Abstract
Diabetes is characterized by several poorly understood phenomena including dysfunctional wound healing and impaired vasculogenesis. P53, a master cell cycle regulator, is upregulated in diabetic wounds and has recently been shown to play regulatory roles in vasculogenic pathways. We have previously described a novel method to topically silence target genes in a wound bed with siRNA. We hypothesized that silencing p53 results in improved diabetic wound healing and augmentation of vasculogenic mediators. Paired 4-mm stented wounds were created on diabetic db/db mice. Topically applied p53 siRNA, evenly distributed in an agarose matrix, was applied to wounds at post-wound day 1 and 7 (matrix alone and nonsense siRNA served as controls). Animals were sacrificed at post-wound days 10 and 24. Wound time to closure was photometrically assessed, and wounds were harvested for histology, immunohistochemistry, and immunofluorescence. Vasculogenic cytokine expression was evaluated via western blot, RT-PCR, and ELISA. ANOVA/t-test was used to determine significance (p<=0.05). Local p53 silencing resulted in faster wound healing with wound closure at 18 ± 1.3d in the treated group versus 28 ± 1.0d in controls. The treated group demonstrated improved wound architecture at each time point while demonstrating near complete local p53 knockdown. Moreover, treated wounds showed a 1.92 fold increase in CD31 endothelial cell staining over controls. Western blot analysis confirmed near complete p53 knockdown in treated wounds. At day 10, VEGF secretion (ELISA) was significantly increased in treated wounds (109.3 ± 13.9 pg/ml) versus controls (33.0 ± 3.8 pg/ml) while RT-PCR demonstrated a 1.86 fold increase in SDF-1 expression in treated wounds versus controls. This profile was reversed after treated wounds healed and prior to closure of controls (day 24). Augmented vasculogenic cytokine profile and endothelial cell markers are associated with improved diabetic wound healing in topical gene therapy with p53 siRNA.
Keywords: siRNA, wound, diabetes, p53
Introduction
The pathogenesis of impaired wound healing in diabetes is multi-factorial and incompletely elucidated. Previous investigations have implicated decreased angiogenic growth factors,(1; 2) a prolonged inflammatory state,(3; 4) impaired vasculogenesis,(5-7) and altered apoptosis.(8) Many of these impairments center around the effects of hyperglycemia on the hypoxic response via hypoxia inducible factor-1 (HIF-1) and p53. HIF-1α is the master regulator of the hypoxic response, inducing downstream angiogenic growth factors.(9) Regarded as a cell cycle guardian, the tumor suppressor p53 is a transcriptional activator of apoptotic activity, controlling the fate of damaged cells by detecting and arresting the cell cycle before damage to DNA is converted to inherited mutation.(10) Diabetic wounds are characterized by increased apoptosis and recent data have identified a correlation between elevated levels of p53 in diabetic wounds and an increase in apoptosis.(11) Additonally, hyperglycemia has been shown to increase cellular apoptosis through upregulation of p53-mediated mechanisms.(12; 13) Moreover, evidence has shown an intricate relationship between hypoxia, levels of p53, and levels of HIF-1. Specifically, in conditions of severe hypoxia that approach anoxia, accumulating p53 competes with HIF-1α for binding to p300, a transcriptional coactivator, resulting in repression of HIF-1 transcriptional responses towards gene activation and ultimately, HIF-1α degradation.(14) Evidence also supports a direct interaction between HIF-1α and p53, whereby HIF-1α is degraded by accumulation of p53.(15)
Impaired wound healing in diabetes is characterized by impaired vascular response to ischemia.(16) Endothelial progenitor cells (EPCs) have been identified as circulating effectors of adult neovasculogenesis that reside in bone marrow, and are subsequently mobilized to peripheral blood upon stimuli, including tissue ischemia and local release of cytokines and growth factors.(17; 18) There is a reduction in the number of circulating EPCs in diabetes, which has been implicated in peripheral vascular complications.(19) Ultimately, progenitor cell trafficking to sites of ischemia has been shown to be largely HIF-1α mediated.(20)
We hypothesize that elevated levels of p53 in diabetic wounds leads to impaired healing, which may be mediated through the interaction between p53 and HIF-1α resulting in blunted neovascularization. Moreover, we hypothesize that local silencing of p53 results in improved wound healing associated with the restoration of angiogenic cues.
