Abstract
The aim was to evaluate the clinical outcome of hypofractionated stereotactic radiotherapy (SRT) with CyberKnife for nonfunctioning pituitary adenoma. From October 2000 to March 2009, 100 patients with nonfunctioning pituitary adenoma were treated with hypofractionated SRT. Forty-three patients were male, and 57 were female. The patient's ages ranged from 16 to 82 years (median, 59 years). Five patients were medically inoperable, and 1 refused surgery; the remaining 94 were recurrent cases or those receiving postoperative adjuvant SRT. No patients had a history of previous cranial radiotherapy. Tumor volume ranged from 0.7 to 64.3 mL (median, 5.1 mL). The marginal doses were 17.0 to 21.0 Gy for the 3-fraction schedule and 22.0 to 25.0 Gy for the 5-fraction schedule. Toxicities were evaluated with the Common Terminology Criteria for Adverse Events version 4.0. The median follow-up period for living patients was 33 months (range, 18–118.5 months). The 3-year overall survival and local control rates were 98% and 98%, respectively. In-field and out-field tumor regrowth were observed in 3 and 2 patients, respectively. Transient cyst enlargement occurred in 3 cases. A post-SRT grade 2 visual disorder occurred in 1 patient. Symptomatic post-SRT hypopituitarism was observed in 3 of 74 patients who had not received hormone replacement therapy after surgery. CyberKnife SRT involving 21 Gy in 3 fractions or 25 Gy in 5 fractions is safe and effective for surgical treatment of nonfunctioning pituitary adenoma. Hypofractionated SRT appears useful for protecting the visual nerve and neuroendocrine function, especially for tumors located near the optic pathways and large tumors.
Keywords: CyberKnife, hypofractionated stereotactic radiotherapy, nonfunctioning, optic pathway, pituitary adenoma
Pituitary adenoma (PA) is a benign tumor that occurs mainly in adults between 20 and 50 years of age and constitutes about 10–20% of all intracranial tumors.1,2 PA is divided into functioning and nonfunctioning varieties, and the purpose and method of treatment differ for the two entities. Treatment for functioning PA aims to prevent the excessive secretion of anterior pituitary lobe hormone. On the other hand, treatment of nonfunctioning PA is typically intended to control tumor volume and prevent or reverse visual disorders and endocrinopathies. Tumors that cause visual symptoms are treated primarily with transsphenoidal surgery or craniotomy; and if patients are asymptomatic, a wait-and-see approach may be taken. Nonfunctioning PA is not necessarily treated by immediate radiotherapy (RT) after resection, unlike functioning PA. However, several studies have reported recurrences in about 20–50% of cases treated with surgery alone.3–5
Radiotherapy is considered if residual or recurrent tumors invade the cavernous sinus or in cases in which repeated surgeries have resulted in fibrosis and inoperability. In the past, conventional RT was used to treat such cases.6–8 Considering the proximity of organs at risk (OARs) such as the optic nerve, optic chiasm, and brain stem, the use of stereotactic irradiation is increasing. Outcomes of Gamma Knife stereotactic radiosurgery (SRS) have been reported in many papers.9–11 However, while the targeting accuracy and dose fall-off of Gamma Knife treatment are excellent, it may not be appropriate for tumors that are large or adjacent to optic pathways because the dose limitation for these structures is thought to be 8–10 Gy in a single session.12–14 The sparing of normal tissues, especially late-responding tissues presumably with a low α/β ratio (≤3 Gy), such as the optic pathways and brain stem, may be more efficiently expected by using lower daily doses with fractionated radiation than with SRS.15,16 More recently, several reports have indicated promising outcomes with stereotactic radiotherapy (SRT) and proton therapy using conventional fractionation.17–19 To achieve increased local control while maintaining low optic pathway toxicity, we started protocol-based hypofractionated SRT with the CyberKnife system for PA in 2000. We used hypofraction-ation instead of more conventional fractionation because the use of a lower fractional dose results in poorer dose distribution in the CyberKnife system. Usually, 100–200 beams are employed in one treatment, but since low monitor-unit beams are susceptible to errors of 3% or more, the number of beams has to be reduced with decreasing daily dose. This could lead to poorer dose distribution, so we used 3 or 5 fractions. In the present study, we analyzed the safety and efficacy of hypofractionated SRT with CyberKnife for treatment of nonfunctioning PA at our institutions.
