: 10.1115/1.2798045. [DOI] [PubMed] [Google Scholar]
- 13.Lauer L, Ingebrandt S, Scholl M, Offenhausser A. IEEE Trans Biomed Eng. 2001;48:838–842. doi: 10.1109/10.930910. [DOI] [PubMed] [Google Scholar]
- 14.Tien J, Nelson CM, Chen CS. Proc Natl Acad Sci U S A. 2002;99:1758–1762. doi: 10.1073/pnas.042493399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tourovskaia A, Barber T, Wickes BT, Hirdes D, Grin B, Castner DG, Healy KE, Folch A. Langmuir. 2003;19:4754–4764. [Google Scholar]
- 16.Chiu DT, Jeon NL, Huang S, Kane RS, Wargo CJ, Choi IS, Ingber DE, Whitesides GM. Proc Natl Acad Sci U S A. 2000;97:2408–2413. doi: 10.1073/pnas.040562297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinfeld D, Kahler KH, Hockberger PE. J Neurosci. 1988;8:4098–4120. doi: 10.1523/JNEUROSCI.08-11-04098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irimia D, Karlsson JOM. Biomed Microdev. 2003;5:185–194. [Google Scholar]
- 19.Flounders AW, Brandon DL, Bates AH. Biosensors & Bioelectronics. 1997;12:447–456. doi: 10.1016/s0956-5663(96)00064-4. [DOI] [PubMed] [Google Scholar]
- 20.Sorribas H, Padeste C, Tiefenauer L. Biomaterials. 2002;23:893–900. doi: 10.1016/s0142-9612(01)00199-5. [DOI] [PubMed] [Google Scholar]
- 21.Douvas A, Argitis P, Misiakos K, Dimotikali D, Petrou PS, Kakabakos SE. Biosensors & Bioelectronics. 2002;17:269–278. doi: 10.1016/s0956-5663(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 22.Doh J, Irvine DJ. J Am Chem Soc. 2004;126:9170–9171. doi: 10.1021/ja048261m. [DOI] [PubMed] [Google Scholar]
- 23.Dunn JC, Tompkins RG, Yarmush ML. Biotechnol Prog. 1991;7:237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 24.Guguen-Guillouzo C, Guillouzo A. Mol Cell Biochem. 1983;53–54:35–56. doi: 10.1007/BF00225245. [DOI] [PubMed] [Google Scholar]
- 25.Nelson CM, Raghavan S, Tan JL, Chen CS. Langmuir. 2003;19:1493–499. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.