Materials & Methods
Animals
All experiments were performed in accordance with New York University Animal Care and Use Committee guidelines (IACUC Protocol #061104-01). Adult diabetic db/db (BKS.Cg-m+/+Leprdb/J, no. 642) female mice were obtained from Jackson Labs (Bar Harbor, ME) aged 10 to 12 weeks. The db/db mouse is homozygous for a genetic mutation in the leptin receptor (Leprdb) and demonstrates type 2 diabetic pathologic findings, including peripheral insulin resistance, hyperglycemia (serum glucose concentration >350 g/dl), obesity, and impairment in wound healing by 3 to 8 weeks of age. Six animals (n=6) were utilized in each experimental group.
Wound Model
Experiments utilized a stented-wound healing model described previously.(21; 22) Animals were anesthetized, shaved, and prepared according to standard sterile procedure. A 4-mm punch biopsy tool was used to create two circular, full thickness cutaneous wounds (which extended through the panniculus carnosus) bilaterally on the shaved dorsal skin of diabetic mice. A donut-shaped silicone splint (Grace Bio-Labs; Bend, OR), with an external diameter of 12 mm and an internal diameter of 8 mm was centered on the wound and affixed using cyanoacrylate adhesive (Elmer's, Inc.; Columbus, OH) and interrupted 6-0 nylon sutures (Ethicon). These silicione stents prevent wound contraction, ensuring healing by secondary intention. Furthermore, wound margins were tattooed using India ink. A semiocclusive dressing (Tegaderm, 3M; St. Paul, MN) was applied to cover the wound and splint. Animals were monitored daily for security of wound stents; threatened stents were reinforced with a replacement suture at the tattooed wound margin. Dressing changes occurred every 7 days and as needed. Wounds were digitally photographed (Canon Powershot A590; Tokyo, Japan) every other day from time of wounding; subsequently, obtained digital photographs were analyzed photometrically for granulation and closure using Adobe Photoshop photometric software (Adobe Systems Incorporated; San Jose, CA).
RNA Interference
Small interfering (si) RNA for p53 (Qiagen, Inc.; Valencia, CA) was transfected into animal wounds using a previously described agarose delivery system.(23) Briefly, siRNA against p53 was complexed with liposomal transfection reagent and incorporated into a cooling (<37°C) 0.4% (w/v) liquid agarose mixture. The dose of siRNA and ratio of liposomal transfection reagent was established in preliminary studies. The optimal formulation was determined to be the least concentration of siRNA necessary for efficacy and most favorable carrier matrix handling properties. Ultimately, 20 pmol siRNA was complexed with 0.5 μl Lipofectamine 2000 (Invitrogen; Carlsbad, CA). The agarose gel containing siRNA was applied at post-wounding day 1, and post-wounding day 8, based on previous delivery studies.(23) A no treatment arm using these transfection doses in the same model have been published, and show no difference between the sham (saline) treated and no treatment group.(23)
Immunoblots
Wounds were harvested and homogenized using a Polytron tissue homogenizer (Kinematica, Bohemia, NY). Tissue homogenate was placed in NE-PER (Pierce; Rockford, IL) for cell lysis, and extracted protein was quantified using the BCA Protein Assay (Pierce). 50 μg of protein (from tissue) or 20 μg (from cell culture) was loaded onto a 7.5% SDS PAGE gel (Biorad; Hercules, CA) and transferred to a PVDF membrane (Biorad). The membrane was blocked for 1 hour with Casein in TBS (Pierce), and incubated in 1:250 monoclonal mouse anti-HIF-1α (BD Transduction Laboratories; Lexington KY) or 1:1000 monoclonal mouse serine 15 phosphorylated p53 (Cell Signaling; Danvers, MA) for 12 hours. 1:5000 anti-Lamin A/C (BD Transduction Laboratories) and 1:2500 anti-β-Actin (Novus; Littleton, CO) were used as nuclear and cytoplasmic loading controls respectively. All secondary antibodies used were HRP conjugates (Cell Signaling). Protein expression was detected on Hyperfilm (Amersham Biosciences; Sunnyvale, CA) with ECL plus reagent (Amersham Biosciences). Bands were analyzed quantitatively densitometrically (Kodak 1D; Rochester, NY). Western blots were done in triplicate at minimum.