Materials and Methods
Study Design, Patient Eligibility, and Characteristics
This was a prospective study based on protocols designed by the clinical study committees of the Yokohama CyberKnife Center and Okayama Kyokuto Hospital and was approved by the institutional review boards. The eligibility criteria were as follows: (1) histologically confirmed or image-diagnosed PA with endocrinological findings indicating nonfunctioning PA; (2) recurrent cases, patients receiving postoperative adjuvant SRT, medically inoperable patients, and patients who refused surgical resection; (3) no prior RT or chemotherapy for cranial disease; and (4) willingness to provide written informed consent. In general, patients were deemed to be medically inoperable if they had poor pulmonary function, a history of major cardiovascular disease, or severe diabetes mellitus or were elderly (≥80 years old). From October 2000 to March 2009, 100 patients with nonfunctioning PA were treated with SRT using CyberKnife. The patient and tumor characteristics are summarized in Table 1.
Table 1.
Patient and tumor characteristics
| Characteristics | Number of patients |
|---|---|
| Total | 100 |
| Age (years), median (range) | 59 (16–82) |
| Gender, male/female | 43/57 |
| KPS 100/90/80/70 | 86/6/7/1 |
| After surgery | 94 |
| Refusal of surgery or medical inoperability | 6 |
| Tumor volume (cc), median (range) | 5.1 (0.7–64.3) |
| Pre-SRT visual disorders | 42 |
| Pre-SRT hormone replacement | 26 |
| Interval between final operation and SRT (months) | |
| Median (range) | 11 (1–156) |
Abbreviation: SRT, stereotactic radiotherapy.
Treatment Protocols
SRT was delivered in either 3 or 5 fractions, with the 5-fraction schedule being used for young patients (<30 years old) and those with tumors that were large (≥15 cc) or adjacent to optic pathways (distance, <2 mm). Radiation doses were prescribed at the margin (95% volume border of the planning target volume [PTV]). The planned dose was either 21 Gy in 3 fractions or 25 Gy in 5 fractions; when the doses delivered to the OARs (optic nerve, chiasm, and brain stem) exceeded these levels, the marginal doses were reduced. Thus, the maximum doses allowed for these OARs were 21 Gy in 3 fractions and 25 Gy in 5 fractions. All irradiation was given once a day, 3 to 5 days a week.
CyberKnife System
The CyberKnife system (Accuray) is equipped with a 6-MV photon-beam accelerator, a robotic arm that can be moved in 6 dimensions of freedom, and a target locating system (TLS). The robotic arm allows beam delivery from over 1200 positions around the patient. Beams are delivered through circular collimators ranging from 5 to 60 mm in diameter. The TLS consists of an orthogonal X-ray system, and the X-rays are registered to digitally reconstructed radiographs derived from planning CT scans. During treatment, control algorithms dynamically reposition the robotic arm based on the target movement detected by the TLS. Frequent TLS checks throughout treatment detect target motion in real time, thus enabling frameless stereotactic irradiation. The geometrical accuracy of our institution is less than 0.5 mm.20 The geometrical accuracy of and clinical experiences with the CyberKnife system have been reported by several investigators.21,22
Treatment Planning
Radiation treatment was planned using a CT-based 3D treatment planning system (Ontarget [Accuray; until December 2008] or Multiplan [Accuray; from January 2009). Patients were lightly restrained with a custom-made thermoplastic face mask (WFR/Aquaplast), and 1.5-mm-thick CT images were taken after the administration of iopamidol (Oypalomin 370 syringe, Fuji Pharma, or Iopamiron 370, Bayer Schering Pharma; 2 mL/kg of body weight) through a 22-gauge catheter inserted into a forearm vein using a power injector (Stellent Sx, Medrad) at a rate of 1 mL/second. The scans were performed at 30 s after the injection of iopamidol. The CT images used for treatment planning were acquired using a scanner (Somatom-Emotion Duo, Siemens Medical Systems), and the scanning parameters were as follows: detector configuration, 1.5 × 2 mm; slice thickness, 1.5 mm; scan time, 1.0 s; 130 kV; 190 mA; field of view, 30 cm; and matrix, 512 × 512. Next, MRI was performed by a 1.5-T system (Magnetom Symphony, Siemens Medical Systems) with an 8-channel phased-array coil. T1-weighted contrast-enhanced images were obtained after the injection of meglumine gadopentetate (Magnevist Syringe, Bayer Schering Pharma; 0.2 mL/kg of body weight). The imaging parameters were as follows: repetition time/echo time, 724/11 ms; flip angle, 80 degrees; number of signals acquired, 2; field of view, 192 × 220; matrix, 179 × 256; acquisition time, 263 s; slice thickness, 2.0 mm; and slice interval, 0.5 mm. Then, the MRI images were fused with the CT images using Ontarget or Multiplan.