RNA Extraction and Analysis
Total mRNA was isolated and purified from tissue homogenate using an RNeasy RNA tissue extraction kit (Qiagen; Valencia, CA). Quality and quantity were assessed using an Agilent 2100 Bioanalyzer (Agilent; Santa Clara, CA), and 5 μg of total RNA was reversed transcribed using a QuantiTect Reverse Transcription kit (Qiagen) according to the manufacturer's instructions. Real-time RT-PCR was performed with 1 ng of reverse-transcribed cDNA per sample using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems; Foster City, CA). SYBR green was used to detect PCR products (SYBR Green PCR Master Mix, Applied Biosystems). All reactions were performed in triplicate. Custom designed primers for p53 (5′-TGTAATAGCTCCTGCATGGG-3′ 3′-TTCTGTACGGCGGTCTCTCCC-5′), SDF-1 (5′-TCAGCCTGAGCTACAGATGC-3′ 3′–AGGGCACAGTTTGGAGTGTT-5′), VEGF (5′-TCTTCAAGCCATCCTGTGTG-3′ 3′- CTGCATGGTGATGTTGGACT-5′), HIF-1 (5′-TGATGACCAGCAACTTGAGG-3′ 3′- TGGGGCATGGTAAAAGAAAG-5′) and 18S (5′-CGGCTACCAGATCCAAGGAA-3′ 3′-GCTGGAATTACCGCGGCTG-5′) were synthesized. Standard curves were generated using 18S as the internal control, with relative amounts of mRNA normalized to 18S mRNA. Values are expressed as fold increases relative to the reference sample (untreated control).
ELISA
Tissue homogenate from wounds were centrifuged for 5 minutes at 5000×g. Samples were then plated and assayed using a commercially available mouse VEGF ELISA Immunoassay kit (RND Systems; Minneapolis, MN) per the manufacturer's instructions. Optical density of samples was read at 450 nm with a wavelength correction of 540 nm. VEGF quantity was determined by plotting against a concurrently run standard curve.
Immunohistochemistry
Serial 5-mm paraffin sections were analyzed by immunofluorescent histochemistry against CD31 using a rat anti-mouse CD31 primary antibody (MEC 13.3; BD Biosciences; San Diego, CA; 1:50 dilution) and rabbit anti-rat IgG biotinylated secondary antibody (Vector Labs; Burlingame, CA; 1:2000 dilution). Addition of horseradish peroxidase-streptatividin (Vector Labs; 1:500 dilution) and 3,3′-diaminobenzidine tetrahydrochloride completed the detection, and hematoxylin provided counterstaining. Skin sections on slides were digitized to an image with dimensions of 1712 × 1368 pixels at 200× magnification. Nikon NIS Elements software (Nikon; Melville, NY) was used to segment and quantify the number and average cross-sectional area of CD31-positive vessels across five nonconsecutive tissue sections for each wound. Furthermore, tissue architecture was evaluated using hematoxylin and eosin staining of paraffin sections.
Statistical Analyses
All mean values are expressed as ± standard error of mean (SEM). One-way ANOVA tests with post-hoc Tukey-Kramer analyses or Students t-tests were utilized to determine statistical significance. A p-value < 0.05 was considered statistically significant.
Results
Silencing p53 produces consistent gene knockdown
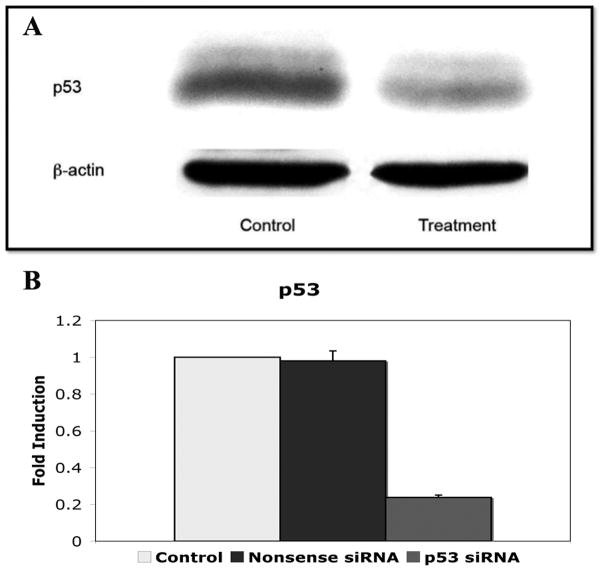
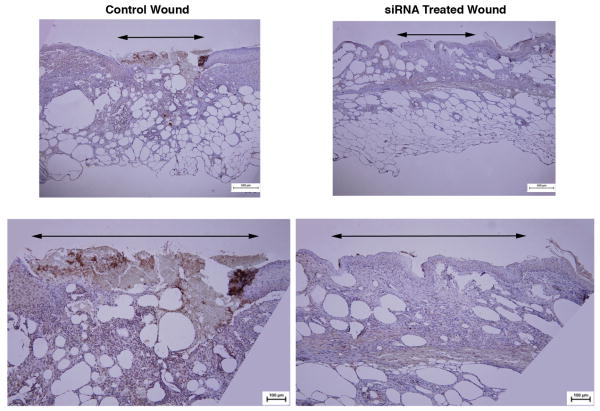
Murine wounds transfected with siRNA against p53 and harvested at day 10 post-wounding showed marked knockdown of p53 protein expression as measured by western blot (Figure 1a). In addition, p53 mRNA levels of wound homogenates demonstrated a 76.1% reduction in p53 siRNA treated wounds compared to 1.9% decrease in p53 levels in nonsense siRNA treated wounds when compared to no treatment controls (0.24 ± 0.01 vs. 0.98 ± 0.05) (Figure 1b). Homogenate of spleen and liver from the same animals revealed no significant difference in expression from control animals (data not shown). Immunohistochemical analysis of p53 expression in wounds showed near complete knockdown in treatment areas at day 10 post-wounding (Figure 2).
Figure 1.
a) Western blot of wound tissue homogenate displaying marked knockdown of p53 after topical application of agarose matrix containing siRNA against p53. b) p53 mRNA expression in p53 siRNA treated wounds compared to nonsense siRNA treated wound and nontreated control wounds.
Figure 2.
Immunohistochemical staining for p53 demonstrates reduced expression of p53 within p53-silenced wound beds compared to nonsense-treated wounds at post-wounding day 10.
Silencing of p53 Increases Gene Expression of HIF-1 and HIF-1 Dependent Cytokines
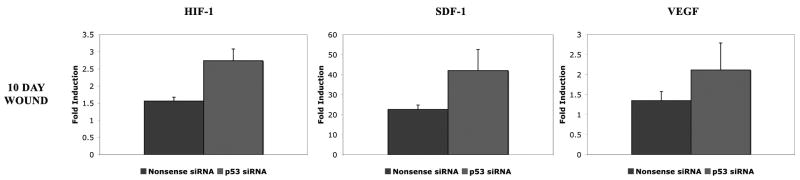
In order to determine the effects of p53 silencing on vasculogenic cytokine expression during diabetic wound healing, stented wounds in db mice were harvested for RNA extraction following application of p53 siRNA or nonsense siRNA with relative expression compared to non-wounded, non-treated skin. At 10 days post-wounding, there was a 1.76 fold increase in HIF-1 mRNA expression (2.74 ± 0.11 vs. 1.56 ± 0.34) in p53 siRNA treated wounds compared to nonsense siRNA treated wounds (Figure 3). Furthermore, there was a 1.86 fold increase in SDF mRNA expression in p53-silenced wounds compared to nonsense treated (42.08 ± 10.56 vs. 22.66 ± 2.20) (Figure 3). VEGF mRNA analysis revealed a 1.57 fold increase in p53 silenced wounds compared to nonsense siRNA treated wounds (2.12 ± 0.68 vs. 1.35 ± 0.23) (Figure 3).