The lesions visible on CT and/or MRI were taken as the gross tumor volume (GTV). Taking the direction of tumor invasion into consideration, the clinical target volume (CTV) was adjusted based on information from the preoperative images and the planning CT and/or MRI. The PTV was equal to the CTV. Conformal treatment plans were designed for all cases using an inverse planning algorithm that involved setting dose constraints to minimize the irradiation delivered to critical structures such as optic pathways and the brain stem. The doses were calculated on the basis of the ray tracing algorithm.
Follow-Up Evaluation and Statistical Analysis
After SRT, the patients were followed at 1, 3, 6, and 12 months during the first year, and at intervals of 6 or 12 months thereafter. Regular follow-up studies included brain MRI, visual perception tests, and examinations of hormonal levels. The local responses to SRT were classified according to the Response Evaluation Criteria in Solid Tumors. The rates of overall survival, local control, and disease-free survival were calculated using the Kaplan–Meier method. Out-field recurrence apart from PTV was not included in the calculation of local control rates. Differences between pairs of Kaplan–Meier curves were examined using the log-rank test. Values of P < .05 were considered to be statistically significant. Statistical analyses were carried out with StatView version 5 (SAS Institute). Toxicities were evaluated with the Common Terminology Criteria for Adverse Events version 4.0.
Results
Treatment Characteristics
SRT was given in 3 or 5 fractions. The 3-fraction and 5-fraction schedules were delivered to 83 and 17 patients, respectively. Forty-eight patients received lower doses than planned because the doses delivered to the optic pathways exceeded the maximum doses. A summary of the treatments is shown in Table 2. A conformity and homogeneity index was calculated according to the Radiation Therapy Oncology Group 1993 criteria.23
Table 2.
Treatment characteristics and dose volume analyses of 100 patients
| Characteristic | |
|---|---|
| Marginal dose D95 (Gy) | 21.0 (17.0–25.0) |
| Fraction number 3/5 | 83/17 |
| Prescribed isodose (%) | 81.6 (61.0–91.4) |
| Conformity indexa | 1.4 (1.1–2.1) |
| Homogeneity indexa | 1.2 (1.1–1.6) |
| Optic nerve maximum dose (Gy) | 19.9 (1.4–25.0) |
| Optic chiasm maximum dose (Gy) | 20.3 (2.6–25.0) |
| Pituitary stalk maximum dose (Gy) | 12.5 (2.5–25.0) |
| Brain stem maximum dose (Gy) | 14.0 (3.0–25.0) |
| Collimator (mm) | |
| 7.5/10.0/12.5/15.0/20.0/25.0 | 3/13/18/17/15/4 |
Abbreviation: D95, dose delivered to 95% of the planning target volume.
*Data are presented as absolute numbers or the median (range).
aConformity index, prescribed isodose volume/target volume; Homogeneity index, maximum dose within the target volume/prescribed dose, according to the Radiation Therapy Oncology Group.23 Radiation doses were prescribed at the margin (95% volume border of the planning target volume).