Figure 3.
Vasculogenic cytokine (HIF-1, SDF, and VEGF) mRNA expression in p53 siRNA treated wounds compared to nonsense siRNA treated wounds at post-wounding day 10. All cytokines demonstrate an upregulation of expression in p53-silenced wounds compared to nonsense siRNA treated wounds.
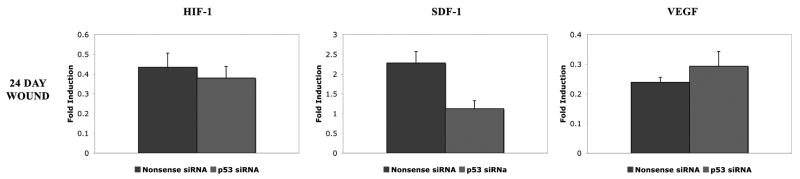
At day 24 post-wounding after a total of two applications (the last at post-wounding day eight), and six days after wound closure in the treated groups, angiogenic chemokine profiles demonstrated a reversal from that seen at earlier time points. HIF-1 mRNA levels (0.38 ± 0.06 vs. 0.43 ± 0.07 fold-induction) in p53-silenced wounds were similar to nonsense siRNA treated controls (Figure 4). Meanwhile, SDF mRNA was relatively decreased in p53-silenced wounds compared to nonsense treated wounds (1.12 ± 0.20 vs. 2.28 ± 0.29 fold, respectively; p<0.05) (Figure 4). VEGF mRNA levels in p53-silenced wounds also were similar to nonsense wounds (0.29 ± 0.05 vs. 0.24 ± 0.02 fold) (Figure 4).
Figure 4.
Vasculogenic cytokine (HIF-1, SDF, and VEGF) mRNA expression in p53 siRNA treated wounds compared to nonsense siRNA treated wounds at post-wounding day 24. Wounds no longer demonstrate the increase in vasculogenic cytokines observed in the open wound at the earlier time point.
p53 Silencing Increases Local Angiogenic Cytokine Release
Similarly, these wounds were harvested for protein extraction following application of p53 siRNA or nonsense siRNA. Wounds harvested at post-wounding day 10 demonstrated a greater than three-fold increase in VEGF in p53-silenced wounds (109.3 ± 13.9 pg/ml) compared to nonsense siRNA-treated controls (33.0 ± 3.8 pg/ml), (p<0.05) as determined by ELISA.
Topical p53 Silencing Improves Wound Vascularity
CD31 immunofluorescent endothelial staining was increased in p53-silenced wounds (3.48 ± 0.15% positive cells/hpf) compared to nonsense siRNA-treated wounds (1.81 ± 0.08% positive cells/hpf), demonstrating increased vascularity in p53-silenced wounds (p<0.05) at comparable time points and prior to wound closure (Figure 5).
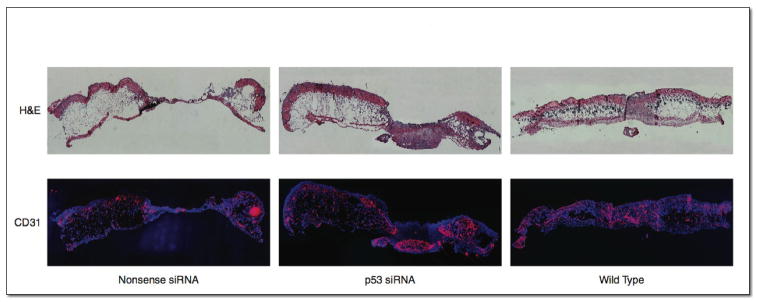
Figure 5.
H&E (top), and CD31 (bottom) immunofluorescent stained histologic sections of dorsal wound beds with nonsense control or p53 siRNA treatment, demonstrating 7.63 fold increase in CD31 staining in p53 siRNA treated wounds compared to controls.
P53 Silencing Improves Diabetic Wound Closure
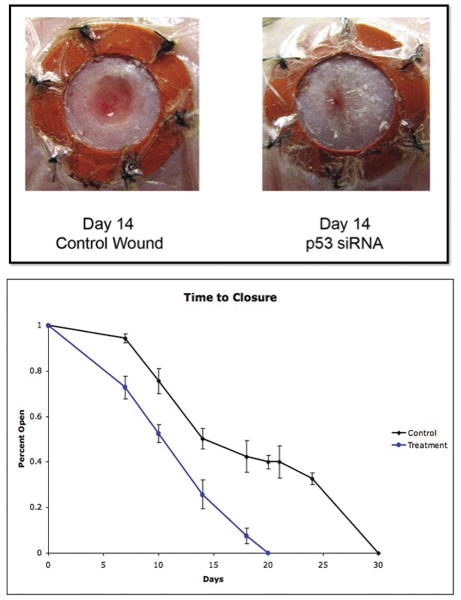
Diabetic wounds treated with p53 siRNA evidenced a much more vigorous wound healing response with earlier development and accumulation of granulation tissue. Moreover, treated groups had a greatly decreased time to closure compared to nonsense siRNA-treated controls. Wounds treated with p53 siRNA developed robust granulation tissue by day 10 and were closed by 18 ± 1.3 days. In contrast, by day 10, nonsense siRNA-treated wounds demonstrated minimal granulation tissue with ultimate wound closure occurring at 28 ± 1 days (p<0.05), nearly two thirds longer than the p53 siRNA-treated wounds (Figure 6). Interestingly, a prior report indicates that stented wounds in nondiabetic C57/BL6 background mice experience wound closure at 12.7 ± 1.0 days which suggests a partial improvement towards the normal phenotype in the p53 siRNA-treated group.(22)
Figure 6.
Photometric image of diabetic stented wounds at day 14 (top) and graph of time to closure (bottom) in p53 siRNA-treated versus nonsense-treated siRNA wounds.
P53 Silencing in Diabetic Wounds is temporary
In healed wounds at 30 and 60 days, p53 levels in p53 siRNA-treated and nonsense siRNA-treated wounds returned to equally low levels (mRNA, immunohistochemistry; data not shown). Furthermore, re-wounded formerly p53 siRNA-treated and nonsense siRNA-treated mice showed similar p53 levels in their healing wounds.
Discussion
Diabetes has long been characterized by increased cellular stress. Hypoxia and hyperglycemic conditions likely bear some responsibility for this phenomenon. Multiple factors, including increased apoptosis, decreased vascular recovery, an aberrant inflammatory response, and delayed cellular turnover in the diabetic wound contribute to impaired wound healing. Here, we have demonstrated that diabetic wound healing is enhanced through local p53 silencing with concomitant improvement of neovascularization. Diabetes and the hyperglycemic state are characterized by an increase in p53 activity both in diabetic fibroblasts and in diabetic wounds, with a resultant increase in apoptosis.(8) The relationship between HIF-1, p53, and apoptosis is complex, and has not been fully elucidated. HIF-1 can either induce or inhibit apoptosis, depending on the cellular context.(24) The severity of hypoxia along with the amount of associated genotoxic injury is suggested to modulate HIF-1 related pathways.(25) Moreover, p53 regulates HIF-1 levels.(14; 15; 26-28) With severe hypoxia, there is accumulation of p53, resulting in repression and eventual destabilization of HIF-1. The mechanisms responsible for this observation are incompletely defined, with proposals that include direct interaction,(29; 30) indirect interaction via Mdm2,(30), and competition between p53 and HIF-1 for the shared transcriptional co-activator p300.(15; 28) HIF-1 levels are decreased in the diabetic state,(2) and accordingly, fibroblasts in diabetic tissue produce diminished amounts of HIF-1 dependent angiogenic growth factors required for ischemic neovascularization, such as VEGF and SDF-1, leading to vascular impairments in ischemic settings.(31-33)
Increasing evidence implicates p53 at a central axis, affecting various avenues, particularly neovascularization, through its activity such that ultimately, impaired wound healing in diabetes is incurred. In response to ischemia, fibroblasts in normal tissue produce HIF-1 dependent angiogenic growth factors required for neovascularization, such as SDF-1 and VEGF.(34) Inadequate production of these cytokines in the diabetic state leads to impaired neovasculogenesis in ischemic settings.(31-33) Here, we demonstrate that targeted, local silencing of p53 within the wound bed effectively reduces p53, with an associated increase in HIF-1 dependent angiogenic growth factors in-vivo. This treatment also results in improved tissue vascularity in the wound bed and ultimately, improved wound healing and reduced time to closure.