Survival and Local Control
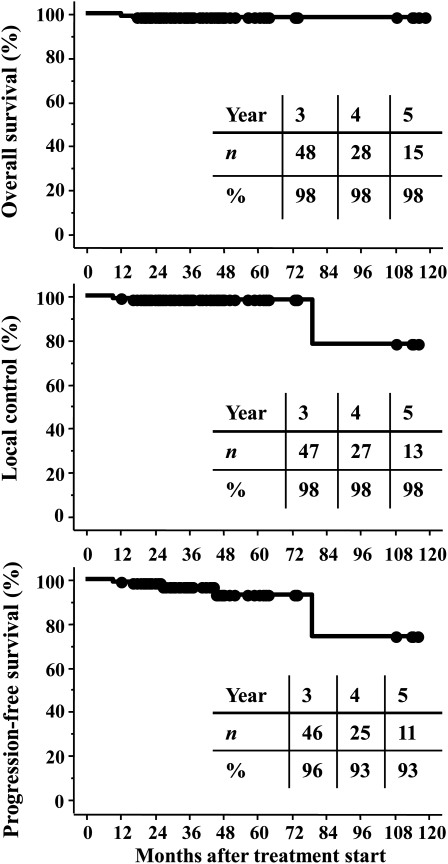
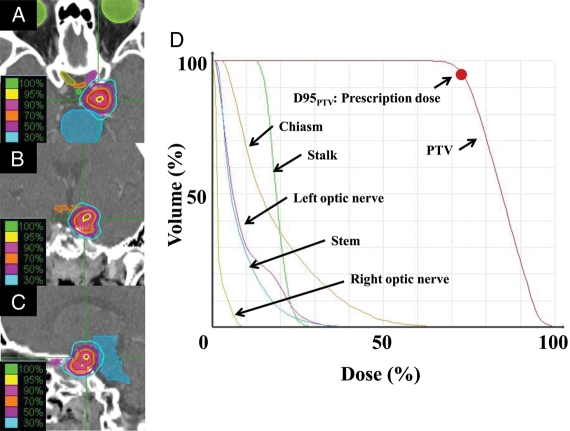
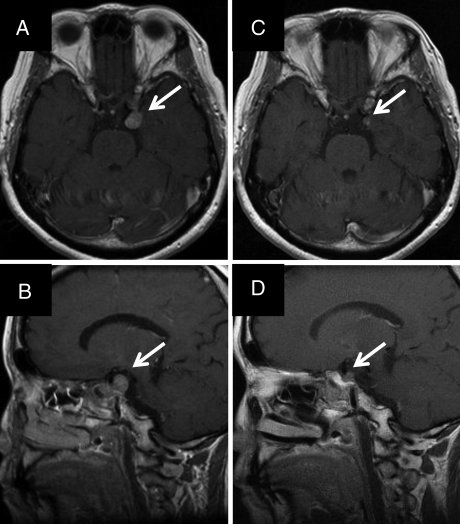
All patients were observed for a minimum of 1.5 years or until death. The median duration of follow-up was 33 months for all patients (range, 18–118.5 mo for living patients; 12–118.5 mo for all patients). One patient showed a complete response, 29 patients showed a partial response, 65 showed stable disease, and 5 showed progressive disease at the latest follow-up. The 3-year overall survival rate was 98% (95% confidence interval [CI]: 95–100%). The 3-year local control and progression-free survival rates were 98% (CI: 95–100%) and 96% (CI: 92–100%), respectively (Fig. 1). In-field tumor regrowth was observed in 3 patients at 10, 16, and 80 months, respectively. Out-field tumor regrowth was observed in 2 patients at 27 and 45 months, respectively; both developed at surgical margins far from the PTV, so they were not included in local recurrence. Figure 2 shows an example of a treatment planning and dose volume histogram for the PTV and OARs including the chiasm, optic nerves, pituitary stalk, and brain stem. The tumor volume in this patient was 1.6 cc, and she was treated with 21 Gy in 3 fractions. She had been treated with transsphenoidal surgery 3 times. However, recurrent tumors had invaded the left cavernous sinus and could not be totally removed, so SRT was administered. She had grade 2 visual disorder, visual field constriction, and paralysis of the left oculomotor nerve before surgery. After surgery, the visual disorder and visual field constriction improved. Although she suffered paralysis of the left oculomotor nerve before SRT, she obtained a partial response, and the local tumor was controlled without late complications at 22 months after treatment (Fig. 3).
Fig. 1.
Overall survival, local control, and progression-free survival curves.
Fig. 2.
Example of hypofractionated stereotactic radiotherapy planning using CyberKnife for a patient with nonfunctioning pituitary adenoma. (A) Isodose distribution in the transverse plane. (B) Isodose distribution in the coronal plane. (C) Isodose distribution in the sagittal plane. (D) Dose volume histogram used for planning.
Fig. 3.
(A) Gadolinium-enhanced transverse MRI before stereotactic radiotherapy. (B) Gadolinium-enhanced sagittal MRI before stereotactic radiotherapy. (C) Gadolinium-enhanced transverse MRI at 22 months after stereotactic radiotherapy. (D) Gadolinium-enhanced sagittal MRI at 22 months after stereotactic radiotherapy.
Complications
Table 3 summarizes the adverse events observed in this study. A post-SRT grade 2 visual disorder was observed in 1 patient at 36 months. In this patient, the maximum doses delivered to the optic nerve and chiasm were 20.8 and 20.7 Gy, respectively, in 3 fractions. However, the possibility of glaucoma unrelated to SRT could not be ruled out in this patient. Post-SRT hypopituitarism was observed in 3 patients who received no hormone replacement therapy after surgery, and the maximum doses delivered to the pituitary in these patients were 20.4, 20.5, and 20.9 Gy in 3 fractions. No radiation-induced brain necrosis or paralysis of the oculomotor or abducens nerve was observed. Transient cyst enlargement occurred in 3 patients at 3, 6, and 9 months, who then developed transient slight visual field disturbance. However, their symptoms improved as their cysts diminished at 9, 9, and 12 months.