Since p53 mutations have been implicated in many tumorogenic scenarios, the delivery system described herein silences p53 expression not only temporarily, but also locally. No systemic p53 knockdown was observed, suggesting a safe topical delivery of siRNA against p53. Previously published findings of this targeted therapy have shown no systemic spread, with unaffected liver, lung, and spleen homogenates.(23) Additionally, there is a return of normal p53 expression after wound closure. Interestingly, the angiogenic chemokines seen upregulated in the open wound at day 10 in the p53-silenced group have become quiescent at post-wounding day 24 as the wounds experienced accelerated closure compared to their nonsense siRNA treated controls. These data suggest, that without the open wound delivering hypoxic cues for angiogenic chemokines to initiate neovascularization from the local milieu (fibroblasts, endothelial cells), there is no longer the same stimulus in the epithelialized wound. There were only two applications of p53 siRNA (post-wounding day 1 and day 8) as the improved closure at day 18 in the p53-silenced wound negated the need for an additional dose.
Impaired diabetic wound healing remains a fundamental problem clinically with enormous implications. Our findings assist in discerning a potential mechanism for impaired diabetic wound healing, and proposes a novel potential therapeutic that improves diabetic wound closure and wound vascularity.
Acknowledgments
This work was in part supported by grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Young Investigator Award: John Paul Tutela, the second author of this manuscript, received a Young Investigator Award at the annual wound healing society meeting in Dallas, TX, April 28, 2009.
Phuong D. Nguyen was also an Young Investigator Award winner for a related project, “Mediators of Increased Apoptosis in Stressed Diabetic Fibroblasts P53 Control of BAX and BCL-2” at the same time. The award was presented in Limoges by John Paul Tutela at the European Tissue Repair Society, Limoges, France, August 26, 2009
Statement of Financial Interest: We hereby certify that, to the best of our knowledge, no financial support or benefits have been received by any co-author, by any member of our immediate family or any individual or entity with whom or with which we have a significant relationship from any commercial source which is related directly or indirectly to the work which is reported in the article.
References
- 1.Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, Pereira T, Yla-Herttuala S, Poellinger L, Brismar K, Catrina SB. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105:19426–19431. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53:3226–3232. doi: 10.2337/diabetes.53.12.3226. [DOI] [PubMed] [Google Scholar]
- 3.Goova MT, Li J, Kislinger T, Qu W, Lu Y, Bucciarelli LG, Nowygrod S, Wolf BM, Caliste X, Yan SF, Stern DM, Schmidt AM. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am J Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan MJ, Ceradini DJ, Gurtner GC. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal. 2005;7:1476–1482. doi: 10.1089/ars.2005.7.1476. [DOI] [PubMed] [Google Scholar]
- 6.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capla JM, Grogan RH, Callaghan MJ, Galiano RD, Tepper OM, Ceradini DJ, Gurtner GC. Diabetes impairs endothelial progenitor cell-mediated blood vessel formation in response to hypoxia. Plast Reconstr Surg. 2007;119:59–70. doi: 10.1097/01.prs.0000244830.16906.3f. [DOI] [PubMed] [Google Scholar]