Table 3.
Complications related to stereotactic radiotherapy
| Adverse event | Number of patients |
|
|---|---|---|
| Evaluable | With complications | |
| Grade 2 visual disorders | 58 | 1 |
| Hypopituitarism | 74 | 3 |
| Brain necrosis | 100 | 0 |
| Oculomotor nerve paralysis | 100 | 0 |
| Abducens nerve paralysis | 100 | 0 |
| Transient cyst enlargement | 100 | 3 |
Toxicities were evaluated according to the Common Terminology Criteria for Adverse Events version 4.0.
Discussion
Table 4 shows representative RT results for nonfunctioning PA.9–11,17–19 A variety of RT techniques, beams, machines, and dose specifications were used at each institution. Representative reports of Gamma Knife SRS indicate 5-year local control rates of 92–97% with a median follow-up period of 5 years or longer.9–11 Although the median follow-up period was shorter (about 3 years), local control rates were 98% at 3 to 5 years in the present study. The incidences of visual disorders and hypopituitarism were also comparable. In the present study, about 15% of the patients had large tumors (>15 cc) that would not normally be treated with Gamma Knife SRS or tumors that were adjacent to the optical pathways. Despite the presence of such difficult cases, our results for hypofractionated SRT compare favorably with those reported previously. This favorable outcome may have been caused by our use of hypofractionation. In Gamma Knife SRS, the dose delivered to the optic pathways is usually limited to 8–10 Gy, whereas we were able to safely administer 21 Gy in 3 fractions or 25 Gy in 5 fractions in most cases. Although linear quadratic formalism is not applicable to these fractionation schedules,24,25 21 Gy in 3 fractions corresponds to 13.1 Gy in 1 fraction, assuming an α/β ratio of 3 Gy, and 25 Gy in 5 fractions corresponds to 12.7 Gy. The actual efficacy of hypofractionation is considered to be about 15% higher.24 Therefore, it could be said that 21 Gy in 3 fractions corresponds to 15.1 Gy in 1 fraction, and 25 Gy in 5 fractions corresponds to 14.6 Gy. In vivo, a higher effect of fractionation is expected, especially when reoxygenation of hypoxic cells takes place,26,27 but this is probably not the case for benign tumors such as PA. At any rate, the biological effects of our fractionation schedules on tumors appear to be stronger than those of the 8–10 Gy dose used in Gamma Knife treatment, which may account for the favorable tumor control rate observed in our study.
Table 4.
Representative reported results of SRS, FSRT, FPT, and HSRT for nonfunctioning pituitary adenoma
| Author, year | Therapy, device | Patient number | Marginal or total dose (mean, range) | Treatment outcome (local control rate, visual disorders, hypopituitarism) | Median follow-up (months) |
|---|---|---|---|---|---|
| Kobayashi 20099 | SRS, GK | 60 | 14.1 Gy/1 Fr (none) | 97% (≥3y), 4%, 8% | 63 |
| Mingione 200610 | SRS, GK | 90 | 18.5 Gy/1 Fr (5.0–25.0 Gy/1Fr) | 92% (4y), 0%, 25% | 45 |
| Pollock 200811 | SRS, GK | 62 | 16.0 Gy/1 Fr (11.0–20.0 Gy/1Fr) | 95 % (3y, 7y), 0%, 32% | 64 |
| Snead 200817 | FSRT, Linac | 59 | 45.0 Gy/25 Fr (43.0–50.4 Gy/25 Fr) | 98% (10y), 1%, 35% (including functioning PA) | 80 |
| Minniti 200618 | FSRT, Linac | 67 | 45.0 Gy/25 Fr (45.0–50.0 Gy/25–30 Fr) | 98% (3y), 1%, 22% (including functioning PA) | 32 |
| Ronson 200619 | FPT, Proton | 24 | 50.4 CGE/28Fr or 54.0 CGE/30Fr (50.4–55.9 CGE/28–30 Fr) | 100% (4y), 23%, 28% (including functioning PA) | 47 |
| This study 2010 | HSRT, CK | 100 | 21.0 Gy/3Fr or 25.0 Gy/5 Fr (17.0–25.0 Gy/3–5 Fr) | 98 % (3y), 1%, 2% | 33 |
Abbreviations: SRS, stereotactic radiosurgery; FSRT, fractionated stereotactic radiotherapy; FPT, fractionated proton therapy; HSRT, hypofractionated stereotactic radiotherapy; GK, Gamma Knife; Fr, fractions; y, years; PA, pituitary adenoma; CK, CyberKnife; CGE, cobalt-gray equivalent.
Fractionated radiation is also known to reduce the incidence of complications involving late-responding normal tissues. The effect of fractionation is especially high when the α/β ratio is low. Therefore, if the α/β ratio of the optic nerve is lower than that of PA, our fractionation schedules are expected to be more effective than the single fraction used in SRS. Unfortunately, no data are available about the α/β ratio of PA, although the α/β ratio for the optic nerve is considerably low (<3 Gy). Mayo et al.28 proposed a model for estimating the maximum tolerable dose for visual function based on several reported datasets. However, they also reported a lack of good outcomes for hypofractionated SRT. On the other hand, Adler et al.29 reported promising preliminary outcomes with regard to visual field preservation for hypofractionated SRT. Considering that only one visual complication was observed in the present study and that this may not necessarily have been caused by radiation, our criteria for the maximum doses delivered to the optic pathways of 21 Gy in 3 fractions and 25 Gy in 5 fractions are considered to be appropriate. Paradoxically, this fact suggests that the α/β ratio of the optic pathway is considerably low (probably lower than 3 Gy). We are still seeking appropriate fractionation schedules. If the α/β ratio of the optic nerve is lower than that of PA, the use of a higher fraction number may be more efficient. Although the CyberKnife system used in the present study was not suitable for many-fraction treatment, use of 6 or more fractions has now become possible with the currently available updated Multiplan planning system. We will plan to evaluate 8- to10-fraction treatment in the near future.
During planning, we did not add a setup margin. The PTV was defined as the CTV. The margins around the GTV should be defined with caution due to the proximity of OARs such as the optic pathways and brain stem. It is especially important to minimize the margin for recurrent and postresection residual cases because the OARs may be more vulnerable to irradiation. Interestingly, postradiation hypopituitarism developed less often in this study than in previous studies.9–11,17–19 The reasons for this are unclear; however, in addition to the use of hypofractionation, possible explanations include differences in GTV contouring, margin setting, apparatus, and dose specifications. The CyberKnife system maintains very high dose conformity and homogeneity within the targeted volume, so the nontumorous parts of the pituitary received much lower doses than the PTV in many cases. Such highly sophisticated irradiation might have contributed to the low incidence of hypopituitarism, but careful follow-up evaluation for hypopituitarism as well as for visual disorders and brain necrosis remains critical. In recent years, several reports have indicated the effectiveness of 3-T head MRI, particularly in the sellar region.30 Using 3-T MRI, high-resolution anatomical and physiological images of the optic pathways can be obtained.31 In addition, proton magnetic resonance spectroscopy can provide valuable information on biodistribution of metabolites such as N-acetylaspartate and choline-containing compounds. So, if the accuracy improves, it may become possible to distinguish between tumor and scar tissue.32 Furthermore, diffusion tensor tractography can depict pathways of nerve fibers and can be fused with treatment planning images taken previously.33 In the case of nonfunctioning PA adjacent to the optical pathways, better planning is required, so SRT using these modalities should be a topic of future investigation.
In conclusion, CyberKnife SRT involving 21 Gy in 3 fractions or 25 Gy in 5 fractions is safe and effective for the treatment of nonfunctioning PA. To protect the visual nerve and neuroendocrine function, hypofractionated SRT seems preferable, especially for tumors located near the optic pathways and for large tumors. Further investigation of hypofractionated SRT for nonfunctioning PA is warranted to confirm our findings. Longer follow-up is necessary to prove the superiority over Gamma Knife treatment. Cases undergoing hypofractionated SRT have not been reported often, and we expect that our outcomes will contribute to establishing a standard treatment for nonfunctioning PA.
Funding
None.
Acknowledgments
The authors are grateful to Mr Kosaku Inada, Mr Manabu Senda, Mr Kohei Okawa, and Ms Kumiko Ogawa for their valuable help in this research.
Conflict of interest statement. None declared.