- 8.Jazayeri L, Callaghan MJ, Grogan RH, Hamou CD, Thanik V, Ingraham CR, Capell BC, Pelo CR, Gurtner GC. Diabetes increases p53-mediated apoptosis following ischemia. Plast Reconstr Surg. 2008;121:1135–1143. doi: 10.1097/01.prs.0000302499.18738.c2. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 10.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 11.Kane CD, Greenhalgh DG. Expression and localization of p53 and bcl-2 in healing wounds in diabetic and nondiabetic mice. Wound Repair Regen. 2000;8:45–58. doi: 10.1046/j.1524-475x.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- 12.Keim AL, Chi MM, Moley KH. Hyperglycemia-induced apoptotic cell death in the mouse blastocyst is dependent on expression of p53. Mol Reprod Dev. 2001;60:214–224. doi: 10.1002/mrd.1080. [DOI] [PubMed] [Google Scholar]
- 13.Ortega-Camarillo C, Guzman-Grenfell AM, Garcia-Macedo R, Rosales-Torres AM, Avalos-Rodriguez A, Duran-Reyes G, Medina-Navarro R, Cruz M, Diaz-Flores M, Kumate J. Hyperglycemia induces apoptosis and p53 mobilization to mitochondria in RINm5F cells. Mol Cell Biochem. 2006;281:163–171. doi: 10.1007/s11010-006-0829-5. [DOI] [PubMed] [Google Scholar]
- 14.Schmid T, Zhou J, Brune B. HIF-1 and p53: communication of transcription factors under hypoxia. J Cell Mol Med. 2004;8:423–431. doi: 10.1111/j.1582-4934.2004.tb00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 16.Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554–560. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 17.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 18.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 19.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 20.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 21.Galiano RD, Michaels J, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- 22.Michaels J, Churgin SS, Blechman KM, Greives MR, Aarabi S, Galiano RD, Gurtner GC. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen. 2007;15:665–670. doi: 10.1111/j.1524-475X.2007.00273.x. [DOI] [PubMed] [Google Scholar]
- 23.Thanik VD, Greives MR, Lerman OZ, Seiser N, Dec W, Chang CC, Warren SM, Levine JP, Saadeh PB. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 2007;14:1305–1308. doi: 10.1038/sj.gt.3302986. [DOI] [PubMed] [Google Scholar]
- 24.Hammond EM, Giaccia AJ. The role of p53 in hypoxia-induced apoptosis. Biochem Biophys Res Commun. 2005;331:718–725. doi: 10.1016/j.bbrc.2005.03.154. [DOI] [PubMed] [Google Scholar]
- 25.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fels DR, Koumenis C. HIF-1alpha and p53: the ODD couple? Trends Biochem Sci. 2005;30:426–429. doi: 10.1016/j.tibs.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Blagosklonny MV, An WG, Romanova LY, Trepel J, Fojo T, Neckers L. p53 inhibits hypoxia-inducible factor-stimulated transcription. J Biol Chem. 1998;273:11995–11998. doi: 10.1074/jbc.273.20.11995. [DOI] [PubMed] [Google Scholar]
- 28.Schmid T, Zhou J, Kohl R, Brune B. p300 relieves p53-evoked transcriptional repression of hypoxia-inducible factor-1 (HIF-1) Biochem J. 2004;380:289–295. doi: 10.1042/BJ20031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansson LO, Friedler A, Freund S, Rudiger S, Fersht AR. Two sequence motifs from HIF-1alpha bind to the DNA-binding site of p53. Proc Natl Acad Sci U S A. 2002;99:10305–10309. doi: 10.1073/pnas.122347199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J Biol Chem. 2003;278:13595–13598. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- 31.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frank S, Hubner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995;270:12607–12613. doi: 10.1074/jbc.270.21.12607. [DOI] [PubMed] [Google Scholar]
- 33.Lerman OZ, Galiano RD, Armour M, Levine JP, Gurtner GC. Cellular dysfunction in the diabetic fibroblast: impairment in migration, vascular endothelial growth factor production, and response to hypoxia. Am J Pathol. 2003;162:303–312. doi: 10.1016/S0002-9440(10)63821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake CJ, LaRue A, Ferrara N, Little CD. VEGF regulates cell behavior during vasculogenesis. Dev Biol. 2000;224:178–188. doi: 10.1006/dbio.2000.9744. [DOI] [PubMed] [Google Scholar]