References
- 1.Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613–619. doi: 10.1002/cncr.20412. doi:10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol. 2006;8:27–37. doi: 10.1215/S1522851705000323. doi:10.1215/S1522851705000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Losa M, Mortini P, Barzaghi R, et al. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg. 2008;108:525–532. doi: 10.3171/JNS/2008/108/3/0525. doi:10.3171/JNS/2008/108/3/0525. [DOI] [PubMed] [Google Scholar]
- 4.Brochier S, Galland F, Kujas M, et al. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: a study of 142 patients. Eur J Endocrinol. 2010;163:193–200. doi: 10.1530/EJE-10-0255. doi:10.1530/EJE-10-0255. [DOI] [PubMed] [Google Scholar]
- 5.Park P, Chandler WF, Barkan AL, et al. The role of radiation therapy after surgical resection of nonfunctional pituitary macroadenomas. Neurosurgery. 2004;55:100–106. doi: 10.1227/01.neu.0000126885.71242.d7. doi:10.1227/01.NEU.0000126885.71242.D7. [DOI] [PubMed] [Google Scholar]
- 6.Brada M, Rajan B, Traish D, et al. The long-term efficacy of conservative surgery and radiotherapy in the control of pituitary adenomas. Clin Endocrinol. 1993;38:571–578. doi: 10.1111/j.1365-2265.1993.tb02137.x. doi:10.1111/j.1365-2265.1993.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki R, Murakami M, Okamoto Y, et al. The efficacy of conventional radiation therapy in the management of pituitary adenoma. Int J Radiat Oncol Biol Phys. 2000;47:1337–1345. doi: 10.1016/s0360-3016(00)00503-4. doi:10.1016/S0360-3016(00)00503-4. [DOI] [PubMed] [Google Scholar]
- 8.Erridge SC, Conkey DS, Stockton D, et al. Radiotherapy for pituitary adenomas: long-term efficacy and toxicity. Radiother Oncol. 2009;93:597–601. doi: 10.1016/j.radonc.2009.09.011. doi:10.1016/j.radonc.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T. Long-term results of stereotactic gamma knife radiosurgery for pituitary adenomas. Specific strategies for different types of adenoma. Prog Neurol Surg. 2009;22:77–95. doi: 10.1159/000163384. doi:10.1159/000163384. [DOI] [PubMed] [Google Scholar]
- 10.Mingione V, Yen CP, Vance ML, et al. Gamma surgery in the treatment of nonsecretory pituitary macroadenoma. J Neurosurg. 2006;104:876–883. doi: 10.3171/jns.2006.104.6.876. doi:10.3171/jns.2006.104.6.876. [DOI] [PubMed] [Google Scholar]
- 11.Pollock BE, Cochran J, Natt N, et al. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int J Radiat Oncol Biol Phys. 2008;70:1325–1329. doi: 10.1016/j.ijrobp.2007.08.018. doi:10.1016/j.ijrobp.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Tishler RB, Loeffler JS, Lunsford LD, et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215–221. doi: 10.1016/0360-3016(93)90230-s. [DOI] [PubMed] [Google Scholar]
- 13.Leber KA, Berglöff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43–50. doi: 10.3171/jns.1998.88.1.0043. doi:10.3171/jns.1998.88.1.0043. [DOI] [PubMed] [Google Scholar]
- 14.Stafford SL, Pollock BE, Leavitt JA, et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177–1181. doi: 10.1016/s0360-3016(02)04380-8. doi:10.1016/S0360-3016(02)04380-8. [DOI] [PubMed] [Google Scholar]
- 15.Shigematsu N, Kunieda E, Kawaguchi O, et al. Indications of stereotactic irradiation for brain lesions. Acta Oncol. 2000;39:597–603. doi: 10.1080/028418600750013276. doi:10.1080/028418600750013276. [DOI] [PubMed] [Google Scholar]
- 16.Hoban PW, Jones LC, Clark BG. Modeling late effects in hypofrationated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43:199–210. doi: 10.1016/s0360-3016(98)00369-1. doi:10.1016/S0360-3016(98)00369-1. [DOI] [PubMed] [Google Scholar]
- 17.Snead FE, Amdur RJ, Morris CG, et al. Long-term outcomes of radiotherapy for pituitary adenomas. Int J Radiat Oncol Biol Phys. 2008;71:994–998. doi: 10.1016/j.ijrobp.2007.11.057. doi:10.1016/j.ijrobp.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 18.Minniti G, Traish D, Ashley S, et al. Fractionated stereotactic conformal radiotherapy for secreting and nonsecreting pituitary adenomas. Clin Endocrinol. 2006;64:542–548. doi: 10.1111/j.1365-2265.2006.02506.x. doi:10.1111/j.1365-2265.2006.02506.x. [DOI] [PubMed] [Google Scholar]
- 19.Ronson BB, Schulte RW, Han KP, Loredo LN, Slater JM, Slater JD. Fractionated proton beam irradiation of pituitary adenomas. Int J Radiat Oncol Biol Phys. 2006;64:425–434. doi: 10.1016/j.ijrobp.2005.07.978. doi:10.1016/j.ijrobp.2005.07.978. [DOI] [PubMed] [Google Scholar]
- 20.Inoue H, Omori R, Sato K, et al. The accuracy of the CyberKnife. J Jpn Soc Ther Radiol Oncol. 2003;15:177–185. [Google Scholar]
- 21.Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP. An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery. 2003;52:140–146. doi: 10.1097/00006123-200301000-00018. discussion 146–147 doi:10.1097/00006123-200301000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Antypas C, Pantelis E. Performance evaluation of a CyberKnife G4 image-guided robotic stereotactic radiosurgery system. Phys Med Biol. 2008;53:4697–4718. doi: 10.1088/0031-9155/53/17/016. doi:10.1088/0031-9155/53/17/016. [DOI] [PubMed] [Google Scholar]
- 23.Shaw E, Kline R, Gillin M, et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27:1231–1239. doi: 10.1016/0360-3016(93)90548-a. doi:10.1016/0360-3016(93)90548-A. [DOI] [PubMed] [Google Scholar]
- 24.Iwata H, Shibamoto Y, Murata R, et al. Estimation of errors associated with use of linear-quadratic formalism for evaluation of biologic equivalence between single and hypofractionated radiation doses: an in vitro study. Int J Radiat Oncol Biol Phys. 2009;75:482–488. doi: 10.1016/j.ijrobp.2008.12.093. doi:10.1016/j.ijrobp.2008.12.093. [DOI] [PubMed] [Google Scholar]
- 25.Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. doi:10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 26.Shibamoto Y, Kitakabu Y, Murata R, et al. Reoxygenation in the SCCVII tumor after KU-2285 sensitization plus single or fractionated irradiation. Int J Radiat Oncol Biol Phys. 1994;29:583–586. doi: 10.1016/0360-3016(94)90461-8. doi:10.1016/0360-3016(94)90461-8. [DOI] [PubMed] [Google Scholar]
- 27.Tomita N, Shibamoto Y, Ito M, et al. Biological effect of intermittent radiation exposure in vivo: recovery from sublethal damage versus reoxygenation. Radiother Oncol. 2008;86:369–374. doi: 10.1016/j.radonc.2007.08.007. doi:10.1016/j.radonc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(suppl):28–35. doi: 10.1016/j.ijrobp.2009.07.1753. [DOI] [PubMed] [Google Scholar]
- 29.Adler JR, Jr, Gibbs IC, Puataweepong P, et al. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2008;62(suppl):733–743. doi: 10.1227/01.neu.0000316277.14748.63. [DOI] [PubMed] [Google Scholar]
- 30.Pinker K, Ba-Ssalamah A, Wolfsberger S, Mlynarik V, Knosp E, Trattnig S. The value of high-field MRI (3T) in the assessment of sellar lesions. Eur J Radiol. 2005;54:327–334. doi: 10.1016/j.ejrad.2004.08.006. doi:10.1016/j.ejrad.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Satogami N, Miki Y, Koyama T, Kataoka M, Togashi K. Normal pituitary stalk: high-resolution MR imaging at 3T. AJNR Am J Neuroradiol. 2010;31:355–359. doi: 10.3174/ajnr.A1836. doi:10.3174/ajnr.A1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chernov MF, Kawamata T, Amano K, et al. Possible role of single-voxel (1)H-MRS in differential diagnosis of suprasellar tumors. J Neurooncol. 2009;91:191–198. doi: 10.1007/s11060-008-9698-y. doi:10.1007/s11060-008-9698-y. [DOI] [PubMed] [Google Scholar]
- 33.Masutani Y, Aoki S, Abe O, Hayashi N, Otomo K. MR diffusion tensor imaging: recent advance and new techniques for diffusion tensor visualization. Eur J Radiol. 2003;46:53–66. doi: 10.1016/s0720-048x(02)00328-5. doi:10.1016/S0720-048X(02)00328-5. [DOI] [PubMed] [Google Scholar]